Abstract
Background
Tumor irradiation combined with adjuvant treatments, either vascular targeted or immunomodulatory, is under intense investigation. Gene electrotransfer of therapeutic genes is one of these approaches. The aim of this study was to determine, whether gene electrotransfer of plasmid encoding shRNA for silencing endoglin, with vascular targeted effectiveness, can radiosensitize melanoma B16F10 tumors.
Materials and methods
The murine melanoma B16F10 tumors, growing on the back of C57Bl/6 mice, were treated by triple gene electrotransfer and irradiation. The antitumor effect was evaluated by determination of tumor growth delay and proportion of tumor free mice. Furthermore, histological analysis of tumors (necrosis, apoptosis, proliferation, vascularization, presence of hypoxia and infiltration of immune cells,) was used to evaluate the therapeutic mechanisms.
Results
Gene electrotransfer of plasmid silencing endoglin predominantly indicated vascular targeted effects of the therapy, since significant tumor growth delay and 44% of tumor free mice were obtained. In addition, irradiation had minor effects on radioresistant melanoma, with 11% of mice tumor free. The combined treatment resulted in excellent effectiveness with 88% of mice tumor free, with more than half resistant to secondary tumor challenge, which was observed also with the plasmid devoid of the therapeutic gene. Histological analysis of tumors in the combined treatment group, demonstrated similar mode of action of the gene electrotransfer of plasmid encoding shRNA for silencing endoglin and devoid of it, both through the induction of an immune response.
Conclusions
The results of this study indicate that irradiation can in radioresistant melanoma tumors, by release of tumor associated antigens, serve as activator of the immune response, besides directly affecting tumor cells and vasculature. The primed antitumor immune response can be further boosted by gene electrotransfer of plasmid, regardless of presence of the therapeutic gene, which was confirmed by the high radiosensitization, resulting in prolonged tumor growth delay and 89% of tumor free mice that were up to 63% resistant to secondary challenge of tumor. In addition, gene electrotransfer of therapeutic plasmid for silencing endoglin has also a direct effect on tumor vasculature and tumors cells; however in combination with radiotherapy this effect was masked by pronounced immune response.
Keywords: gene therapy, electrotransfer, plasmid, irradiation, immune response, melanoma
Introduction
Electroporation is used as drug delivery system for molecules with hampered transmembrane transport.1 It is effective for delivery of smaller molecules, as chemotherapeutics in electrochemotherapy (ECT)1-3 and also for larger molecules, as are plasmids in gene electrotransfer (GET). GET is recently getting a lot of scientific consideration and it is used for enhanced DNA delivery into various tissue types (i.e. skin, liver, kidney etc.), as well as into tumors.4-6 Its effectiveness was first demonstrated in a wide range of preclinical studies and has thereafter proceeded to clinical oncology, veterinary and human. The results of clinical trials of GET of plasmid encoding human IL-127 and antiangiogenic plasmid AMEP8, are promising. In addition to GET of therapeutic plasmids, some plasmids devoid of therapeutic genes have also resulted in good antitumor effectiveness.9,10 This phenomenon was observed in different tumors models and by using different plasmids. It was attributed to immune sensing of the introduced DNA as a DAMP (Damage Associated Molecular Pattern), which switch leads to activation of an immune response.11
Radiotherapy is one of the principal treatment modalities for primary tumors and their metastases.12 Nowadays, irradiation is widely investigated for its associated effects on priming antitumor immunity.13 There is growing evidence of irradiation’s effect on the antitumor immune response, inducing immunogenic cell death and generating danger signals. An important danger signal after irradiation is DNA released from the nucleus of dying cells. This DNA is recognized by the immune system as a DAMP, and can promote the activation of immune response against irradiated cells.14,15
Studies combining tumor irradiation with GET of different therapeutic plasmid demonstrated tumor radiosensitization.16-18 A promising approach to target tumors and its microenvironment with a combined treatment modality is through destructing abnormal tumor blood vessels. One of the promising targets, gaining on its value due to different signaling pathways from VEGF, is endoglin. It is a TGF-β coreceptor and has already demonstrated good antitumor and antimetastatic effectiveness in different tumor models when targeted with GET of plasmid.18–22 In particular, GET of shRNA for silencing endoglin in B16F10 melanoma mice tumors that express high levels of endoglin, resulted in up to 58% of tumor cures.23
Tissue specific eukaryotic promoters are tightly regulated and mainly drive expression of transgene in specific cell types, although minimal unspecific expression in non-targeted tissue can also occur.24 We constructed a plasmid containing a tissue specific promoter for endothelin and encoding shRNA for silencing endoglin. This plasmid was tested in a previous study, where the effectiveness of the plasmid with tissue specific promoter was compared to a plasmid with constitutive promoter in a tumor model that does not express targeted molecule, endoglin. In vivo, the effectiveness of the GET of the plasmids was comparable and resulted in significant radiosensitization, which resulted in prolonged tumor growth delay with nearly 50% of tumor free mice. Thus, the aim of this current study was to determine, whether GET of plasmid, with tissue specific promoter and encoding shRNA for silencing endoglin, can radiosensitize melanoma B16F10 tumors, which express targeted molecule, endoglin, and possibly have also some immunomodulatory effectiveness.
Materials and methods
Cell lines and plasmids
Murine melanoma cell line B16F10 (American Type Culture Collection, Manassas, VA, USA) was cultured in advanced minimum essential medium (AMEM, Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 5% fetal bovine serum (FBS, Gibco), 10 mM/L L-glutamine (GlutaMAX, Gibco), 100 U/mL penicillin (Grünenthal, Aachen, Germany) and 50 mg/mL gentamicin (Krka, Novo mesto, Slovenia) in a 5% CO2 humidified incubator at 37°C.
The plasmid with tissue specific promoter for endothelial cells, encoding shRNA for silencing endoglin (pET-antiCD105; TS plasmid) was used in experiments as the therapeutic plasmid.18 The control plasmid, encoding shRNA with no homology to any gene in the mouse genome and with constitutive CMV promoter, was used as a negative control (pControl).25 Amplification of both plasmids was performed in a competent E.coli (JM107; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA).
All plasmids were isolated using JETSTAR 2.0 ENDOTOXIN-FREE Plasmid MEGA Kit (Genomed, Löhne, Germany) and diluted in endotoxin free water to a concentration of 1 μg/μL (in vitro experiments) and 4 μg/μL (in vivo experiments). Concentrations of plasmids were measured with a spectrophotometer at 260 nm (Epoch Microplate Spectrophotometer, Take3 Micro-Volume Plate, BioTek, Bad Friedrichshall, Germany) and purity of plasmid was determined by agarose gel electrophoresis and measurements of the absorbance ratio at 260 and 280 nm.
Experimental animals
All animal experiments were conducted in accordance with the guidelines for animal experiments of the EU Directive and the permission obtained from the Ministry of Agriculture and the Environment of the Republic of Slovenia (Permission No. 344011/2015/16), which was given, based on the approval of the National Ethics Committee for Experiments on Laboratory Animals).
Female C57Bl/6 mice, 6-8-week old, purchased from Envigo Laboratories (Udine, Italy), were used in the study. Before the experiment, mice were subjected to an adaptation period of 2 weeks. Animals were maintained under specific pathogen-free conditions at a constant room temperature, humidity and a 12 h light/dark cycle. Food and water were provided ad libitum. For induction of subcutaneous tumors, a suspension of 1 × 106 B16F10 cells in 0.1 ml of physiological saline was injected subcutaneously into the back of the mice. The animals bearing tumors of 40 mm3 were randomly divided into experimental groups and subjected to a specific experimental protocol. The tumor measurements were completed when the tumors reached 350 mm3, and mice were humanely sacrificed.
Experimental groups and the number of animals in each of them were as follows and as described in table Table 1: triple injection of endotoxin-free water alone (control group; CTRL) or of pControl or TS plasmids alone, or in combination with triple application of electric pulses alone (3 × EP) or combined with plasmids (3 × GET (pControl); 3 × GET (TS)). Furthermore, the remaining groups were also the ones in combination with irradiation (IR) and other therapies described above, which are three injections of plasmids (pControl + IR; TS + IR) and in combination with electric pulses (3 × EP + IR; 3 × GET (pControl) + IR; 3 × GET (TS) + IR).
Table 1.
Response of B16F10 melanoma to different treatment modalities
| Therapeutic group | DT (days) | TGD (days) | TF | SC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | AM | SEM | AM | SEM | n | % | n | % | |||
| CTRL | 8 | 1.2 | ± | 0.1 | 0.0 | ± | 0.1 | 0 | 0 | - | - |
| pControl | 8 | 1.9 | ± | 0.2 | 0.7 | ± | 0.2 | 0 | 0 | - | - |
| TS | 8 | 2.2 | ± | 0.3 | 1.0 | ± | 0.3 | 0 | 0 | - | - |
| 3 × EP | 8 | 3.1 | ± | 0.3 | 2.0 | ± | 0.3 | 0 | 0 | - | - |
| 3 × GET (pControl) | 8 | 9.4 | ± | 6.5 | 8.2 | ± | 2.5 | 0 | 0 | - | - |
| 3 × GET (TS) | 9 | 9.5 | ± | 3.2 | 8.6 | ± | 3.0 | 4 | 44 | 3 | 75 |
| IR | 9 | 1.8 | ± | 0.3 | 0.7 | ± | 0.3 | 0 | 0 | - | - |
| pControl + IR | 9 | 2.5 | ± | 0.3 | 1.3 | ± | 0.3 | 1 | 11 | 1 | 100 |
| TS + IR | 8 | 4.0 | ± | 1.2 | 2.8 | ± | 1.2 | 0 | 0 | - | - |
| 3 × EP+IR | 9 | 4.3 | ± | 1.3 | 3.2 | ± | 1.3 | 2 | 22 | 0 | 0 |
| 3 × GET (pControl) + IR | 9 | 36.0 | ± | n/a | 34.9 | ± | n/a | 8 | 89 | 5 | 63 |
| 3 × GET (TS) + IR | 8 | 32.0 | ± | n/a | 30.8 | ± | n/a | 7 | 88 | 4 | 57 |
AM = Arithmetic mean; DT = Tumor doubling time; Groups: (CTRL = control; EP = electric pulses; GET = gene electrotransfer; IR = irradiation; TS = pETantiCD105); n = Number of all mice in the group; n/a = Not applicable; SC = Mice resistant to secondary challenge; SEM = Standard error; TF = Tumor free mice; TGD = Tumor growth delay
In vivo GET
In vivo GET of plasmid into subcutaneous tumors was performed 3 times every second day (on days 0, 2 and 4). 12.5 μL (4 μg/μL) of plasmid (150 μg in total) in endotoxin-free H2O was injected intratumorally 10 min before 8 square electric pulses with a voltage-to-distance ratio of 600 V/cm, a pulse duration of 5 ms, and a frequency 1 Hz were applied. Electric pulses were generated by electric pulse generator ELECTRO CELL B10 (Betatech, L’Union, France) and delivered through 2 parallel stainless steel electrodes with 2 or 4 mm distance between them, depending on the tumor volume. After 4 pulses, electrodes were turned for 90° for 4 additional pulses to assure GET to entire tumor.
Irradiation of tumors
Tumors were locally irradiated with a single dose of 15 Gy on day 1 from the beginning of the experiment, at a dose rate of 2.16 Gy/min, using a Darpac 2000 X-ray unit (Gulmay Medical Ltd., Shepperton, UK) operating at 220 kV, 10 mA, with 1.8-mm aluminum filtration. During irradiation, mice were restrained in special lead holders with apertures for irradiation of the tumors. Due to the fixed size of the apertures, some healthy tissue (3 – 5 mm of skin surrounding the tumor) was exposed to the irradiation as well.
Tumor growth
The therapeutic potential in vivo was assessed by measuring the tumor size every second day and calculating tumor volume according to the formula for ellipsoid: V=axbxc π/6, where a, b and c represent perpendicular tumor diameters.21,26 The tumor growth curves were drawn as arithmetic means (AM) with bars representing standard errors (SEM).
The tumor growth delay for each experimental group was calculated as the difference in tumor doubling times of experimental and control groups. Tumor doubling time is the number of days in which the initial tumor volume (40-50 mm3) doubles. Mice that remained tumor free for 70 days were termed tumor free and local tumor control was deemed to have been achieved (Table 1). The weight of the mice was followed as a general index of systemic toxicity, and acute skin reaction in the whole irradiated field around the tumor was evaluated as described elsewhere.17
Tumor challenge of tumor free mice
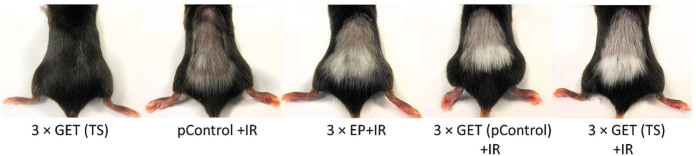
After 70 days, when mice were designated as tumor free, they were challenged with a subcutaneous injection of 1 × 106 B16F10 cells in 0.1 ml of physiological saline in the right flank. Mice that at least 20 days after the challenge remained tumor free were marked as resistant to secondary challenge (Table 1, Figure 2). The growing tumors were measured twice a week and when volume of 150 mm3 was reached mice were sacrificed and tumors were collected for further histological analysis as described below.
Figure 2.
Immune response of melanoma tumors is observed by vitiligo effect.
CTRL = control; EP = electric pulses; GET = gene electrotransfer; IR = irradiation; TS = pET-antiCD105
Histology
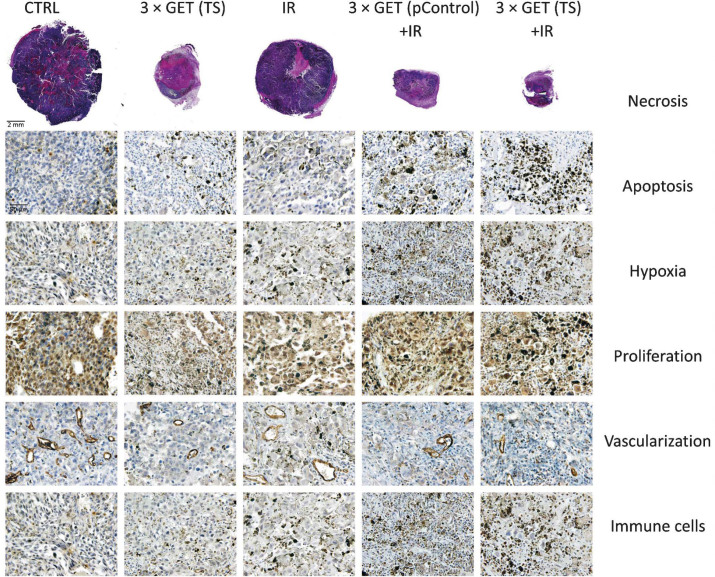
After therapies, at day 6, three mice from each experimental group were sacrificed. The tumors were excised, fixed in IHC zinc fixative (BD Biosciences, San Diego, CA, USA) and embedded in paraffin. Six consecutive 2-μm thick sections were cut from each paraffin block and stained as followed. To estimate the percent of the area of tumor necrosis, the first section was stained with hematoxylin and eosin. The other five sections were used for immunohistochemical (IHC) staining to evaluate percentage of hypoxic cells, cells in apoptosis, immune cells, proliferating cells and the number of blood vessels. To determine hypoxic cells, rabbit polyclonal antibodies against HIF-1-alpha (ab2185, Abcam, Cambridge, MA, USA) at dilution 1:3500, were used. In addition, apoptosis was evaluated with help of cleaved Caspase-3 (Asp175., Cell signaling Technology, Danvers, MA, USA) at dilution 1:1500, whereas immune cells (NK and CTL) were stained with help of Granzyme B (ab4059, Abcam) at dilution 1:1250. For staining proliferating cells, rabbit monoclonal antibodies against Ki-67 (clone SP6, Thermo Fisher Scientific) at dilution 1:1200 were used. The last section was stained for determination of the number of blood vessels, by using primary rabbit polyclonal antibodies against CD31 (ab28364, Abcam) at dilution 1:1000. For these sections, a peroxidase-conjugated streptavidin–biotin system (Rabbit specific HRP/DAB detection IHC kit, ab64261, Abcam) was used as the colorogenic reagent followed by hematoxylin counterstaining. From each slide and each feature (apoptosis, hypoxia, proliferation, vascularisation and immune cells), five randomly selected viable parts of each tumor were observed and captured under the light microscopy, by DP72 CCD camera (Olympus, Hamburg, Germany) connected to a BX-51 microscope (Olympus) under 40× magnification (numerical aperture 0.85). The viable parts were analyzed in blind fashion and the results were presented as the percent (hypoxia, apoptosis, proliferation) of the cells or the number of cells (immune cells) or the structures (vascularization) positive to IHC staining. The percentage of necrosis was contributed to the tumors as whole and was also evaluated in blind fashion, as previously described.18
In addition, tumors that grew up to 150 mm3 after secondary challenge of tumors, were excised, fixed and embedded in paraffin, as described above. Furthermore, from each tumor sections were cut and stained with hematoxylin and eosin to determinate morphological changes of tumor cells.
Statistical analysis
All data were tested for normality of distribution with the Shapiro-Wilk test. The differences between the experimental groups were statistically evaluated by one-way analysis of variance (one-way ANOVA) followed by a Holm-Sidak test for multiple comparison. A P-value of less than 0.05 was considered to be statistically significant. SigmaPlot Software (Systat Software, Chicago, IL, USA) was used for statistical analysis and graphical representation.
Results
Gene electrotransfer of plasmid silencing endoglin indicates a vascular targeted effects of the therapy
GET of either plasmids (pControl, TS) to melanoma tumors had statistically significant antitumor effectiveness compared to untreated tumors, which resulted in 8.2 ± 2.5 and 8.6 ± 3.0 days of tumor growth delay, respectively (Table 1). However, GET (TS) resulted in 44% of tumor free mice and 75% of them were resistant to secondary challenge, whereas in the GET (pControl) group no tumor free mice were obtained. Histological analysis (Table 2) of GET (TS) group demonstrated reduction of vascularization (14.9 ± 1.1%) and proliferating cells (49.5 ± 3.8%), whereas hypoxia (46.3 ± 3.0%) levels were increased and statistically significant to at least pertinent control groups (CTRL, pControl, TS and 3 × GET (pControl)). The levels of necrosis, apoptosis and number of infiltrating immune cells in the tumors were comparable in both GET treatment modalities (Figure 1) and statistically significant to at least CTRL, pControl and TS. These results indicated the vascular targeted effects of the GET (TS), used for silencing endoglin.
Table 2.
Immunohistological analysis of tumors
| Therapeutic group | Necrosis (%) | Apoptosis (%) | Hypoxia (%) | Proliferation (%) | Vascularization (n of structures) | Immune cells (n of cells) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | SEM | AM | SEM | AM | SEM | AM | SEM | AM | SEM | AM | SEM | |||||||
| CTRL | 25.0 | ± | 2.6 | 11.3 | ± | 1.6 | 4.7 | ± | 1.0 | 92.2 | ± | 1.3 | 51.6 | ± | 3.2 | 7.9 | ± | 0.9 |
| pControl | 20.0 | ± | 2.6 | 11.6 | ± | 1.0 | 5.4 | ± | 1.0 | 92.1 | ± | 1.2 | 47.1 | ± | 4.7 | 8.3 | ± | 1.1 |
| TS | 21.7 | ± | 2.6 | 11.4 | ± | 1.5 | 5.5 | ± | 0.7 | 92.7 | ± | 1.0 | 50.5 | ± | 4.2 | 7.9 | ± | 0.7 |
| 3 × EP | 13.3 | ± | 3.6 | 18.7 | ± | 2.7 | 7.8 | ± | 0.8 | 74.4 | ± | 1.7 | 39.9 | ± | 2.6 | 14.3 | ± | 1.7 |
| 3 × GET (pControl) | 53.3 | ± | 7.9 | 19.5 | ± | 3.0 | 15.3 | ± | 2.9 | 75.6 | ± | 2.4 | 35.3 | ± | 4.2 | 15.1 | ± | 1.6 |
| 3 × GET (TS) | 65.8 | ± | 7.6 | 26.7 | ± | 2.2 | 46.3 | ± | 3.0 | 49.5 | ± | 3.8 | 14.9 | ± | 1.1 | 19.3 | ± | 1.7 |
| IR | 21.7 | ± | 4.2 | 18.4 | ± | 1.8 | 30.2 | ± | 2.4 | 53.3 | ± | 3.5 | 30.1 | ± | 2.3 | 26.8 | ± | 2.6 |
| pControl + IR | 37.5 | ± | 8.0 | 18.7 | ± | 1.9 | 32.3 | ± | 2.5 | 54.5 | ± | 4.7 | 24.5 | ± | 1.8 | 26.4 | ± | 2.1 |
| TS + IR | 43.3 | ± | 8.0 | 19.9 | ± | 1.5 | 31.6 | ± | 2.3 | 52.0 | ± | 2.9 | 23.8 | ± | 1.6 | 26.4 | ± | 2.3 |
| 3 × EP + IR | 25.0 | ± | 4.8 | 31.0 | ± | 2.0 | 51.7 | ± | 2.6 | 59.4 | ± | 4.0 | 28.9 | ± | 2.3 | 29.3 | ± | 2.1 |
| 3 × GET (pControl) + IR | 65.0 | ± | 7.6 | 38.7 | ± | 3.1 | 58.0 | ± | 2.8 | 46.0 | ± | 4.8 | 18.9 | ± | 2.0 | 45.8 | ± | 3.0 |
| 3 × GET (TS) + IR | 79.2 | ± | 4.2 | 50.3 | ± | 2.4 | 66.7 | ± | 1.6 | 46.7 | ± | 4.8 | 12.8 | ± | 1.0 | 46.5 | ± | 2.1 |
AM = Arithmetic mean; Groups: (CTRL = control; EP = electric pulses; GET = gene electrotransfer; IR = irradiation; TS = pET-antiCD105); n = Number of structures or cells; SEM = Standard error
Figure 1.
Histological sections of melanoma tumors on day 6 after the beginning of the therapy.
CTRL = control; EP = electric pulses; GET = gene electrotransfer; IR = irradiation; TS = pET-antiCD105
Irradiation, alone or combined either with plasmids injection or electric pulses, had minor effect on radioresistant melanoma tumors
Irradiation monotherapy with 15 Gy had minor effects on radioresistant melanoma tumor. The tumor growth delay was moderate in the groups of irradiation alone or in combination with injection of plasmids (from 0.7 ± 0.3 to 2.8 ± 1.2 days) and up to 11% of tumor free mice were obtained in the group of irradiation in combination with injection of plasmid pControl (Table 1). The results of histological analysis (Table 2, Figure 1) indicates an immunological effect of the irradiation alone or in combination with plasmids injection, since the number of infiltrating immune cells in the tumors was statistically significantly higher in comparison to control groups (CTRL, pControl, TS) and groups applying electric pulses (alone or combined with injection of plasmids (GET (pControl) and GET (TS)), and the tumor free mice were resistant to secondary challenge. Furthermore, irradiation alone caused the reduction in proliferating cell levels (53.3 ± 3.5%) and in tumor vascularization (30.1 ± 2.3%), whereas apoptosis (18.4 ± 1.8%) and hypoxia (30.2 ± 2.4%) levels were elevated. All of these results were statistically significant compared to CTRL, pControl and TS groups.
The application of electric pulses to tumors in combination with tumor irradiation resulted in better antitumor effectiveness than observed in each of these two treatment modalities alone. The tumor growth delay in combined treatment was 3.2 ± 1.3 days and 22% of tumor free mice were obtained (Table 1). The histological analysis of combined treatment demonstrated statistically significant higher levels of apoptosis (31.0 ± 2.0%) and hypoxia (51.7 ± 2.6%) in comparison to the groups of irradiation and electric pulses alone. Among these three groups no statistically significant differences were observed in the levels of necrosis. Furthermore, the analysis of levels of proliferating cells, the number of tumor blood vessels and immune cells infiltrating in the tumors, resulted in similar and moderate effectiveness of the combined treatment (3 × EP + IR) and irradiation (alone or combined with injection of plasmids), which statistically significantly differed from group of electric pulses alone. Nevertheless, in this combined treatment modality (3 × EP + IR) no tumor free mice were resistant to secondary challenge of tumors.
Combination of GET and irradiation exerts pronounced antitumor effects
The groups combining GET of plasmids (pControl, TS) and irradiation resulted in pronounced therapeutic effectiveness, with up to 89% and 88% of tumor free mice, respectively, and from those up to 63% and 57% of mice was resistant to secondary tumor challenge, respectively (Table 1). The histological analysis, of tumors excised 6 days after treatment (Table 2, Figure 1), indicated on similar mode of action of these combined treatment modalities (3 × GET (pControl or TS) + IR), since the elevation of necrosis (65.0 ± 7.6%, 79.2 ± 4.2%), number of immune cells (45.8 ± 3.0%, 46.5 ± 2.1%), reduction of tumor vascularization (18.9 ± 2.0%, 12.8 ± 1.0%) and proliferation (46.0 ± 4.8%, 46.7 ± 4.8%), did not differ between these groups. Only two statistically significant differences between the therapeutic groups combining GET (pControl, TS) and irradiation were observed which were the level of hypoxia (58.0 ± 2.8%, 66.7 ± 1.6%), and apoptosis (38.7 ± 3.1%, 50.3 ± 2.4%).
The high number of infiltrating immune cells (Table 2) in these tumors indicated on important mode of this therapeutic action; the highest and statistically significantly increased number of immune cells was observed in the groups combining GET of plasmids and irradiation, followed by all the remaining groups that included irradiation (alone, TS, pControl and 3 × EP). In these groups, the immune cells infiltration was comparable and statistically significant to all of the other remaining groups. Furthermore, the immune cells infiltration was the lowest in the groups that included electric pulses (alone or with plasmids alone). Additionally, all of the mice that were tumor free after the therapies including irradiation, either alone or in combination with other modalities, had fur discoloration, known as vitiligo, indicating immune response (Figure 2). This was further confirmed with high number of mice that were resistant to secondary challenge in tumor free mice, with the exception of the group combining electric pulses and irradiation (Table 1). In these groups, in which tumors grew after secondary challenge, the growth rate and histology of the tumors were the same as after the initial induction of tumors (data not shown).
In addition, the safety of the treatment (irradiation alone or in combination with other modalities) was proven, since no body weight loss over 10%, or any other side effects were observed, except for the temporary hair loss in the irradiated area, without skin desquamation (data not shown).
Discussion
The results of this study indicate a dual effect of GET of plasmid encoding shRNA for silencing endoglin, the direct and the indirect, both having a radiosensitizing effect. The direct effect was on the tumor vasculature and also on melanoma tumor cells, whereas the indirect was observed with the use of plasmid devoid of therapeutic gene, through boosting the immune response in tumors. Furthermore, irradiation had mainly affected melanoma cells, although some effect on vasculature could also be noticed. In addition, the higher number of infiltrating immune cells in all of the groups combined with irradiation indicated an important role of the immune system. All of these effects had synergistic action and, in combined treatment modality of GET and irradiation, resulted in increased radiosensitizing effectiveness of melanoma tumors that resulted in prolonged tumor growth delay, which led to 88% of tumor free mice, of which 57% were resistant to secondary challenge of tumors.
Dual effectiveness of GET
The first direct effect of GET (TS) was on tumor vasculature, which was significantly reduced after the treatment. This vascular effect can be ascribed to the specificity of the plasmid for endothelial cells and to the endoglin silencing. This direct effect on tumor vasculature was also observed in other studies using GET of plasmid for silencing endoglin on melanoma22,23 and other tumor models.18,19,21 The vascular targeted effects in these studies were first confirmed in non-endoglin expressing tumors, i.e. murine mammary adenocarcinoma, by endothelial-specific promoter18,21 and non-specific, constitutive promoter18–21, that resulted in pronounced antitumor effectiveness. In melanoma tumor model, B16F10-luc, the GET of plasmids silencing endoglin resulted in significant antimetastatic effectiveness.22 Furthermore, GET of plasmid silencing endoglin, with constitutive promoter, performed on small melanoma B16F10 tumor model (4 mm3 at the beginning of therapy) confirmed vascular targeted effects, which resulted in prolonged tumor growth delay and tumor free mice (58%), also due to nonspecific nature of constitutive plasmid that was used in this study silenced endoglin also in melanoma cells.23 Nevertheless, in the current study, which was done on bigger tumors (40 mm3), in addition to significant increased level of hypoxia, also decreased number of proliferating tumor cells was observed. This was attributed to the second direct effect of GET (TS), that is silencing of endoglin also in melanoma cells, since it is known that plasmid with tissue specific promoters can be leaky and can also be transcribed in non-targeted tissue.27 Therefore, GET (TS) has dual direct effects; primarily by targeting vasculature and secondly by inhibition of proliferation of melanoma cells. These two effects together resulted in 44% of tumor free mice, from which 75% were resistant to secondary challenge of tumor cells.
Nevertheless, high level of infiltrating immune cells in the tumors, indicated also an indirect effect of this treatment, through the stimulation of immune response. This is also supported by the data obtained with GET of the plasmid devoid of therapeutic gene (pControl). In comparison to GET of therapeutic plasmid, TS, no tumor cures were obtained, however, in other measured parameters, infiltration of immune cells into the tumors, levels of necrosis and apoptosis as well as in tumor growth delay no statistically significant differences were obtained between these groups. Thus, to a certain degree, a similar mode of antitumor action can be ascribed to the GET of plasmid DNA, mainly because the values of these parameters were statistically different from pertinent control groups. Antitumor effectiveness of GET of non-therapeutic (control) plasmids was also observed in other studies9,10, and the authors indicated that effectiveness was due to involvement of immune system. Namely, the plasmid introduced during GET can act as a DAMP that is recognized through different sensors (Pattern Recognition Receptors), leading to the activation of the signal transduction cascade that ultimately triggers the production of type 1 interferons and other cytokines.28 These act as a link between the innate and adaptive immune response29 and can induce the adaptive immune response against the introduced DNA and consequently the transfected cells. Furthermore, similar immune response can also be triggered by the stress30 that is produced during the transfection procedure, like mechanical stress, heat, and ROS, that have all been previously reported after electroporation.31,32
Priming effect of irradiation
To target primary tumors, as was done in this study, irradiation is one of the most used treatment modalities.33 This therapeutic approach alone or in combination with injection of plasmids, in radioresistant melanoma tumor model, resulted in moderate tumor growth delay, which was attributed to decreased proliferation of melanoma cells, increased levels of apoptosis and necrosis. Further histological analysis indicated on the radiation damage of the tumor vasculature, as a second effect of the irradiation, as seen in other studies.34,35 This vascular damage was less pronounced than in group of GET (TS), although in comparison to GET (TS), irradiation monotherapy resulted in higher infiltration of the immune cells in the tumors. Furthermore, in the group combining irradiation and injection of therapeutic plasmid, one mouse was tumor free, which was also resistant to secondary challenge of tumor. This indicates a priming effect of irradiation combined with introduction of foreign DNA, through boosting the immune response. When irradiated cells die, they release their antigens in the context of the danger signal (DAMPS), which result in the priming of a tumor specific immune response against the released antigens. Therefore, irradiated tumors can sometimes act as a powerful individualized in situ vaccine13, which is manifested as the abscopal effect of the irradiation. This was confirmed in in vivo studies indicating that irradiation can induce in vivo priming of T cells to exogenous model antigens engineered to be expressed by tumors.13 In melanoma tumor model, priming of antitumor T cells to the model antigen ovalbumin, was more effective when single, 15 Gy dose was used, rather than 3 Gy given in 5 consecutive days.36 Further on, another group also showed induction of antitumor T-cell responses with other antigen expression, when single 20 Gy dose was applied, but not by 5 Gy given 4 times.37
The combination of electric pulses and irradiation exerted moderate antitumor effectiveness that resulted in 22% of tumor free mice. Similar results were obtained in our previous studies on murine mammary adenocarcinoma18, sarcoma17 and Ehrlich-Lettre ascites38, where also 20%, 27% and 54% of tumor free mice were observed, respectively. Therefore we can assume that electroporation of tumors contributes to radioresponsiveness of melanoma, most probably due to generation of ROS32,39 after application of the electric pules, which also resulted in significantly elevated levels of hypoxia in our histological analysis.
Immune boosting and radiosensitization by plasmid DNA
The combined treatment modalities (combination of GET (TS or pControl) with irradiation) exerted excellent, highly statistically significant antitumor effect in comparison to control groups, which resulted in 88% and 89% of tumor free mice, respectively, of which 57% and 63% of mice were resistant to secondary challenge of tumors, respectively. The results of histological analysis were similar between groups of combined treatment modality (GET + IR), regardless of the applied plasmid. The only two differences were on the levels of apoptosis and hypoxia. The analysis of the presence of the immune cells showed on the highest number of the immune cells in the groups of GET of plasmids and irradiation, followed by irradiation groups (alone or combined with injections of plasmids or combined with electric pulses). Therefore, it can be presumed that the priming effect of irradiation can be boosted by GET of the plasmid DNA to fully exert vaccinating effect. Additionally, fur discoloration, vitiligo, an immune-mediated destruction of normal melanocytes that has also been recognized as a positive prognostic indicator for treatment response40,41, was also observed in this study in all the groups where tumor free mice were observed (except 3 × GET (TS)). In addition, majority of the tumor free mice were resistant to secondary challenge of tumors, further indicating on the development of the immune memory. The tumors in experimental groups, which were resistant to secondary challenged of tumor, were therefore radiosensibilized through GET of plasmid, since in the group combining electric pulses and irradiation, no mice resulted resistant to secondary challenge of tumor. The GET of plasmids in combination with irradiation presumably generated many danger signals that collectively define immunogenic cell death.13
Furthermore, the immunogenicity of tumor might also play an important role in combined treatment modality.42 In the current study we obtained significant antitumor effectiveness and up to 89% of tumors free mice, that were up to 63% resistant to secondary challenge of tumors, when combining GET and irradiation in melanoma tumor model. Furthermore, in our previous study combining the same treatment modality on a different tumor model, murine mammary adenocarcinoma TS/A, we achieved up to 44% of tumors free mice with therapeutic plasmid silencing endoglin and 20% tumor free mice with the control plasmid.18 We can presume that the boosting of immune response depends on the tumor type, melanoma being more immunogenic that mammary adenocarcinoma TS/A. Nevertheless, the mechanisms of these therapies are not fully elucidated. Currently, we presume that there is involvement of DNA sensors, ROS and specific immune response after irradiation, but further studies are needed.
Conclusions
The results of this study indicate that irradiation can in radioresistant mice tumors, such as melanoma, by release of tumor associated antigens serve as the target of the immune response. This can be further boosted by GET of plasmid, with or without therapeutic gene, which was confirmed by the equal radiosensitization resulting in prolonged tumor growth delay and up to 89% of tumor free mice, which kept immune memory to melanoma cells. In addition, GET of therapeutic plasmid silencing endoglin has also direct effect on vasculature and tumors cells; however in combination with radiotherapy this effect was masked by pronounced immune response.
Acknowledgements
The authors would like to acknowledge M. Lavric and S. Kranjc for their help with cell cultures and tumor transplantation, U. Lampreht Tratar and M. Ota for help with IHC, as well as V. Todorovic for useful tips. The research was supported by Slovenian Research Agency (P3-0003, J3-4211, J3-6793) and conducted in the scope of LEA EBAM (French-Slovenian European Associated Laboratory: Pulsed Electric Fields Applications in Biology and Medicine) and is a result of networking efforts within the COST TD1104 Action.
Footnotes
Conflict of interest: The authors declare no conflict of interest
References
- 1.Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D.. Electroporationbased technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622. [DOI] [PubMed] [Google Scholar]
- 2.Hribernik A, Cemazar M, Sersa G, Bosnjak M, Snoj M.. Effectiveness of electrochemotherapy after IFN-α adjuvant therapy of melanoma patients. Radiol Oncol. 2016;50:21–7. doi: 10.1515/raon-2015-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campana LG, Clover AJ, Valpione S, Quaglino P, Gehl J, Kunte C. et al. Recommendations for improving the quality of reporting clinical electro-chemotherapy studies based on qualitative systematic review. Radiol Oncol. 2016;50:1–13. doi: 10.1515/raon-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cemazar M, Golzio M, Sersa G, Rols MP, Teissié J.. Electrically-assisted nucleic acids delivery to tissues in vivo: where do we stand? Curr Pharm Des. 2006;12:3817–25. doi: 10.2174/138161206778559740. [DOI] [PubMed] [Google Scholar]
- 5.Heller R, Heller LC.. Gene electrotransfer clinical trials. Adv Genet. 2015;89:235–62. doi: 10.1016/bs.adgen.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Heller LC, Heller R.. Electroporation gene therapy preclinical and clinical trials for melanoma. Curr Gene Ther. 2010;10:312–7. doi: 10.2174/156652310791823489. [DOI] [PubMed] [Google Scholar]
- 7.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK. et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896–903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spanggaard I, Snoj M, Cavalcanti A, Bouquet C, Sersa G, Robert C. et al. Gene electrotransfer of plasmid antiangiogenic metargidin peptide (AMEP) in disseminated melanoma: safety and efficacy results of a phase I first-in-man study. Hum Gene Ther Clin Dev. 2013;24:99–107. doi: 10.1089/humc.2012.240. [DOI] [PubMed] [Google Scholar]
- 9.Heller L, Todorovic V, Cemazar M.. Electrotransfer of single-stranded or double-stranded DNA induces complete regression of palpable B16.F10 mouse melanomas. Cancer Gene Ther. 2013;20:695–700. doi: 10.1038/cgt.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Znidar K, Bosnjak M, Cemazar M, Heller LC.. Cytosolic DNA sensor upregulation accompanies DNA electrotransfer in B16.F10 melanoma cells. Mol Ther Nucleic Acids. 2016;5:e322. doi: 10.1038/mtna.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmet CJ, Ishii KJ.. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–91. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 12.Baskar R, Ann-Lee K, Yeo R, Yeoh KW.. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–9. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demaria S, Golden EB, Formenti SC.. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1–8. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 14.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:543–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng L, Liang H, Fu S, weichselbaum R.R, Fu YX.. From DNA damage to nucleic acid sensing: A strategy to enhance radiation therapy. Clin Cancer Res. 2016;22:20–5. doi: 10.1158/1078-0432.CCR-14-3110. [DOI] [PubMed] [Google Scholar]
- 16.El Kaffas A, Tran W, Czarnota GJ.. Vascular strategies for enhancing tumour response to radiation therapy. Technol Cancer Res Treat. 2012;11:421–32. doi: 10.7785/tcrt.2012.500265. [DOI] [PubMed] [Google Scholar]
- 17.Sedlar A, Kranjc S, Dolinsek T, Cemazar M, Coer A, Sersa G.. Radiosensitizing effect of intratumoral interleukin-12 gene electrotransfer in murine sarcoma. BMC cancer. 2013;13:38. doi: 10.1186/1471-2407-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stimac M, Kamensek U, Cemazar M, Kranjc S, Coer A, Sersa G.. Tumor radio-sensitization by gene therapy against endoglin. Cancer Gene Ther. 2016;23:214–20. doi: 10.1038/cgt.2016.20. [DOI] [PubMed] [Google Scholar]
- 19.Dolinsek T, Markelc B, Bosnjak M, Blagus T, Prosen L, Kranjc S. et al. Endoglin silencing has significant antitumor effect on murine mammary adenocarcinoma mediated by vascular targeted effect. Curr Gene Ther. 2015;15:228–44. doi: 10.2174/1566523215666150126115501. [DOI] [PubMed] [Google Scholar]
- 20.Dolinsek T, Markelc B, Sersa G, Coer A, Stimac M, Lavrencak J. et al. Multiple delivery of siRNA against endoglin into murine mammary adenocarcinoma prevents angiogenesis and delays tumor growth. PLoS One. 2013;8:e58723. doi: 10.1371/journal.pone.0058723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stimac M, Dolinsek T, Lampreht U, Cemazar M, Sersa G.. Gene electrotransfer of plasmid with tissue specific promoter encoding shRNA against endoglin exerts antitumor efficacy against murine TS/A tumors by vascular targeted effects. PLoS One. 2015;10:e0124913. doi: 10.1371/journal.pone.0124913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tesic N, Kamensek U, Sersa G, Kranjc S, Stimac M, Lampreht U. et al. Endoglin (CD105) Silencing mediated by shrna under the control of endothelin-1 promoter for targeted gene therapy of melanoma. Mol Ther Nucleic Acids. 2015;4:e239. doi: 10.1038/mtna.2015.12. [DOI] [PubMed] [Google Scholar]
- 23.Dolinsek T, Sersa G, Prosen L, Bosnjak M, Stimac M, Razborsek U. et al. Electrotransfer of plasmid DNA encoding an anti-mouse endoglin (CD105) shRNA to B16 melanoma tumors with low and high metastatic potential results in pronounced anti-tumor effects. Cancers. 2015;8:3. doi: 10.3390/cancers8010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadakis ED, Nicklin S a, Baker a H, White SJ.. Promoters and control elements: designing expression cassettes for gene therapy. Curr Gene Ther. 2004;4:89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- 25.Bosnjak M, Dolinsek T, Cemazar M, Kranjc S, Blagus T, Markelc B. et al. Gene electrotransfer of plasmid AMEP, an integrin-targeted therapy, has antitumor and antiangiogenic action in murine B16 melanoma. Gene Ther. 2015;22:578–90. doi: 10.1038/gt.2015.26. [DOI] [PubMed] [Google Scholar]
- 26.Tomayko MM, Reynolds CP.. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–54. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 27.Kamensek U, Tesic N, Sersa G, Kos S, Cemazar M.. Tailor-made fibroblast-specific and antibiotic-free interleukin 12 plasmid for gene electrotransfermediated cancer immunotherapy. Plasmid. 2016;89:9–15. doi: 10.1016/j.plasmid.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Dempsey A, Bowie AG.. Innate immune recognition of DNA: A recent history. Virology. 2015;479:146–52. doi: 10.1016/j.virol.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiarella P, Fazio VM, Signori E.. Electroporation in DNA vaccination protocols against cancer. CurrDrug Metab. 2013;14:291–9. doi: 10.2174/1389200211314030004. [DOI] [PubMed] [Google Scholar]
- 30.Muralidharan S, Mandrekar P.. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol. 2013;94:1167–84. doi: 10.1189/jlb.0313153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarek M.. Membrane electroporation: A molecular dynamics simulation. Biophys J. 2005;88:4045–53. doi: 10.1529/biophysj.104.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markelc B, Tevz G, Cemazar M, Kranjc S, Lavrencak J, Zegura B. et al. Muscle gene electrotransfer is increased by the antioxidant tempol in mice. Gene Ther. 2012;19:312–20. doi: 10.1038/gt.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolus NE.. Basic review of radiation biology and terminology. J Nucl Med Technol. 2001;29:67–73. [PubMed] [Google Scholar]
- 34.Watters D.. Molecular mechanisms of ionizing radiation-induced apoptosis. Immunol Cell Biol. 1999;77:263–71. doi: 10.1046/j.1440-1711.1999.00824.x. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A. et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 36.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM.. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sersa G, Kranjc S, Cemažar M.. Improvement of combined modality therapy with cisplatin and radiation using electroporation of tumors. Int J Radiat Oncol Biol Phys. 2000;46:1037–41. doi: 10.1016/s0360-3016(99)00464-2. [DOI] [PubMed] [Google Scholar]
- 39.Bonnafous P, Vernhes MC, Teissié J, Gabriel B.. The generation of reactive-oxygen species associated with long-lasting pulse-induced electropermeabilisation of mammalian cells is based on a non-destructive alteration of the plasma membrane. Biochim Biophys Acta. 1999;1461:123–34. doi: 10.1016/s0005-2736(99)00154-6. [DOI] [PubMed] [Google Scholar]
- 40.Maio M.. Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23:10–4. doi: 10.1093/annonc/mds257. [DOI] [PubMed] [Google Scholar]
- 41.Ratterman M, Hallmeyer S, Richards J.. Sequencing of new and old therapies for metastatic melanoma. Curr Treat Options Oncol. 2016;17:1–9. doi: 10.1007/s11864-016-0427-z. [DOI] [PubMed] [Google Scholar]
- 42.Blankenstein T, Coulie PG, Gilboa E, Jaffee EM.. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12:307–13. doi: 10.1038/nrc3246. [DOI] [PMC free article] [PubMed] [Google Scholar]