Abstract
Background:
Camellia sinensis, the most consumed and popular beverages worldwide, and Eugenia uniflora, a Brazilian native species, have been already confirmed to have beneficial effects in the treatment of diabetes mellitus. However, their potential acting together against an enzyme linked to this pathology has never been exploited.
Objective:
The aim of this study was to evaluate the inhibitory properties of individual and combined ethanolic extracts of the leaves of C. sinensis and E. uniflora over alpha-glucosidase, a key digestive enzyme used on the Type 2 diabetes mellitus (T2DM) control. In addition, their inhibitory activity against 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) and peroxyl radicals was also assayed.
Materials and Methods:
Enzyme inhibition and antioxidant potential were assessed based on in vitro assays. Total phenolic compounds, carotenoids, and chlorophylls A and B were achieved using spectrophotometric methods.
Results:
E. uniflora was almost 40 times more active on alpha-glucosidase than C. sinensis and combined extracts showed a significant synergistic effect with an obtained IC50 value almost 5 times lower than the theoretical value. C. sinensis extract was twice more active than E. uniflora concerning DPPH•, in contrast, E. uniflora was almost 10 times more effective than C. sinensis on inhibition of peroxyl radicals with a significant synergistic effect for combined extracts. The extracts activities may be related with their phytochemicals, mainly phenolic compounds, and chlorophylls.
Conclusion:
Combined C. sinensis and E. uniflora ethanolic extracts showed synergistic effect against alpha-glucosidase and lipid peroxidation. These herbal combinations can be used to control postprandial hyperglycemia and can also provide antioxidant defenses to patients with T2DM.
SUMMARY
Alfa-glucosidase and antioxidant Interaction between Camellia sinensis L. Kuntze and Eugenia uniflora L. ethanolic extracts was investigated.
Extracts showed synergistic effect over alpha-glucosidase and peroxyl radicals.
Total phenolic, carotenoids and chlorophylls A and B can be responsible by the observed activities.
Extracts could be used as alternative to control postprandial hyperglycemia.
Extracts could increase antioxidant defenses to patients with T2DM.
Abbreviations Used: T2DM: Type 2 diabetes mellitus; DPPH: 2,2-diphenyl-1-picrylhydrazyl radical; PNPG: 4-Nitrophenyl β-D-glucuronide; LOO: Lipid peroxidation; SEM: Standard error of the mean; CAE: Chlorogenic acid equivalent
Key words: Additive effect, antihyperglycemic effect, antiradical activity, diabetes, phytochemicals, synergistic effect
INTRODUCTION
Diabetes mellitus is becoming a major public health concern, with high social and health-care costs since it affects over 387 million people worldwide causing 4.9 million deaths in 2014 (1 death each 7 s) according to the International Diabetes Federation. In Brazil, this pathology is present in around 13 million people between 20 and 79 years old.[1] Type 2 diabetes mellitus (T2DM) accounts for 90% of cases of diabetes and is characterized by individuals with postprandial hyperglycemia associated with low production of insulin, resistance to insulin, or both. One strategy used to control T2DM is the use of inhibitors of digestive enzymes such as alpha-glucosidase that is present in the intestine which catalyzes' the digestion of complex carbohydrates, converting them into easily digestible monosaccharides.[2] Inhibitors of this enzyme are used by individuals with T2DM to promote a decrease in glucose uptake and consequently a reduction in blood sugar levels. Different glucosidase inhibitors are currently used in patients with T2DM, namely acarbose, the first alpha-glucosidase inhibitor that is produced by fermentation of actinomycetes called Actinoplanes sp. and miglitol which is synthesized starting from the naturally occurring 1-deoxynojirimycin as a lead structure.[3] Since then, several studies have been performed in different species aiming to find new sources of inhibitors of this enzyme due to increase cases of T2DM in the world. Researchers have proposed different species as natural sources of alpha-glucosidase inhibitors including Camellia sinensis L. Kuntze (green tea), the most consumed and popular beverages worldwide,[4] and Eugenia uniflora L. (Brazilian Pitanga), a Brazilian native species that, shows in recent years an interesting potential as source of bioactive compounds. Both species have been already confirmed to have beneficial effects in the treatment of diabetes mellitus. C. sinensis tea consumption was effective against type 2 diabetes in a retrospective cohort studies in Japan and Taiwan.[5,6] Moreover, different studies also showed the positive effect of C. sinensis on T2DM prevention and treatment that was related with their phytochemicals mainly flavonols,[7] methylxanthine alkaloids,[8] and polysaccharides[9] by different mechanisms including inhibition of glucosidases.[10]E. uniflora leaves have been empirical used in the T2DM treatment and also showed inhibitory properties against alpha-glucosidase.[11,12,13,14,15] E. uniflora leaves are source of macrocyclic hydrolysable tannin dimers (eugeniflorins D1 and D2), oenothein B, 1,2,4,6-tetra-O-galloyl-fl-o-glucose, gallocatechin and myricitrin, compounds that may be responsible for the species activity.[16] Besides, it is well known that leaves also contain chlorophylls and carotenoids pigments, and these phytochemicals compounds can also confer good antioxidant activities for both species, which is an additional feature for the treatment of patients with T2DM which have their antioxidants defenses altered.
It is well established that when compounds with different properties are combined, numerous interactions can occur toward each other which can result in effects different from the formers. These effects can be classified as synergistic, antagonistic, or additive. The combination of extracts with different composition and the presence of synergistic effect is of great interest from the pharmacological point of view since the biological effect of the combined product is greater than the sum of individual agents.[17] Thus, smaller quantities of extracts are required to achieve the desirable effect which may improve the health-promoting properties of both products. Thus, the aim of this work was to determine the alpha-glucosidase inhibitory activity, and antioxidant effect of combined ethanolic extracts of C. sinensis L. Kuntze and E. uniflora L. leaves to assess their potential use in the T2DM treatment. In addition, the phytochemicals present in both extract were also determined and their relation with the observed biological effects discussed.
MATERIALS AND METHODS
Chemicals
Reference compounds and reagents were purchased from different suppliers. 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), iron sulfate heptahydrate, linoleic acid, Tris-HCl, phosphate buffer, alpha-glucosidase (type I from baker's yeast), and 4-nitrophenyl α-D-glucopyranoside (PNP-G) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ethanol was purchased from Synth (Diadema, SP, Brazil), ethyl ether, dichloromethane, ascorbic acid, phosphoric acid hydrogen peroxide solution (30% w/w) was from Impex (Diadema, SP, Brazil) and Acarbose (Glucobay®) was from Bayer Pharma AG (Leverkusen, Germany).
Samples
C. Sinensis (green tea), from a commercial brand, was purchased from local market in Pelotas and deposited in the laboratory of Food Science and Technology under the number: CS-01. E. uniflora leaves from purple genotype (plant identification PIT102, deposit number: ECT450) were collected from the Active Germplasm Bank of native fruits at Embrapa Clima Temperado (Brazil, 31°40'47”S, 52°26'24”W), on November 21, 2014 and identified by the PhD Gustavo Heiden Curator of the Embrapa Clima Temperado Herbarium. After collection, the sample was transported immediately to the laboratory, where it was placed in oven at 37°C until constant weight. Samples were powdered and sieved (<1 mm) for further extraction.
Preparation of extracts
Samples were extracted with ethanol 95% using 1 g of sample/100 ml of solvent, at 200 rpm, during 60 min. Extracts were filtered through paper filter (Whatman n°4) and further evaporated under pressure at 40°C. Ethanolic extracts yields were 14.47% ± 2.47% and 6.87% ± 1.08% for C. sinensis and E. uniflora, respectively. Samples were redissolved in ethanol leading to extracts with concentration of 3.5 mg/ml, which were further stored at −20°C until analysis.
Alpha-glucosidase inhibition and antioxidant potential
General
To evaluate the antihyperglycemic and antioxidant potential of leaves extracts in vitro assays were performed by spectrophotometric methods using an Amersham, ultraviolet-visible Ultrospec-3100 Pro Amersham Bioscience spectrophotometer. The IC50 values were calculated using at least 5 concentrations for each extract, and combined extracts (1:1). Three extracts were prepared for each sample and assays were performed in triplicate (n = 9) and expressed as ± standard error of the mean (SEM).
Alpha-glucosidase inhibitory activity
The effect on alpha-glucosidase was assessed using a procedure previously reported with a slightly modification.[18] Briefly, 20 µl of extract or ethanol (control) was added to a vial with 100 µl of PNP-G (3.25 mM) in phosphate buffer (pH 7.0). The reaction was initiated by the addition of 100 µl of enzyme (9.37 U/ml in phosphate buffer, pH 7.0), and vials were incubated at 37°C for 10 min. The reaction was stopped by adding 0.600 ml of Na2 CO3 (1M), and the absorbance at 405 nm was measured. Acarbose was used as positive control (85-1360 µg/ml).
2,2-diphenyl-1-picrylhydrazyl radical scavenging activity
The hydrogen atoms or electrons donation ability of the extracts was measured from the bleaching of purple-colored methanol solution of DPPH• by adaptation of the methods reported in the literature.[19,20] Briefly, 50 µl of each extract or ethanol (control) were added to 200 µl of a 0.6 mM DPPH• methanol solution. The reaction was mixed and incubated in the dark for 30 min at room temperature; samples were read at 515 nm.
Lipid peroxidation inhibition
Lipid peroxidation (LOO•) was measured according to the method described in the literature.[21] Briefly, the reaction mixture contained 50 μl of extract or ethanol (control), 250 μl linoleic acid (20 mM), 150 μl Tris-HCl (100 mM, pH 7.5), and 50 μl FeSO4.7H2O (4 mM). Linoleic acid peroxidation was initiated by the addition of 50 μl of ascorbic acid (5 mM) followed by incubation for 60 min at 37°C. The addition of 1.5 ml of ethanol-ether (3:1, v/v) and vortexing for 1 min allowed the separation of conjugated dienes in the organic layer that was spectrophotometrically measured at 233 nm.
Calculation of effects
Theoretical effects values for alpha-glucosidase and antioxidant activities of the mixtures were calculate as weighted mean experimental IC50 values [Table 1] and considering the additive contributions of 50% individual extracts as follows:
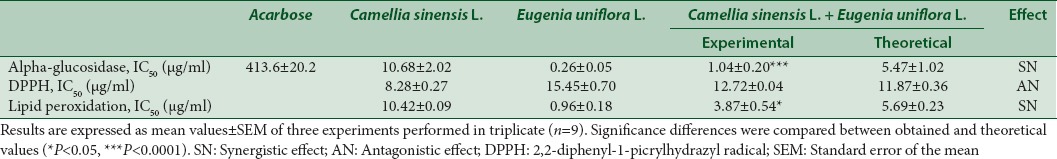
Table 1.
IC50 values for alpha-glucosidase, 2,2-diphenyl-1-picrylhydrazyl radical, and lipid peroxidation inhibition of individual and combined ethanolic extracts of Camellia sinensis L. Kuntze and Eugenia uniflora L
Theoretical IC50 = IC50C. sinensis × 0.50 + IC50E. uniflora × 0.50
The classification in additive, synergistic, or antagonistic effects was performed by comparison of obtained IC50 values with the theoretical IC50 value according to literature.[22,23] The interaction was considered additive when theoretical IC50 and experimental IC50 values show differences lower than 5%; for synergistic effect, the experimental IC50 values are more than 5% lower than theoretical values, and antagonistic effect when experimental IC50 values was more than 5% higher when compared with theoretical values as can be seen in Figure 1.[24]
Figure 1.
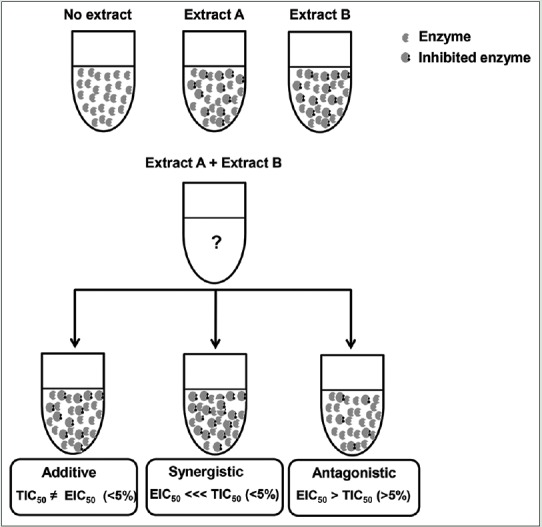
Classification in additive, synergistic, or antagonistic effects using the inhibition of alpha-glucosidase as example. TIC50 = Theoretical IC50 and EIC50 = Experimental IC50
Phytochemical analysis
Total phenolic compounds
Total phenolic content was measured according to the Folin-Ciocalteu method adapted from Swain and Hillis.[25] Briefly, 50 μl aliquot of the extract and the control (50 μl of ethanol) were each combined with 250 μL of 0.25 N Folin-Ciocalteau reagent. After 3 min reaction, 500 μl of Na2 CO3 (1N) was added, the mixtures were incubated for 2 h at room temperature, the absorbance was measured at 725 nm, and the results were expressed as mg of chlorogenic acid equivalents per 100 g of sample (CAE mg/100 g of sample) using a chlorogenic acid (0–0.4 mg/ml) standard curve.
Total carotenoids and chlorophylls content
Carotenoid, chlorophyll A, and chlorophyll B contents were assessed according to literature.[26] The ethanolic extracts were analyzed at different wavelengths and quantified according to the following equations:
Chlorophyll A = 13.36A664 − 5.19 A649
Chlorophyll B = 27.43A649 − 8.12 A664
Carotenoids = (1000A470 − 2.13Chlorophyll A − 97.63Chlorophyll B)/209
where A470, A649, and A664 are the absorbance's read at 470 nm, 649 nm, and 664 nm, respectively. Results are expressed as µg/g of sample.
Statistical analysis
Data are reported as mean ± SEM of three extracts analyzed in triplicate (n = 9). One-way analysis of variance was employed to compare the means related to the evaluated parameters. Significant differences were considered when P < 0.05.
RESULTS
Alpha-glucosidase inhibition
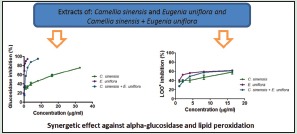
A dose response inhibitory effect over alpha-glucosidase was observed for both extracts and their combination as can be observed in Figure 2. The inhibition of 50% of the enzyme (IC50) was lower for E. uniflora, followed by combined extracts and C. sinensis [Table 1]. The combination was found to be synergistic since the experimental IC50 values were more than 5% lower than theoretical value [Table 1]. Both extracts and their combination showed IC50 lower than acarbose (positive control).
Figure 2.
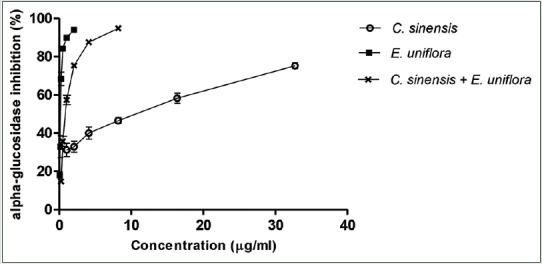
Effect of different concentrations of individual and combined extracts of Camellia sinensis and Eugenia uniflora on alpha-glucosidase
2,2-diphenyl-1-picrylhydrazyl radical scavenging activity
All ethanolic extracts, under the assays conditions, inhibit the free radical DPPH• in a concentration-dependent way [Figure 3a] with IC50 values ranging from 8.28 to 15.45 µg/ml [Table 1]. C. sinensis was almost 2 times more effective than E. uniflora. The extracts combination showed an antagonistic effect on DPPH• since the experimental value of IC50 was 6.68%, which was higher than 5%, when compared with the theoretical IC50 value [Table 1].
Figure 3.
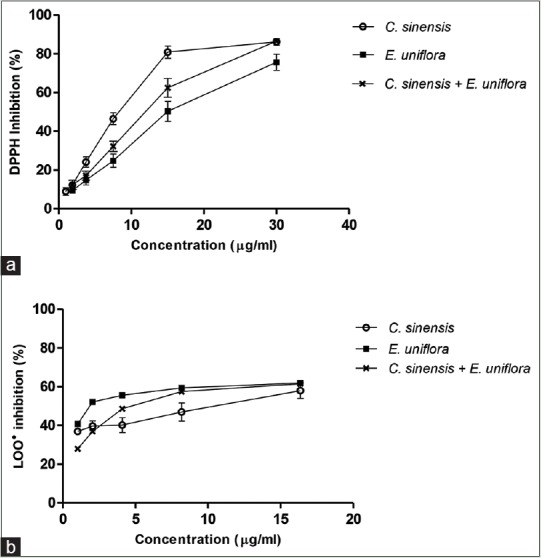
Effect of different concentrations of individual and combined extracts of Camellia sinensis and Eugenia uniflora on 2,2-diphenyl-1-picrylhydrazyl radical (a) and lipid peroxidation (b)
Lipid peroxidation inhibition
C. sinensis and E. uniflora ethanolic leaves extracts and their combination were evaluated as inhibitors of LOO•. Figure 3b displays the dose-dependent behavior observed for all extracts. E. uniflora was 10 times more effective than C. sinensis with IC50 values of 0.96 and 10.42 µg/ml [Table 1], respectively. A synergistic effect was observed when combining both extracts on the LOO• inhibition, with an experimental value of IC50 3.87 µg/ml, which is lower than the theoretical value and lower than the IC50 found for C. sinensis alone.
Phytochemicals
Total phenolic compounds
The total phenolic content of the C. sinensis and E. uniflora extracts was measured, and the results were expressed as milligram of CAE per 100 g dried weight sample. As can be observed on Table 2, the total phenolic compounds in C. sinensis (812.58 ± 117.15 mg of CAE/100 g of sample) and E. uniflora (663.82 ± 107.58 mg of CAE/100 g of sample) were not significantly different.
Table 2.
Total phenolic, carotenoids, chlorophylls (A and B) contents in ethanolic extracts of Camellia sinensis L. Kuntze and Eugenia uniflora L

Total carotenoids
The total content of carotenoids in ethanolic extracts of C. sinensis and E. uniflora is presented in Table 2. C. sinensis (0.13 ± 0.03 µg/g of sample) was 2-fold richer in these compounds than E. uniflora (0.07 ± 0.02 µg/g of sample).
Chlorophylls
The contents of chlorophylls in the extracts are presented in Table 2. C. sinensis also showed higher concentrations of chlorophyll A (0.96 ± 0.16 mg/g of sample) than E. uniflora (0.60 ± 0.09 mg/g of sample), conversely, E. uniflora had higher amounts of chlorophyll B (0.46 ± 0.11 mg/g of extract), almost 2 times, when compared to C. sinensis (0.18 ± 0.02 mg/g of sample).
DISCUSSION
Hyperglycemia, typical in patients with T2DM, is characterized by the abnormal increase in the level of fasting and postprandial blood glucose. Thus, controlling postprandial hyperglycemia is a major therapeutic approach for T2DM management. Different strategies can be used to decrease the high levels of glucose after a carbohydrate-rich meal, inhibit the enzymes responsible for their digestion is an example. Thus, in this study, we evaluated the potential of C. sinensis and E. uniflora and their combination as inhibitors of alpha-glucosidase, an enzyme present in the human body responsible for the carbohydrate breakdown.
Both extracts were tested at same concentration; however, E. uniflora achieved almost 100% of alpha-glucosidase inhibition with lower amounts. As consequence, the IC50 found for C. sinensis was almost 40 times higher than the IC50 of E. uniflora. C. sinensis and E. uniflora ethanolic extracts were 40 and 1500 times more effective than the positive control acarbose, respectively. C. sinensis showed an IC50(10.68 µg/ml) almost 30 times lower than reported for a hydro-alchoholic extract (IC50 = 299 µg/ml).[27] In addition, this value is slightly higher than the IC50 reported in the literature for C. sinensis water extracts 4.42 µg/ml, but lower than 2040 µg/ml reported by other authors.[28,29] This variation was expected since samples, extraction procedure and enzymatic protocols used were not the same. The alpha-glucosidase inhibitory value for E. uniflora (0.26 µg/ml) [Table 1] was lower than the value described in the literature.[14] These authors have studied different fractions of leaves ethanolic extract, instead of whole extract, and have found that 4 fractions were capable of inhibiting almost 50% of the enzyme at a concentration of 100 µg/ml. Nevertheless, both extracts were more effective on alpha-glucosidase inhibition than extracts of Bauhinia species, commonly used to treat T2DM.[30]
Concerning the interaction between C. sinensis and E. uniflora ethanolic leaves extracts on the inhibitory activity of alpha-glucosidase, the value obtained was almost 400 times more effective than acarbose. A similar result was observed for the herbal mixture of Allium sativum plus Lagerstroemia speciosa on the inhibition of alpha-glucosidase, where their combination was also more effective than the positive control miglitol.[31]
Since the alpha-glucosidase inhibition by combined extracts was greater than the individual extracts, lower amounts of extract were needed to achieve the biological effect. This fact can be very beneficial to human health, due to the increased therapeutic effect and the reduced toxicity. As far as we are concerned, this is the first report about the interaction of C. sinensis and E. uniflora ethanolic leaves extracts and their inhibitory effect against the alpha-glucosidase enzyme.
As recent studies shows that T2DM patients have an increased free-radical production and reduced antioxidant defense, which leads to different health complications, supplementation with antioxidants agents can be useful in their treatment. Therefore, the antiradical potential of C. sinensis, E. uniflora, and combined extracts was evaluated.
Different in vitro methods can be used to measure the efficiency of natural antioxidant compounds either as pure or as plant mixtures. Owing to the complex nature of extracts and their mechanisms of action, a single method is not capable to provide a comprehensive view of their antioxidant profile. In addition, the comparison of results with those found in the literature is very difficult since the assays conditions cannot be exactly the same and the relative effectiveness of antiradical compound is highly dependent on their concentration, test system, time, and selected assay. Thus, the radical-scavenging activity of C. sinensis and E. uniflora and their combination was assessed by means of two assays: DPPH• and peroxyl radicals.
The hydrogen atoms or electrons donation ability of C. sinensis and E. uniflora ethanolic leaves extracts and their combination was measured from the bleaching of purple colored methanol solution of DPPH•. This assay is frequently used for the screening of the antiradical activity of different matrix and isolated compounds, due to its reproducibility, simplicity, and fastness. The present study showed better results on the inhibitory effect over the free radical DPPH• when comparing the IC50 found for C. sinensis with those reported in the literature for ethanolic extract (IC50 = 10.35 ± 0.14 μg/ml), water extracts (IC50 = 15.63 and 60.00 µg/ml), and hydroalcoholic extracts (IC50 = 201.3 µg/ml).[29,32,33] Antagonistic effect occurs when the combination of extracts reduces the observed activity relatively to extracts tested alone, indicating the E. uniflora and C. sinensis combined extracts had a negative effect over DPPH• inhibition. Nevertheless, this is the first report of the DPPH• inhibitory effect of C. sinensis and E. uniflora combined extracts, as far as we known.
Peroxyl radicals (LOO•) are the product of oxidation of lipids after the attack of reactive oxygen species. In individuals with diabetes, this product is present in higher amounts when compared to nondiabetic ones.[34] This radical species can cause alterations on cell membrane lipids, process that can result in cell damage, death, and neoplasia which are probably involved in the complications of diabetes and also in the incidence of several chronic and degenerative diseases. Concerning E. uniflora extracts, results found under the assay conditions tested in the present study showed inhibitory properties 6–60 times lower than reported in the literature.[32,35]E. uniflora ethanolic extracts inhibited the LOO• in rat's brain and liver homogenates at concentrations ranging from 6.3 to 50 µg/ml.[35] In addition, the administration of an aqueous extract (IC50 = 60.00 µg/ml) to Type 1 non obese diabetic mices reduces in 76% the serum LOO• when compared to the untreated and by 69% when compared with acute diabetic animals.[32] In respect to C. sinensis, the IC50 value found was 30 times lower than that reported for an ethanolic extract (IC50 = 333.29 ± 17.90 µg/ml).[36] Moreover, a statistically significant decrease on LOO• markers in diabetic patients treated with green tea extract capsule was also reported (200 mg of standardized extract of C. sinensis L. leaves, adjusted to 70% polyphenols) after 9 months or after 18 months on a follow-up study.[37]
A synergistic effect was observed when combining both extracts on the LOO• inhibition. The combination of both extracts had a positive effect on the studied biological property. Thus, inhibition of LOO• can be achieved at low concentrations reducing the amounts of individual extracts needed, which can improve, for instance, the protection of membrane cells against oxidative injuries.
Different biological activities have been attributed to phenolic compounds, including the alpha-glucosidase and antioxidant activities studied here in. It is well stablished by different studies that the total phenolic composition of a matrix gives an idea of how rich this product is in antioxidants since these parameters are closely related. The total phenolic composition found for C. sinensis was similar to those found by other authors while for E. uniflora higher values were reported.[38,39]
Although different studies suggest a correlation between the total amounts of phenolic compounds and the biological activity, this was not observed in the present study. Our result indicates that probably, the type and amounts of individual phenolic compounds present in these matrices drives the inhibitory effect. C. sinensis is known to have high flavonoid content, primarily catechins such as (−)-epigallocatechin gallate, (−)-epigallocatechin, (−)-gallocatechin, and (+)-catechin.[40] Conversely, E. uniflora leaves extract is poorly characterized with only two studies reporting its composition. One report of identification of myricetin and quercetin derivatives in a fraction obtained from ethanolic extract and other about macrocyclic hydrolysable tannin dimers (eugeniflorins D1 and D2), oenothein B, 1,2,4,6-tetra-O-galloyl-fl-o-glucose, gallocatechin, and myricitrin isolated from the leaves.[16,41] Thus, further study is needed, such as bio-guided fractionation, to determine the phenolic compound or group of compounds, responsible for the observed biological activity. Nevertheless, the amounts of phenolic compounds provided by both extracts can be partially responsible for the alpha-glucosidase inhibition and can also offer good antioxidant protection for patients with T2DM.
Other important phytochemicals are carotenoids and chlorophylls; these compounds are colorful pigments abundant in fruits and vegetables. Carotenoids are considered important bioactive compounds for human's health as scientific studies demonstrate their important role in reducing the risk of degenerative diseases.[42] Different studies have demonstrated significant decrease of plasma antioxidants by carotenoids (α- and γ-tocopherol, α and β-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin) in the progression of diabetes and its associated complications such as endothelial dysfunction and atherosclerosis.[43,44,45] The amounts of carotenoids in C. sinensis was similar to those found in literature, and these are the first report about the amounts of carotenoids in E. uniflora leaves extracts.[46] As far as we are concerned carotenoids have never been studied as inhibitors of alpha-glucosidase; however, as previously stated, they are important phytochemicals that could prevent the development of degenerative diseases. This fact is mainly because carotenoids are important antioxidant compounds, they have been reported as active toward peroxyl radicals, a deleterious radical species that can be in the origin of different degenerative diseases, but with no activity against DPPH• radicals.[47]
Regarding chlorophylls and their related compounds, they are among the best candidates for the chemicals responsible for the general protection afforded by vegetables. The main benefits of chlorophylls are their anticarcinogenic activity, related to their antioxidant effects; and their contribution to a positive hematological status due to the similarity between chlorophyll structure and hemoglobin. The total content of chlorophylls was proposed as quality parameter for C. sinensis and the concentration found among 14 samples ranged from 1.18 ± 0.16 to 1.98 ± 0.11 mg/g of sample.[48] Thus, the value found in the present study was in agreement with the reported value. No report was found about the chlorophyll content in E. uniflora as far as we known. Although there are no studies reporting the inhibition of alpha-glucosidase by chlorophylls, different studies showed that natural matrices rich in these types of compounds have an important role in diabetes.[49] The chlorophylls effect over DPPH• and LOO• have been reported.[50] Chlorophyll A and B inhibited 40% of DPPH• at a concentration of 0.18 m/mg for both compounds. This value is lower than the concentrations of chlorophylls in both extracts [Table 2], indicating that these compounds can partially explain their DPPH• inhibition. The same behavior was also observed in the reduction on LOO• for both compounds, where chlorophyll A and B showed IC50 values of 4.40 and 23.59 µg/g, respectively.[50]
Taken together, these results indicates that the inhibitory effect on alpha-glucosidase and LOO• activities is likely mediated by the contribution of several or multiple bioactive compounds of the extracts, which is confirmed when extracts are combined, and a synergistic effect was observed.
CONCLUSION
The ethanolic extracts of E. uniflora and C. sinensis and their combination showed remarkable inhibitory activity over alpha-glucosidase, being 40–1500 times more effective than acarbose. A synergistic effect was found in the interaction of E. uniflora and C. sinensis over alpha-glucosidase and LOO•. These results may be partially explained by the presence and combination of phenolic compounds, chlorophylls A and B, and carotenoids. This study indicates that individual and combined extracts may be used for the treatment of diabetes mellitus, by inhibiting the enzyme, and also protecting the T2DM patients by improving their antioxidant status.
Financial support and sponsorship
Financial support of CNPq/ Science Without Borders Program project “Frutas Nativas do Brasil: potencial anti-hiperglicimiante e antioxidante. Juliana Vinholes thanks the Science without Borders Program (CNPq) for the Young Talent attraction fellowship.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Juliana Vinholes
Juliana Vinholes, Graduated in Chemistry from the Federal University of Pelotas (2002) and PhD in Chemistry from the University of Aveiro (2013). I have experience in analytical chemistry, focused on the development of chromatographic methods for primary and secondary metabolites analysis. In addition, I am also involved in the evaluation of biological potential of isolated compounds and natural matrices.
REFERENCES
- 1.Brazilian Diabetes Society (SBD) There are 13.4 million people with diabetes in Brazil. 2013. [Last retrieved on 2013 Aug 10]. Available from: http://www.diabetes.org.br/sala-denoticias/2364-sao-134-milhoes-de-pessoas-portadoras-de-diabetes-no-brasilbetes-no-Brasil .
- 2.Li DQ, Qian ZM, Li SP. Inhibition of three selected beverage extracts on alpha-glucosidase and rapid identification of their active compounds using HPLC-DAD-MS/MS and biochemical detection. J Agric Food Chem. 2010;58:6608–13. doi: 10.1021/jf100853c. [DOI] [PubMed] [Google Scholar]
- 3.Grabley S, Thiericke R. Bioactive agents from natural sources: Trends in discovery and application. Adv Biochem Eng Biotechnol. 1999;64:101–54. doi: 10.1007/3-540-49811-7_4. [DOI] [PubMed] [Google Scholar]
- 4.Tadesse A, Hymete A, Bekhit AA, Mohammed SF. Quantification of total polyphenols, catechin, caffeine, L-theanine, determination of antioxidant activity and effect on antileishmanial drugs of ethiopian tea leaves extracts. Pharmacognosy Res. 2015;7(Suppl 1):S7–14. doi: 10.4103/0974-8490.157991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iso H, Date C, Wakai K, Fukui M, Tamakoshi A. JACC Study Group. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554–62. doi: 10.7326/0003-4819-144-8-200604180-00005. [DOI] [PubMed] [Google Scholar]
- 6.Wu CH, Lu FH, Chang CS, Chang TC, Wang RH, Chang CJ. Relationship among habitual tea consumption, percent body fat, and body fat distribution. Obes Res. 2003;11:1088–95. doi: 10.1038/oby.2003.149. [DOI] [PubMed] [Google Scholar]
- 7.Bahmani M, Golshahi H, Saki K, Rafieian-Kopaei M, Delfan B, Mohammadi T. Medicinal plants and secondary metabolites for diabetes mellitus control. Asian Pac J Trop Dis. 2014;4:S687–92. [Google Scholar]
- 8.Satoh T, Igarashi M, Yamada S, Takahashi N, Watanabe K. Inhibitory effect of black tea and its combination with acarbose on small intestinal a-glucosidase activity. J Ethnopharmacol. 2015;161:147–55. doi: 10.1016/j.jep.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Boros K, Jedlinszki N, Csupor D. Theanine and caffeine content of infusions prepared from commercial tea samples. Pharmacogn Mag. 2016;12:75–9. doi: 10.4103/0973-1296.176061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Yang Z, Wei X. Sugar compositions, a-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int J Biol Macromol. 2010;47:534–9. doi: 10.1016/j.ijbiomac.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 11.de Souza GC, Haas AP, von Poser GL, Schapoval EE, Elisabetsky E. Ethnopharmacological studies of antimicrobial remedies in the South of Brazil. J Ethnopharmacol. 2004;90:135–43. doi: 10.1016/j.jep.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Santos MM, Nunes MG, Martins RD. Empirical use of medicinal plants for diabetes treatment. Rev Bras Planta Med. 2012;14:327–34. [Google Scholar]
- 13.Trojan-Rodrigues M, Alves TL, Soares GL, Ritter MR. Plants used as antidiabetics in popular medicine in Rio Grande do Sul, Southern Brazil. J Ethnopharmacol. 2012;139:155–63. doi: 10.1016/j.jep.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 14.Arai I, Amagaya S, Komatsu Y, Okada M, Hayashi T, Kasai M, et al. Improving effects of the extracts from Eugenia uniflora on hyperglycemia and hypertriglyceridemia in mice. J Ethnopharmacol. 1999;68:307–14. doi: 10.1016/s0378-8741(99)00066-5. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura T, Kasai M, Hayashi T, Arisawa M, Momose Y, Arai I, et al. a-glucosidase inhibitors from paraguayan natural medicine, ñangapiry, the leaves of Eugenia uniflora. Pharm Biol. 2000;38:302–7. doi: 10.1076/1388-0209(200009)3841-AFT302. [DOI] [PubMed] [Google Scholar]
- 16.Mei-Hsien L, Saori N, Ling-Ling Y, Kun-Ying Y, Tsutomu H, Takashi Y, et al. Two macrocyclic hydrolysable tannin dimers from Eugenia uniflora. Phytochemistry. 1997;44:1343–9. [Google Scholar]
- 17.Gallucci MN, Oliva M, Asero C, Dambolena J, Luna A, Zygadlo J, et al. Antimicrobial combined action of terpenes against the food-borne microorganisms Escherichia coli, Staphylococcus aureus and Bacillus cereus. Flavour Frag J. 2009;24:348–54. [Google Scholar]
- 18.Ferreres F, Vinholes J, Gil-Izquierdo A, Valentão P, Gonçalves RF, Andrade PB. In vitro studies of a-glucosidase inhibitors and antiradical constituents of Glandora diffusa (Lag.) D.C. Thomas infusion. Food Chem. 2013;136:1390–8. doi: 10.1016/j.foodchem.2012.09.089. [DOI] [PubMed] [Google Scholar]
- 19.Vinholes J, Grosso C, Andrade PB, Gil-Izquierdo A, Valentão P, Pinho PG, et al. In vitro studies to assess the antidiabetic, anti-cholinesterase and antioxidant potential of Spergularia rubra. Food Chem. 2011;129:454–62. doi: 10.1016/j.foodchem.2011.04.098. [DOI] [PubMed] [Google Scholar]
- 20.Vinholes J, Gonçalves P, Martel F, Coimbra MA, Rocha SM. Assessment of the antioxidant and antiproliferative effects of sesquiterpenic compounds in in vitro Caco-2 cell models. Food Chem. 2014;156:204–11. doi: 10.1016/j.foodchem.2014.01.106. [DOI] [PubMed] [Google Scholar]
- 21.Grosso C, Vinholes J, Silva LR, de Pinho PG, Gonçalves RF, Valentão P, et al. Chemical composition and biological screening of Capsella bursa-pastoris. Rev Bras Farmacogn. 2011;21:635–43. [Google Scholar]
- 22.Pereira C, Calhelha RC, Barros L, Queiroz MJ, Ferreira IC. Synergisms in antioxidant and anti-hepatocellular carcinoma activities of artichoke, milk thistle and borututu syrups. Ind Crops Prod. 2014;52:709–13. [Google Scholar]
- 23.Nedamani ER, Mahoonak AS, Ghorbani M, Kashaninejad M. Antioxidant properties of individual vs. combined extracts of rosemary leaves and oak fruit. J Agric Sci Technol. 2014;16:1575–86. [Google Scholar]
- 24.Yeh P, Tschumi AI, Kishony R. Functional classification of drugs by properties of their pairwise interactions. Nat Genet. 2006;38:489–94. doi: 10.1038/ng1755. [DOI] [PubMed] [Google Scholar]
- 25.Swain T, Hillis WE. The phenolic constituents of Prunus domestica. I. – The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–8. [Google Scholar]
- 26.Sumanta N, Haque CI, Nishika J, Suprakash R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res J Chem Sci. 2014;4:63–9. [Google Scholar]
- 27.Ramírez G, Zavala M, Pérez J, Zamilpa A. In vitro screening of medicinal plants used in Mexico as antidiabetics with glucosidase and lipase inhibitory activities. Evid Based Complement Alternat Med. 2012;2012:701261. doi: 10.1155/2012/701261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao J, Xu P, Wang Y, Wang Y, Hochstetter D. Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against a-amylase and a-glucosidase in vitro. Molecules. 2013;18:11614–23. doi: 10.3390/molecules180911614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh J, Jo SH, Kim JS, Ha KS, Lee JY, Choi HY, et al. Selected tea and tea pomace extracts inhibit intestinal a-glucosidase activity in vitro and postprandial hyperglycemia in vivo. Int J Mol Sci. 2015;16:8811–25. doi: 10.3390/ijms16048811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreres F, Gil-Izquierdo A, Vinholes J, Silva ST, Valentão P, Andrade PB. Bauhinia forficata Link authenticity using flavonoids profile: Relation with their biological properties. Food Chem. 2012;134:894–904. doi: 10.1016/j.foodchem.2012.02.201. [DOI] [PubMed] [Google Scholar]
- 31.Kesavanarayanan KS, Sathiya S, Ranju V, Sunil AG, Ilavarasan R, Saravana Babu C, et al. In vitro cytotoxic, antioxidative and alpha-glucosidase inhibitory potential of a herbal mixture comprised of Allium sativum and Lagerstroemia speciosa. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 3):58–68. [PubMed] [Google Scholar]
- 32.Schumacher NS, Colomeu TC, de Figueiredo D, Carvalho Vde C, Cazarin CB, Prado MA, et al. Identification and antioxidant activity of the extracts of Eugenia uniflora leaves. characterization of the anti-inflammatory properties of aqueous extract on diabetes expression in an experimental model of spontaneous type 1 diabetes (NOD Mice) Antioxidants (Basel) 2015;4:662–80. doi: 10.3390/antiox4040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tewari I, Sharma L, Lal Gupta G. Synergistic antioxidant activity of three medicinal plants Hypericum perforatum, Bacopa monnieri, and Camellia Sinensis. Indo Am J Pharm Sci Res. 2014;4:2563–8. [Google Scholar]
- 34.Seghrouchni I, Drai J, Bannier E, Rivière J, Calmard P, Garcia I, et al. Oxidative stress parameters in type I, type II and insulin-treated type 2 diabetes mellitus; insulin treatment efficiency. Clin Chim Acta. 2002;321:89–96. doi: 10.1016/s0009-8981(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 35.Kade IJ, Ibukun EO, Nogueira CW, da Rocha JB. Sun-drying diminishes the antioxidative potentials of leaves of Eugenia uniflora against formation of thiobarbituric acid reactive substances induced in homogenates of rat brain and liver. Exp Toxicol Pathol. 2008;60:365–71. doi: 10.1016/j.etp.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Lassed S, Amrani A, Altun M, Zama D, Demirtas I, Benayache F, et al. In vitro antioxidant, inhibition of oxidative DNA damage and antiproliferative activities of ethanolic green tea (Camellia sinensis) extract. Int J Pharm Sci Rev Res. 2015;35:36–42. [Google Scholar]
- 37.Spadiene A, Savickiene N, Ivanauskas L, Jakstas V, Skesters A, Silova A, et al. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J Food Drug Anal. 2014;22:505–11. doi: 10.1016/j.jfda.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira VP, Knor FJ, Vellosa JC, Beltrame FL. Determination of phenolic compounds and antioxidant activity of green, black and white teas of Camellia sinensis (L.) Kuntze, Theaceae. Rev Bras Planta Med. 2014;16:490–8. [Google Scholar]
- 39.Santos RM, Oliveira MS, Ferri PH, Santos SC. Seasonal variation in the phenol content of Eugenia uniflora L. leaves. Rev Bras Planta Med. 2011;13:85–9. [Google Scholar]
- 40.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–33. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rattmann YD, De Souza LM, Malquevicz-Paiva SM, Dartora N, Sassaki GL, Gorin PA, et al. Analysis of flavonoids from Eugenia uniflora leaves and its protective effect against murine sepsis. J Evid Based Complement Altern Med. 2012;2012:9. doi: 10.1155/2012/623940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55:207–16. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Polidori MC, Mecocci P, Stahl W, Parente B, Cecchetti R, Cherubini A, et al. Plasma levels of lipophilic antioxidants in very old patients with type 2 diabetes. Diabetes Metab Res Rev. 2000;16:15–9. doi: 10.1002/(sici)1520-7560(200001/02)16:1<15::aid-dmrr71>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Polidori MC, Stahl W, Eichler O, Niestroj I, Sies H. Profiles of antioxidants in human plasma. Free Radic Biol Med. 2001;30:456–62. doi: 10.1016/s0891-5849(00)00345-2. [DOI] [PubMed] [Google Scholar]
- 45.Valabhji J, McColl AJ, Richmond W, Schachter M, Rubens MB, Elkeles RS. Total antioxidant status and coronary artery calcification in type 1 diabetes. Diabetes Care. 2001;24:1608–13. doi: 10.2337/diacare.24.9.1608. [DOI] [PubMed] [Google Scholar]
- 46.Kumar N, Rai R, Bera B. Impact of organic farming on the biochemical constituents and quality parameters of Darjeeling tea (Camellia sinensis (L) O Kuntze) Two and a Bud. 2012;59:50–5. [Google Scholar]
- 47.Müller L, Fröhlich K, Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011;129:139–48. [Google Scholar]
- 48.Ošťádalová M, Tremlová B, Pokorná J, Král M. Chlorophyll as an indicator of green tea quality. Acta Vet Brno. 2014;83:S103–9. [Google Scholar]
- 49.Bar-Sela G, Cohen M, Ben-Arye E, Epelbaum R. The medical use of wheatgrass: Review of the gap between basic and clinical applications. Mini Rev Med Chem. 2015;15:1002–10. doi: 10.2174/138955751512150731112836. [DOI] [PubMed] [Google Scholar]
- 50.Hsu C, Chao P, Hu S, Yang C. The Antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Food Nutr Sci. 2013;4:1–8. [Google Scholar]


