Abstract
Background
The role of 18F-fluorodeoxyglucose positron emission computed tomography (18F-FDG PET/CT) is increasing in the diagnosis of polymyalgia rheumatica (PMR), one of the most common inflammatory rheumatic diseases. In addition to other locations, increased 18F-FDG accumulation has been detected in the praepubic region in some patients. However, a deeper description and pathophysiological explanation of this increased praepubic accumulation has been lacking. The aim of the presented study is to confirm a decrease in praepubic 18F-FDG accumulation in response to therapy and to describe potential correlations to other 18F-FDG PET/CT scan characteristics during the course of disease. As a secondary objective, we describe the pathological aspects of the observed praepubic 18F-FDG uptake.
Patients and methods
A retrospective review of patients with newly suspected PMR undergoing baseline and follow up 18F-FDG PET/CT between February 2010 and March 2016 is given. Those with a visually detected presence of praepubic 18F-FDG accumulation were further analysed. The uptake was assessed visually and also semi-quantitatively in the defined region of interest by calculation of target-to-liver ratios. Other regions typical for PMR were systematically described as well (shoulders, hips, sternoclavicular joints, ischiogluteal bursae, spinous interspaces).
Results
Twenty-three out of 89 screened patients (26%) presented with initial praepubic 18F-FDG PET/CT positivity, 15 of whom also underwent follow up 18F-FDG PET/CT examination. Five out of 15 patients presented with increased 18F-FDG accumulation in large arteries as a sign of giant cell arteritis. During follow up examination, decrease in 18F-FDG accumulation caused by therapeutic intervention was observed in all evaluated locations in all analysed patients and no new positivity was indicated, including periarticular, extraarticular tissues or target large vessels. Praepubical accumulation of 18F-FDG was diminished in all patients (15/15, 100%) after treatment with steroids.
Conclusions
Increased praepubic 18F-FDG uptake in patients with PMR is relatively common and this region should be systematically evaluated during differential diagnosis of rheumatic and malignant disease. Praepubic inflammation is probably related to enthesitis and tenosynovitis at the origin of pectineus and adductor longus muscles ventrally from the pubis.
Key words: positron emission tomography, polymyalgia rheumatica, enthesitis, tenosynovitis, fluorodeoxyglucose
Introduction
Polymyalgia rheumatica (PMR) is the most common inflammatory rheumatic disease in patients older than 50 years, with a higher incidence in women. PMR shares many pathogenetic and epidemiological features with giant cell arteritis (GCA)1, and 50% of patients with GCA also develop PMR symptomatology.2 The typical symptoms of PMR are bilateral aching of the shoulder girdle, neck and hip girdle, and morning stiffness lasting for 30 minutes or more. These symptoms are probably related to inflammation of the subacromial, subdeltoid and trochanteric bursae, and the glenohumeral or hip joints.3,4 The diagnosis of PMR is made primarily on clinical grounds and is bolstered by laboratory evidence of an acute phase reaction. There is no single diagnostic test for PMR, but several diagnostic and classification criteria have been suggested by some groups.5–9 Each set of criteria has advantages and disadvantages. A PMR-associated ultrasound lesion(s) in the shoulders and/or hips is currently acknowledged as diagnostic criteria for the scoring algorithm in the differential diagnosis of PMR.10 However, additional imaging methods for assessing rheumatic diseases are warranted.
Prolonged febrile illness with concomittant non-specific symptoms can be also a sign of PMR as well as GCA. Thus, patients may be referred during differential diagnostics of inflammatory or malignant disease to whole body positron emission tomography (PET) or a combination of PET with computed tomography (PET/CT) using 18F-fluorodeoxyglucose (18F-FDG).11–14 Both GCA and PMR have their own characteristic 18F-FDG PET/CT features, which may occur in a non-mutually exclusive manner.
PMR presents with increased 18F-FDG accumulation in periarticular areas of shoulder and hip girdle and of sternoclavicular joints.15–17 The other location with metabolically active inflammation in PMR patients are the extraarticular synovial structures (bursae). It appears that 18F-FDG detection of extraarticular bursitis using PET/CT might be routinely achievable for PMR patients, with reasonable sensitivity (85.7%) and specificity (88.2%), by considering high 18F-FDG uptake in at least 2 of 3 locations (ischial tuberosity, greater trochanter, spinous processes).18
Contrasting at least in part with PMR, GCA imaging typically reveals increased avidity in the wall of whole aorta, including its branches (subclavian and brachial arteries, brachiocephalic trunk, common iliac arteries and femoral arteries).19–21 Importantly, from the clinical point of view, 18F-FDG uptake in pertinent locations decreases in response to effective treatment in both PMR and GCA. Thus, 18F-FDG PET/CT evaluation may be used for monitoring therapy and for follow up.15,19,22,23
In our previous study, increased 18F-FDG accumulation was detected in the praepubic region in some patients.17 However, a deeper description and pathophysiological explanation of this increased praepubic accumulation is needed. The aim of the present study is to confirm a decrease of praepubic 18F-FDG accumulation in response to treatment and to describe potential correlations to other 18F-FDG PET/CT scan characteristics and to the course of disease, and thereby to support the validation of the praepubic avidity within the general 18F-FDG PET/CT features of PMR. As a secondary objective, we provide a description of the pathological aspect of the observed praepubic 18F-FDG uptake.
Patients and methods
Patients with suspected new or relapsed PMR who underwent 18F-FDG PET/CT examination at Masaryk Memorial Cancer Institute in Brno between February 2010 and March 2016 were retrospectively screened for visually detected presence of praepubic 18F-FDG accumulation. Patients for whom follow up 18F-FDG PET/CT was performed during corticosteroid therapy within the clinical remission phase were eligible for further analysis. All patient had to meet ACR 2012 diagnostic criteria for PMR. Patients who were previously treated for known PMR (with at least 15 months from the termination of therapy) were eligible as well. All patients initially provided their signed informed consent with participation on further retrospective studies and this analysis was approved by the institutional review board. Treatment consisted by prednisone, methylprednisolone or methotrexate in various dosages as listed in Table 1.
Table 1.
Imaging and laboratory results of all patients. Reported baseline treatment was initiated several days after baseline PET/CT
| No | Disease status |
Date of baseline (B) and follow up (FU) examination |
Time to control exam (months) |
B/FU praepubic to liver uptake ratio |
B/FU FW (mm/h) |
B/FU CRP (mg/dL) |
B/FU treatment (mg/day) |
B/FU region of positivity of other PMR areas (target-to- liver ratio) |
B/FU vascular positivity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | R | 11-18-2014 | 4.0 | 1.134 | 44 | 23 | P 15 | S 2.23, H 1.25, Scl 1.13, Isch 1.45 |
no positivity |
| 3-20-2015 | 0.576 | 16 | 1.4 | P 5 | H 1.11 | no positivity | |||
| 2 | N | 2-23-2010 | 48.7 | 1.234 | 120 | 49 | P 15 | S 1.65, H 1.24, Scl 1.24, Isch 1.17, L 1.14 |
no positivity |
| 3-15-2014 | 0.812 | 26 | 16.9 | M 8 | no positivity | no positivity | |||
| 3 | N | 9-1-2014 | 3.2 | 2.148 | 120 | 137 | P 60 | S 1.87, Scl 1.45, H 2.31, Isch 1.65, L 1.12, Th 1.14, C 1.24 |
no positivity |
| 12-8-2014 | 0.992 | 35 | 16.7 | P 10 | S 1.36 | no positivity | |||
| 4 | N | 7-24-2010 | 6.1 | 1.891 | 120 | 56.8 | P 40 | S 1.78, Scl 1.54, H 1, Isch 2.21, L 1.08 |
no positivity |
| 1-25-2011 | 0.345 | 30 | 5.7 | P 2.5 | no positivity | no positivity | |||
| 5 | N | 3-13-2014 | 12.0 | 1.772 | 60 | 28.5 | P 20 | S 2.02, H 1.47, Scl 1.24, Isch 2.02, L 1.23 |
V3/6 |
| 3-13-2015 | 0.987 | 7 | 1 | P 0 | no positivity | V1/6 no positivity |
|||
| 6 | N | 10-14-2013 | 16.9 | 1.298 | 54 | 45 | P 20 | S1.78, H1.88, Scl 1.95, L 1.25, Isch 1.16 |
|
| 3-13-2015 | 0.537 | 6 | 5.3 | P 2.5 | no positivity | no positivity | |||
| 7 | R | 1-3-2014 | 13.1 | 1.302 | 77 | 78.3 | M 16 | S 1.78, H 1.87, Scl 1.35, Isch 1.65, L 1.12 |
V4/6 |
| 2-6-2015 | 0.403 | 5 | 1.9 | M 2 | no positivity | no positivity | |||
| 8 | N | 6-9-2012 | 33.1 | 1.835 | 50 | 76 | P 30 | S 2.21, H 2.16, Scl 1.8, Isch 1.78, L 1.2 |
V4/6 |
| 3-13-2015 | 0.54 | 14 | 3.1 | P 0 | H 1.23 | V2/6 | |||
| 9 | N | 6-3-2015 | 3.1 | 1.502 | 60 | 41.5 | P 15 | S 2.36, H 2.24, Scl 2.03, Isch 2.04, C 1.11 |
no positivity |
| 9-4-2015 | 0.76 | 16 | 13.4 | P 10 | no positivity | no positivity no positivity |
|||
| 10 | N | 1-8-2014 | 21.7 | 1.209 | 80 | 78.3 | P 60 | S 1.84, H1.78, Scl 1.69, Isch 1.57, L 1.23 |
|
| 10-30-2015 | 0.395 | 10 | 2.5 | P 7.5 | no positivity | no positivity | |||
| 11 | R | 3-4-2015 | 8.1 | 1.546 | 62 | 16.5 | M 48 + MTX 10/ week M 4 + |
S 2.66, H 2.74, Scl 2.0, Isch 1.72, C 1.47, L 1.48 |
V5/6 |
| 11-5-2015 | 0.811 | 30 | 3.3 | MTX 10/ week |
H 1.14, C 1.08, S 1.11, Scl 1.07 S 2.27, H 2.06, Scl 2.16, Isch |
V3/6 | |||
| 12 | N | 2-6-2015 | 8.3 | 1.789 | 120 | 98.7 | M 32 | 3.03, L 1.45 | no positivity |
| 10-16-2015 | 0.97 | 40 | 7.5 | M 8 | Scl 1.14, Isch 1.21 | no positivity | |||
| 13 | N | 1-23-2015 | 14 | 1.123 | 70 | 67.5 | P 15 | S 1.78, H 1.87, Scl 1.69, Isch 1.57, L 1.24 |
no positivity |
| 3-24-2016 | 0.441 | 6 | 1.4 | P 5 | no positivity | no positivity no positivity |
|||
| 14 | N | 9-16-2015 | 5.8 | 1.282 | 74 | 52 | P 20 | S 2.44, H 3.04, Scl 2.07, L 1.21 | |
| 3-9-2016 | 0.41 | 24 | 3.2 | P 7.5 | no positivity | no positivity | |||
| 15 | N | 10-26-2015 | 3.2 | 1.892 | 80 | 118.8 | P 30 | S 2.93, H 2.97, Isch 3.22, C 1.44, L 1.24, Th 1.17, Scl 1.79 |
V4/6 |
| 2-1-2016 | 0.678 | 5 | 1.3 | P20 | no positivity | no positivity |
B/C = baseline and control; C, L, Th = cervical, lumbal, thoracic interspinous space; FW = Fåhræus-Westergren test; H = hip; M = methylprednisolone; Isch = ischiogluteal bursae; MTX = methotrexate; N = newly diagnosed; P = prednisone; R = relapse; S = shoulder; Scl = sternoclavicular joint; V = vascular uptake with number indicating presence in regions from 6 measured
18F-FDG PET/CT examination was performed utilizing the hybrid scanner Biograph 64 HR+ Siemens Erlangen, Germany. CT scan was performed in low dose CT (25 mAs eff/120 kV) as well as diagnostic or contrast enhanced CT scan (160 mAs eff/12 kV) (intravenous Iomeron 400, BRACCO, Milan, Italy). All patients had standard preparation prior to examination, including restriction of physical activity for 12 h, fasting for at least 6 h, capillary glycemia below 10 mmol/L (180 mg/dL) prior to 18F-FDG administration and peroral hydration with 500-1,000 mL of plain water. 18F-FDG (UJV Rez, Czech Republic) was administered in a dose range of 327-434 (median 366 MBq) in the baseline study and in a dose range of 301-400 (median 362 MBq) in the follow up examination. After an in vivo accumulation time of 55 to 75 minutes, whole body scanning from the proximal third of thighs to the skull base was performed in baseline as well as follow up study. All images were iteratively reconstructed and corrected for attenuation.
18F-FDG uptake was assessed visually and also semi-quantitatively in the defined region of interest (ROI) with calculation of target-to-liver ratios. Liver 18F-FDG uptake (SUVmax) was used as a reference base (measured within the ROI located in the centrum of the right liver lobe). Praepubic 18F-FDG accumulation was semiquantitavely assessed as SUVmax within elliptic ROI located through both preapubic regions while keeping in the safe distance from the bladder based on the investigator discretion. Praepubic-to-liver ratio (SUVmax) was subsequently calculated.
Other regions typical for PMR were systematically described with measurement of SUVmax: shoulders, hips and sternoclavicular joints and extraarticular sites – in ischial tuberosity and between spinous processes where ischiogluteal and interspinous bursae are often presented, respectively. A target-to-liver ratio higher than 1.0 was considered positive in all mentioned regions. For paired organs, the higher value (from the right or left site) was used for target-to-liver calculation. 18F-FDG uptake (SUVmax) within the typical sites for GCA was also systematically evaluated, namely in the walls of following arteries: thoracic aorta, abdominal aorta, brachial and subclavian arteries, iliac and femoral arteries. An artery wall-to-liver ratio higher than 1.0 was also considered positive. The number of positive vascular regions out of six evaluated regions is reported. Again, for paired structures, the higher value (from the right or left site) was used for target-to-liver calculation. The same scan evaluation was performed for follow up scans.
Results
Patients characteristic
From 89 screened patients, 23 (26%) presented with initial praepubic 18F-FDG PET/CT positivity. 15 patients, 10 women and 5 men with a median age of 70 years, range 53 to 78, underwent also follow up 18F-FDG PET/CT and met inclusion criteria for the presented analysis (Table 2). Twelve out of 15 patients were classified as newly diagnosed; prior to the initiation of corticosteroid therapy, the other three patients had been previously treated for known PMR (with at least 15 months from the termination of therapy). Laboratory values for FW and CRP were obtained for all patients, ± 14 days around 18F-FDG PET/CT (Table 3). Patient number 5 had accompanied giant cell arteritis confirmed histologically by temporal artery biopsy.
Table 2.
Patients’ baseline characteristics
| Characteristics | Numbers (%) n= 15 (100%) |
|---|---|
| Sex, n (%) | |
| females | 10 (66.7 %) |
| males | 5 (33.3 %) |
| Age at the disease onset | |
| median (min-max) | 70 years (53-78) |
| Time to follow up exam | |
| median (min-max) | 8 months (3-49) |
Table 3.
Changes in analysed laboratory parameters between baseline and follow up examination
| Baseline median (min-max) |
Follow up median (min-max) |
p-value median (min-max) |
|
|---|---|---|---|
| CRP | 57 mg/l (17-137) | 3 mg/l (1-17) | 0.001 |
| FW | 74 mm/hod (44-120) | 16 mm/hod (5-40) | 0.001 |
Baseline 18F-FDG PET/CT characteristics
18F-FDG PET/CT characteristic including 18F-FDG dosage are summarized in Table 4. Increased 18F-FDG accumulation (positivity) was observed at baseline in all patients in the praepubic region, with praepubic-to-liver ratios higher than 1.0. This accumulation was obvious by visual evaluation only, as presented in Figure 1, and this finding was always accompanied by additional positivity in 18F-FDG PET/CT scans (Table 1). Other sites with increased accumulation were as follow: around the shoulder girdle in all 15 patients, around the hip girdle in 15 patients, and around sternoclavicular joints in 14 out of 15 patients. 18F-FDG PET/CT positivity was observed also in extraarticular synovial structures, in ischiogluteal bursa in 14 patients and between spinous processes of the vertebrae in 14 patients, most commonly within lumbal region in 13 patients.
Table 4.
Nuclear medicine data at baseline and follow up examination
| Baseline median (min-max) |
Follow up median (min-max) |
p-value median (min-max) |
|
|---|---|---|---|
| 18F-FDG | 366 MBq (327-434) | 362 MBq (301-400) | 0.271 |
| Praepubic / liver 18F-FDG uptake | 1.50 (1.12-2.15) | 0.58 (0.35-0.99) | 0.001 |
Figure 1.
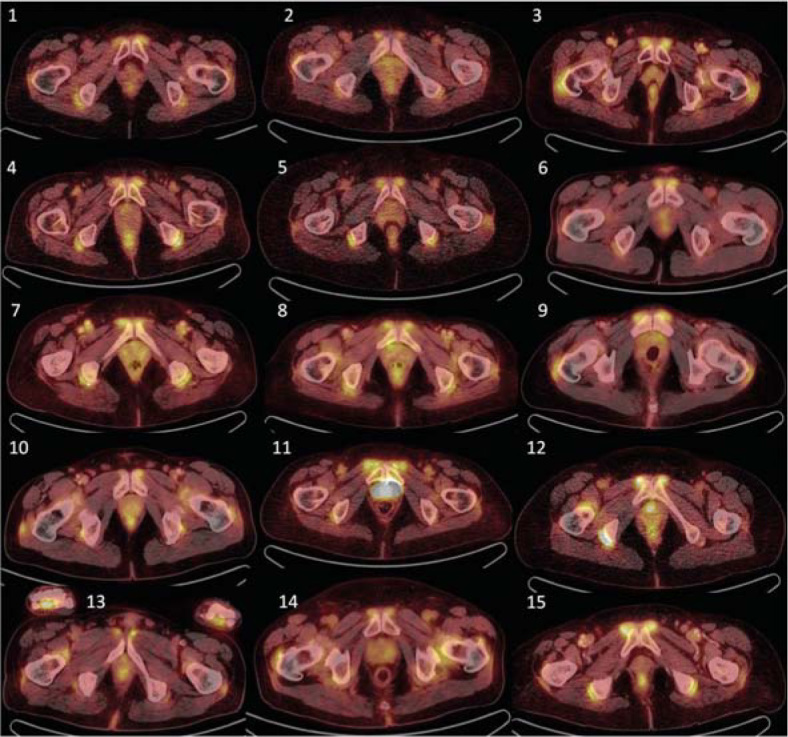
Initial 18FDG-PET/CT examination of all 15 examined patients, showing transversal planes through maximal praepubic uptake. Visually detectable accumulation can be observed in all patients.
Five out of 15 patients presented with increased 18F-FDG accumulation in large arteries as a sign of large vessel vasculitis of GCA. In those with large vessel vasculitis, 18F-FDG PET/CT positivity was at least in 3 of 6 evaluated vascular regions. Increased values of FW and CRP were detected in all patients during initial examination and were correlated to newly diagnosed PMR or to its relapse (Table 3).
Follow up 18F-FDG PET/CT characteristics
Given the retrospective nature of this study, it was not possible to keep a strict interval between baseline and follow up 18F-FDG PET/CT scans. Follow up examinations were timed by clinical purposes rather than by experimental needs. Follow up 18F-FDG PET/CT (median time 8 months, range 3-49) revealed continuing positivity around the shoulder girdle in only 2/15 patients, around hip joints in 3/15, around the sternoclavicular joint in 2/15, in extraarticular synovial structures in ischiogluteal bursae in 1/15 and in interspinous regions of cervical vertebrae in 1/15 patients. In three out of 15 patients, positivity in continuous large vessels was observed, maximally in 3 vessel regions out of 6 measured. In all evaluated locations in all analysed patients, a decrease in 18F-FDG accumulation (target-to-liver ratio) was observed, and no new positivity was indicated, including periarticular, extraarticular tissues or target large vessels. Praepubic accumulation of 18F-FDG was diminished in all patients after treatment with steroids. Praepubic-to-liver ratio was lower than 1.0 post therapy and this decrease in accumulation was clear by visual assessment (Figure 2).
Figure 2.
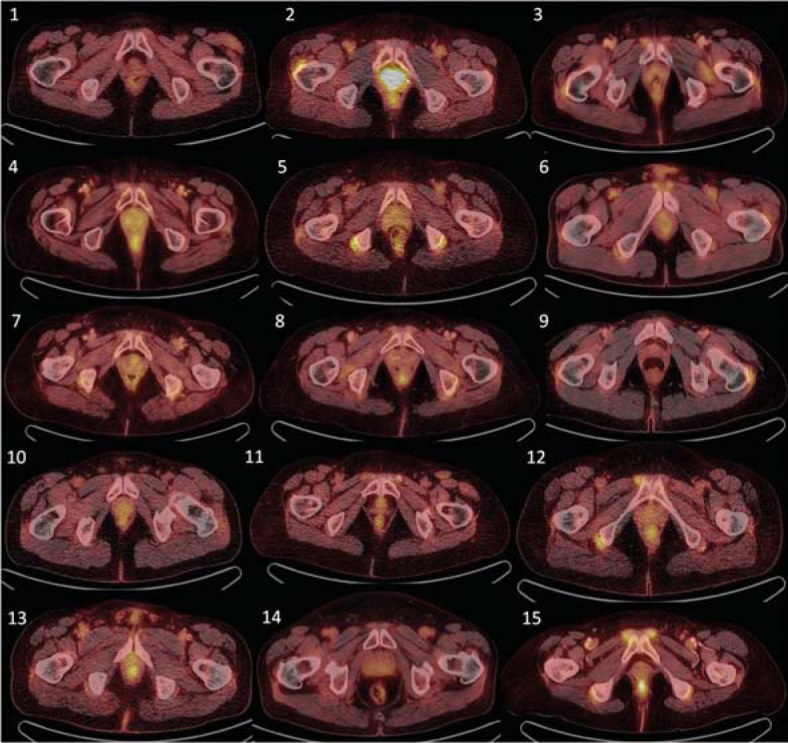
Control 18FDG-PET/CT examination showing corresponding transversal slices as in Figure 1. Compared with Figure 1, decrease or complete diminishment of 18FDG accumulation is observable in all patients.
During follow up 18F-FDG PET/CT examination, all patients were undergoing steroid treatment and they reported subjective improvement in health condition, and disease remission was confirmed by attending rheumatologist. The laboratory signs of inflammation, FW, and CRP were decreased as well (Table 3).
Discussion
In the presented retrospective analysis of 18F-FDG PET/CT findings in patients with proven PMR, 25.8% patients presented with praepubic 18F-FDG uptake with fifteen patients from our cohort being able to undergo follow up 18F-FDG PET/CT examination. We were not able to perform follow up 18F-FDG PET/CT scan in a prospectively defined phase of treatment; instead, the aim was to obtain 18F-FDG PET/CT study during disease remission. During this follow up 18F-FDG PET/CT exam, praepubic accumulation of 18F-FDG was diminished in all 15 patients after treatment with steroids.
In our previous study, praepubic positivity was described only in 5/67 (8%) PMR patients representing only a relatively rare sign of the disease.17 However, 32/67 (48%) patients were examined on an older PET scanner with the rest undergoing examination in a newer hybrid PET/CT scanner with better image resolution. Lower detectability of targed lesions may have occured on 18F-FDG PET scanner alone and also underevaluation of accumulation quantification may result in false negativity (praepubic-to-liver ratio < 1.0). Even if visual accumulation was clearly positive, no patient from our previous cohort met the criterion for positivity. Given the above findings, it is reasonable to speculate that the percentage of positive preapubic accumulation in patients with active PMR would be higher when examined on a hybrid 18F-FDG PET/CT rather than a PET camera. The overall number of patients with initial praepubic 18F-FDG PET/CT positivity was 23/89 (25.8%). Thus, praepubic accumulation should not be considered a constant and frequent sign of 18F-FDG PET/CT results as it is in periarticular 18F-FDG accumulation in shoulders and hips. In cohorts from previous studies, accumulation in the shoulder groin girdle was observed in 33/35 (94%), in 12/14 (86%) and in 58/67 (87%) and in the hips in 31/35 (89%), in 12/14 (86%) and in 47/67 (70%) PMR patients, respectively.15–17
In a recently published report of 15 patients with PMR and of 9 patients with Elderly-Onset Rheumatoid Arthritis (EORA), praepubic 18F-FDG uptake was recommended as one of the signs to enable differentiation between these two conditions. 24 The incidence of praepubic 18F-FDG uptake (pectineus enthesitis) was relatively high with 9 patients from 15 evaluated which may be related to sample size bias. Patients with PMR developed significantly higher uptake compared to those with EORA.24 Patients in the present study represent a retrospectively described cohort of patients with praepubic 18F-FDG PET/CT positivity that includes post-treatment follow up examination. In light of these findings, it seems possible that increased praepubic accumulation may have been present in previously published case reports including those where axial slices indicating ischiogluteal bursitis were published.18,25-27 Sondag et al. observed 18F-FDG uptake in 11/50 (22%) patients; however, the cohort was a mixture of those with and without treatment with 22/50 (44%) with administration of steroids.28 Mackie et al. published the first MRI findings of inflammation in the front of the symphysis in patients with PMR in 2015.29 A similar observation was observed in our cohort (patient number 11); data not shown.
It is difficult to exactly determine the pathological background of increased 18F-FDG uptake in the praepubic region in patients with PMR. The described accumulation seems to be relatively bordered and with spheric shape in some patients, while with blurry margins in others. MRI may be helpful in further evaluation of this region. Based on the MRI finding presented by Mackie et al. and Wakura et al., we assume another type of extraarticular inflammation is responsible for the observed PET findings.24,30 Namely, it appears to represent features of enthesitis and tenosynovitis of pectineus and adductor longus muscles. There is probably no bursa in the locations under the tendons of these muscles, which is also supported by findings on MRI, where no fluid collection with evidence of thickened wall suggestive for bursitis was observed. However, some reports have recently suggested a combination of PMR and tenosynovitis in other locations, namely in the long head of biceps brachii 31 or in extensor tenosynovitis of the hand32 or in the vicinity of the enthesis of the rectus femoris.24
Our observations confirm that 18F-FDG PET/CT examination seems to be an advantageous one-step diagnostic modality for detecting different variants of PMR, for assessing extent and severity, and for excluding occult malignancy. The follow up exam may be useful in monitoring disease activity including the praepubic location. It is conceivable that the praepubic region will become part of targeted examination for other imaging strategies as is US or MRI and that clinical significance and correlations will be further discovered.
Conclusions
Increased praepubic tracer accumulation is becoming an integral part of 18F-FDG PET/CT evaluation of polymyalgia rheumatica, and is probably a correlate of enthesitis and tenosynovitis at the origin of pectineus and adductor longus muscles ventrally from the pubis. The findings described here were consistently presented in combination with other periarticular accumulations (shoulder and hip girdle and surrounded bursae, sternoclavicular joint) and other extraarticular bursae at some distance from joints such as the ischiogluteal bursae and interspinous bursae in spine. Some patients presented with signs of large vessels vasculitis of GCA. In accordance with other 18F-FDG PET/CT positive locations, praepubic accumulation was decreased in relation to PMR treatment. Our findings support the clinical value of 18F-FDG PET/CT examination of patients with suspected or proved PMR.
Acknowledgments and funding
This work was supported in part by the Ministry of Health, Czech Republic – Conceptual Development of Research Organization (MMCI 00209805, FNBr, 65269705) and project MEYS-NPS I-LO1413.
Disclosure: No potential conflicts of interest were disclosed.
References
- 1.Nesjet G,, Nesjet R. In: Vasculitis (2nd ed.) Ball GV,, Bridges SL Jr, editors. Oxford University Press; 2008. Giant cell arteritis and polymyalgia rheumatica; pp. 307–16. [Google Scholar]
- 2.Pipitone N,, Salvarani C.. Update on polymyalgia rheumatica. Eur J Intern Med. 2013;24:583–9. doi: 10.1016/j.ejim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Salvarani C,, Cantini F,, Olivieri I,, Barozzi L,, Machioni L,, Niccoli L,. et al. Proximal bursitis in active polymyalgia rheumatica. Ann Intern Med. 1997;127:27–31. doi: 10.7326/0003-4819-127-1-199707010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Cantini F,, Nicoli L,, Nannini C,, Padula A,, Olivieri I,, Boiardi L,. et al. Inflammatory changes of the hip synovial structures in polymyalgia rheumatica. Clin Exp Rheumatol. 2005;23:462–8. [PubMed] [Google Scholar]
- 5.Chuang TY,, Hunder GG,, Ilstrup DM,, Kurland LT.. Polymyalgia rheumatica: a 10-year epidemiologic and clinical study. Ann Intern Med. 1982;97:672–80. doi: 10.7326/0003-4819-97-5-672. [DOI] [PubMed] [Google Scholar]
- 6.Hamrin B.. Polymyalgia arteritica. Acta Med Scand. 1972;533:1–131. [PubMed] [Google Scholar]
- 7.Bird HA,, Esselinckx W,, Dixon AS,, Mowat AG,, Wood PH.. An evaluation of criteria for polymyalgia rheumatica. Ann Rheum Dis. 1979;38:434–9. doi: 10.1136/ard.38.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones JG,, Hazleman BL.. Prognosis and management of polymyalgia rheumatica. Ann Rheum Dis. 1981;40:1–5. doi: 10.1136/ard.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healey LA.. Long-term follow-up of polymyalgia rheumatica: evidence for synovitis. Semin Arthritis Rheum. 1984;13:322–8. doi: 10.1016/0049-0172(84)90012-X. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta B,, Cimmino MA,, Maradit-Kremers H,, Schmidt WA,, Schirmer M,, Salvarani C,. et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2012;71:484–92. doi: 10.1136/annrheumdis-2011-200329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaudemans AWJM,, Signore A.. FDG-PET/CT in infections: the imaging method of choice? Eur J Nucl Med Mol Imaging. 2010;37:1986–91. doi: 10.1007/s00259-010-1587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaruskova M,, Belohlavek O.. Role of FDG-PET and PET/CT in the diagnosis of prolonged febrile states. Eur J Nucl Med Mol Imaging. 2006;33:913–8. doi: 10.1007/s00259-006-0064-z. [DOI] [PubMed] [Google Scholar]
- 13.Kubota K,, Nakamoto Y,, Tamaki N,, Kanegae K,, Fukuda H,, Kaneda T,. et al. FDG-PET for the diagnosis of fever of unknown origin: a Japanese multi-center study. Ann Nucl Med. 2011;25:355–64. doi: 10.1007/s12149-011-0470-6. [DOI] [PubMed] [Google Scholar]
- 14.Vaidyanathan S,, Patel CN,, Scarsbrook AF,, Chowdhury FU.. FDG PET/CT in infection and inflammation-current and emerging clinical applications. Clin radiol. 2015;70:787–800. doi: 10.1016/j.crad.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Blockmans D,, De Ceuninck L,, Vanderschueren S,, Knockaert D,, Mortelmans L,, Bobbaers H.. Repetitive 18-fluorodeoxyglucose positron emission tomography in isolated polymyalgia rheumatica: a prospective study in 35 patients. Rheumatology. 2007;46:672–7. doi: 10.1093/rheumatology/kel376. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita H,, Inoue M,, Takahashi Y,, Kano T,, Mimori A.. The natural history of asymptomatic positron emission tomography: positive giant cell arteritis after a case of self-limiting polymyalgia rheumatica. Mod Rheumatol. 2012;22:942–6. doi: 10.1007/s10165-012-0689-7. [DOI] [PubMed] [Google Scholar]
- 17.Rehak Z,, Vasina J,, Nemec P,, Fojtik Z,, Koukalova R,, Bortlicek Z,. et al. Various forms of 18F-FDG PET and PET/CT findings in patients with polymyalgia rheumatica. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:629–36. doi: 10.5507/bp.2015.026. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita H,, Kubota K,, Takahashi Y,, Minaminoto R,, Morooka M,, Ito K,. et al. Whole-body fluorodeoxyglucose positron emission tomography/computed tomography in patiens with active polymyalgia rheumatica: evidence for distinctive bursitis and large-vessel vasculitis. Mod Rheumatol. 2012;22:705–11. doi: 10.1007/s10165-011-0581-x. [DOI] [PubMed] [Google Scholar]
- 19.Blockmans D,, De Ceuninck L,, Vanderschueren S,, Knockaert D,, Mortelmans L,, Bobbaers H.. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study in 35 patients. Arthritis Rheum. 2006;55:131–7. doi: 10.1002/art.21699. [DOI] [PubMed] [Google Scholar]
- 20.Lensen KDF,, Comans EFI,, Voskuyl AE,, Van der Laken CJ,, Brouwer E,, Zwijnenburg AT,. et al. Large-Vessel Vasculitis: Interobserver Agreement and Diagnostic Accuracy of 18 F-FDG-PET/CT. Biomed Res Int. 2015;2015:914692. doi: 10.1155/2015/914692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs M,, Briel M,, Daikeler T,, Walker UA,, Rasch H,, Berg S,. et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging. 2012;39:344–53. doi: 10.1007/s00259-011-1967-x. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi Y,, Nakayama S,, Terada Y.. Clinical implication of FDG-PET/CT in monitoring disease activity in large-vessel giant cell arteritis linked with secondary polymyalgia rheumatica. Case Reports in Internal Medicine. 2014;1:6–9. doi: 10.5430/crim.v1n1p6. [DOI] [Google Scholar]
- 23.Glaudemans AWJM,, de Vries EFJ,, Galli F,, Dierckx RAJO,, Slart RHJA,, Signore A.. The use of 18F-FDG-PET/CT for Diagnosis and Treatment Monitoring of Inflammatory and Infectious Diseases. Clinical and Developmental Immunology. 2013;2013:623036. doi: 10.1155/2013/623036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakura D,, Kotani T,, Takeuchi T,, Komori T,, Yoshida 1, Makino S,. et al. Differentiation between Polymyalgia Rheumatica (PMR) and Elderly-Onset Rheumatoid Arthritis Using 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: Is Enthesitis a New Pathological Lesion in PMR? PLoS One. 2016;11:e0158509. doi: 10.1371/journal.pone.0158509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toriihara A,, Seto Y,, Yoshida K,, Umehara I,, Nakagawa T,, Tassei MD,. et al. F-18 FDG PET/CT of Polymyalgia Rheumatica. Clin Nucl Med. 2009;34:305–6. doi: 10.1097/RLU.0b013e31819e51fd. [DOI] [PubMed] [Google Scholar]
- 26.Kotani T,, Komori T,, Kanzaki Y,, Takeuchi T,, Wakura D,, Iimori A,. et al. FDG-PET/CT of polymyalgia rheumatica. Mod Rheumatol. 2011;21:334–6. doi: 10.1007/s10165-010-0382-7. [DOI] [PubMed] [Google Scholar]
- 27.Park JS,, Pyo JY,, Park HJ,, Lee HS,, Kang Y,, Kang MI,. et al. Typical 18-FDG-PET/CT Findings of Polymyalgia Rheumatica: A Case Report. Journal of Rheumatic Diseases. 2013;20:113–7. doi: 10.4078/jrd.2013.20.2.113. [DOI] [Google Scholar]
- 28.Sondag M,, Guillot X,, Verhoeven F,, Blagosklonov O,, Prati C,, Boulahdour H. et al. Utility of 18F-fluoro-dexoxyglucose positron emission tomography for the diagnosis of polymyalgia rheumatica: a controlled study. Rheumatology. 2016;55:1452–7. doi: 10.1093/rheumatology/kew202. [DOI] [PubMed] [Google Scholar]
- 29.Mackie SL,, Pease CT,, Fukuba E,, Harris E,, Emery P,, Hodgson R,. et al. Wholebody MRI of patients with polymyalgia rheumatica identifies a distinct subset with complete patient-reported response to glucocorticoids. Ann Rheum Dis. 2015;74:2188–92. doi: 10.1136/annrheumdis-2015-207395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackie SL,, Mc Gonagle DG.. Response to:’A relationship between extracapsular involvement and response to steroid treatment in polymyalgia rheumatica: too soon to conclude?’by Yang. et al. Ann Rheum Dis. 2016;75:e17. doi: 10.1136/annrheumdis-2015-208962. [DOI] [PubMed] [Google Scholar]
- 31.Ruta S,, Rosa J,, Navarta DA,, Saucedo C,, Catoggio LJ,, Monaco RG,. et al. Ultrasound assessment of new onset bilateral painful shoulder in patiens with polymyalgia rheumatica and rheumatoid arthritis. Clin Rheumatol. 2012;31:1383–7. doi: 10.1007/s10067-012-2016-2. [DOI] [PubMed] [Google Scholar]
- 32.Cimmino MA,, Parodi M,, Zampogna G,, Barbieri F,, Garlaschi G.. Polymyalgia rheumatica is associated with extensor tendon tenosynovitis but not with synovitis of the hands: a magnetic resonance imaging study. Rheumatology. 2011;50:494–9. doi: 10.1093/rheumatology/keq367. [DOI] [PubMed] [Google Scholar]


