Abstract
Introduction:
Cholesterol lowering activity of Mangifera indica L. has been determined by earlier researchers and kernel, leaf and bark have shown significant activity. However, the specific cholesterol lowering activity of leaf methanol extract has not been determined.
Materials and Methods:
The present study involved evaluation of cholesterol lowering potential of methanol extract of M. indica leaves using high cholesterol diet model in albino Wistar rats. The acute oral toxicity at a dose of 5000 mg/ kg body weight was also determined in female albino Wistar rats. Phytoconstituents Iriflophenone 3-C-β-D-glucoside and mangiferin were quantified in methanol extracts of different varieties of mango leaves using high performance liquid chromatography.
Results and Discussion:
Significant cholesterol lowering activity was observed with methanol extract of M. indica leaves, at dose of 90 mg/kg body weight in rats and it was also found to be safe at dose of 5000 mg/kg rat body. Iriflophenone 3-C-β-D-glucoside and mangiferin were found to be in the range of 1.2 to 2.8% w/w and 3.9 to 4.6% w/w, respectively which along with 3 β taraxerol and other sterols could be contributing to the cholesterol lowering activity of mango leaves extract.
Conclusions:
The phytosterols rich extract of Mangifera indica leaves is a good source of nutraceutical ingredient that have the potential to lower serum cholesterol levels.
SUMMARY
The Mangifera indica leaves methanolic extract showed significant cholesterol lowering activity in high cholesterol diet induced hypercholesterolaemia model in rats when evaluated at a dose of 90 mg/kg rat body weight. The extract was found to contain Iriflophenone 3-C-β-D-glucoside and mangiferin which along with 3 β taraxerol and other sterols could be contributing to the cholesterol lowering activity.
Key words: Hypercholesterolemia, Mangifera indica, mangiferin, mango leaves
INTRODUCTION
High cholesterol is the 6th risk factor for death in the world.[1] Diets high in saturated fat, physical inactivity, and genetics can increase cholesterol levels. Cholesterol increases the risks of heart disease, stroke, and other vascular diseases. Globally, one-third of ischemic heart disease is attributable to high blood cholesterol. Hypercholesterolemia is the root cause for atherosclerosis and other cardiac complications. Individuals with elevated low-density lipoprotein (LDL) cholesterol are prone to the development of coronary heart disease through multiple stages of the process. Lowering of serum LDL cholesterol is the primary target of therapy. A number of clinical trials on cholesterol-lowering therapy using 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors (statins [STs])[2] are published. STs are drugs of first choice in hypercholesterolemic patients, especially in those at high cardiovascular risk, some of them are intolerant to STs.[3] Nutraceuticals are borderline devices between nutrients and drugs providing a supplementation of particular nutrients with beneficial effects on health. Nutraceuticals derived from plants have been suggested to improve plasma lipid profile.[4] Extracts of gum ghatti of Anogeissus latifolia, Sida rhomboidea, soy protein, grape seeds, garlic, ginger, and citrus peel have been assessed for hypocholesterol activity.[5,6,7,8,9,10,11] In particular, many herbal extracts are studied for controlling the cholesterol level. One such plant is Mangifera indica L. (Family; Anacardiaceae) commonly known as Mango, a large evergreen tree of tropical and subtropical region of India and other Asian countries.[12]M. indica is one of the most famous of all tropical fruiting trees.[13] There are more than thousand varieties of mango trees all over the world. Most parts of the tree (fruit, seed, pulp, stem bark, root, and leaves) have shown medicinal properties.[14] In ayurvedic literature, different parts of this plant have been recommended as a remedy for various ailments. It is reported as antidiabetic,[15] anti-oxidant,[16] antiviral,[17] cardiotonic,[18] hypotensive,[19] anti-inflammatory,[20] antibacterial,[21] antifungal,[22] anthelmintic,[23] antiparasitic,[24] antitumor,[25] anti-HIV,[25] anti-bone resorption,[26] antispasmodic,[27] antipyretic,[27] antidiarrheal,[28] anti-allergic,[29] immunomodulation,[30] hypolipidemic,[31] hepatoprotective,[31] antimicrobial,[32] and gastroprotective.[33] Phytochemical studies on various parts of M. indica L. revealed that it contains phenolic acids, phenolic esters, flavonols, etc., Mangiferin, a natural C-glucoside xanthone,[34] has been reported from various parts of M. indica and it had been studied for many pharmacological activities such as antidiabetic, rheumatoid arthritis, anti-inflammatory, hypolipidemic, cardiotonic, and antioxidant activities.[35,36,37,38,39,40] The leaves of M. indica have been studied for antidiabetic properties using normoglycemic, glucose-induced hyperglycemia and streptozotocin-induced diabetic mice.[41] The aqueous extract of the leaves of M. indica has been reported to possess hypoglycemic activity.[15] Flavonoid-rich fraction of kernels of M. indica, leaf, and bark extract have shown anti-atherogenic activity and excretion of cholesterol through feces.[42] The ethanolic extract of immature leaf has been assessed for favorable hypolipidemic and hepatoprotective activities.[31] However, there is no report on the use of methanol extract of leaf for hypocholesterol activity. Hence, the present study involving the development of standardized methanol extract of M. indica leaf for hypercholesterolemia was undertaken. The oral toxicity study of leaf methanol extract at 5000 mg/kg body weight was also determined using female Wistar rats.
MATERIALS AND METHODS
Chemicals
Cholesterol AR and cholic acid were purchased from HiMedia Laboratories, Mumbai, India; ezetimibe tablet was from Lupin Ltd., Mumbai, India, and atorvastatin tablet from Ranbaxy Laboratories Ltd., Gurgaon, India; cholesterol estimation kits and triglycerides estimation kits were purchased from Bhat Bio-Tech India (P) Ltd., Bangalore, India.
Plant material
Fresh leaves of M. indica (Sindoora variety) were collected from Krishnagiri district, Tamil Nadu, India, and identified by Dr. P. Santhan, Taxonomist, Natural Remedies Pvt. Ltd, Bengaluru. A voucher specimen was deposited in the Agronomy Department of Natural Remedies Pvt. Ltd. The leaves were washed with water to remove mud and dusts. Further, the material was dried at room temperature, followed by hot air oven at not >40°C. The leaves were made into coarse powder for the extraction.
Preparation of standardized methanol extract
Leaves of Sindoora variety (5 kg) were extracted 3 times with methanol under the conditions of reflux for 3 h. The methanol extracts were filtered, combined, and concentrated at 60°C under vacuum using a rotary evaporator. The final powdered form of methanol extract was analyzed by high-performance liquid chromatography (HPLC) and used for hypocholesterol study.
High-performance liquid chromatography quantification of Iriflophenone3-C-β-D-glucoside and Mangiferin in the methanol extract
The HPLC instrument consisted of Shimadzu SIL-10A Autoinjector, sample cooler, two CTO-10A pumps, CTO-10A column oven, SPD-M10AVP diode array detector, and SCL 10AVP central unit (Shimadzu Ltd, Kyoto, Japan). A purospher star Hiber® (250 mm × 4.6 mm, 5 μm, Merck, Damstadt, Germany) was used. The gradient mobile phase consisted of water (solvent A) and acetonitrile (solvent B). The flow rate of the effluent was 1.5 ml/min, with run time of 52 min. Analysis was performed at room temperature using a gradient elution program: 0–5 min, 5–17% phase B; 5–10 min, 17–18% phase B; 10–15 min, 18–20% phase B; 15–20 min, 20–22% phase B; 20–25 min, 22–25% phase B; 25–30 min, 25–95% phase B; 30–34 min, 95–5% phase B; the detector wavelength was 280 nm and the volume of injection was 20 µL.
Animals and diet for inducing hypercholesterolemia
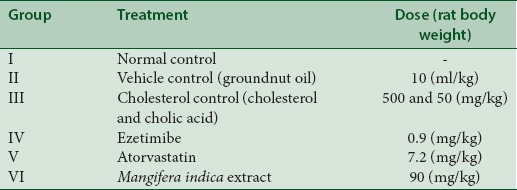
Male albino Wistar rats were divided into six groups. Six rats were randomly allotted to each group. Group I served as normal control. Group II was administered with groundnut oil (10 ml/kg) and Group III was treated with cholesterol at the dose of 500 mg/kg and cholic acid at the dose of 50 mg/kg in groundnut oil. Group IV was administered with ezetimibe at the dose of 0.9 mg/kg and Group V was treated with atorvastatin at 7.2 mg/kg rat body weight. Group VI was treated with the extract of M. indica at 90 mg/kg [Table 1]. Demineralized water was used as a vehicle for administration of reference standards and test substance. All the treatments were given daily by oral gavage for 42 days.
Table 1.
Study design
Individual animal body weight was recorded at initiation of the study and mean group body weights were calculated thereafter weekly, until the end of the experiment. Plasma levels of cholesterol and triglycerides were estimated at weekly intervals, till the end of the study period.
Acute toxicity study
Acute oral toxicity test was performed as per OECD-423 guidelines. Healthy adult female rats, acclimatized to laboratory conditions for 1 week before dosing, were used in this study. Animals were randomly assigned to the cages, and the individual animal was fur marked with picric acid. The females were nulliparous and not pregnant.
The rats were deprived of feed overnight before and 3 h after the administration of the test substance. Water was not withheld during this period. The test substance, solubilized in demineralized water, was administered by gavage to rats for 14 days using an intubation needle of appropriate size fitted into a syringe.[43]
Biochemical estimations
Total cholesterol
Serum total cholesterol was estimated by cholesterol oxidase-phenol amino antipyrine method using commercial kit.
Statistical analysis
The data were analyzed using one-way ANOVA followed by Bonferroni method as post hoc test. In case of heterogeneous data, after transformation Dunnett T3 method was used. All values were reported as mean ± standard error of mean. The statistical significance was set at P < 0.05.
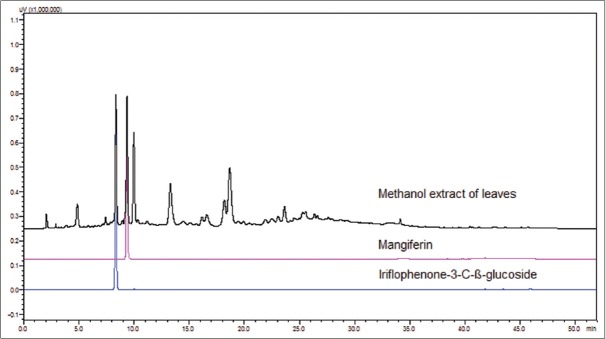
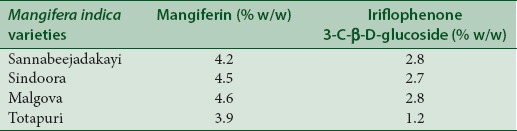
Mangiferin and iriflophenone-3-C-β-glucoside quantified in leaf methanol extract by HPLC [Figure 1] in different varieties of mango were found to be ranging 3.9–4.6% w/w and 1.2–2.8% w/w, respectively [Table 2]. The content of 3β-taraxerol was also measured using previously described method[34] and was found to be 0.40–0.49% w/w.
Figure 1.
High-performance liquid chromatography chromatograms overlay of standards and Mangifera indica leaf methanol extract
Table 2.
Details of amount of iriflophenone 3-C-β-D-glucoside and mangiferin in different varieties of mango leaves
The mean body weight of experimental rats at weekly intervals is represented in Figure 2. On day 0, no significant difference in body weight was observed in all the groups. There was no significant difference in body weight observed in cholesterol control rats when compared to vehicle control rats on different weekly time intervals of the study period.
Figure 2.
Effect of Mangifera indica extract on body weight of animals
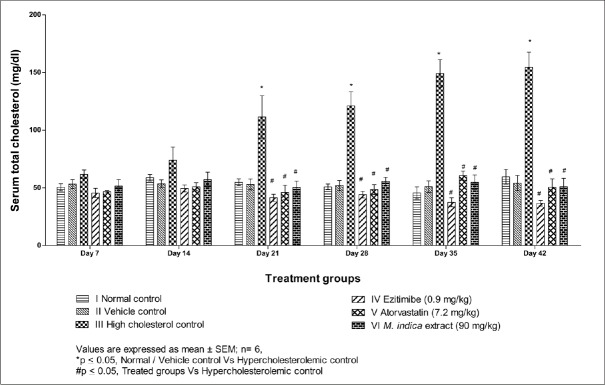
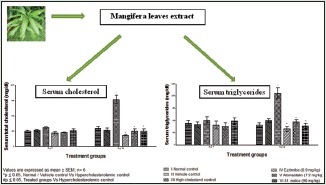
The mean plasma cholesterol levels at weekly intervals are presented in Table 3 and Figure 3. The plasma cholesterol was significantly increased in cholesterol control from day 21 to day 42 with a nonsignificant increase observed during day 7 to day 14 when compared to vehicle control. A significant decrease in plasma cholesterol was observed on treatment with an extract of M. indica and all other substances from day 21 to day 42 as compared to cholesterol control rats.
Table 3.
Effect of extract of Mangifera indica on plasma cholesterol in albino Wistar rats
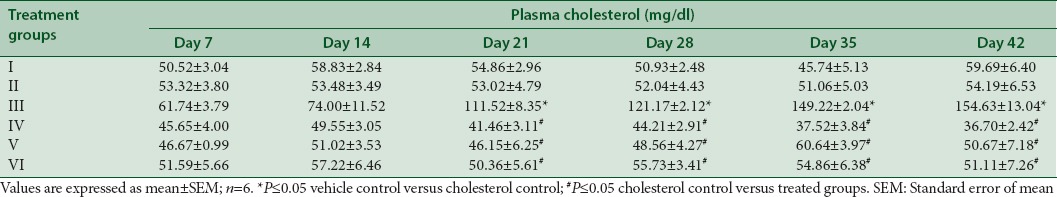
Figure 3.
Effect of extract of Mangifera indica leaves on serum total cholesterol level in albino Wistar rats
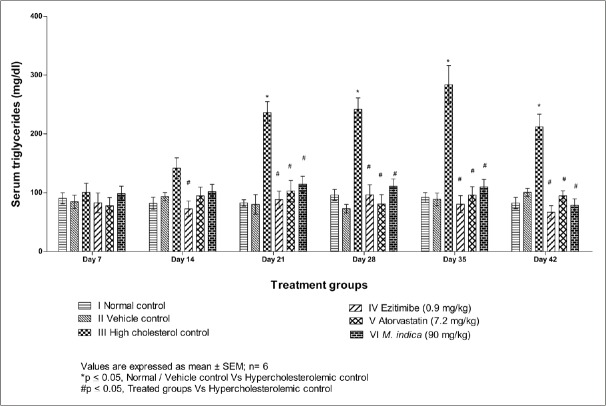
The mean plasma triglycerides levels at different time intervals are presented in Table 4 and Figure 4. The plasma triglyceride was significantly increased in cholesterol control rats from day 21 to day 42 as compared to vehicle control rats. A significant decrease in plasma triglycerides was observed on treatment with an extract of M. indica and all other substances from day 21 to day 42 as compared to cholesterol control rats.
Table 4.
Effect of extract of Mangifera indica on plasma triglycerides in albino Wistar rats
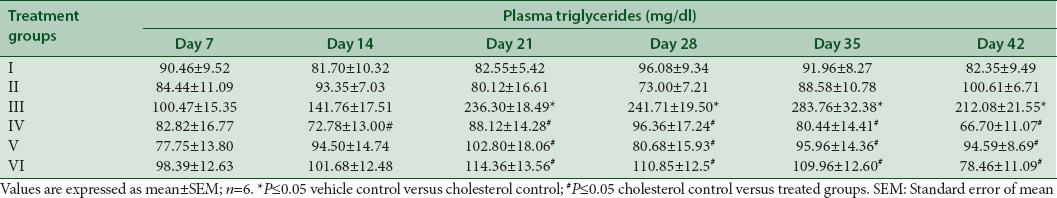
Figure 4.
Effect of extract of Mangifera indica leaves on serum triglycerides level in albino Wistar rats
Observations of clinical signs were made at 10 min, 30 min, 1 h, 2 h, 4 h, and 6 h after dosing on day 0 and once daily thereafter for 14 days at approximately same time. Cage-side observations included changes in the skin, fur, eyes, and mucous membrane. It also included respiratory, circulatory, autonomic and central nervous system, and somato motor activity and behavioral pattern. Particular attention was directed to the observation of tremors, convulsion, salivation, diarrhea, lethargy, sleep, and coma.
In acute oral toxicity studies, the test animals did not show any significant physical changes and behavioral patterns. There was no mortality even after 14 days observation, and there were no abnormalities found in the organs after the sacrifice of animals when compared to the control at the end of 14 days of general observation [Table 5].
Table 5.
Effect of extract of Mangifera indica on mortality of albino Wistar rats in acute oral study

DISCUSSION
M. indica has been reported to possess antidiabetic and hypolipidemic activities.[41,42,44] Phytochemical analysis of M. indica has shown the presence of many bioactive compounds such as flavonoids and phenolics. In our previous study, identification of bioactive compounds through BAGF, we have reported that ethyl acetate fraction of M. indica leaf methanolic extract has comparatively higher potency in cholesterol esterase inhibition assay, and further fractionation of this extract yielded 3β-taraxerol enriched fraction, which inhibited cholesterol esterase in vitro with an IC50 of 0.86 µg/ml.[45] In the present in vivo study, the leaf methanol extract of M. indica has shown a significant reduction in serum cholesterol level in albino Wistar rats. The dose of 90 mg/kg body weight was chosen to consider the human dose of 1.0 g/day. This single dose study has shown reduction of 14% serum cholesterol level compared to 17% and 63% reduction caused by atorvastatin and ezetimibe, respectively. In case of triglycerides, the reduction due to extract was 31% compared to 58% and 11% caused by atorvastatin and ezetimibe, respectively. The standards used in the study were ezetimibe and atorvastatin that are known to act by different mechanism in the regulation of cholesterol. Ezetimibe, a potent inhibitor of cholesterol absorption, acts at the brush border of the small intestine to inhibit intestinal absorption of dietary and biliary cholesterol across the intestinal wall and decreasing the delivery of intestinal cholesterol to the liver. However, HMG-CoA reductase inhibitors (STs) act by blocking the HMG-CoA reductase enzyme, which catalyzes the rate-limiting step in de novo cholesterol synthesis. The compounds such as mangiferin, iriflophenone derivatives are reported to show antidiabetic activity.[46] The results of the present study have shown that extract treated groups had significantly reduced the levels of cholesterol, and the activity was comparable with the activity of standards used in the study. The chemical constituents present in the extract have shown hypocholesterol activity by different mechanisms. The water-soluble flavonoids present in the extract might be contributing for HMG-CoA reductase inhibition,[35] whereas the nonpolar sterol like 3β-taraxerol present in the extract might be competing with cholesterol for absorption. Thereby, the different constituents present in the extract could contribute to the reduction of cholesterol and triglycerides level in the study.
The nontoxic nature of methanol extract of M. indica was confirmed from the acute oral toxicity study as per Organisation for Economic Co-operation and Development (OECD) guidelines. The results indicated that the methanol extract of M. indica is nontoxic at the dose of 5000 mg/kg body weight of animal and test animals have shown normal behavior during a period of 14 days.
CONCLUSION
The methanol extract of M. indica showed a significant cholesterol-lowering activity at 90 mg/kg from day 21 to day 42 of 6 weeks treatment period, and a significant decrease in plasma triglycerides was also observed on treatment with extract. The 3β-taraxerol, mangiferin, and iriflophenone-3-C-β-glucoside quantified in leaf methanol extract by HPLC and were found to be 0.49% w/w, 4.6% w/w, and 2.37% w/w, respectively. The methanol extract of M. indica leaf was found to be safe after oral administration of single dose of 5000 mg/kg body weight to female albino Wistar rats.
Financial support and sponsorship
Financial support for this study was partially provided by the National Medicinal Plants Board (New Delhi).
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Shekhar Michael Dethe
Shekhar M. Dethe, is a Senior Scientist at Natural Remedies R and D Centre, Bangalore. He has a Doctorate Degree in Biochemistry. His research specialization includes studies in Mixed Function Oxidase System, In vitro enzyme inhibition assays, Herb-drug interaction, Bioavailability and in vitro absorption studies. He has authored more than 20 peer-reviewed papers in scientific journals.
Acknowledgments
The authors would like to thank the National Medicinal Plants Board (New Delhi) for partial financial support.
REFERENCES
- 1. [Last accessed on 2015 Jan 10]. Available from: http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_part 2.pdf .
- 2.Cleeman JI. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Pisciotta L, Bellocchio A, Bertolini S. Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Health Dis. 2012;11:123. doi: 10.1186/1476-511X-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannarino MR, Ministrini S, Pirro M. Nutraceuticals for the treatment of hypercholesterolemia. Eur J Intern Med. 2014;25:592–9. doi: 10.1016/j.ejim.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Parvathi KM, Ramesh CK, Krishna V, Paramesha M, Kuppast IJ. Hypolipidemic activity of gum ghatti of Anogeissus latifolia. Pharmacogn Mag. 2009;5:11. [Google Scholar]
- 6.Devkar RV, Ramachandran AV, Patel DK, Patel KA, Patel UK, Thounaojam MC, et al. Assessment of lipid lowering effect of Sida rhomboidea. Roxb methanolic extract in experimentally induced hyperlipidemia. J Young Pharm. 2009;1:233. [Google Scholar]
- 7.Zhong F, Liu J, Ma J, Shoemaker CF. Preparation of hypocholesterol peptides from soy protein and their hypocholesterolemic effect in mice. Food Res Int. 2007;40:661–7. [Google Scholar]
- 8.Yamakoshi J, Kataoka S, Koga T, Ariga T. Proanthocyanidin-rich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 1999;142:139–49. doi: 10.1016/s0021-9150(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 9.Yeh YY, Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: Human and animal studies. J Nutr. 2001;131:989S–93S. doi: 10.1093/jn/131.3.989S. [DOI] [PubMed] [Google Scholar]
- 10.Bhandari U, Sharma JN, Zafar R. The protective action of ethanolic ginger (Zingiber officinale) extract in cholesterol fed rabbits. J Ethnopharmacol. 1998;61:167–71. doi: 10.1016/s0378-8741(98)00026-9. [DOI] [PubMed] [Google Scholar]
- 11.Bok SH, Lee SH, Park YB, Bae KH, Son KH, Jeong TS, et al. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: Cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J Nutr. 1999;129:1182–5. doi: 10.1093/jn/129.6.1182. [DOI] [PubMed] [Google Scholar]
- 12.Khare CP. An encyclopedia of medicinal plants of India. Heidelberg, Germany: Springer-Verlag; 2004. p. 151. [Google Scholar]
- 13.Shah KA, Patel MB, Patel RJ, Parmar PK. Mangifera indica (mango) Pharmacogn Rev. 2010;4:42–8. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma PC. Database on Medicinal Plants Used in Ayurveda. Vol. 2. New Delhi: Central Council for Research in Ayurveda & Sidha; 2001. pp. 8–28. [Google Scholar]
- 15.Aderibigbe AO, Emudianughe TS, Lawal BA. Evaluation of the antidiabetic action of Mangifera indica in mice. Phytother Res. 2001;15:456–8. doi: 10.1002/ptr.859. [DOI] [PubMed] [Google Scholar]
- 16.Sultana B, Hussain Z, Asif M, Munir A. Investigation on the antioxidant activity of leaves, peels, stems bark, and kernel of mango (Mangifera indica L.) J Food Sci. 2012;77:C849–52. doi: 10.1111/j.1750-3841.2012.02807.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhu XM, Song JX, Huang ZZ, Wu YM, Yu MJ. Antiviral activity of mangiferin against herpes simplex virus type 2 in vitro. Zhongguo Yao Li Xue Bao. 1993;14:452–4. [PubMed] [Google Scholar]
- 18.Prabhu S, Jainu M, Sabitha KE, Devi CS. Cardioprotective effect of mangiferin on isoproterenol induced myocardial infarction in rats. Indian J Exp Biol. 2006;44:209–15. [PubMed] [Google Scholar]
- 19.Ronchi SN, Brasil GA, do Nascimento AM, de Lima EM, Scherer R, Costa HB, et al. Phytochemical and in vitro and in vivo biological investigation on the antihypertensive activity of mango leaves (Mangifera indica L.) Ther Adv Cardiovasc Dis. 2015;9:244–56. doi: 10.1177/1753944715572958. [DOI] [PubMed] [Google Scholar]
- 20.Garrido G, González D, Lemus Y, García D, Lodeiro L, Quintero G, et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG) Pharmacol Res. 2004;50:143–9. doi: 10.1016/j.phrs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Doughari JH, Manzara S. In vitro antibacterial activity of crude leaf extracts of Mangifera indica Linn. Afr J Microbiol Res. 2008;2:67–72. [Google Scholar]
- 22.Kanwal Q, Hussain I, Latif Siddiqui H, Javaid A. Antifungal activity of flavonoids isolated from mango (Mangifera indica L.) leaves. Nat Prod Res. 2010;24:1907–14. doi: 10.1080/14786419.2010.488628. [DOI] [PubMed] [Google Scholar]
- 23.García D, Escalante M, Delgado R, Ubeira FM, Leiro J. Anthelminthic and antiallergic activities of Mangifera indica L. stem bark components Vimang and mangiferin. Phytother Res. 2003;17:1203–8. doi: 10.1002/ptr.1343. [DOI] [PubMed] [Google Scholar]
- 24.Perrucci S, Fichi G, Buggiani C, Rossi G, Flamini G. Efficacy of mangiferin against Cryptosporidium parvum in a neonatal mouse model. Parasitol Res. 2006;99:184–8. doi: 10.1007/s00436-006-0165-4. [DOI] [PubMed] [Google Scholar]
- 25.Guha S, Ghosal S, Chattopadhyay U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy. 1996;42:443–51. doi: 10.1159/000239478. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Miyahara T, Tezuka Y, Namba T, Nemoto N, Tonami S, et al. The effect of Kampo formulae on bone resorption in vitro and in vivo. I. Active constituents of Tsu-kan-gan. Biol Pharm Bull. 1998;21:1322–6. doi: 10.1248/bpb.21.1322. [DOI] [PubMed] [Google Scholar]
- 27.Awe SO, Olajide OA, Oladiran OO, Makinde JM. Antiplasmodial and antipyretic screening of Mangifera indica extract. Phytother Res. 1998;12:437–8. [Google Scholar]
- 28.Sairam K, Hemalatha S, Kumar A, Srinivasan T, Ganesh J, Shankar M, et al. Evaluation of anti-diarrhoeal activity in seed extracts of Mangifera indica. J Ethnopharmacol. 2003;84:11–5. doi: 10.1016/s0378-8741(02)00250-7. [DOI] [PubMed] [Google Scholar]
- 29.Rivera DG, Balmaseda IH, León AA, Hernández BC, Montiel LM, Garrido GG, et al. Anti-allergic properties of Mangifera indica L. extract (Vimang) and contribution of its glucosylxanthone mangiferin. J Pharm Pharmacol. 2006;58:385–92. doi: 10.1211/jpp.58.3.0014. [DOI] [PubMed] [Google Scholar]
- 30.Makare N, Bodhankar S, Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol. 2001;78:133–7. doi: 10.1016/s0378-8741(01)00326-9. [DOI] [PubMed] [Google Scholar]
- 31.Hossain MS, Ahmed M, Islam A. Hypolipidemic and hepatoprotective effects of different fractions of ethanolic extract of immature leaves of Mangifera indica (Linn.) in alloxan induced diabetic rats. IJPSR. 2010;1:132. [Google Scholar]
- 32.Engels C, Knödler M, Zhao YY, Carle R, Gänzle MG, Schieber A. Antimicrobial activity of gallotannins isolated from mango (Mangifera indica L.) kernels. J Agric Food Chem. 2009;57:7712–8. doi: 10.1021/jf901621m. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho AC, Guedes MM, de Souza AL, Trevisan MT, Lima AF, Santos FA, et al. Gastroprotective effect of mangiferin, a xanthonoid from Mangifera indica, against gastric injury induced by ethanol and indomethacin in rodents. Planta Med. 2007;73:1372–6. doi: 10.1055/s-2007-990231. [DOI] [PubMed] [Google Scholar]
- 34.Ichiki H, Miura T, Kubo M, Ishihara E, Komatsu Y, Tanigawa K, et al. New antidiabetic compounds, mangiferin and its glucoside. Biol Pharm Bull. 1998;21:1389–90. doi: 10.1248/bpb.21.1389. [DOI] [PubMed] [Google Scholar]
- 35.Luczkiewicz P, Kokotkiewicz A, Dampc A, Luczkiewicz M. Mangiferin: A promising therapeutic agent for rheumatoid arthritis treatment. Med Hypotheses. 2014;83:570–4. doi: 10.1016/j.mehy.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Leiro J, Arranz JA, Yáñez M, Ubeira FM, Sanmartín ML, Orallo F. Expression profiles of genes involved in the mouse nuclear factor-kappa B signal transduction pathway are modulated by mangiferin. Int Immunopharmacol. 2004;4:763–78. doi: 10.1016/j.intimp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Dineshkumar B, Mitra A, Manjunatha M. Studies on the anti-diabetic and hypolipidemic potentials of mangiferin (Xanthone Glucoside) in streptozotocin-induced type 1 and type 2 diabetic model rats. Int J Adv Pharm Sci. 2010;1:75–85. [Google Scholar]
- 38.Sato T, Kawamoto A, Tamura A, Tatsumi Y, Fujii T. Mechanism of antioxidant action of pueraria glycoside (PG)-1 (an isoflavonoid) and mangiferin (a xanthonoid) Chem Pharm Bull (Tokyo) 1992;40:721–4. doi: 10.1248/cpb.40.721. [DOI] [PubMed] [Google Scholar]
- 39.Prabhu S, Jainu M, Sabitha KE, Devi CS. A study on the beneficial effect of mangiferin on isoproterenol induced mycocardial infarction rat model. Pharmacogn Mag. 2005;1:92. [Google Scholar]
- 40.Muruganandan S, Gupta S, Kataria M, Lal J, Gupta PK. Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology. 2002;176:165–73. doi: 10.1016/s0300-483x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 41.Bhowmik A, Khan LA, Akhter M, Rokeya B. Studies on the anti-diabetic effects of Mangifera indica stem-barks and leaves on non-diabetic, type 1 and type 2 diabetic model rats. Bangladesh J Pharm. 2009;4:110–4. [Google Scholar]
- 42.Anila L, Vijayalakshmi NR. Flavonoids from Emblica officinalis and Mangifera indica -effectiveness for dyslipidemia. J Ethnopharmacol. 2002;79:81–7. doi: 10.1016/s0378-8741(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 43.Sahgal G, Ramanathan S, Sasidharan S, Mordi MN, Ismail S, Mansor SM. Brine shrimp lethality and acute oral toxicity studies on Swietenia mahagoni (Linn.) Jacq. seed methanolic extract. Pharmacognosy Res. 2010;2:215–20. doi: 10.4103/0974-8490.69107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diego AM, Ripoll C, Ilic N, Alexander P, Cristin A, Raskin I. Inhibition of lipid metabolic enzymes using Mangifera indica extracts. J Food Agric Environ. 2006;4:21–6. [Google Scholar]
- 45.Gururaja GM, Mundkinajeddu D, Dethe SM, Sangli GK, Abhilash K, Agarwal A. Cholesterol esterase inhibitory activity of bioactives from leaves of Mangifera indica L. Pharmacognosy Res. 2014;7:355–62. doi: 10.4103/0974-8490.159578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pranakhon R, Aromdee C, Pannangpetch P. Effects of iriflophenone 3-C-ß-glucoside on fasting blood glucose level and glucose uptake. Pharmacogn Mag. 2015;11:82–9. doi: 10.4103/0973-1296.149711. [DOI] [PMC free article] [PubMed] [Google Scholar]