Abstract
Background:
Matricaria chamomilla is an aromatic plant with antioxidant, anticancer, and anti-inflammatory properties. However, the inhibitory role of M. chamomilla on migration and invasion of human breast cancer cells remains unclear.
Objective:
This study investigated the methods to evaluate these anticancer mechanisms of M. chamomilla on human breast cancer MCF-7 and MDA-MB-468 cell lines.
Materials and Methods:
The cells were treated with hydroalcoholic extract of M. chamomilla at different concentrations (50–1300 μg/mL) for 24, 48, and 72 h in a culture medium containing 10% fetal bovine serum. This study quantified the 50% growth inhibition concentrations (IC50) by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay; apoptosis and necrosis through Hoechst 33342/propidium iodide staining; cell proliferation and clone formation by clonogenic assay as well as cellular migration, invasion, and attachment. After 24, 48, and 72 h of treatment, the IC50levels were 992 ± 2.3 μg/mL, 893 ± 5.4 μg/mL, and 785 ± 4.8 μg/mL against MDA-MB-468, respectively, and 1288 ± 5.6 μg/mL, 926 ± 2.5 μg/mL, and 921 ± 3.5 μg/mL, against MCF-7, respectively. Furthermore, increasing the extract concentrations induced cellular apoptosis and necrosis and decreased cellular invasion or migration through 8 μm pores, colonization and attachment in a dose-dependent manner.
Results:
It indicated time- and dose-dependent anti-invasive and antimigrative or proliferative and antitoxic effects of hydroalcoholic extract of aerial parts of chamomile on breast cancer cells.
Conclusion:
This study demonstrated an effective plant in preventing or treating breast cancer.
SUMMARY
Antioxidant compounds in Matricaria chamomilla have anticancer effects.
Hydroalcoholic extract of M. chamomilla controls cellular proliferation and apoptosis induction.
Hoechst 33342/propidium iodide staining suggested that the extract induces apoptosis more than necrosis.
Hydroalcoholic extract of M. chamomilla prevents colonization and cellular migration of human breast cancer MDA-MB-468 and MCF-7 cell lines in a time- and dose-dependent manner.
M. chamomilla has low cytotoxic effects on natural cells.
Abbreviations Used: IARC: International Agency for Research on Cancer; WHO: World Health Organization; FBS: Fetal bovine serum; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; DMSO: Dimethyl sulfoxide; PI: Propidium iodide; LN: Live cells with normal nucleus; LA: Live cells with apoptized nucleus; DN: Dead cells with normal nucleus; DA: Dead cells with apoptized nucleus; BSA: Bovine serum albumin; ANOVA: Analysis of variance; IC50: 50% growth inhibition concentration; GSE: Grape seed extract
Key words: Apoptosis, attachment, breast cancer, invasive, migration
INTRODUCTION
Report of the International Agency for Research on Cancer (IARC), an agency of the WHO, indicates the preference of preventive actions on controlling breast and cervical cancer. The most common cancers are lung, breast, and colorectal cancers. Breast cancer is the first leading cause of cancer death in women in 140 countries. It contributes to one-fourth of all types of cancer in women. Generally, cancer has an increasing trend in developing countries due to lifestyle change such as diet change.[1,2]
Human has discovered remedial effects of many plants for many years and has used them to treat many diseases. It has been articulated that most plants have chemoprotective properties. Allium species members including garlic, boll, and shallot as well as members of Asteraceae family including chamomile and artemisia are among these plants, which are rich sources of chemoprotective phytochemicals such as phytosterols, flavonoids, carotenoids, and terpenoids. They act as antioxidants and destroy free radicals and stimulate immune system. They control cancer development through nitrosation and formation of DNA adducts with carcinogens and related metabolic paths.[3]
Phytochemicals in Matricaria chamomilla flower extract include different acids such as tartaric acid, citric acid, and succinic acid. It has other compounds such as myristin, proazolene, luteolin, and coumarin derivatives as well as different flavonoids such as flavones and flavonols. Its floret contains rutin, apigenin, and free quercetin.[4] Maximum levels of phenols and flavonoids have been observed in its alcoholic extract while the minimum ones have been observed in its aqueous extract.[5] Oxygen free radicals contribute to the pathophysiology of many diseases including cancer and inflammation.[6] According to findings, the phenolic compounds in M. chamomilla act as antioxidants, remove free radicals, and prevent collagen degradation by superoxide anionic radicals.[7]
Methanolic and aqueous extracts of M. chamomilla have the least antiproliferative effect on normal cells while significantly affect biological ability of different cancer cells.[8] Studies suggest that bisabolol oxide A, a compound of M. chamomilla, together with fluorouracil-5, exhibits antiproliferative action on K562 cell line in blood cancer.[9] Another study showed that M. chamomilla and Calendula officinalis teas have cytotoxic effects on cancer cells.[10]
Researches on phytochemicals with the ability of preventing the emergence of a number of tumors are being increased and food derivatives, which are almost nontoxic, have attracted special attentions. M. chamomilla has effective compounds with antiproliferative and cytotoxic effects and has been traditionally used in Iran as a remedial herb to treat some diseases. However, little is known about anticancer mechanisms of M. chamomilla on preventing the migration and invasion of breast cancer cells. Therefore, this study tries to assess these effects on breast cancer cell lines by different methods.
MATERIALS AND METHODS
Plant collection
The aerial parts of M. chamomilla L were supplied from Royan Borom Darou (Iran). Its scientific name was also confirmed by this company. The plant was collected from a region located at 34°29' N and 51°4'49” E. The extract prepared through soaking method. To this end, M. chamomilla was grinded softly using an electric mill, and then 10 g of the resulted powder was mixed with 100 mL ethylic alcohol 70%. Then, the mixture was placed in a shaker and rotated at 200 rpm.
After 24 h, the contents of the shaker were filtered through a Whatman filter paper. The solution was then dried at room temperature and weighed; it was kept at −20°C. The desired concentrations were solved in RPMI (GIBCO Laboratories, Grand Island, NY) culture medium containing 10% fetal bovine serum (FBS). The solution was filtered through a 0.22 µm filter and different concentrations of 50–1300 µg/mL were prepared.
Cell culture
MCF-7 and MDA-MB-468 cell lines were supplied by the Pasteur Institute of Iran and were cultured in RPMI medium containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin within an incubator at 37°C with 95% humidity and 5% CO2.
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay
The cells were exposed to different concentrations of M. chamomilla hydroalcoholic extract (50–1300 μg/mL) for 24, 48, and 72 h at three iterations. Control specimens were also prepared at three iterations at the same time, which contained the same cells and an extract-free culture medium. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test.[11] Briefly, 1 × 106 cells were placed in a 96-well culture plate and were treated with the extract for 24, 48, and 72 h. They were then kept in GIBCO MTT solution (5 mg/mL in phosphate-buffered saline [PBS]) for 4 h until formation of formazan crystals; the crystals were solved in 100 μL dimethyl sulfoxide (DMSO), and the absorbance was read at 570 nm by a Microplate Reader (BioTek EL × 800).
Detection of apoptosis by fluorescence microscopy
Fluorescence microscope and Hoechst 33342/propidium iodide (PI) staining were used to quantify nucleus change and cellular death at different concentrations of the extract and to determine apoptosis or necrosis cause of cellular death dose-dependently. First, a total number of 2 × 105 cells were cultured in a 6-well culture plate and were treated with different concentrations of M. chamomilla hydroalcoholic extract. After 24 h, the cells were separated from the plate by trypsin and were washed with ice-cold PBS buffer. Using 10 μg/mL Hoechst stain, the cells were incubated in a 37°C incubator for 7 min and then stained with 2.5 μg/mL PI for 15 min in darkness. The cellular suspension was then placed on a glass slide, and the number of cells was counted by a fluorescence microscope (Olympus B × 51).
The maximum absorbance of Hoechst 33342/PI occurs at ~ 460 nm and >575 nm, respectively. Four fields per slide and 200 cells per field were counted. The number of live cells with normal nucleus (LN), live cells with apoptized nucleus (LA), dead cells with normal nucleus (DN), and dead cells with apoptized nucleus (DA) was counted, and the percentage of apoptized and necrotized cells was carried out at three iterations.
In this regard, healthy live cells are characterized with blue chromatin and organized pattern while apoptized live cells have light blue, highly condensed or fragmented nucleus. DN are characterized with pink chromatin and organized pattern, and DA have light pink, highly condensed or fragmented chromatin. The percentage of apoptized and necrotized cells was derived from the following equation:
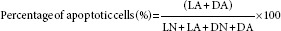
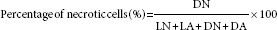
Clonogenic assay
In this method, 1000 cells were incubated in a 6-well culture plate. After 24 h, each well was treated with different concentrations of the extract (50–400 μg/mL). After 9 days, the cells were separated from the well's medium and were washed by PBS. They were then fixed with methanol solution (methanol/acetic acid = 3/1) and placed in a 37°C incubator for 5 min. The fixative solution was then removed from the wells. Crystal violet (0.5%) was used to stain the cells for 15 min. Then, it was washed with water. In each well, the number of colonies with more than 50 cells was counted using stereomicroscope (Olympus SZX16).
Cell migration assay
A total number of 5 × 104 cells of MCF-7 were treated with the extract at 644 µg/mL and 1288 µg/mL concentrations in 24-cell plates for 24 h for three iterations. The same procedure was applied on MDA-MB-468 cell line at 469 µg/mL and 992 µg/mL concentrations. Then, the cells were separated by trypsin and transferred to Millicell wells (EMD Millipore Corporation, Billerica, MA) with a pore size of 8 µm containing 300 µL serum-free medium. Then, the Millicell wells were placed in 24-cell plates containing 750 µL culture medium, containing 10% FBS, for 16 h at 37°C. Culture medium inside the Millicell wells was then removed and the wells were washed with PBS. Using formaldehyde (3.7% in PBS), the cells were fixed for 2 min at room temperature. Thereafter, the wells were washed with PBS and the cells were permeabilized by methanol 100% for 20 min at room temperature. After this step, methanol was removed and the Millicell wells were washed with PBS. The cells were stained with crystal violet 0.5% for 20 min in darkness and the wells were washed. Nonmigrated cells scraped off the wells' bottom with cotton swabs, and migrated cells were counted in 8 fields; then, the average of cell migration was obtained.[12]
Invasion assay
A total number of 5 × 104 cells of MCF-7 cell line were treated with the extract at 644 µg/mL and 1288 µg/mL concentrations in 24-cell plates for 24 h for three iterations. The same procedure was applied on MDA-MB-468 cell line at 469 µg/mL and 992 µg/mL concentrations. This was practiced using Millicell wells with a pore size of 8 μm covered with Matrigel.[13] Then, the Millicell wells were placed in 24-cell plates containing 750 µL culture medium with 10% FBS for 16 h at 37°C. Culture medium and the Matrigel inside the Millicell wells were removed and the wells were washed with PBS. Using formaldehyde (3.7% in PBS), those cells that crossed Matrigel and the wells were fixed for 2 min at room temperature. The wells were washed with PBS and the cells were permeabilized by methanol 100% for 20 min at room temperature. In the next step, the wells were washed with PBS. The cells were stained with crystal violet 0.5% for 20 min in darkness and the wells were washed. Nonmigrated cells were scraped off the wells' bottom with cotton swabs and migrated cells were counted in 8 fields; then, the average of cell migration was obtained.[12]
Wound healing assay
A total number of 2 × 105 cells were poured in 6-cell plates. When the density of cells raised to 80%, to starve the cells, the culture medium containing 10% FBS was replaced by another culture medium with 2% FBS. After 24 h of starvation, cell layer on the bottom of the plate was scratched slowly by the head of a 1 mm sampler. Then, the cells were washed with PBS and a culture medium containing 10% serum was put on them. Control well had no extract while other wells were treated with different concentrations of M. chamomilla extract. The scratched zone of cells was assessed after 0, 24, 48, and 72 h of scratching. ImageJ was used to evaluate the extent of healing. To create a defined area, the length and width of the scratch were measured and healing extent was calculated using the following equation.[14]
Percent closure
-
Determine the surface area of the defined wound area [Figure 1].
Total surface area = width (µm) × length
-
Determine the surface area of the migrated cells into the wound area.
Migrated cell surface
Area = length of cell migration (µm) × length
Percent closure (%) = migrated cell surface area/total surface area × 100
Figure 1.
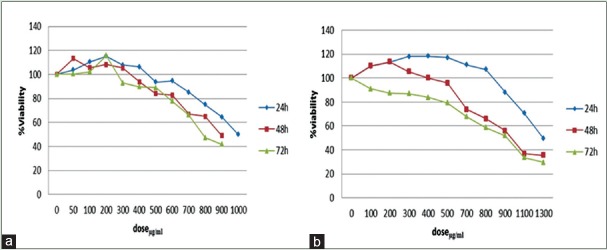
(a) Mean proliferation percentage of MDA-MB-468 cell line after 24, 48, and 72 h of treatment with different concentrations of hydroalcoholic extract of aerial parts of Matricaria chamomilla; (b) Mean proliferation percentage of MCF-7 cell line after 24, 48, and 72 h of treatment different concentrations of hydroalcoholic extract of aerial parts of Matricaria chamomilla
Attachment assay
Cell lines were treated with different concentrations of M. chamomilla extract. They were incubated at 37°C for 24 h and suspended using trypsin. Then, 96-well plates were covered by laminin (10 µg/mL). After an hour, the wells were washed at first by 0.1% bovine serum albumin (BSA) and then by 0.5% BSA. Then, cellular suspension was added to the wells and was incubated for 30 min. Then, it was fixed by paraformaldehyde and stained by crystal violet. Attached cells were counted using invert microscope. This test was iterated for 3 times.
Statistical survey
Statistical analyses were performed using SPSS (version 19, US). Data were analyzed with analysis of variance (ANOVA), following post hoc Tukey test. Significance level was set at P < 0.05.
RESULTS
3-(4,5-dimethylthiazol-2-yl)-2,5 -diphenyltetrazolium bromide assay
Figure 1 shows the results of exposure of breast cancer MCF-7 and MDA-MB-468 cell lines to hydroalcoholic extract of M. chamomilla by MTT method after 24, 48, and 72 h. This study showed that in the presence of M. chamomilla, 50% growth inhibition concentrations (IC50) occur in MDA-MB-468 cell line after 24, 48, and 72 h at concentrations 758 ± 4.8 µg/mL, 893 ± 5.4 µg/mL, and 992 ± 2.3 µg/mL, respectively, and in MCF-7 cell line at concentrations 921 ± 3.5 µg/mL, 926 ± 2.5 µg/mL, and 1288 ± 5.6 µg/mL, respectively.
Cellular morphology
Morphological results associated with MDA-MB-468 cell line after 24 h of treatment show that as the extract concentration exceeds 550 µg/mL, nucleus apoptosis increases significantly so that the extract concentration was 200 µg/mL, nucleus apoptosis and necrosis were about 2.1% and 0.2%, respectively; while when the extract concentration raised to 500 µg/mL, nucleus apoptosis and necrosis raised to 9.3% and 5.7%, respectively. However, when the extract concentration was 1000 µg/mL, nucleus apoptosis and necrosis were 30.2% and 17.3%, respectively [Figure 2].
Figure 2.
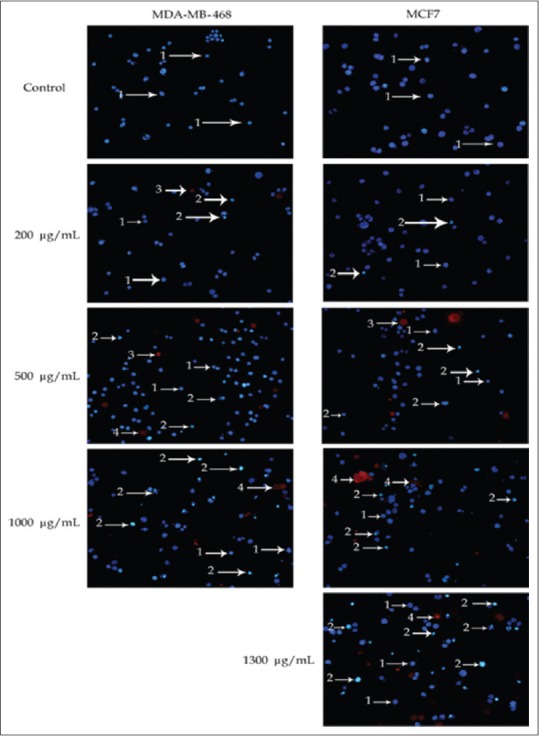
Images for morphological changes of MDA-NF-468 and MCF-7 cancer cells stained by Hoechst 33342/propidium iodide fluorescence. The cells were treated with chamomile extract for 24 h. Images are from a large fluorescence microscope with ×20 magnification. Arrows show – 1: Live cells with normal nucleus, 2: Live cell with apoptized nucleus, 3: Dead cell with normal nucleus, and 4: Dead cell with apoptized nucleus
Regarding MCF-7 cell line, cellular morphology results, after 24 h of treatment, showed no apoptosis and necrosis in the control group. When the extract concentration was 200 µg/mL, nucleus apoptosis and necrosis were 0.4% and 0.1%, respectively; while when the extract concentration raised to 500 µg/mL, nucleus apoptosis and necrosis raised to 4.7% and 1.7%, respectively. These values were, respectively, 16.4% and 7% and 22.1% and 36.8% for concentrations of 1000 µg/mL and 1300 µg/mL [Figure 2]. Cell process change was evident in this cell line at higher concentrations of the extract. These data showed dose-dependent responses.
Clone formation by exposure to the extract
According to clonogenic assay results, the number of colonies, formed due to the exposure of cells to the extract, decreased as the extract concentration increased so that after 9 days of exposure, the number of colonies decreased as the extract concentration increased. Figure 3 shows the percentage of colonies, following 9 days of treatment with different concentrations of the extract compared with control. Figure 4 shows that the number of colonies inside culture plate decreases after 9 days of treatment with different concentrations of the extract. Furthermore, Figure 4 shows the extract affected colonies in a larger magnification.
Figure 3.
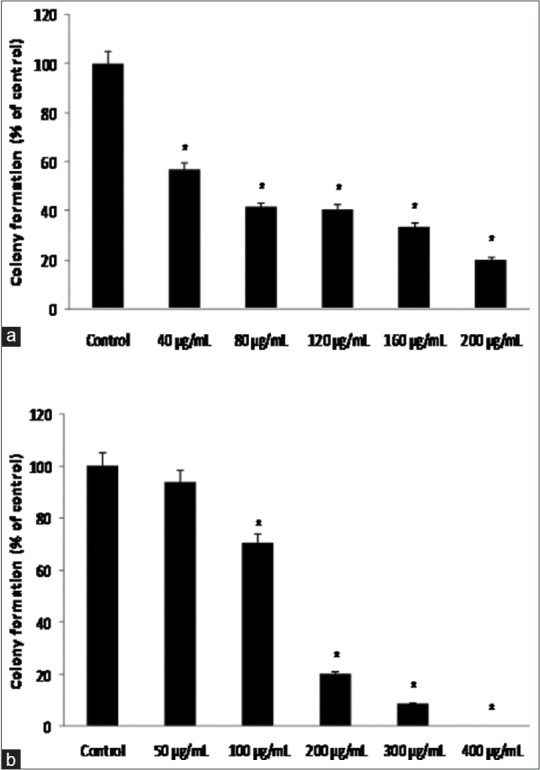
Mean colonization percentage of MCF-7; and MDA-MB-468 to (a) MCF-7 and (b) MDA-MB-468 cell lines after 9 days of treatment with hydroalcoholic extract of aerial parts of chamomile at different concentrations compared with control. *Indicates a significant difference at the level of P < 0.05 compared with control
Figure 4.
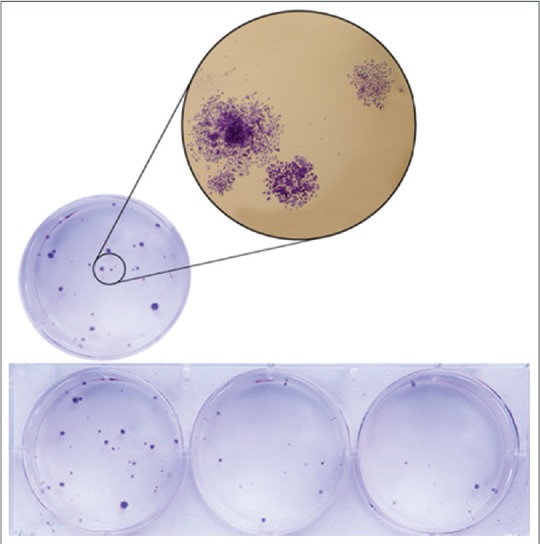
Number of colonies decreased as the extract concentration increased and colonization with larger magnification
Wound healing determination in cancer cell lines
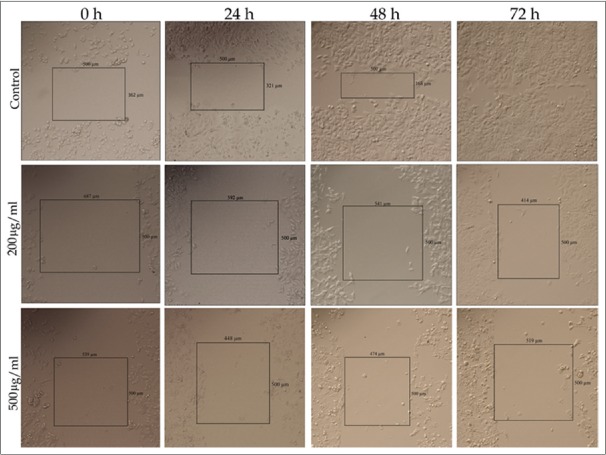
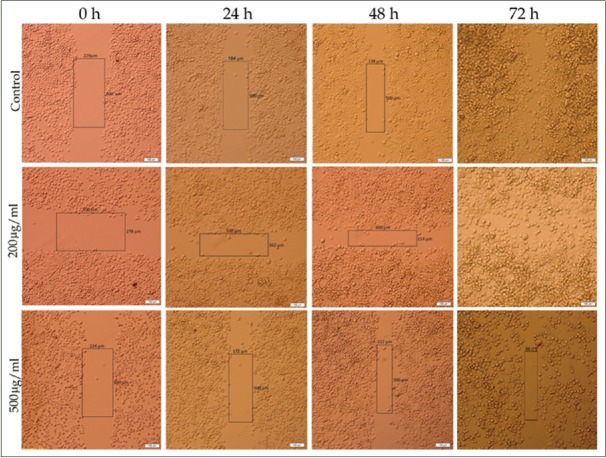
The results of wound healing assay indicate that cancer cells migration plays a significant role in pathological processes such as formation of tumors in internal organs. Wound healing assay was used to survey the effects of M. chamomilla extract on cells migration. Figure 5 shows that following increases in concentration and time, cellular migration and wound healing decrease in MDA-MB-468 and MCF-7 cell lines, compared with control, which indicated dose- and time-dependent manner. Figures 6 and 7 show cellular migration percentage compared with control after 24, 48, and 72 h of treatment with the extract.
Figure 5.
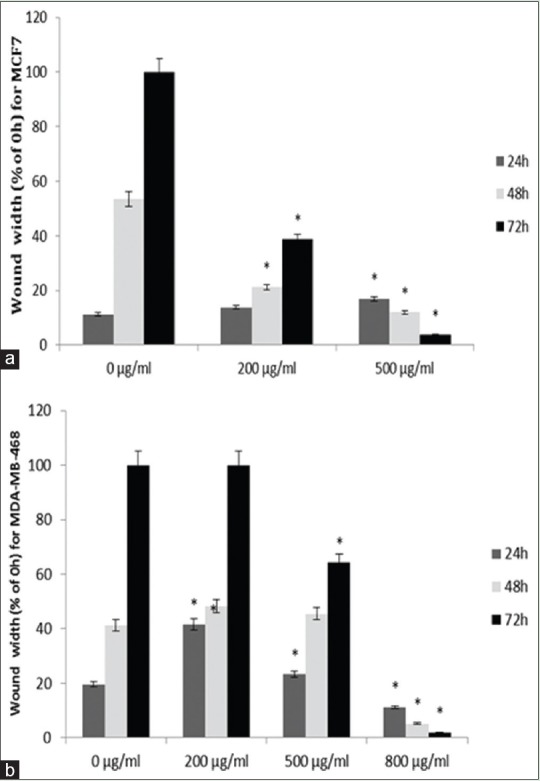
Mean of MCF-7; and MDA-MB-468 with (a) MCF-7 and (b) MDA-MB-468 cell lines migration percentage compared with the control group after 24, 48, and 72 h of treatment with different concentrations of chamomile extract. *Indicates a significant difference at the level of P < 0.05 compared with control
Figure 6.
MCF-7 cell line cellular migration rate at different concentrations of hydroalcoholic extract of chamomile with ×10 magnification
Figure 7.
MDA-MB-468 cell line cellular migration rate at different concentrations of hydroalcoholic extract of chamomile with ×10 magnification
Cellular migration and invasion under the influence of Matricaria chamomilla extract
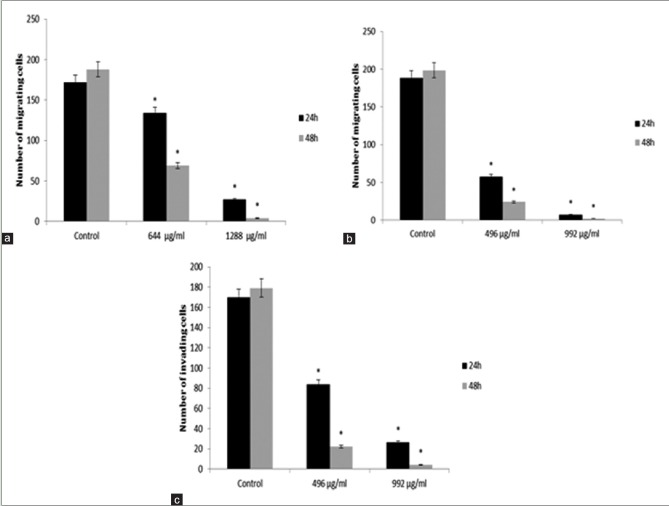
The antiproliferation effects of M. chamomilla extract on MCF-7 and MDA-MB-468 cell lines suggest that cellular migration and invasion decrease as time and concentration increase. MCF-7 cell line is not invasive and this study showed invasion neither in the control group nor in the cells treated with different concentrations of the extract. Figure 8a and b compares cellular migration rate through 8 µm pores of Millicell with control after 24 and 48 h. Figure 8c shows cellular invasion rates of MDA-MB-468 and MCF-7 cell lines after 24 and 48 h of treatment with different concentrations of the extract [Figure 9].
Figure 8.
(a) Mean migration of MCF-7; (b) MDA-MB-468; and (c) mean migration of MDA-MB-468 cell lines after 24 and 48 h of treatment with different concentrations of chamomile extract. *Indicates a significant difference at the level of P < 0.05 compared with control
Figure 9.
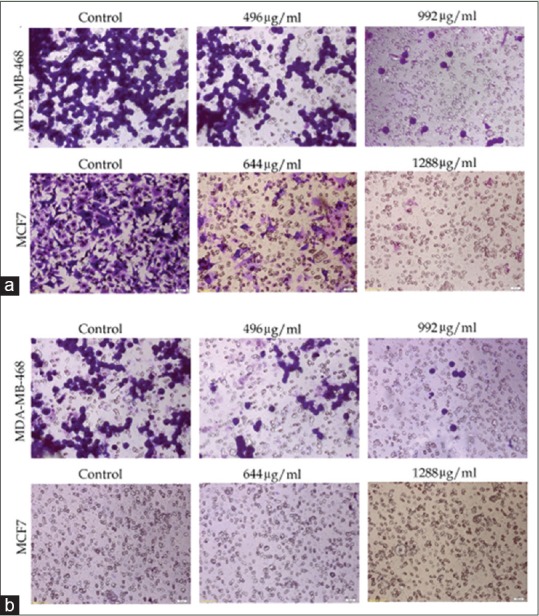
Cells migration and invasion rate through wells with 8 μm pores. (a) migration rate of cell lines under the influence of different concentrations of hydroalcoholic extract of aerial parts of chamomile; (b) invasion rate of cell lines under the influence of different concentrations of hydro-alcoholic extract of aerial parts of chamomile (no invasion was seen in MCF-7 cell line)
Cellular attachment under the influence of hydroalcoholic extract of Matricaria chamomilla
Laminin was used to evaluate cellular attachment. As shown in Figure 10, cellular attachment decreases significantly in MCF-7 and MDA-MB-468 cell lines at IC50 and half of IC50 concentrations. According to the curves, cellular attachment of MCF-7 cell line has been considerably decreased compared with the control group at IC50 concentration. The same trend is seen in MDA-MB-468 cell line.
Figure 10.
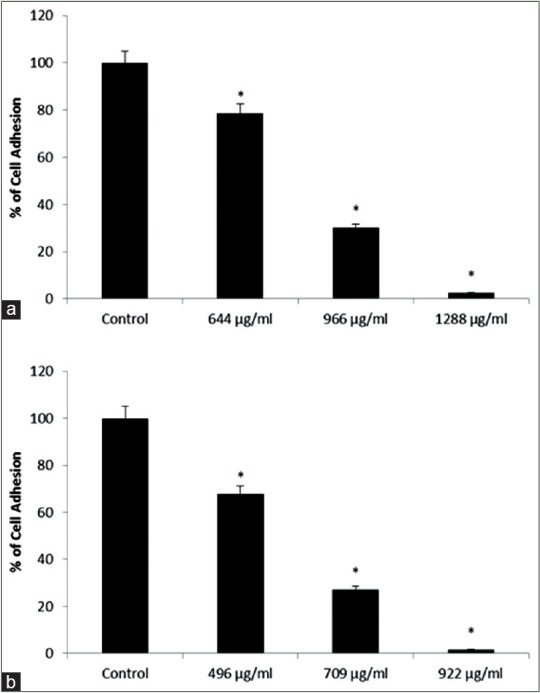
(a and b) Indicate mean cellular attachment percentage in MCF-7 and MDA-MB-468 cell lines, respectively, compared with the control group at different concentrations of chamomile extract. *Indicates a significant difference at the level of P < 0.05 compared with control
DISCUSSION
Breast cancer is being increasingly developed across the world.[1] This is one of the most important causes that made us study breast cancer. The reports of the IARC of WHO indicate the priority of preventive actions on controlling breast cancer in women.[1]
M. chamomilla is considered as an anticancer plant due to the following facts. It contains various groups of flavonoids and plant phenols that prevent cancer.[15,16] It has been widely used for several years as a traditional remedy and its effects on deferent human diseases have been realized. Not only it is used as a drinking but also it is an essential component in many healthy diets such as fruit and vegetable diets.[15,16] Antioxidant compounds in M. chamomilla have anticancer effects.[17] This study showed that hydroalcoholic extract of M. chamomilla controls cellular proliferation and apoptosis induction, depending on concentration or dose and time, and prevents colonization and cellular migration in MCF-7 and MDA-MB-468 cell lines.
The current study on the death pattern of cancer cells treated with M. chamomilla extract showed that the hydroalcoholic extract of M. chamomilla induces more apoptosis than necrosis in breast cancer cells. Metabolic effects of M. chamomilla in its tea form showed apparent changes in bioresponses so that drinking M. chamomilla tea may promote health.[18] Another study showed that M. chamomilla has high amounts of flavonoids and its glicon and apigenin have superior anticancer effects and can result in selective prevention of cell proliferation and apoptosis in cancer cells. This study showed IC50 levels in the presence of M. chamomilla after 24, 48, and 72 h of treatment. However, it has reported that low concentrations of flavonoids increase MCF-7 cell line proliferation.[19] In this study, the raise of proliferation was seen after 24 h of treatment with the extract at concentrations 0–800 µg/mL for MCF-7 cell line and 0–400 µg/mL for MDA-MB-468 cell line. However, MTT cell toxicity test showed that in the event of lower concentrations and less time, not only the extract has toxic effects on breast cancer cells but also it results in their proliferation. On the other hand, even at the same low concentration, toxic effect increased as time increased and this stopped cancer cells proliferation. Previous studies showed that some natural compounds such as essential oils, flavonoids, and terpenoids could significantly increase cellular proliferation at low concentrations.[20]
In recent years, the antiproliferative effects of flavonoids on different cancer cells have been approved by studies. Quercetin effect on MCF-7 cell line was studied and it was suggested that this compound affects cell cycle and results in the cell line apoptosis.[21] This agrees with the current study results performed by Hoechst 33342/PI staining method. It suggested that the extract induces apoptosis more than necrosis. One reason may be the effect of this extract on DNA of breast cancer cells.
Metastasis is a dynamic and complex biological event. Its success depends on the capability of cancer cells in segregation from adjacent cells so that they can target extracellular matrix and basal membrane and travel to distant tissues via blood and lymphatic flows and proliferate there and form secondary tumors.[22,23] One of the most important objectives of this study is to inhibit the migration of cancer cells through Matrigel, by which we can control the destruction of extracellular matrix and stop migration of cells through extracellular matrix. As it was mentioned before, this process plays a significant role in metastasis. This study suggests that hydroalcoholic extract of M. chamomilla has the capability to control breast cancer cells invasion at concentrations of 496 µg/mL and 644 µg/mL for MDA-MB-468 and MCF-7 cell lines, respectively.
It is many years that taxol has been discovered and prescribed as an effective drug for treating many malignant diseases. Both in vivo and in vitro studies have been carried out on taxol cytotoxic effects, and all of them have approved that it is a toxic drug for cancer cells. It has been realized that taxol kills cancer cells by stopping cell cycle.[24] Examined the effect of 2–20 nM taxol on MCF-7 cell line (human breast cancer), HeLa cell line (human cervical cancer), A549 cell line (human lung cancer), U373 cell line (human astrocytes cancer), HT-29 cell line (human colon cancer), OVG-1 cell line (human ovarian cancer), and PC-Sh and C-Zd cell lines (human pancreas cancer), they concluded that the IC50 of taxol varies from 2.5 nM to 7.5 nM, depending on cell type, and its cytotoxicity increases by 5–500 times in different cells as treatment time increases from 24 h to 72 h.[24]
This study evaluated the effects of M. chamomilla extract and approved its cytotoxic effects on human breast cancer MCF-7 and MDA-MB-468 cell lines and IC50 were determined. According to the data, taxol is a stronger substance for treating different cancers including breast cancer; however, since M. chamomilla extract is less toxic for human natural cells,[10] its active compounds may be used, by conducting more research in future, as a replacement for taxol as well as for other chemical substances which are toxic to natural cells.
A study has assessed the cytotoxic effects of grape seed extract (GSE) on the invasion and migration of MDA-MB231 cell line.[25] Their results showed that in comparison with the control group, cellular migration decreased significantly, following 24 h of treatment with GSE (25 µg/mL). They repeated the test with Matrigel to investigate the effect of GSE on cellular invasion and obtained similar results. At a concentration of 25 µg/mL, GSE significantly affects MDA-MB231 cell line invasion. This study demonstrated that the invasion of MCF-7 and MDA-MB-468 cell lines significantly decreases at concentrations of 1288 µg/mL and 992 µg/mL, respectively. Comparing this study with the above one reveals that GSE can better influence the migration and invasion of breast cancer cell lines than M. chamomilla extract; however, considering the fact that the latter has lower cytotoxic effects on natural cell than the former,[26] the remedial role of M. chamomilla extract in preventing different cancers, especially breast cancer, becomes more evident.[25]
PC-3 cells exposed to the aqueous extract of M. chamomilla exhibit signs of antiproliferative effect ranging from 3% to 25% at a concentration of 25-800 µg/mL. Similarly, when the same cells were exposed to the methanolic extract of M. chamomilla at concentrations 25–800 µg/mL, they exhibited 4% to 74% antiproliferative effect. Simultaneous exposure of PC-3 cells to aqueous and methanolic extracts of M. chamomilla (200 µg/mL) for 24 h induces apoptosis.[25] According to research, stewed extracts of M. chamomilla and C. officinalis showed cytotoxic effects on target cancer cells where toxic effect of C. officinalis was more than that of M. chamomilla.
Stewed M. chamomilla has less toxic effects on peripheral blood mononuclear cells while those of C. officinalis are higher. It has conducted a study and showed that M. chamomilla extract has a very low toxic effect on human natural cells.[10] It has showed that a given concentration of M. chamomilla extract is very toxic to cancer cells while has no toxic effect on normal cells.[27] It can be concluded from the studies that a given concentration is available which is very toxic for MCF-7 and MDA-MB-468 cell lines but has a very weak effect on normal cells.
Research on the apigenin content of the extract show that apigenin is an important factor in the plant's anticancer action.[8] This study showed the antiproliferative effect of M. chamomilla on human cancer cells at concentrations 400–1300 µg/mL. The same observations have been reported in studies on other plants of the family at different concentrations. From available information, let us argue that some extracts show more effects than separated components. It should be noted, however, that some compounds available in plants' extracts may play a special role in their final biological activity. Extracts of other plants such as Glycyrrhiza glabra, soybean, green tea, Chinese traditional plants, and red and white wine have exhibited anticancer effects. The findings agree with studies results.[26,28,29]
This study revealed that hydroalcoholic extract of M. chamomilla increases cellular apoptosis and necrosis and decreases cellular proliferation or migration, colonization, invasion, and attachment.
The findings suggest that M. chamomilla extract can prevent the migration and invasion of MCF-7 and MDA-MB-468 cell lines in a time- and dose-dependent manner. Based on the findings, it appears that the plant plays an effective role in preventing or treating breast cancer. However, other related studies required to be designed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Reza Mahmoudi
Reza Mahmoudi obtained Ph.D. of Anatomy and Embryology from Tehran University of Medical Sciences in 2005. He is working as Professor at Yasuj University of Medical sciences and Cellular and Molecular Research Center, Yasuj, Iran. He has more than 20 years of teaching and research experience. His area of research includes assisted reproductive technology, cell culture and herbal medicine study. He has published and presented papers in reputed national and international journals and conferences. He is members of Iranian society for reproductive medicine and Iranian Association of Anatomy.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. 2012. GLOBOCAN v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Harirchi I, Kolahdoozan S, Karbakhsh M, Chegini N, Mohseni SM, Montazeri A, et al. Twenty years of breast cancer in Iran: Downstaging without a formal screening program. Ann Oncol. 2011;22:93–7. doi: 10.1093/annonc/mdq303. [DOI] [PubMed] [Google Scholar]
- 3.Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999;70(3 Suppl):491S–9S. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- 4.Ganzera M, Schneider P, Stuppner H. Inhibitory effects of the essential oil of chamomile (Matricaria recutita L.) and its major constituents on human cytochrome P450 enzymes. Life Sci. 2006;78:856–61. doi: 10.1016/j.lfs.2005.05.095. [DOI] [PubMed] [Google Scholar]
- 5.Haghi G, Hatami A, Safaei A, Mehran M. Analysis of phenolic compounds in Matricaria chamomilla and its extracts by UPLC-UV. Res Pharm Sci. 2014;9:31–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Dreher D, Junod AF. Role of oxygen free radicals in cancer development. Eur J Cancer. 1996;32A:30–8. doi: 10.1016/0959-8049(95)00531-5. [DOI] [PubMed] [Google Scholar]
- 7.Cemek M, Kaga S, Simsek N, Büyükokuroglu ME, Konuk M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J Nat Med. 2008;62:284–93. doi: 10.1007/s11418-008-0228-1. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava JK, Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J Agric Food Chem. 2007;55:9470–8. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- 9.Ogata-Ikeda I, Seo H, Kawanai T, Hashimoto E, Oyama Y. Cytotoxic action of bisabololoxide A of German chamomile on human leukemia K562 cells in combination with 5-fluorouracil. Phytomedicine. 2011;18:362–5. doi: 10.1016/j.phymed.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Matic IZ, Juranic Z, Savikin K, Zdunic G, Nadvinski N, Godevac D. Chamomile and marigold tea: Chemical characterization and evaluation of anticancer activity. Phytother Res. 2013;27:852–8. doi: 10.1002/ptr.4807. [DOI] [PubMed] [Google Scholar]
- 11.Rahimi HR, Rasouli M, Jamshidzadeh A, Farshad S, Firoozi MS, Taghavi AR, et al. New immunological investigations on Helicobacter pylori-induced gastric ulcer in patients. Microbiol Immunol. 2013;57:455–62. doi: 10.1111/1348-0421.12056. [DOI] [PubMed] [Google Scholar]
- 12.Dinicola S, Mariggiò MA, Morabito C, Guarnieri S, Cucina A, Pasqualato A, et al. Grape seed extract triggers apoptosis in Caco-2 human colon cancer cells through reactive oxygen species and calcium increase: Extracellular signal-regulated kinase involvement. Br J Nutr. 2013;110:797–809. doi: 10.1017/S0007114512006095. [DOI] [PubMed] [Google Scholar]
- 13.Chen PN, Hsieh YS, Chiang CL, Chiou HL, Yang SF, Chu SC. Silibinin inhibits invasion of oral cancer cells by suppressing the MAPK pathway. J Dent Res. 2006;85:220–5. doi: 10.1177/154405910608500303. [DOI] [PubMed] [Google Scholar]
- 14.Shin HA, Shin YS, Kang SU, Kim JH, Oh YT, Park KH, et al. Radioprotective effect of epicatechin in cultured human fibroblasts and zebrafish. J Radiat Res. 2014;55:32–40. doi: 10.1093/jrr/rrt085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanden Berghe W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of our epigenomes. Pharmacol Res. 2012;65:565–76. doi: 10.1016/j.phrs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Watson RR, Preedy VR, Zibadi S, editors. Polyphenols in Human Health and Disease. Amsterdam: Elsevier; 2013. pp. 1289–1307. [Google Scholar]
- 17.Di Domenico F, Foppoli C, Coccia R, Perluigi M. Antioxidants in cervical cancer: Chemopreventive and chemotherapeutic effects of polyphenols. Biochim Biophys Acta. 2012;1822:737–47. doi: 10.1016/j.bbadis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Tang H, Nicholson JK, Hylands PJ, Sampson J, Holmes E. A metabonomic strategy for the detection of the metabolic effects of chamomile (Matricaria recutita L.) ingestion. J Agric Food Chem. 2005;53:191–6. doi: 10.1021/jf0403282. [DOI] [PubMed] [Google Scholar]
- 19.Le Bail JC, Varnat F, Nicolas JC, Habrioux G. Estrogenic and antiproliferative activities on MCF-7 human breast cancer cells by flavonoids. Cancer Lett. 1998;130:209–16. doi: 10.1016/s0304-3835(98)00141-4. [DOI] [PubMed] [Google Scholar]
- 20.Kaji T, Kaga K, Miezi N, Hayashi T, Ejiri N, Sakuragawa N. Possible mechanism of the stimulatory effect of Artemisia leaf extract on the proliferation of cultured endothelial cells: Involvement of basic fibroblast growth factor. Chem Pharm Bull (Tokyo) 1990;38:2494–7. doi: 10.1248/cpb.38.2494. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Zhang M, Yu L, Zhao Y, He N, Yang X. Antitumor activities of quercetin and quercetin-5',8-disulfonate in human colon and breast cancer cell lines. Food Chem Toxicol. 2012;50:1589–99. doi: 10.1016/j.fct.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Bullock MD, Sayan AE, Packham GK, Mirnezami AH. MicroRNAs: Critical regulators of epithelial to mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in cancer progression. Biol Cell. 2012;104:3–12. doi: 10.1111/boc.201100115. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–64. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Liebmann JE, Cook JA, Lipschultz C, Teague D, Fisher J, Mitchell JB. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br J Cancer. 1993;68:1104–9. doi: 10.1038/bjc.1993.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinicola S, Pasqualato A, Cucina A, Coluccia P, Ferranti F, Canipari R, et al. Grape seed extract suppresses MDA-MB231 breast cancer cell migration and invasion. Eur J Nutr. 2014;53:421–31. doi: 10.1007/s00394-013-0542-6. [DOI] [PubMed] [Google Scholar]
- 26.Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung JW, et al. Chemopreventive properties of the ethanol extract of Chinese licorice (Glycyrrhiza uralensis) root: Induction of apoptosis and G1 cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett. 2005;230:239–47. doi: 10.1016/j.canlet.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava JK, Pandey M, Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009;85:663–9. doi: 10.1016/j.lfs.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. In vitro and in vivo antitumorigenic activity of a mixture of lysine, proline, ascorbic acid, and green tea extract on human breast cancer lines MDA-MB-231 and MCF-7. Med Oncol. 2005;22:129–38. doi: 10.1385/MO:22:2:129. [DOI] [PubMed] [Google Scholar]
- 29.Sebastian KS, Thampan RV. Differential effects of soybean and fenugreek extracts on the growth of MCF-7 cells. Chem Biol Interact. 2007;170:135–43. doi: 10.1016/j.cbi.2007.07.011. [DOI] [PubMed] [Google Scholar]