Abstract
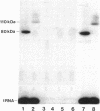
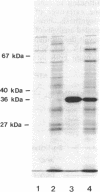
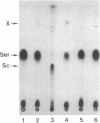
The selD gene from Escherichia coli, whose product is involved in selenium metabolism, has been cloned and sequenced. selD codes for a protein of 347 amino acids with a calculated molecular weight of 36,687. Analysis of the selD gene product through expression of the gene in the phage T7 promoter/polymerase system confirmed the predicted molecular weight of the protein. Gene disruption experiments demonstrated that the SelD protein is required both for the incorporation of selenium into the modified nucleoside 5-methylaminomethyl-2-selenouridine of tRNA and for the biosynthesis of selenocysteine from an L-serine residue esterbonded to tRNA(Ser)(UCA). tRNA(Ser)(UCA) has been purified, aminoacylated with L-serine, and used as a substrate for the development of an in vitro system for selenocysteine biosynthesis. Efficient formation of selenocysteinyl-tRNA(Ser)(UCA) was achieved by using extracts in which both the selD and the selA gene products were overproduced. The results demonstrate that selenocysteine is synthesized from L-serine bound to tRNA(UCA) and they are in accord with SelD functioning as a donor of reduced selenium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett E. L., Jackson C. E., Fukumoto H. T., Chang G. W. Formate dehydrogenase mutants of Salmonella typhimurium: a new medium for their isolation and new mutant classes. Mol Gen Genet. 1979;177(1):95–101. doi: 10.1007/BF00267258. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Böck A., Stadtman T. C. Selenocysteine, a highly specific component of certain enzymes, is incorporated by a UGA-directed co-translational mechanism. Biofactors. 1988 Oct;1(3):245–250. [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cox J. C., Edwards E. S., DeMoss J. A. Resolution of distinct selenium-containing formate dehydrogenases from Escherichia coli. J Bacteriol. 1981 Mar;145(3):1317–1324. doi: 10.1128/jb.145.3.1317-1324.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. P., Sommer R., Polke C., Beck E., Schaller H. Structure of the orgin of DNA replication of bacteriophage fd. Proc Natl Acad Sci U S A. 1978 Jan;75(1):50–53. doi: 10.1073/pnas.75.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Mandrand-Berthelot M. A. Escherichia coli formate-to-nitrate respiratory chain: genetic analysis. Biochem Soc Trans. 1982 Dec;10(6):478–480. doi: 10.1042/bst0100478. [DOI] [PubMed] [Google Scholar]
- Holmes W. M., Hurd R. E., Reid B. R., Rimerman R. A., Hatfield G. W. Separation of transfer ribonucleic acid by sepharose chromatography using reverse salt gradients. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1068–1071. doi: 10.1073/pnas.72.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G. F., Ames B. N. Isolation and characterization of a selenium metabolism mutant of Salmonella typhimurium. J Bacteriol. 1988 Feb;170(2):736–743. doi: 10.1128/jb.170.2.736-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee B. J., Worland P. J., Davis J. N., Stadtman T. C., Hatfield D. L. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989 Jun 15;264(17):9724–9727. [PubMed] [Google Scholar]
- Leinfelder W., Forchhammer K., Zinoni F., Sawers G., Mandrand-Berthelot M. A., Böck A. Escherichia coli genes whose products are involved in selenium metabolism. J Bacteriol. 1988 Feb;170(2):540–546. doi: 10.1128/jb.170.2.540-546.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinfelder W., Stadtman T. C., Böck A. Occurrence in vivo of selenocysteyl-tRNA(SERUCA) in Escherichia coli. Effect of sel mutations. J Biol Chem. 1989 Jun 15;264(17):9720–9723. [PubMed] [Google Scholar]
- Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988 Feb 25;331(6158):723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- Lyons L. B., Zinder N. D. The genetic map of the filamentous bacteriophage f1. Virology. 1972 Jul;49(1):45–60. doi: 10.1016/s0042-6822(72)80006-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Maruyama N., Hitaka T., Sukenaga Y. The detection of natural opal suppressor seryl-tRNA in Escherichia coli by the dot blot hybridization and phosphorylation by a tRNA kinase [corrected] . FEBS Lett. 1989 Apr 24;247(2):345–348. doi: 10.1016/0014-5793(89)81367-5. [DOI] [PubMed] [Google Scholar]
- Mizutani T. Some evidence of the enzymatic conversion of bovine suppressor phosphoseryl-tRNA to selenocysteyl-tRNA. FEBS Lett. 1989 Jul 3;250(2):142–146. doi: 10.1016/0014-5793(89)80707-0. [DOI] [PubMed] [Google Scholar]
- Nader W. F., Edlind T. D., Huettermann A., Sauer H. W. Cloning of Physarum actin sequences in an exonuclease-deficient bacterial host. Proc Natl Acad Sci U S A. 1985 May;82(9):2698–2702. doi: 10.1073/pnas.82.9.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecher A., Zinoni F., Böck A. The seleno-polypeptide of formic dehydrogenase (formate hydrogen-lyase linked) from Escherichia coli: genetic analysis. Arch Microbiol. 1985 May;141(4):359–363. doi: 10.1007/BF00428850. [DOI] [PubMed] [Google Scholar]
- Roy K. L., Söll D. Purification of five serine transfer ribonucleic acid species from Escherichia coli and their acylation by homologous and heterologous seryl transfer ribonucleic acid synthetases. J Biol Chem. 1970 Mar 25;245(6):1394–1400. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C., Davis J. N., Zehelein E., Böck A. Biochemical and genetic analysis of Salmonella typhimurium and Escherichia coli mutants defective in specific incorporation of selenium into formate dehydrogenase and tRNAs. Biofactors. 1989 Mar;2(1):35–44. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer A. J., Stadtman T. C. Biosynthesis of 5-methylaminomethyl-2-selenouridine, a naturally occurring nucleoside in Escherichia coli tRNA. Arch Biochem Biophys. 1986 Aug 1;248(2):540–550. doi: 10.1016/0003-9861(86)90507-2. [DOI] [PubMed] [Google Scholar]
- Wittwer A. J., Tsai L., Ching W. M., Stadtman T. C. Identification and synthesis of a naturally occurring selenonucleoside in bacterial tRNAs: 5-[(methylamino)methyl]-2-selenouridine. Biochemistry. 1984 Sep 25;23(20):4650–4655. doi: 10.1021/bi00315a021. [DOI] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]