Abstract
Objective:
The aim of this study is to determine the phytochemical composition, antifungal activity of Mentha piperita essential oil (MPE) against Fusarium sporotrichioides.
Methods:
The phytochemical composition was conducted by gas chromatography mass spectrometry (GC MS) analysis and mycelia growth inhibition was determined by minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC), the morphological characterization was observed by scanning electron microscopy. Finally, the membrane permeability was determined by the release of extracellular constituents, pH, and total lipid content.
Result:
In GC MS analysis, 22 metabolites were identified such as menthol, l menthone, pulegone, piperitone, caryophyllene, menthol acetate, etc. The antifungal activity against targeted pathogen, with MIC and MFC 500 μg/mL and 1000 μg/mL, respectively. The MPE altered the morphology of F. sporotrichoides hyphae with the loss of cytoplasm content and contorted the mycelia. The increasing concentration of MPE showed increase in membrane permeability of F. sporotrichoides as evidenced by the release of extracellular constituents and pH with the disruption of cell membrane indicating decrease in lipid content of F. sporotrichoides.
Conclusion:
The observed results showed that MPE exhibited promising new antifungal agent against Fusarium sporotrichioides.
SUMMARY
F. sporotrichioides, filamentous fungi contaminate to corn and corn--based products
F. sporotrichioides mainly responsible for the production of T-2 toxin
Phytochemical composition was conducted by gas chromatography--mass spectrometry analysis
Mentha piperita essential oil (MPE) is commonly known as peppermint
The F. sporotrichioides growth was inhibited by MPE (minimum inhibitory concentration, minimum fungicidal concentration)
Morphological observation by scanning electron microscope.
Abbreviations Used: Cfu: Colony forming unit; DMSO: Dimethyl sulfoxide, °C: Degree celsius; F. Sporotrichoides: Fusarium sporotrichioides; EOs: Essential oils; M: Molar, g: Gram/gravity, mg: Milligram; μg: Microgram, ml: Milliliter; mm: Millimeter, min: Minutes; M. piperita: Mentha piperita, MIC: Minimum inhibitory concentration; MFC: Minimum fungicidal concentration; MAE: Mentha arvensis essential oil; Na2SO4: Sodium sulfate; pH: Potential Hydrogen; PDB: Potato Dextrose Broth; SEM: Scanning electron microscope
Key words: Fusarium sporotrichioides, Mentha piperita essential oil, gas chromatography-mass spectrometryGC-MS, Mentha piperita essential oil, scanning electron microscope
INTRODUCTION
Fusarium sporotrichioides, a filamentous fungi of the section sporotrichiella, necrotrophic pathogen associated with Fusarium head blight of cereals.[1,2,3] F. sporotrichioides, Fusarium acuminatum, Fusarium Sambucinum, and Fusarium poae are predominant species widespread in the soil and on plants, throughout the cold and cool regions of the world. They invade cereal crops and are responsible for the production toxic metabolites such as type A trichothecenes: T-2 toxin, HT-2 toxin, T-2 tetraol, T-2 triol, scirpentriol (STO), and diacetoxyscirpenol which may contaminate animal and human food.[4,5,6,7,8,9,10,11,12,13] Trichothecene is sesquiterpenoid highly toxic; T-2 toxin and HT-2 toxin are potent inhibitor of protein synthesis and are highly cytotoxic to eukaryotes.[14] T-2 toxin, HT-2 toxin found to be contaminants in wheat, maize, barley, oats, rice, beans, and soybeans as well as in some cereal-based products and have played a role in plant diseases. These toxins are an important agricultural problem due to their detrimental effects on both human and animal health.[15,16,17] Trichothecene has acute symptoms in man associated with high-level intake of trichothecenes include necrotic lesions of the oral cavity, esophagus, and stomach, nausea, vomiting, abdominal pain, diarrhea, dizziness, headache, and marked leukopenia.[18,19] Within the outbreak of the so-called “alimentary toxic aleukia” documented.[18,20] Therefore, control of F. sporotrichioides growth in agricultural products is essential to reduce food-borne illness. Several low-molecular organic acids antifungal chemical have been used to control the growth of F. sporotrichioides during grain storage. These chemical fungicides show serious health issues in humans and animals due to their toxicity by the migration of chemical residue into food chain and they also produce resistance in fungi.[21,22,23] Hence, natural plant components may act as an alternative to these chemical preservatives and fungicides.
Plants essential oils have long been researched for their antibacterial, antifungal, antiviral, and antioxidant properties. Essential oils and their principle compounds, such as menthol, eugenol, cinnamaldehyde, thymol, carvacrol, terpineol, and citral, have been considered to be effective against many filamentous fungi. The several studies on essential oils cinnamon, citral, Litsea cubeba, Zingiber officinale, clove, eucalyptus, anise, peppermint, spearmint, and camphor oils show inhibitory activity against pathogenic microorganisms. cinnamon essential oil showed potent antifungal activity against mycotoxigenic fungi Aspergillus flavus,[24] citral, octanal, and a-terpineol volatile oils against Geotrichum citri-aurantii[25] and clove and Cinnamon oils were found to be effective against A. flavus which is responsible for aflatoxin production in maize under favorable conditions.[26,27,28] Growth inhibition and morphological alterations of Fusarium verticillioides and inhibition of fumonisin B1 by Z. officinale, Rosmarinus officinalis L., cinnamon essential oil reported by Xing et al., 2014,[29] Yamamoto-Ribeiro et al., 2013[30] da Silva Bomfim et al., 2015.[31]
Mentha piperita essential oil (MPE) is commonly known as peppermint. Leaves of mint plant are aromatic perennial herb cultivated in most part of the world. It is used as folk medicine and frequently used in herbal tea and for culinary purpose to add flavor and aroma. The essential oil of M. piperita rich in menthol, l-menthone, pulegone, piperitone, menthol acetate, piperitenone menthone, carvone, menthofuran, isomenthone, menthyl acetate, isopulegol, menthol, 3-octanol, pulegone; hence, it also used as natural antioxidants.[32,33,34,35] MPE have shown potent inhibitory activity against several microorganisms such as Penicillium digitatum, A. flavus, Aspergillus niger, Candida albicans, Saccharomyces cerevisiae Mucor spp., and Fusarium oxysporum,[33,36] further MPE reported as antifungal agent for several mycotoxigenic speceies by Silva et al., 2012;[37] A. flavus and A. parasiticus, Freire et al., in 2012[38] reported against Aspergillus ochraceous, Colletotrichum gloeosporioides, Colletotrichum musae, F. oxysporum, Fusarium semitectum. Currently, it has been widely used in cosmetic, food and pharmaceutical industries no studies of which we are aware have reported the effects of MPE on F. sporotrichioides. Therefore, the present study was undertaken to evaluate the effects of MPE against the growth of F. sporotrichioides. Morphological alteration was investigated by scanning electron microscopy.
MATERIALS AND METHODS
Preparation of essential oil
The plant was collected from herbal farm, Mysore, India. Leaves are washed thoroughly, dried in shade, and powdered. The essential oil was obtained from hydrodistillation using a Clevenger-type apparatus in accordance with the method recommended by the European Pharmacopoeia.[39] The extraction was performed for 180 min and 200 g of powder yield the 1000 μL of oil. The oil obtained was stored at 4°C and protected from light for subsequent use and chemical analysis.
Gas chromatography-mass spectrometry analysis of oil
The GC-MS analyses were performed in EI mode (70 eV) with an Agilent 7890 GC system, equipped with model 5975 mass selective detector (Agilent Technologies, USA). SGE BPX5 fused silica capillary columns (30 m × 0.32 mm i.d., 0.25 µm film thickness) were employed for separation; the column oven temperature was raised linearly from 80°C (hold for 2 min) to 280°C (hold for 5 min) at 20°C/min. Helium was used as carrier gas at constant flow of 1.2 mL/min. The samples were analyzed in splitless mode at injection temperature of 250°C, EI source temperature 230°C, and quadrupole analyzer at 150°C, ionization current at 235 eV.
Culture and preparation of Fusarium sporotrichioides
F. sporotrichioides Microbial Type Culture Collection 1894[40] was used as the test organism and was obtained from the Department of Microbiology, Defence Food Research Laboratory, Mysore. The fungus was purified and harvested at 37°C on potato dextrose broth (PDB). The spores concentration was adjusted to 5 × 105 CFU/mL using a hemocytometer.
Minimum inhibitory concentration and minimum fungicidal concentration
Minimum inhibitory concentration (MIC) determination was performed using 96-well microtiter plates. The F. sporotrichioides fungal inocula in 96-well microtiter plates were treated with MPE at different concentrations and incubated for 24 h; then, absorbance was measured at 575 nm. Fluconazole was used as the standard and also to compare the cell viability under inverted microscope. The minimum fungicidal concentration (MFC) was regarded as the lowest concentration that prevented growth of the fungus following 72 h incubation at 28°C in a fresh potato dextrose agar (PDA) plate, indicating more than 99.5% killing of the original inocula.[41]
Scanning electron microscopy
The 24 h old fungal culture on PDB treated with MPE at varied concentrations of control, MIC and MFC were used for scanning electron microscope (SEM) observation. The F. sporotrichioides cells were fixed in glutaraldehyde in 0.1 M PBS for 30 min, washed with 0.1 M PBS, and dehydrated by immersing in ice-cold ethanol for 10 min. The dehydrated samples were smeared on silver stub like a thin film and were coated by cathodic spraying (Polaron gold). The SEM observations were made using a ZEISS Instrument (EHT = 15.00 kV, signal A = VPSE G3).
Release of cellular constituents
The release of cellular constituents into the supernatant was measured following Paul et al.[42] method. The 2 days old mycelia from PDB were collected by centrifugation at 4000 g for 20 min, washed 3 times, and resuspended in 100 mL phosphate buffer saline (pH 7.0). The resulting suspension was treated with MPE with MIC and MFC for 0, 30, 60, and 120 min. Then, 2 mL of sample was collected and centrifuged at 12,000 g for 2 min, and 1 mL of supernatant was used to measure the cellular constituents in spectrophotometer at 260 nm.
Measurement of extracellular pH
The extracellular pH was measured by Shao et al.[43] method. The extracellular pH of F. sporotrichioides was determined by eutech pH meter. The fungal suspension 105 CFU/mL were added to 20 mL PDB and incubated in a moist chamber at 28°C ± 2°C for 2 days. The mixtures were centrifuged at 4000 g for 20 min, and the resulting pellet was collected, washed for 2–3 times with sterilized double-distilled water, and resuspended in 20 mL sterilized double-distilled water. After the addition of the MPE, the extracellular pH was determined.
Determination of lipid content
Total lipid content of F. sporotrichioides cells with the MPE at various concentrations (control, MIC, and MFC) was determined using phosphovanillin method.[44,45] The 2-day-old mycelia from 50 mL PDB were collected and centrifuged at 4000 g for 10 min. Then, the samples are lyophilized. About 0.1 g of dry mycelia were homogenized with liquid nitrogen and extracted with 4.0 mL of methanol: chloroform: water mixture (2:1:0.8, v/v/v) with vigorous shaking for 30 min. The resulting sample was centrifuged at 4000 g for 10 min. The lower phase containing lipids was mixed with 0.2 mL saline solution and centrifuged at 4000 g for 10 min. To the lipid mixture add 0.2 mL chloroform and 0.5 mL of H2 SO4 and warm in a boiling water bath for 10 min. After that, 3 mL phosphovanillin was added and shaken vigorously, and then incubated at room temperature for 10 min. The absorbance was measured at 520 nm. Cholesterol was used as a standard.
RESULTS
Gas chromatography-mass spectrometry analysis
Phytochemical analysis was carried out by gas chromatography-mass spectrometry to determine the chemical composition of MPE. A total of 22 metabolites were identified. The chemical formulas and mass of the detected compounds chromatogram are presented in Table 1 and Figure 1.
Table 1.
Phytochemical constituents of Mentha piperita essential oil by gas chromatography-mass spectrometry analysis
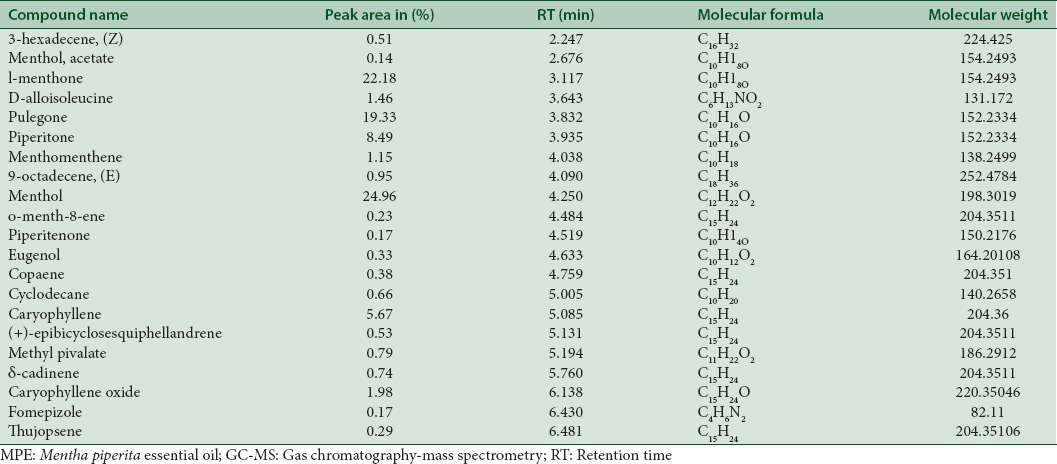
Figure 1.
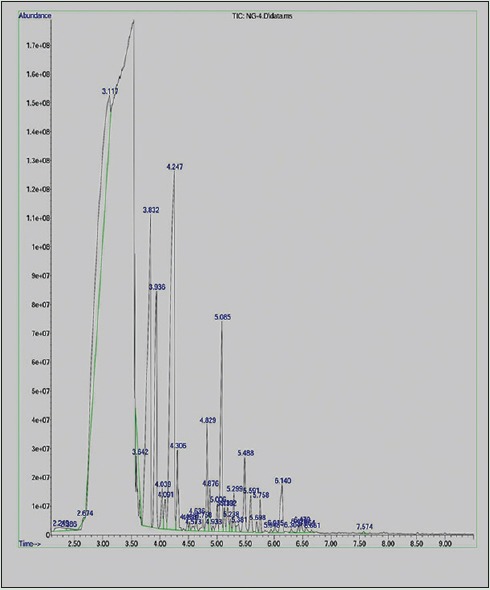
Chromatogram of Mentha piperita essential oil
Minimum inhibitory concentration
The antifungal activity of MPE was estimated using cell viability assay with fluconazole as the standards. The MIC and MFC values of MPE are shown in Table 2 and Figure 2. MPE at a concentration of 500 µg/mL inhibited mycelial growth; whereas at 1000 µg/mL (MFC), it induced fungicidal activity.
Table 2.
Minimum inhibitory concentrations and minimum fungicidal concentrations of Mentha piperita essential oil

Figure 2.
Inverted microscopic observation of Fusarium sporotrichioides: (a) Control, (b) fluconozole, (c) 500 μg/mL Mentha piperita essential oil, (d) 1000 μg/mL Mentha piperita essential oil
Scanning electron microscopy
The effect of the MPE on the morphology of F. sporotrichioides as examined through SEM is shown in Figure 3. The control fungus grown on PDA had normal tubular hyphae with regular morphology [Figure 3a and b]. After 48 h of treatment using the MIC and MFC of the MPE treated fungal mycelia showed distorted, shrunken morphology [Figure 3c and d].
Figure 3.
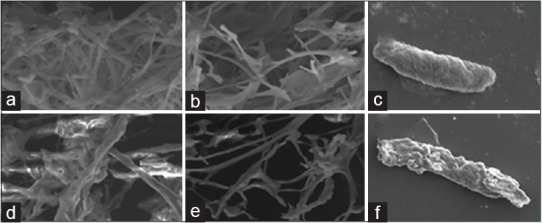
Scanning electron microphotography of Fusarium sporotrichioides: (a-c) control/untreated; (d-f) treated with Mentha piperita essential oil
Release of cellular constituents
The results of the release of cell constituents when F. sporotrichioides was treated with MPE at three different concentrations (control, MIC, and MFC) for 0, 30, 60, and 120 min, respectively, are shown in Figure 4. A significant increase in the release of cell constituents was observed immediately after the treatment with the MPE at different concentrations compared to control.
Figure 4.
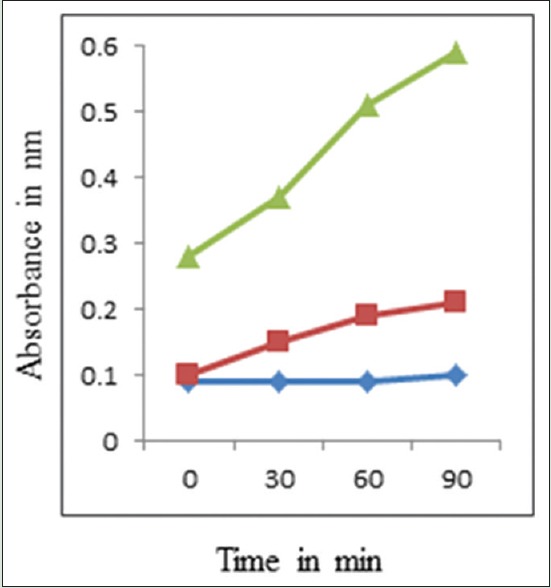
Effects of the Mentha piperita essential oil on the 260 nm absorbing material release of Fusarium sporotrichioides ([◊]: control; [◼]: Minimum inhibitory concentration; [◂]: Minimum fungicidal concentration)
Measurement of extracellular pH
The extracellular pH of F. sporotrichioides cells is shown in Figure 5. A gradual increase in extracellular pH was observed in control in 30, 60, and 90 min. The extracellular pH values in the F. sporotrichioides suspensions after incubation with MPE at MIC and MFC for 30 min were 4.70 and 4.55, respectively, which were significantly lower than that of the control (5.5). The extracellular pH in F. sporotrichioides suspensions incubation with MPE at MIC and MFC for 60 min was 4.90 and 4.60, respectively, which were significantly lower than that of the control (5.8). And finally, the extracellular pH in F. sporotrichioides suspensions incubation with MPE at MIC and MFC for 90 min was 5.0 and 4.50, respectively, which were significantly lower than that of the control (5.5).
Figure 5.
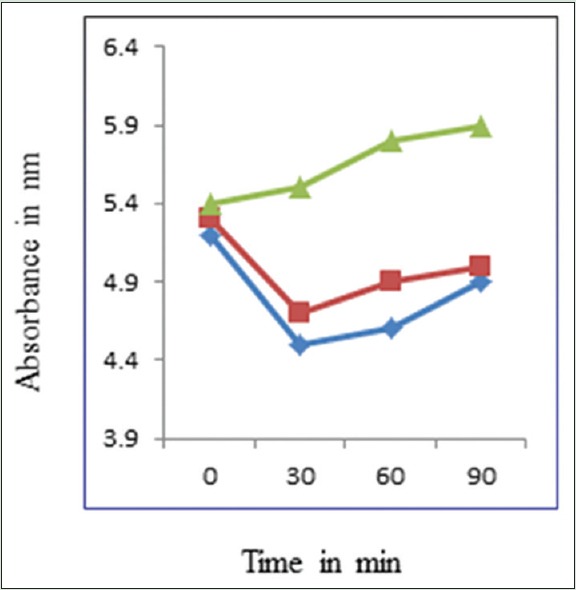
Effects of the Mentha piperita essential oil on the extracellular pH of Fusarium sporotrichioides ([▴]: Control; [◼]: Minimum inhibitory concentration; [◊]: Minimum fungicidal concentration)
Total lipid content
The effect of MPE on the total lipid content of F. sporotrichioides is shown in Table 3. The lipid content of pathogen is decreased after treating with MPE. The total lipid contents of F. sporotrichioides after incubation with MPE at MIC and MFC for 120 min were 120.1 ± 3.5 and 80.4 ± 4.6 mg/g dry weight, respectively, which were significantly lower than that of control (160.2 ± 3.8 mg/g dry weight).
Table 3.
Effect of Mentha piperita essential oil on the total lipid contents of Fusarium sporotrichioides

DISCUSSION
In this study, MPE was found to be effective in inhibiting the growth of F. sporotrichioides. In MPE rich in menthol, l-menthone, pulegone, piperitone, caryophyllene, and menthol acetate, these are the major compounds which are reported in many articles.[32,46] In addition, δ-cadinene, copaene, eugenol, methyl pivalate, menthomenthene, thujopsene, caryophyllene oxide also some of the compound identified in MPE and demonstrated antifungal activity and antibacterial activity.[47,48,49,50,51,52] Further, the anti-inflammatory, cytotoxic effects were reported by Sun et al. 2014.[53] The MIC values of MPE obtained in this study were 500 µg/mL and 1000 µg/mL at this particular concentration; the fungal growth was minimum as compared to untreated fungal cells. The potential mechanisms of volatile compounds as reported could damage the cell and disturb the cellular metabolism.[54] The previous report concluded that volatile compounds act as H + carriers and depletion of adenosine triphosphate takes place and disturb the cellular membrane, by reacting with the active sites of cellular enzymes.[55,56] The present study clearly demonstrates that the antifungal activity of MPE supported by SEM images of MPE showing morphological changes in the fungal hyphae due to the leakage of ions, leakage of molecular substances, and lesions as well as discrepancies in cell metabolism.
Extracellular enzymes are synthesized inside the cell and then secreted outside the cell, where their function is to break down complex macromolecules into smaller units to be taken up by the cell for growth and assimilation. Extracellular pH was determined to explain the release of the cellular material and the changes of membrane permeabilization. Results showed that, after the treatment of the MPE, the release of cell constituents in the fungi suspensions visibly increased compare to control. Meanwhile, after 30 min of exposure, the MPE apparently induced the leakage of intracellular protons as evidenced by the decrease in the values of extracellular pH. These findings suggest the irreversible damage to the cytoplasmic membranes of F. sporotrichioides.
Cell membrane encoding lipids is a major component of the cell. It performs many important functions including increasing the stability of the membrane, reducing the permeability of water-soluble materials, and adjusting the liquidity of the membrane.[57] The decrease in membrane stability and increased in permeability of water-soluble materials suggest the decrease in lipid content.[44] The previous studies regarding volatile compounds have been reported to disrupt the lipid structures of the cells.[58] A significant decrease in lipid content of A. niger and A. flavus cells in fumigated and nonfumigated cells with 1 µL/mL of Cymbopogon citratus essential oil (A. niger) and C. citratus essential oil (A. flavus) were reported by.[44,59] In the present study, the addition of MPE significantly decreased the lipid content of F. sporotrichioides. These results suggest that MPE is capable of acting on the cell membrane structure and disrupt the cell membrane integrity.
CONCLUSION
The results of the present study indicate the antifungal efficacy of MPE, by changes in the morphological alteration, and growth inhibition effects on F. sporotrichioides. These findings suggest that MPE may be used to control F. sporotrichioides. Future studies with its toxic metabolites should be conducted to evaluate the potency of MPE to generate more effective natural fungicides or preventives which is less risk or safe to the environment and health.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Farhath Khanum
Dr. Farhath khanum, Scientist “F” HOD, Biochemistry and Nanoscience Discipline, Defence Food Research Laboratory, Mysore – 570011, Karnataka, India.
REFERENCES
- 1.Edwards SG. Influence of agricultural practices on Fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol Lett. 2004;153:29–35. doi: 10.1016/j.toxlet.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Loogrieco A, Visconti A. On Overview on Toxigenic Fungi and Mycotoxins in Europe. The Netherlands: Kluwer Academic Publisher; 2004. pp. 1–251. [Google Scholar]
- 3.Mielniczuk E, Kiecana I, Perkowski J. Susceptibility of oat genotypes to Fusarium crookwelense Burgess, Nelson and Toussoun infection and mycotoxin accumulation in kernels. Biol Bratisl. 2004;59:809–16. [Google Scholar]
- 4.Brown DW, McCormick SP, Alexander NJ, Proctor RH, Desjardins AE. A genetic and biochemical approach to study trichothecene diversity in Fusarium sporotrichioides and Fusarium graminearum. Fungal Genet Biol. 2001;32:121–33. doi: 10.1006/fgbi.2001.1256. [DOI] [PubMed] [Google Scholar]
- 5.Moss MO. Mycotoxin review – 2. Fusarium. Mycologist. 2002;16:156–60. [Google Scholar]
- 6.Mateo JJ, Mateo R, Jiménez M. Accumulation of type A trichothecenes in maize, wheat and rice by Fusarium sporotrichioides isolates under diverse culture conditions. Int J Food Microbiol. 2002;72:115–23. doi: 10.1016/s0168-1605(01)00625-0. [DOI] [PubMed] [Google Scholar]
- 7.Moss MO, Thrane U. Fusarium taxonomy with relation to trichothecene formation. Toxicol. A. 2006. The potential of nonpathogenic Fusarium oxysporum and other biological control organisms for suppressing Fusarium wilt of banana. Plant Pathol. 2004;55:217–23. doi: 10.1016/j.toxlet.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Thrane U, Adler A, Clasen PE, Galvano F, Langseth W, Lew H, et al. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int J Food Microbiol. 2004;95:257–66. doi: 10.1016/j.ijfoodmicro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Niessen L, Schmidt H, Vogel RF. The use of tri5 gene sequences for PCR detection and taxonomy of trichothecene-producing species in the Fusarium section sporotrichiella. Int J Food Microbiol. 2004;95:305–19. doi: 10.1016/j.ijfoodmicro.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Perkowski J, Basinski T. Natural contamination of oat with group A trichothecene mycotoxins in Poland. Food Addit Contam. 2002;19:478–82. doi: 10.1080/02652030110102827. [DOI] [PubMed] [Google Scholar]
- 11.Perkowski J, Kiecana I, Stachowiak J, Basinski T. Natural occurrence of scirpentriol in cereals infected by Fusarium species. Food Addit Contam. 2003;20:572–8. doi: 10.1080/0265203031000100773. [DOI] [PubMed] [Google Scholar]
- 12.Minervini F, Fornelli F, Lucivero G, Romano C, Visconti A. T-2 toxin immunotoxicity on human B and T lymphoid cell lines. Toxicology. 2005;210:81–91. doi: 10.1016/j.tox.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Perkowski J, Wiwart M, Busko M, Laskowska M, Berthiller F, Kandler W, et al. Fusarium toxins and total fungal biomass indicators in naturally contaminated wheat samples from North-Eastern Poland in 2003. Food Addit Contam. 2007;24:1292–8. doi: 10.1080/02652030701416566. [DOI] [PubMed] [Google Scholar]
- 14.Alexander NJ, Hohn TM, McCormick SP. The TRI11 gene of Fusarium sporotrichioides encodes a cytochrome P-450 monooxygenase required for C-15 hydroxylation in trichothecene biosynthesis. Appl Environ Microbiol. 1998;64:221–5. doi: 10.1128/aem.64.1.221-225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkowski J, Kiecana I. Biosynthesis of Fusarium mycotoxins in kernels of spring barley (Hordeum vulgare L.) inoculated with Fusarium crookwellense Burgess, Nelson, Toussoun, F. culmorum (WG Sm.) Sacc. i F. graminearum Schwabe. Bulletin of Plant Breeding and Acclimatization Institute. 1998:69–80. [Google Scholar]
- 16.Hohn TM, Krishna R, Proctor RH. Characterization of a transcriptional activator controlling trichothecene toxin biosynthesis. Fungal Genet Biol. 1999;26:224–35. doi: 10.1006/fgbi.1999.1122. [DOI] [PubMed] [Google Scholar]
- 17.Pascale M, Haidukowski M, Visconti A. Determination of T-2 toxin in cereal grains by liquid chromatography with fluorescence detection after immunoaffinity column clean-up and derivatization with 1-anthroylnitrile. J Chromatogr A. 2003;989:257–64. doi: 10.1016/s0021-9673(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 18.Steyn PS. Mycotoxins, general view, chemistry and structure. Toxicol Lett. 1995;82-83:843–51. doi: 10.1016/0378-4274(95)03525-7. [DOI] [PubMed] [Google Scholar]
- 19.Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001;167:101–34. doi: 10.1016/s0300-483x(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 20.Wannemacher RW, Wiener SL, Sidell FR, Takafuji ET, Franz DR. Trichothecene mycotoxins. Med Aspects Chem Biol Warf. 1997;6:655–76. [Google Scholar]
- 21.Chen PJ, Moore T, Nesnow S. Cytotoxic effects of propiconazole and its metabolites in mouse and human hepatoma cells and primary mouse hepatocytes. Toxicol In Vitro. 2008;22:1476–83. doi: 10.1016/j.tiv.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Tateishi H, Miyake T, Mori M, Sakuma Y, Saishoji T. Effect of application timing of metconazole on Fusarium head blight development and mycotoxin contamination in wheat and barley. J Pestic Sci. 2014;39:1–6. [Google Scholar]
- 23.Scordino M, Sabatino L, Traulo P, Gagliano G, Gargano M, Pantò V, Gambino GL. LC/MS/MS detection of fungicide guazatine residues for quality assessment of commercial citrus fruit. Eur Food Res Technol. 2008;227:1339–47. [Google Scholar]
- 24.Manso S, Cacho-Nerin F, Becerril R, Nerín C. Combined analytical and microbiological tools to study the effect on Aspergillus flavus of cinnamon essential oil contained in food packaging. Food Control. 2013;30:370–8. [Google Scholar]
- 25.Zhou H, Tao N, Jia L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control. 2014;37:277–83. [Google Scholar]
- 26.Sinha KK, Sinha AK, Prasad G. The effect of clove and cinnamon oils on growth of and aflatoxin production by Aspergillus flavus. Lett Appl Microbiol. 1993;16:114–7. [Google Scholar]
- 27.Montes-Belmont R, Carvajal M. Control of Aspergillus flavus in maize with plant essential oils and their components. J Food Prot. 1998;61:616–9. doi: 10.4315/0362-028x-61.5.616. [DOI] [PubMed] [Google Scholar]
- 28.Bullerman LB, Lieu FY, Seier SA. Inhibition of growth and aflatoxin production by cinnamon and clove oils. Cinnamic aldehyde and eugenol. J Food Sci. 1977;42:1107–9. [Google Scholar]
- 29.Xing, Fuguo, Hua H, Selvaraj JN, Zhao Y, Zhou L, et al. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control. 2014;46:343–50. [Google Scholar]
- 30.Yamamoto-Ribeiro MM, Grespan R, Kohiyama CY, Ferreira FD, Mossini SA, Silva EL, et al. Effect of Zingiber officinale essential oil on Fusarium verticillioides and fumonisin production. Food Chem. 2013;141:3147–52. doi: 10.1016/j.foodchem.2013.05.144. [DOI] [PubMed] [Google Scholar]
- 31.da Silva Bomfim N, Nakassugi LP, Faggion Pinheiro Oliveira J, Kohiyama CY, Mossini SA, Grespan R, et al. Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem. 2015;166:330–6. doi: 10.1016/j.foodchem.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Mohaddese M, Kazempour N. Chemical composition and antimicrobial activity of peppermint (Mentha piperita L.) Essential oil. Songklanakarin J Sci Technol. 2014;36:1. [Google Scholar]
- 33.Moghaddam M, Pourbaige M, Tabar HK, Farhadi N, Hosseini SM. Composition and antifungal activity of Peppermint (Mentha piperita) essential oil from Iran. J Essent Oil Bearing Plants. 2013;16:506–12. [Google Scholar]
- 34.Skalicka-Woz´niak K, Walasek M. Preparative separation of menthol and pulegone from peppermint oil (Mentha piperita L.) by high performance counter current chromatography. Phytochem Lett. 2014;10:xciv–viii. [Google Scholar]
- 35.Uribe E, Marín D, Vega-Gálvez A, Quispe-Fuentes I, Rodríguez A. Assessment of vacuum-dried peppermint (Mentha piperita L.) as a source of natural antioxidants. Food Chem. 2016;190:559–65. doi: 10.1016/j.foodchem.2015.05.108. [DOI] [PubMed] [Google Scholar]
- 36.Tyagi AK, Malik A. Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapour phase against food spoiling microorganisms. Food Control. 2011;22:1707–14. [Google Scholar]
- 37.Silva FC, Chalfoun SM, Siqueira VM, Botelho DM, Lima N, Batista LR. Evaluation of antifungal activity of essential oils against potentially mycotoxigenic Aspergillus flavus and Aspergillus parasiticus. Rev Bras Farmacognosia. 2012;22:1002–10. [Google Scholar]
- 38.Freire MM, Jham GN, Dhingra OD, JARDIM C, Barcelos RC, VALENTE V, MOREIRA M. Composition, antifungal activity and main fungitoxic components of the essential oil of Mentha piperita L. J Food Saf. 2012;32:29–36. [Google Scholar]
- 39.Council of Europe. Methods of pharmacognosy. Eur Pharmacopoeia. 1997;3:121–2. [Google Scholar]
- 40.Ramana MV, Balakrishna K, Murali HC, Batra HV. Multiplex PCR-based strategy to detect contamination with mycotoxigenic Fusarium species in rice and finger millet collected from Southern India. J Sci Food Agric. 2011;91:1666–73. doi: 10.1002/jsfa.4365. [DOI] [PubMed] [Google Scholar]
- 41.Talibi I, Askarne L, Boubaker H, Boudyach EH, Msanda F, Saadi B, Aoumar AA. Antifungal activity of some Moroccan plants against Geotrichum candidum the causal agent of postharvest citrus sour rot. Crop Prot. 2012;35:41–6. [Google Scholar]
- 42.Paul S, Dubey RC, Maheswari DK, Kang SC. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food borne pathogens. Food Control. 2011;22:725–31. [Google Scholar]
- 43.Shao X, Cheng S, Wang H, Yu D, Mungai C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J Appl Microbiol. 2013;114:1642–9. doi: 10.1111/jam.12193. [DOI] [PubMed] [Google Scholar]
- 44.Helal GA, Sarhan MM, Abu Shahla AN, Abou El-Khair EK. Effects of Cymbopogon citratus L. essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus ML2-strain. J Basic Microbiol. 2007;47:5–15. doi: 10.1002/jobm.200610137. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Li R, Lu D, Ma S, Yan Y, Li W. A quick isolation method for mutants with high lipid yield in oleaginous yeast. World J Microbiol Biotechnol. 2009;25:921–5. [Google Scholar]
- 46.Saharkhiz MJ, Motamedi M, Zomorodian K, Pakshir K, Miri R, Hemyari K. Chemical composition, antifungal and antibiofilm activities of the essential oil of Mentha piperita L. ISRN Pharm. 2012;2012:718645. doi: 10.5402/2012/718645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samber N, Varma AL, Manzoor N. Evaluation of Mentha piperita essential oil and its major constituents for antifungal activity in Candida spp. Int J Innov Technol Exploring Eng. 2014;3:9404–11. [Google Scholar]
- 48.Moghtader M. In vitro antifungal effects of the essential oil of Mentha piperita L. and its comparison with synthetic menthol on Aspergillus niger. Afr J Plant Sci. 2013;7:521–7. [Google Scholar]
- 49.Mei-Lin T, et al. Chemical composition and biological properties of essential oils of two mint species. Trop J Pharm Res. 2013;12:577–82. [Google Scholar]
- 50.Hadipanah A, Ghahremani A, Aghaee K, Zolfaghar M, Ardalani H. Four Ecotypes of Mentha pipertia in Iran. Phytochemical study. In Biological Forum. 2015;7:1796. [Google Scholar]
- 51.Fahmideh L, Sargazi A, Mehrabi AM, Armin A. Chemical compositions of peppermint (Mentha piperita L.) grown in Isfahan province, Iran. J Biodivers Environ Sci. 2015;7:72–6. [Google Scholar]
- 52.Derwich EL, Benziane ZI, Chabir RA, Taouil RA. In vitro antioxidant activity and GC/MS studies on the leaves of Mentha piperita (Lamiaceae) from Morocco. Int J Pharm Sci Drug Res. 2011;3:130–6. [Google Scholar]
- 53.Sun Z, Wang H, Wang J, Zhou L, Yang P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS One. 2014;9:e114767. doi: 10.1371/journal.pone.0114767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marilena M, Bersani C, Comi G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int J Food Microbiol. 2001;67:187–95. doi: 10.1016/s0168-1605(01)00447-0. [DOI] [PubMed] [Google Scholar]
- 55.Bajpai VK, Sharma A, Baek KH. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food borne pathogens. Food Control. 2013;32:582–90. [Google Scholar]
- 56.Ultee A, Bennik MH, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68:1561–8. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heaton NS, Randall G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011;19:368–75. doi: 10.1016/j.tim.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prashar A, Hili P, Veness RG, Evans CS. Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae. Phytochemistry. 2003;63:569–75. doi: 10.1016/s0031-9422(03)00226-7. [DOI] [PubMed] [Google Scholar]
- 59.Helal GA, Sarhan MM, Abu Shahla AN, Abou El-Khair EK. Effects of Cymbopogon citratus L. essential oil on the growth, lipid content and morphogenesis of Aspergillus niger ML2-strain. J Basic Microbiol. 2006;46:456–69. doi: 10.1002/jobm.200510106. [DOI] [PubMed] [Google Scholar]


