Abstract
Resistance to EGFR kinase inhibitors appears to be invariable in the treatment of non-small cell lung cancer. Several mechanisms have been described. Here, we report the first case of histologic transformation of EGFR mutant lung adenocarcinoma without prior exposure to EGFR inhibition.
Keywords: Squamous NSCLC, adenocarcinoma NSCLC, EGFR
1. INTRODUCTION
Despite initial marked response to EGFR kinase inhibitors in patients with non-small cell lung cancer (NSCLC) patients harboring activating EGFR mutations, drug resistance develops within a median of 12 months. Described resistance mechanisms include secondary mutations within EGFR (e.g. T790M), MET amplification, PI3K pathway hyperactivation, HER2 amplification, AXL overexpression, and epithelial-to-mesenchymal transition. Additionally, in rare cases, EGFR mutant lung adenocarcinoma may undergo histologic transformation to small cell lung cancer and squamous cell cancer after EGFR inhibitor treatment 1,2. Here, we report the first case of histologic transformation of EGFR mutant lung adenocarcinoma without prior exposure to EGFR inhibition.
2. CASE REPORT
During radiographic evaluation of a stage 3A invasive ductal carcinoma of the right breast, a 79-year old woman with no smoking history was found to have a right middle lobe mass was detected (Figure 1A). Biopsy demonstrated a TTF-1- and Napsin A-positive primary lung adenocarcinoma (Figure 2A–2C) harboring a classic EGFR exon 19 deletion (Figure 3). She underwent right middle lobe lobectomy and mediastinoscopy, with a final diagnosis of stage 2 (T2N1M0) disease. The patient received three cycles of adjuvant carboplatin-pemetrexed chemotherapy (Figure 1C). Subsequently she underwent partial right mastectomy, breast and axillary radiation therapy, and started tamoxifen.
Figure 1.
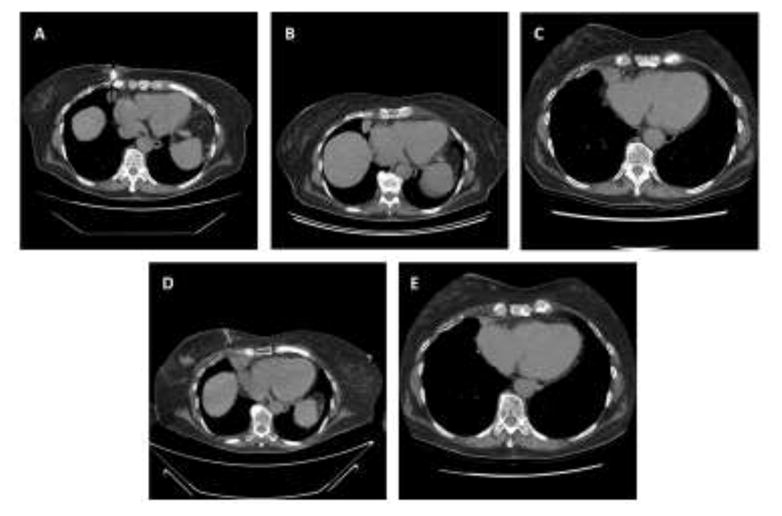
A. computed tomography (CT)-guided biopsy of adenocarcinoma lung lesion at the time of diagnosis. B. chest CT before right middle lobectomy and adjuvant carboplatin-pemetrexed. C. chest CT 5 months after right middle lobectomy and 1 month after last cycle of carboplatin-pemetrexed. D. CT-guided biopsy of squamous cell lung cancer progression. E. chest CT 1 month after initiation of erlotinib with lesion now 1.9 × 0.8 cm, previously 2.4 × 2.0 cm
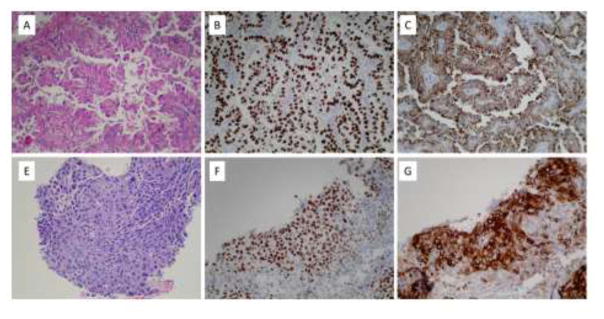
Figure 2.
A. Pulmonary adenocarcinoma, right middle lobe, H&E (200X). B. Pulmonary adenocarcinoma positive for TTF-1 (200X). C. Pulmonary adenocarcinoma positive for Napsin A (200X). Immunohistochemical stains for p63 and CK5/6 are negative (not shown). E. Squamous cell carcinoma, right cardiophrenic angle, H&E (200X). F. Squamous cell carcinoma positive for p63 (200X). G. Squamous cell carcinoma positive for CK5/6 (200X). An immunohistochemical stain for TTF-1 is negative (not shown).
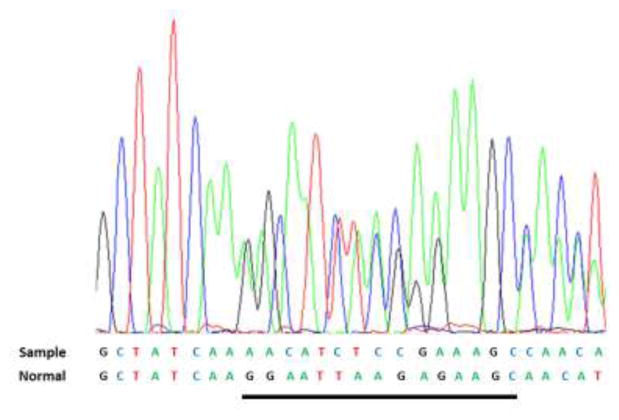
Figure 3.
Colored peaks correspond to the exon 19 DNA sequence indicated as sample. The reference normal sequence is below. The 15 nucleotides deleted in the sample (c2235_2249del) are underlined in the normal. There is no evidence of T790M deletion (not shown)
Approximately 13 months after completing adjuvant chemotherapy, surveillance chest CT demonstrated an enlarging nodule at the right cardiophrenic angle (Figure 1D). Biopsy revealed squamous cell carcinoma (Figure 2E–2F) with no evidence of adenocarcinoma histology. Molecular analysis demonstrated the original EGFR exon 19 deletion and no evidence of T790M mutation (Figure 3). Immunohistochemical analysis of both the original and subsequent lung tumors demonstrated Rb expression (images not shown), suggesting absence of small cell histology. For both the original and subsequent lung tumors, all available tissue underwent histologic review. The patient initiated erlotinib, with partial response lasting six months (Figure 1E).
3. DISCUSSION
To our knowledge, this is the first reported case of histologic transformation of EGFR mutant lung adenocarcinoma without prior exposure to EGFR inhibition. Potential explanations for the histologic transformation described in this case include (1) metaplastic transformation, (2) coexistence of both squamous and adenocarcinoma cells in the original tumor mass, or (3) development of a second primary cancer (unlikely given the maintenance of the original EGFR mutation). This phenomenon may suggest a population of pluripotent EGFR mutant cancer stem cells as the source of resistance. Although we observed morphological and immunohistochemical differences between the initial and recurrence specimens, we cannot rule out the possibility of a mixed tumor because needle biopsies provide limited sampling.
In this case, interval therapies included platinum-pemetrexed chemotherapy, breast irradiation, and the estrogen receptor modulator tamoxifen. Of these, pemetrexed seems the most likely to be associated with selective histologic pressure. Multiple clinical trials have demonstrated preferential efficacy of this agent against non-squamous tumors, which has been attributed to relatively greater expression and activity of thymidylate synthase in squamous cancers. The six-month duration of clinical benefit from EGFR inhibitor treatment of the squamous lung cancer in this case—approximately half the median duration observed in adenocarcinoma cases—is characteristic of EGFR mutant squamous cancers 3.
Recently, histologic transformation has also been reported in anaplastic lymphoma kinase (ALK)-positive NSCLC after ALK inhibitor treatment 4. The present case suggests that lung cancers harboring driver mutations may undergo histologic transformation independent of exposure to kinase inhibitors. Whether these cases have greater propensity than pan-wild type tumors, or their molecular characterization permits a clearer distinction from second primaries, is not clear.
Highlights.
Transformation of EGFR mutant lung adenocarcinoma to squamous cell carcinoma
Histologic transformation without prior EGFR TKI exposure
Histologic transformation is a potential resistance mechanism to EGFR TKIs
Acknowledgments
Funded in part by a National Cancer Institute Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01; to DEG).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As va service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levin PA, Mayer M, Hoskin S, Sailors J, Oliver DH, Gerber DE. Histologic Transformation from Adenocarcinoma to Squamous Cell Carcinoma as a Mechanism of Resistance to EGFR Inhibition. J Thorac Oncol. 2015;10(9):e86–88. doi: 10.1097/JTO.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graziano P, de Marinis F, Gori B, et al. EGFR-Driven Behavior and Intrapatient T790M Mutation Heterogeneity of Non-Small-Cell Carcinoma With Squamous Histology. J Clin Oncol. 2015;33(31):e115–118. doi: 10.1200/JCO.2013.49.5697. [DOI] [PubMed] [Google Scholar]
- 4.Fujita S, Masago K, Katakami N, Yatabe Y. Transformation to SCLC after Treatment with the ALK Inhibitor Alectinib. J Thorac Oncol. 2016;11(6):e67–72. doi: 10.1016/j.jtho.2015.12.105. [DOI] [PubMed] [Google Scholar]