Abstract
Background and Aims:
Post-operative nausea and vomiting (PONV) is one of the most common complications in patients undergoing gynaecological surgeries under spinal anaesthesia (SA). Palonosetron has the unique property of controlling 'delayed chemotherapy-induced nausea and vomiting' when compared to older serotonin antagonists. This study compared the effectiveness of palonosetron with a combination of ramosetron and dexamethasone in preventing PONV.
Methods:
Sixty patients undergoing gynaecological surgeries under SA were randomly allocated into two groups of thirty each, to receive either a combination of 0.3 mg of ramosetron and 8 mg of dexamethasone intravenously (IV) (Group RD) or 0.075 mg of palonosetron IV (Group P). The incidence of PONV, number of complete responders (no nausea, vomiting or use of rescue anti-emetics) and severity of nausea were evaluated during intra- and post-operative period.
Results:
The incidence of complete responders during intraoperative period was 80.0% in Group RD and 76.7% in Group P (P = 0.074) whereas postoperatively at 0–2 h and 2–6 h, it was 73.3% and 83.3% in Group RD respectively as compared to 46.6% and 56.6% in Group P respectively (P = 0.016 and P = 0.024). The incidence of PONV during 24 h of post-operative period was 30.00% in Group RD as compared to 60.00% in Group P (P = 0.0195). Nausea severity score and use of rescue anti-emetics did not vary between the groups.
Conclusion:
Combination of ramosetron and dexamethasone is more effective than palonosetron alone in preventing PONV in patients undergoing gynaecological surgeries under SA.
Keywords: Palonosetron, post-operative nausea and vomiting, ramosetron and dexamethasone
INTRODUCTION
Post-operative nausea and vomiting (PONV) is one of the most troublesome complications which results in patient dissatisfaction and delays discharge from the surgical facility.[1] The incidence of PONV after regional anaesthesia (RA) is around 19%–22%, whereas after general anaesthesia (GA) it is as high as 76%.[2] Although the incidence is less after RA, it can still result in distress for patients and requires intervention.
Manipulation of bowel and stimulation of cervix and uterus play a role in causing PONV after gynaecological surgeries.[3] Several anti-emetics of different pharmacological classes are being used, either alone or in combination for the prevention of PONV. Five-hydroxytryptamine receptor antagonists (5-HT3) are considered as the first-line drugs because of their effectiveness in preventing PONV with minimal adverse effects.
Palonosetron is a newer 5-HT3 antagonist which has been approved by the United States Food and Drug Administration (US FDA) for prevention of PONV in 2008.[4] It has a high receptor binding affinity and its longer half-life prevents PONV for up to 48 h after surgery. Dexamethasone is known to prevent PONV and is found to be more effective in combination with a 5-HT3 receptor antagonist.[5] Studies have shown ramosetron and dexamethasone combination to be more effective in preventing PONV than ramosetron alone.
There are not many studies where palonosetron has been compared with this combination therapy.
Hence, this randomised double-blind study was designed to compare the effects of palonosetron with a combination of ramosetron and dexamethasone in patients operated for gynaecological surgeries under spinal anaesthesia (SA). The primary outcome was to compare the incidence of PONV, and the secondary outcome was to know the number of complete responders, nausea severity score and adverse effects of the drugs studied.
METHODS
After obtaining Ethical Committee approval and written informed consent from patients, 60 women aged 18–60 years, weighing 40–70 kg, belonging to the American Society of Anesthesiologists (ASA) physical Status I or II posted for gynaecological surgeries under SA were included in the study. Patients having contraindications for SA, surgery lasting for >2 h, known allergy to study drugs, those with gastrointestinal disease, history of motion sickness, pregnant and lactating mothers and those who had taken anti-emetic medications within last 24 h were excluded. All the patients who were included in the study had risk factors for PONV like female gender, non-smokers and being treated with tramadol post-operatively for pain relief. They were randomly divided into two groups of thirty each, Group RD and Group P using computer-generated random list. Group RD received 8 mg of dexamethasone, and 0.3 mg of ramosetron IV (total of 4ml) and Group P received 0.075 mg of Palonosetron IV (diluted to a total of 4 ml with normal saline) before subarachnoid block. In the operating room, 18 g iv cannula was secured on the non-dominant hand, and ringer lactate was infused at a rate of 10 ml/kg. Monitors were attached and baseline oxygen saturation (SPO2), electrocardiogram (ECG), heart rate (HR) and non-invasive blood pressure (NIBP) were recorded. The study drugs were prepared by the anaesthesiologist who was involved with the randomisation of patients and was not involved further with the study. Thus, the observer and the patients were kept blinded to the study drugs. Taking all aseptic precautions lumbar puncture was performed at L2–3 or L3–4 intervertebral space, with a 25-gauge Quincke spinal needle in the left lateral position using midline approach. After free flow of cerebrospinal fluid, 15 mg (3 ml) of hyperbaric bupivacaine 0.5% was administered intrathecally, at the rate of 0.2 ml/s and the patients were turned immediately to supine position. Surgery was allowed to start after achieving block height of T8 dermatome. Intraoperatively, continuous ECG, HR, NIBP and SPO2 were monitored. A decrease of mean arterial pressure >20% from the pre-operative value or <90 mm Hg systolic blood pressure was regarded as hypotension. A decrease in HR <50 beats/min was regarded as bradycardia. Hypotension was treated with IV fluids, and mephentermine 6 mg IV and bradycardia was treated with atropine 0.6 mg IV. All the patients were evaluated for nausea and vomiting intraoperatively every 15 min till the end of surgery and postoperatively at 2, 6, 12 and 24 h after surgery, taking the time the patient was shifted to the post-operative ward as T0. Nausea was defined as subjectively unpleasant sensation associated with the urge to vomit. Retching was defined as the laboured, spastic, rhythmic contraction of the respiratory muscles without the expulsion of gastric contents. Vomiting was defined as the forceful expulsion of gastric contents. The severity of nausea was assessed using verbal rating scale as: 0 = none, 1 = mild, 2 = moderate and 3 = severe. Complete response was defined as the absence of nausea, retching, vomiting and no requirement of rescue anti-emetic. Ondansetron 4 mg iv was given as a rescue anti-emetic when there was moderate to severe nausea or vomiting. As a protocol, pain was treated for all patients in post-operative period using tramadol 100 mg iv. The sample size was calculated from the previous study which found complete response to be 83.33% for palonosetron group and taking 95% confidence interval and allowable error as 20%; it was determined to be twenty in each group.[6] Considering the number of dropouts, we decided to enrol thirty patients in each group. IBM SPSS statistical software package for windows (version 17 Illinois, Chicago, USA) was employed for statistical analysis. Independent sample t-test was employed for comparing variables with normal distribution and categorical data analysed by Chi-square test. The P < 0.05 was considered statistically significant.
RESULTS
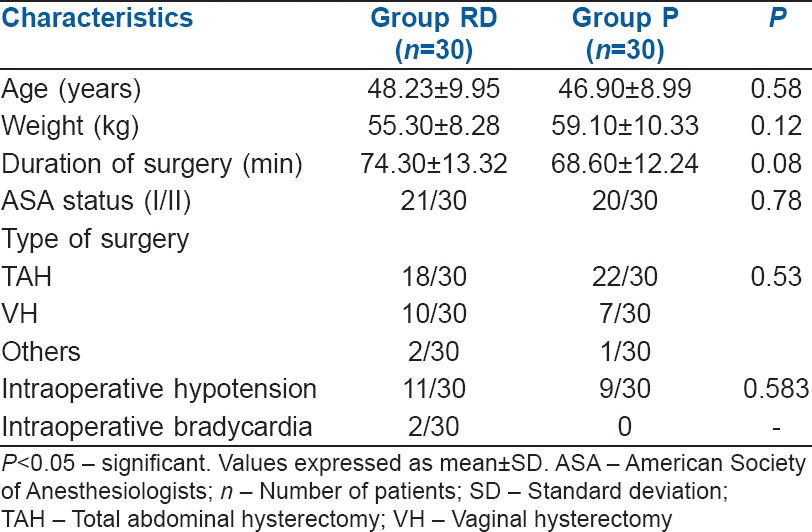
Sixty patients, who were included, successfully completed the study. Both groups were comparable with respect to age, weight, ASA status, type and duration of surgery [Table 1]. Intraoperative hypotension was seen in 11 (36.66%) patients in Group RD when compared to 9 (30.0%) patients in Group P which was statistically insignificant (P = 0.543) [Table 1].
Table 1.
Demographic data
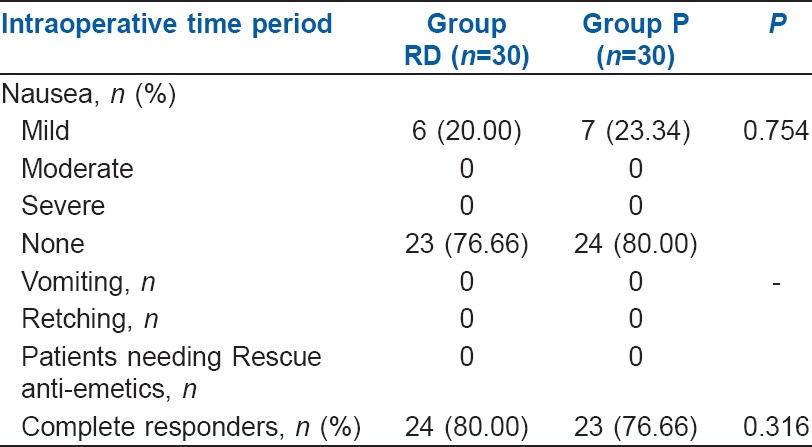
The number of complete responders during the intraoperative period was 24 (80.00%) in Group RD compared to 23 (76.66%) in Group P which was comparable (P = 0.316) [Table 2]. The number of patients having nausea during intraoperative period was 6 (20.00%) in Group RD when compared to 7 (23.34%) in Group P. The difference was found to be non-significant. None had retching, vomiting or received rescue anti-emetics during the intraoperative period [Table 2].
Table 2.
Incidence of intraoperative nausea, vomiting, Number of complete responders and use of rescue anti-emetics
The overall incidence of PONV during 24 h post-operative period was noted to be 9 (30.00%) in Group RD and 18 (60.00%) in Group P. This difference was found to be statistically significant (P = 0.0195). The number of complete responders during 0–2 h and 2–6 h in Group RD was 23 (76.60%) and 25 (83.3%) respectively whereas in Group P it was 14 (46.66%) and 17 (56.66%), respectively. This difference was found to be statistically significant (P = 0.016 and P = 0.024). The incidence of post-operative nausea in Group RD was 6 (20.0%) and 5 (16.6%) respectively whereas in Group P it was 14 (46.6%) and 12 (40.4%) respectively, during 0–2 h and 2–6 h. This difference was found to be statistically significant (P = 0.045 and P = 0.04, respectively) [Table 3 and Figure 1]. The number of complete responders and post-operative nausea during 6–12 h and 12–24 h interval was not significant. The number of patients developing post-operative vomiting and retching, and the number of patients requiring rescue anti-emetics was also not significant during 0–24 h period [Table 3 and Figure 1].
Table 3.
Incidence of post-operative nausea and vomiting, Number of complete responders and use of rescue anti-emetics
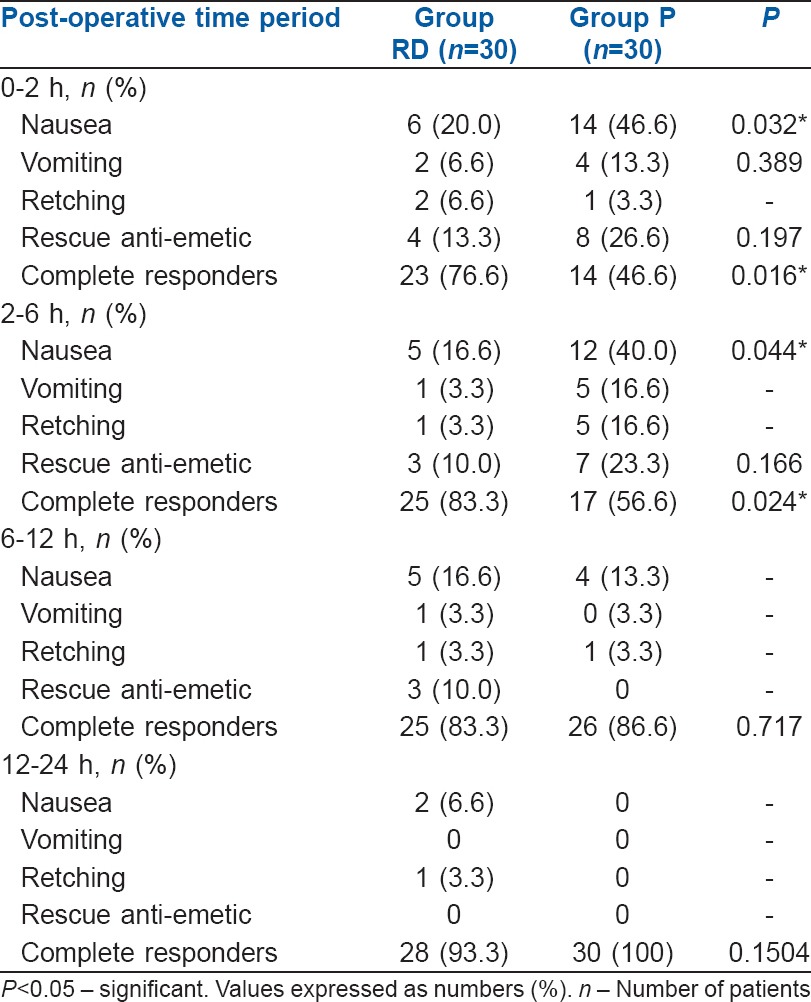
Figure 1.
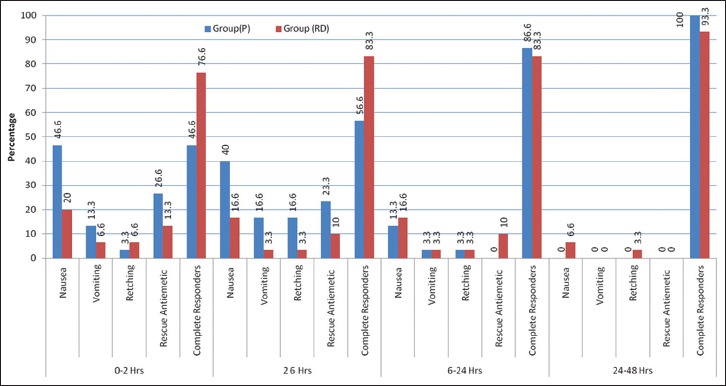
Incidence of post-operative nausea and vomiting, complete responders and use of rescue anti-emetics (original)
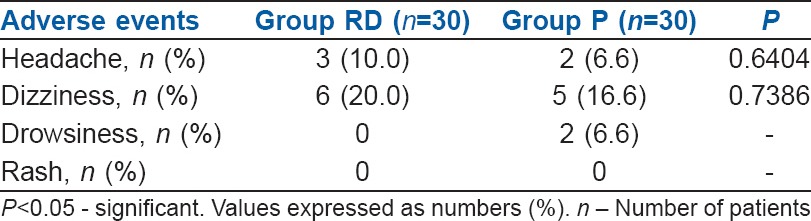
Nausea severity score was comparable in both the groups during intra and post-operative period [Figure 2]. Both the groups of patients had adverse effects such as headache and dizziness, but the incidence was statistically not significant [Table 4].
Figure 2.
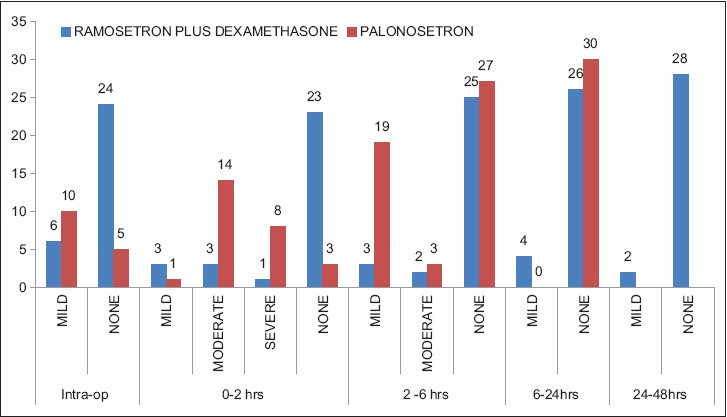
Nausea severity score: The distribution of nausea severity by a four-point verbal rating scale (none, mild, moderate, severe) during intraoperative and post-operative period
Table 4.
Incidence of adverse events
DISCUSSION
This present randomised double-blind study showed that the combination therapy of ramosetron and dexamethasone is more efficient than palonosetron monotherapy in preventing early PONV in patients undergoing gynaecological surgeries under SA. RA is associated with lower incidence of PONV than GA, and it is reported to be around 19%–22%.[6] Our study demonstrated the incidence to be much higher, i.e., as high as 45% which can be attributed to many risk factors in our patients such as female gender, young age (<50 years), non-smoking status, post-operative use of opioids, gynaecological surgery and duration of surgery lasting >30 min. All sixty patients who participated in our study had Apfel simple risk score of three which can increase the incidence of PONV to 60%.[7,8]
For prevention of PONV in high-risk patients, the recent consensus guidelines have suggested the use of either single effective anti-emetic drug or else combination of two drugs belonging to different groups.[6] Since our group of patients had a higher risk score, we decided to use palonosetron monotherapy in one group and compare it with routinely used combination of ramosetron and dexamethasone in our institution to prevent PONV.
We decided to use palonosetron monotherapy in our study, as a study has shown that palonosetron alone was effective in reducing the incidence of PONV than when used in combination with dexamethasone,[9]. According to the previous studies, a dose of 0.075 mg palonosetron given at the beginning of surgery was effective in reducing PONV[10,11] which has also been approved by FDA. Onset of action of palonosetron takes 30 min and so we decided to administer 0.075 mg of palonosetron, which was given at the start of surgery before placement of subarachnoid block.[12]
Ramosetron is a selective 5-HT3 receptor antagonist that exhibits significantly greater binding affinity for 5-HT3 receptors and a slower dissociation rate, resulting in more potent and longer action.[13] Seeing the multifactorial aetiologies combination therapy has been suggested by the recent consensus guidelines.[6] The most commonly used combination is of 5-HT3 receptor antagonists with dexamethasone or droperidol. Several studies have demonstrated that combination therapy of ramosetron and dexamethasone to be effective in reducing the incidence of PONV than ramosetron or dexamethasone alone.[14,15,16] This combination is regularly used in our institution to prevent PONV.
According to studies, dose of 0.3 mg of ramosetron given at the end of surgery and 8 mg dexamethasone given at the beginning of surgery was effective in reducing PONV in patients undergoing surgeries under GA.[6,14,16] Ramosetron has shown to be effective in reducing chemotherapy-induced nausea and vomiting when given 30 min before initiation of chemotherapy while dexamethasone has a slower onset of action and it takes nearly 2 h for its anti-emetic effect.[17,18] Since our study was on patients undergoing surgeries under RA, we decided to administer both the drugs before placement of subarachnoid block.
Our study showed that complete responders were more in ramosetron and dexamethasone combination than in palonosetron group. Ramosetron and dexamethasone in combination reduced the incidence of PONV, pain and shivering in female patients undergoing thyroid surgery.[19] The combination of granisetron and dexamethasone reduced the incidence of PONV.[20] Similar results were demonstrated in our study as well. This shows that the combination therapy of 5-HT3 antagonists with dexamethasone works better than monotherapy.
When different doses of palonosetron were compared with placebo, it has shown that the complete response in 0-2 h and 2-6 h in palonosetron (0.075 mg) group was 45% and 56% respectively which compares with our study. But that incidence was lesser when compared to ramosetron and dexamethasone combination in our study which was 76.6% and 83.3% during 0-2 h and 2-6 h respectively.[10,11] Our study demonstrated that the incidence of nausea was higher in palonosetron group than in the combination of ramosetron and dexamethasone group. This shows that palonosetron, a 5-HT3 antagonist has poor control on nausea like the older generation 5-HT3 antagonists.[6] Adverse effects like headache and dizziness were comparable in both groups in our study.
The limitations of the present study were that the sample size was small and palonosetron was not combined with dexamethasone. Further research is needed to know the efficacy of palonosetron when combined with dexamethasone in prevention of PONV.
CONCLUSION
The combination therapy of ramosetron and dexamethasone is more effective in reducing the incidence of PONV than palonosetron alone in patients undergoing gynaecological surgeries under SA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We acknowledge the assistance and cooperation received from our departmental colleagues, Dr. Gurudatta C.L, Prof and HOD, Department of Anaesthesiology, JSS medical college, without which the study could not have been completed.
REFERENCES
- 1.Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: Results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84:6–10. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 2.Larsson S, Lundberg D. A prospective survey of postoperative nausea and vomiting with special regard to incidence and relations to patient characteristics, anesthetic routines and surgical procedures. Acta Anaesthesiol Scand. 1995;39:539–45. doi: 10.1111/j.1399-6576.1995.tb04115.x. [DOI] [PubMed] [Google Scholar]
- 3.Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–84. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Ho KY, Gan TJ. Pharmacology, pharmacogenetics, and clinical efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Curr Opin Anaesthesiol. 2006;19:606–11. doi: 10.1097/01.aco.0000247340.61815.38. [DOI] [PubMed] [Google Scholar]
- 5.Henzi I, Walder B, Tramèr MR. Dexamethasone for the prevention of postoperative nausea and vomiting: A quantitative systematic review. Anesth Analg. 2000;90:186–94. doi: 10.1097/00000539-200001000-00038. [DOI] [PubMed] [Google Scholar]
- 6.Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 7.Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology. 1999;91:109–18. doi: 10.1097/00000542-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Apfel CC, Greim CA, Haubitz I, Goepfert C, Usadel J, Sefrin P, et al. A risk score to predict the probability of postoperative vomiting in adults. Acta Anaesthesiol Scand. 1998;42:495–501. doi: 10.1111/j.1399-6576.1998.tb05157.x. [DOI] [PubMed] [Google Scholar]
- 9.Park JW, Jun JW, Lim YH, Lee SS, Yoo BH, Kim KM, et al. The comparative study to evaluate the effect of palonosetron monotherapy versus palonosetron with dexamethasone combination therapy for prevention of postoperative nausea and vomiting. Korean J Anesthesiol. 2012;63:334–9. doi: 10.4097/kjae.2012.63.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candiotti KA, Kovac AL, Melson TI, Clerici G, Joo Gan T Palonosetron-Study Group. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo for preventing postoperative nausea and vomiting. Anesth Analg. 2008;107:445–51. doi: 10.1213/ane.0b013e31817b5ebb. [DOI] [PubMed] [Google Scholar]
- 11.Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C Palonosetron-Study Group. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008;107:439–44. doi: 10.1213/ane.0b013e31817abcd3. [DOI] [PubMed] [Google Scholar]
- 12.De Leon A. Palonosetron (Aloxi): A second-generation 5-HT3 receptor antagonist for chemotherapy-induced nausea and vomiting. Proc (Bayl Univ Med Cent) 2006;19:413–6. doi: 10.1080/08998280.2006.11928210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim EJ, Ko JS, Kim CS, Lee SM, Choi DH. Combination of antiemetics for the prevention of postoperative nausea and vomiting in high-risk patients. J Korean Med Sci. 2007;22:878–82. doi: 10.3346/jkms.2007.22.5.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jo YY, Lee JW, Shim JK, Lee WK, Choi YS. Ramosetron, dexamethasone, and their combination for the prevention of postoperative nausea and vomiting in women undergoing laparoscopic cholecystectomy. Surg Endosc. 2012;26:2306–11. doi: 10.1007/s00464-012-2180-0. [DOI] [PubMed] [Google Scholar]
- 15.Ryu JH, Chang JE, Kim HR, Hwang JW, Oh AY, Do SH. Ramosetron vs. ramosetron plus dexamethasone for the prevention of postoperative nausea and vomiting (PONV) after laparoscopic cholecystectomy: Prospective, randomized, and double-blind study. Int J Surg. 2013;11:183–7. doi: 10.1016/j.ijsu.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Jeon Y, Kim H, Kwak KH. Comparison of ramosetron, dexamethasone, and a combination of ramosetron and dexamethasone for the prevention of postoperative nausea and vomiting in Korean women undergoing thyroidectomy: A double-blind, randomized, controlled study. Curr Ther Res Clin Exp. 2010;71:78–88. doi: 10.1016/j.curtheres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JJ, Ho ST, Tzeng JI, Tang CS. The effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for postoperative nausea and vomiting. Anesth Analg. 2000;91:136–9. doi: 10.1097/00000539-200007000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Kang YK, Park YH, Ryoo BY, Bang YJ, Cho KS, Shin DB, et al. Ramosetron for the prevention of cisplatin-induced acute emesis: A prospective randomized comparison with granisetron. J Int Med Res. 2002;30:220–9. doi: 10.1177/147323000203000302. [DOI] [PubMed] [Google Scholar]
- 19.Song YK, Lee C. Effects of ramosetron and dexamethasone on postoperative nausea, vomiting, pain, and shivering in female patients undergoing thyroid surgery. J Anesth. 2013;27:29–34. doi: 10.1007/s00540-012-1473-8. [DOI] [PubMed] [Google Scholar]
- 20.Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Ramosetron vs. granisetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Can J Anaesth. 1999;46:991–3. doi: 10.1007/BF03013138. [DOI] [PubMed] [Google Scholar]


