Abstract
Structurally and functionally, the short-wave-sensitive (S) cone pathways are thought to decline more rapidly with normal aging than the middle- and long-wave-sensitive cone pathways. This would explain the celebrated results by Verriest and others demonstrating that the largest age-related color discrimination losses occur for stimuli on a tritan axis. Here, we challenge convention, arguing from psychophysical data that selective S-cone pathway losses do not cause declines in color discrimination. We show substantial declines in chromatic detection and discrimination, as well as in temporal and spatial vision tasks, that are mediated by S-cone pathways. These functional losses are not, however, unique to S-cone pathways. Finally, despite reduced photon capture by S cones, their postreceptoral pathways provide robust signals for the visual system to renormalize itself to maintain nearly stable color perception across the life span.
1. INTRODUCTION
The photoreceptors of human trichromatic vision are defined according to their photopigment absorption spectra, but the short-wave-sensitive (S) cones and their postreceptoral pathways have several unique properties. Compared to the middle-(M) and long-wave-sensitive (L) cones, the S cones have a more ancient evolutionary origin [1], comprise only about 6% of the total cone population [2], and are distributed differently across the retina [3,4]. Indeed, they leave a small tritanopic area [5–7], or partial chromatic scotoma [8,9], in the central ∼20 arcmin of the fovea centralis. S cones are morphologically different from the M and L cones [10,11], but their isolated receptor response seems to not differ [12], suggesting that it is primarily their postreceptoral circuitry that endows them with their functionally distinct temporal properties [13]. Psychophysically, S-cone pathways are relatively less sensitive to spatial modulation [14,15] and make little contribution to border discrimination [16] and luminance [17], except under specific conditions that unveil a small subtractive signal [18,19]. Ganglion cells carrying S-cone ON signals are morphologically distinct [20] and project to separate laminae, the koniocellular layers, of the lateral geniculate nucleus (LGN) [21]. They have receptive fields that are both large and lacking in spatial opponency [22]. As a result, they have larger spatial summation areas than those mediated by M- and/or L-cones [23,24]. Signals from M- and L-cones are represented equally in the ON and OFF pathways [25,26], but S-cone OFF signals are rare [27,28]. The S cones are more susceptible to photochemical damage from intense light [29,30] and appear to be affected earliest and most severely in many acquired deficiencies of color vision [31,32]. Yet congenital deficiencies affecting them are rare [33]. These and many other features [34,35] notwithstanding, their advantages overshadow their idiosyncrasies, for they transform human vision from a system that could be represented by a plane to a system that must be represented by a three-dimensional space. And, postreceptorally, they form a subsystem for mammalian vision specialized for coding color.
How do the unusual properties of the S-cone pathways manifest themselves in functional vision across the life span? Here, we summarize data that challenge the notion that S cones are more vulnerable to the effects of aging than the other cone types. Indeed, because of their spectral separation, S-cone excitation is not well correlated with that from M and L cones and, as such, they not only extend the visible spectrum, but contribute uniquely to chromatic vision. That is, S cones at their full-width half maximum are separated from their nearest spectral neighbors by 99 nm while the separation between M- and L-cones is only 21 nm [36]. As will be shown, S cones have a pivotal role in signaling changes in the phases of daylight illumination and maintaining stable color perception despite age-related changes in the ocular media that reduce the shortwave light reaching the retina.
2. AGING CONSIDERED AS AN ACQUIRED TRITAN DEFICIENCY
Verriest et al. [37,38] used the Farnsworth Munsell 100-Hue test to measure color discrimination across a wide age range. As shown in Fig. 1 (top left), they found monotonic increases in error scores after adolescence or early adulthood. Not shown in this figure is that the largest error score occurred along a blue–yellow, or tritan, axis, a finding confirmed by other studies [39]. This has engendered the view that aging is associated with a selective decrease in sensitivity of S cones or their postreceptoral pathways.
Fig. 1.
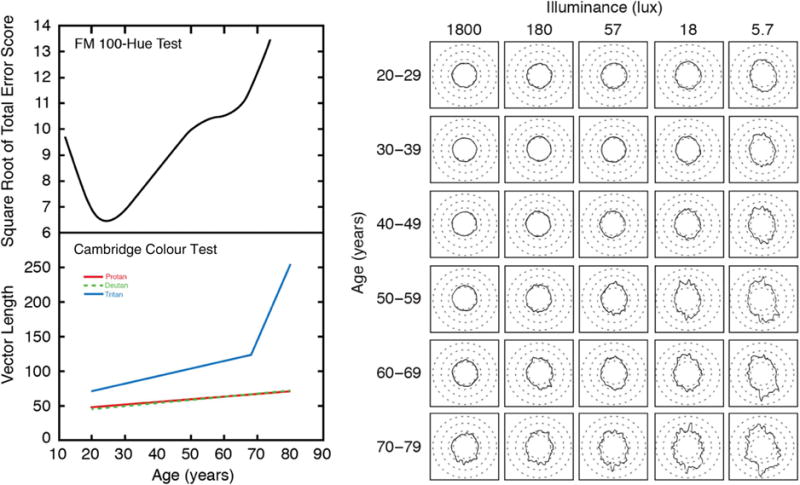
Upper left: Famsworth–Munsell 100-Hue error scores (square root of total error score) fitted for 232 participants. Results were obtained from the right eye tested under illuminant C (200 lux). The smooth curve was fitted by local (LOESS) regression; after Verriest et al. [38]. Lower left: Cambridge Colour Test results fitted to data from 168 trichromats; colors (which overlap for deutan and protan) denote different vectors for cone isolation; from Shinomori et al. [42]. Right: Mean FM 100-Hue Test results as a function of illuminance (columns) and age (rows). Cap position 1 is located at the 12:00 position. After Knoblauch et al. [45].
Selective age-related decreases in discrimination for tritan stimuli can also be seen in more recent data sets using the Cambridge Colour Test [40–42] and the Colour Assessment and Diagnosis Test [43]. Elevations in chromatic thresholds on a tritan line that exceed the age-related elevations along deutan or protan directions in color space are often reported, as shown in Fig. 1 (bottom left). Results such as these have been interpreted as indicating that aging is associated with an acquired deficiency of S cones. Verriest was more cautious. For the FM-100 Hue test, he [37] noted that the step sizes in the blue–green regions are smaller than in other color directions and “thus the test can falsely lead to the diagnosis of a pathological tritan deficiency” (p. 187). He and his colleagues [37,44] also demonstrated that these aging effects could be simulated with filters that selectively absorb short wavelengths, with the implication that senescence of the lens could be, at least in part, responsible for the selective losses observed.
Later, Knoblauch et al. [45] demonstrated that the tritan-like defects of older observers on the FM-100 Hue test were similar to that of younger groups tested at a reduced illuminance level. Their results from 75 observers, each tested at five illuminance levels, are shown in Fig. 1 (right). Rows show different ages, and columns show different light levels. For any given light level, performance changes with age are greatest on a tritan axis, but note that this effect can also be produced within age groups as light level decreases. This could be due to a number of factors, including the construction of the FM-100 Hue test and perhaps a reduced response range of S cones compared to L (and presumably M) cones [46]. More interesting are the changes across panels at a 45° angle; a trade-off can be observed between light level and age. These results set the stage for questioning the widely held conclusion that aging is an acquired deficiency of color vision due to a selective loss in S cones or a postreceptoral pathway.
3. S CONES RECEIVE RELATIVELY FEWER PHOTONS THAN M AND L CONES WITH INCREASING AGE
The quantitative analysis of retinal mechanisms requires a clear understanding of the stimulus reaching the photoreceptors owing to the fact that a significant portion of the light incident on the cornea is lost by reflection, scattering, and absorption. Absorption in the crystalline lens and macular pigment, in particular, produces substantial individual and age-related variation in the intensity and spectral composition of the retinal stimulus. The possibility that variation in prereceptoral absorption contributes to individual differences in color vision has long been recognized. Dalton [47], for example, thought that a blue coloration of his vitreous may be responsible for his dichromacy, while Hering [48] proposed that some types of color vision deficiency might be explained by variations in macular pigmentation. While these views are now regarded as incorrect, it is widely accepted that variation in ocular media absorption can contribute to individual differences in color vision.
A. Ocular Media Density
An important insight about how to measure the ocular media density in the living eye followed from König’s 1894 paper [5] in which he compared scotopic spectral sensitivity specified at the retina with a rhodopsin difference spectrum. The two curves were similar, and he tentatively concluded that spectral sensitivity under scotopic conditions is identical to that of the rhodopsin absorption spectrum filtered by the ocular media. Conversely, measurements of scotopic sensitivity can be used to estimate the ocular media density if the rhodopsin density spectrum is known. Numerous studies have refined these comparisons [49], with the result that König’s conclusion is now incontrovertible.
Ocular media density was quantified for individuals across the life span by measuring scotopic spectral sensitivity functions, as illustrated by Fig. 2 (left). Fortunately, there is a simpler approach once the method is validated. Following van Norren and Vos [49], sensitivity may be measured for pairs of short and long wavelength lights that are equated in terms of rhodopsin photon capture. Differences in log sensitivity between these paired wavelengths must be due to ocular media density; values at other wavelengths may be extrapolated assuming a spectral template. The results from 50 participants between the ages of 1 month and 70 years are shown as open circles in Fig. 2 (right). The data were fitted originally by a linear regression and agreed well with Weale’s [55] later data from excised lenses. However, data from another 50 observers, using psychophysics [53] and sampling more at the upper end of the age range, motivated a more complex model. The two sets of data were fitted well with the two-phase model of Pokorny et al. [54], as shown in Fig. 2 (right). According to this model, which is based on the color-matching functions of Stiles and Burch [56], there is an acceleration in the density of the ocular media after about age 60. The Pokorny et al. model was never proposed to fit data below age 20 years; however, extrapolation of the lower branch, as shown by the dashed line in Fig. 2, fits the data from that age range well. The major importance of findings such as these, however, is not just to demonstrate age-related changes in ocular media density, but also to note that the range at 400 nm is about 1.0 log unit even for observers of the same age. These individual variations often need to be taken into account in understanding vision at short wavelengths. An average correction for different ages is frequently insufficient for understanding individual differences.
Fig. 2.
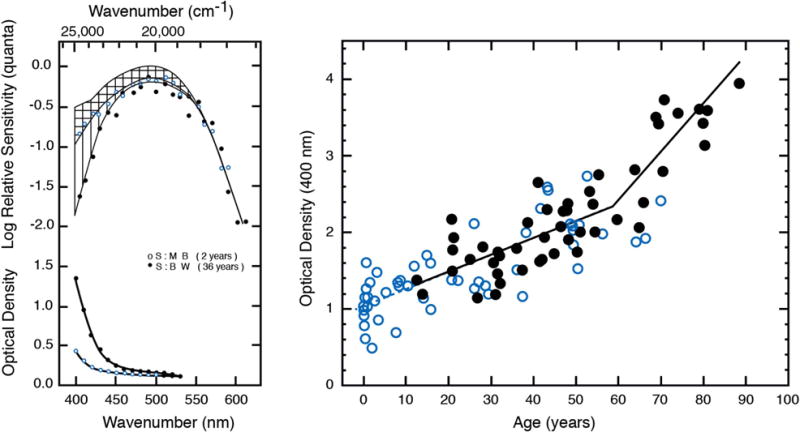
Left: The upper curve shows the human rhodopsin absorption spectrum [50], assuming an optical density of 0.25 [51], compared with the scotopic spectral sensitivity of two observers (middle and bottom curves of the top panel). The difference between spectral sensitivity and the rhodopsin absorption spectrum is denoted by horizontal lines for the child and vertical lines for the adult, and plotted in the bottom panel. These curves represent the ocular media density spectrum (from [52]). Right: Ocular media density based on scotopic sensitivity measured with visually evoked cortical potentials (open circles [52]) and psychophysics (filled circles [53]). The bold bilinear function fitted to the data is taken from the model of Pokorny et al. [54]; the dashed line is extrapolated from the lower branch of the bilinear function for ages 0 to 20 years.
B. Macular Pigment Density and Aging
The central fovea is endowed with a yellow pigment famously, but mistakenly, described in 1799 by Soemmerring [57] as a “foramin,” or hole, through which the choroid could be visualized. Debate ensued for the subsequent 200 years as to its function and even existence [58]. It is now known that the macular pigment (MP) is a mixture of carotenoids derived from the diet [59,60] and is most densely deposited in the retinal nerve fiber layer and inner plexiform layer [61]. The selective absorption of short wavelengths by this yellow pigment has a consequence that Schultze [62] and 19th-century psychophysicists were quick to point out—it reduces sensitivity at short wavelengths and contributes to entoptic phenomena such as Haidinger’s brushes [63] and Maxwell’s spot [8].
A number of psychophysical methods have been developed [64,65] for measuring the MP optical density. A simple method [66] is based on heterochromatic flicker photometry (HFP) with a 460 nm monochromatic standard (the peak of the MP absorption spectrum) presented in temporal counterphase with a series of wavelengths. A short-wave adapting background along with 12–25 Hz flicker of the test stimulus is superimposed to suppress short-wave cones and rods [13] so that the task is mediated by M and L cones. Assumptions underlying this technique are discussed in detail in Appendix A of [53].
Figure 3 (top left) shows results from a young subject having peak MP density ∼0.6. The smooth curve is based on a nomogram for MP scaled to fit the raw data. The spatial distribution of another observer is presented in Fig. 3 (bottom left) and shows a decline in optical density from the fovea radially outward. The foveal density of MP obtained from 50 observers in each of two studies is plotted as a function of age in Fig. 3 (right). The mean value was 0.4, and there was no meaningful change with age from 10 to 90 years. Over a number of studies, age was not found to be a significant predictor of MP density among adults [67], although there is relatively little MP in the infant [48,60,62].
Fig. 3.
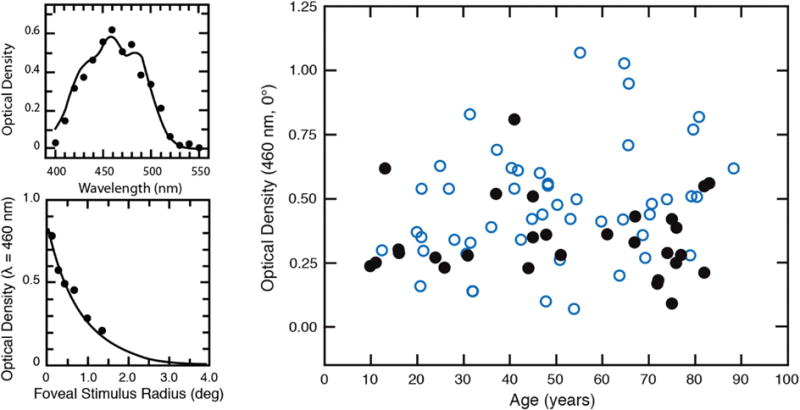
Top left: Data points show the difference spectrum for sensitivity measured foveally and parafoveally under conditions described in the text. The smooth curve is the MP density spectrum from Wyszecki and Stiles [68] adjusted by a scalar for comparison with the data points. Bottom left: Density of the MP plotted as a function of retinal eccentricity fitted with an exponential function. Right: Optical density of MP plotted as a function of age from two studies indicated by different (overlapping) symbols. All data from [53,69].
Psychophysical studies of S-cone sensitivity must often take individual differences in MP optical density into account as it may vary by ∼1.0 log unit at 460 nm in the fovea, thereby significantly altering the sensitivity of S cones specified at the cornea. Further, the spatial distribution of the MP must be taken into account when comparing S-cone sensitivity or color appearance across retinal eccentricity.
C. Implications for Metamerism and S-Cone Photon Capture
In the late 1920s, Wright [70] demonstrated the effects of individual variation in ocular media on matches between a set of individually normalized primaries (460, 530, 650 nm) and a broadband (4800 K) standard. Data were plotted in his (W.D.W.) unit-coordinate system in which the standard “white” plotted near the center of the chromaticity diagram and individual differences in preretinal ocular media were manifest as deviations from this point toward the spectrum locus (∼570–580 nm). Because the deviations between the standard and the spectrum locus in this space did not fall on a straight line, he concluded that there must be variations in both lenticular and macular pigmentation. Wright [71] later noted that the variations were age-related and demonstrated a shift in his own match over 16 years. Subsequent work by Ruddock showed that ocular media density alone can account for these shifts [72]. Wright also noted that it might be difficult to distinguish pigmentation effects from tritanomaly, an observation confirmed by Judd et al. [73]. For tritan matches using wavelengths in which MP is relatively inconsequential, Moreland [74] demonstrated an age-related increase in the amount of short-wave light required for matching along with an increase in the matching range width. He concluded that these results were due solely to decreased retinal illuminance resulting from lenticular and pupil senescence. Note that for this to be so, the shapes but not necessarily the heights of the photoreceptor action spectra (specified at the retina) must be constant across the tested age range (∼20–75 years) for the wavelengths of measurement. This is an important observation for subsequent studies of S-cone aging and can be used to calculate, for example, the loci of dichromatic neutral points (i.e., the wavelength that is metameric to a broadband standard) and how they would shift with aging due to spectral filtering of the broadband standard [75]. Figure 4 shows, for example, the consequences of a match to CIE Illuminant C when measured with short- and middle-wave spectral complementary lights, and the expected shifts due to lenticular senescence for the ages indicated. Note that these data only show metamers, not color appearance.
Fig. 4.
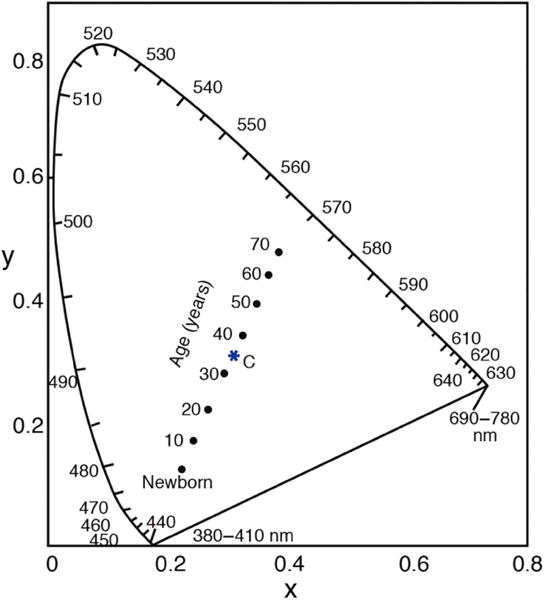
CIE chromaticity diagram for the 1931 CIE 2° observer showing shifts in metameric matches to CIE Illuminant C using 400 and 561.1 nm that result from age-related changes in the ocular media density [76].
Figure 5 illustrates the consequences of ocular media senescence on cone excitation. There is a selective reduction in photons available to S cones compared to M and L cones. This figure shows what happens to the retinal stimulus and not any effects of neural changes with age, to which we now turn. Moreover, age-related reductions in pupil area further increase the effect of ocular media senescence owing to the greater thickness of the lens in its center.
Fig. 5.
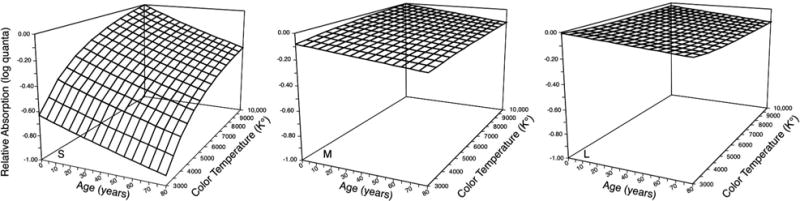
Calculated log relative number of absorbed quanta for S-, M-, and L-cones plotted in separate panels as a function of age-related changes in ocular media density and various phases of daylight illumination (color temperature). Reprinted from [77], with permission from Elsevier.
4. SENSITIVITY CHANGES OF S-CONE MECHANISMS ARE SIMILAR TO THOSE OF M- AND L-CONE MECHANISMS ACROSS THE LIFE SPAN
With the methods described in the previous section, it is possible to quantify cone sensitivities in a manner that separates optical and neural factors. Teller et al. [78] had shown that infants at around two months of age can discriminate wavelength independently of brightness in the Rayleigh region of the spectrum (λ > 540 nm) and at short wavelengths. This indicates that they are at least dichromats, but the identity of the underlying mechanisms is not revealed by discrimination experiments. Attempts to demonstrate an S-cone mechanism in infants were equivocal, and the discrimination data were consistent with mediation by rods and one class of cones [79,80], raising the possibility that S-cone development differs from that of M and L cones. An alternative suggestion was that perhaps the behavioral methods used depend on orienting mechanisms that do not receive input from S cones [81].
Action spectra for infant S-, M-, and L-cone mechanisms were obtained [82–84] using a combination of two-color increment threshold methods [85,86] and double silent substitution [87,88]. The action spectra for various conditions are shown in Fig. 6 and demonstrate the presence of all three classes of photoreceptor in infants with functional connections to the primary visual cortex from which a fixed-criterion response, the visually evoked cortical potential, was recorded. Average sensitivity at short wavelengths was higher in infants than adults, and absolute sensitivity was estimated to be about 10 times lower in infants than in the young adult.
Fig. 6.
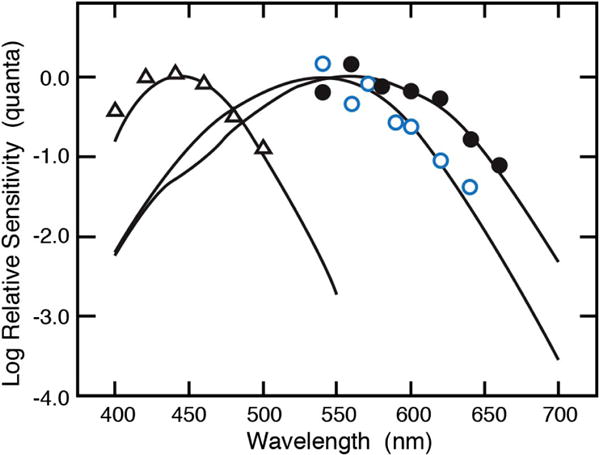
Action spectra of S (triangles), M (open circles), and L (closed circles) cones from individual infants at 4–12 weeks of age [82,83].
A number of studies have investigated increment threshold changes with age, although some early studies [89–91] only tested subjects older than 60 years, while others did not make comparisons with M and L cones. Werner and Steele [92] measured two-color increment thresholds for 76 observers between the ages of 10 and 84 years under conditions dominated by the sensitivity of the S, M, or L cones. Results are shown in Fig. 7. Each panel shows a different cone type, and each datum represents a different person. The age-related loss in sensitivity for each mechanism was greater at short wavelengths, but when averaged across a range of wavelengths, the relative decline was similar for all three mechanisms. There was a linear decrease in log sensitivity as a function of age, and the slopes imply a loss of ∼26% per decade of life. No statistical justification was found for a more complex relationship with age. One may thus think of the older visual system, at this level of processing, as being similar to the young visual system operating at a reduced light level. The data of Knoblauch et al. (Fig. 1, right) demonstrate that this, alone, could produce an age-related increase in the error score on the FM-100 test that is most prominent on a tritan axis.
Fig. 7.
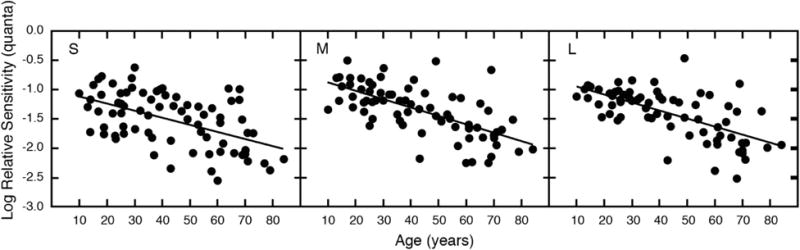
Mean log quantal sensitivity of S-, M-, and L-cone mechanisms plotted as a function of age. Data points represent two-color increment thresholds for individual observers. From [92].
Related results were reported by Knoblauch, Vital-Durand, and Barbur [93]. They used a cone-isolating stimulus with an early version of the Colour Assessment and Diagnosis test and behavioral measures that permitted testing of the full age range. Their results show that the lowest threshold (highest sensitivity) occurs in adolescence and is followed by a parallel rise in threshold for all three cone types with increasing age.
These results are a form of test sensitivity in the Stilesian framework, but “field sensitivities” [94], that is, threshold versus radiance (tvr) functions, are more conducive to quantitative modeling. To that end, tvr functions were measured in three separate studies [53,95,96] designed to control retinal illuminance on an individual basis and to control for age-related changes in pupil area [97] by use of a Maxwellian-view optical system. These data are described more fully in Section 4.B. All studies used a two-alternative, forced-choice method. Unlike previous studies, stimuli were not equated physically for each observer, but physiologically. That is, test and adapting wavelengths were varied in radiance for individual observers to find a set of conditions in which thresholds were measured on the plateau of the tvr function of different detection mechanisms.
Field sensitivities of 50 normal observers, age 18–88 years, were obtained at 0°, 4°, and 8° in the temporal retina. The densities of the ocular media and MP were also measured for each observer using the methods described in Sections 3.A and 3.B, respectively. The functions obtained were well described by Wyszecki and Stiles’s [68] tabulated template, ζ (x), for π mechanisms. Similar threshold elevations were observed for all three cone types and eccentricities as a function of age, consistent with the test sensitivities shown in Fig. 7. These retinally specified thresholds have advantages over corneally specified thresholds in that they do not depend on the wavelength of measurement, and they identify the portion of sensitivity loss that must be ascribed to neural mechanisms, as elaborated in Section 4.B.
A. S-Cone Sensitivity and Relation to the Spatial Distribution of Macular Pigment
The field sensitivities described in the previous section were conducive to addressing a hypothesis about whether MP may protect the S cones from potentially hazardous effects of lifelong exposure to short-wave radiation. Haegerstrom-Portnoy [98] reported sensitivity losses of an S-cone mechanism of an older group of observers, relative to young controls, that were less in the fovea, where the density of the MP is highest, than in the parafovea. However, MP optical density was not measured in that study, and our data do not confirm this result when sensitivity is specified at the retina. Instead, when ocular media and MP density are measured individually, sensitivity losses are similar across the central 8°. Along the same lines, Hammond et al. [99] reported that the age-related loss in an S-cone mechanism (but not an M/L mechanism) was lower in individuals with higher MP density, and this was ascribed to a protective effect of the MP. However, sensitivity was not measured outside the fovea, so the results were inconclusive. Following Hammond et al., Fig. 8 (left) plots S-cone thresholds for two groups according to whether their MP density was above or below the mean of the sample. A regression analysis revealed no significant age × MP interaction, although this would be expected if MP protects the S cones from age-related sensitivity losses.
Fig. 8.
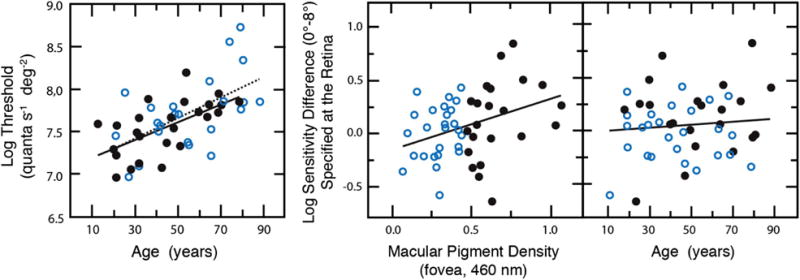
Left panel shows log sensitivity at the fovea for detection by S cones measured on the plateau of individual tvr functions. Filled and unfilled symbols, and solid and dashed lines, represent linear regressions for low- and high-peak MP groups, respectively. Center and right panels show S-cone log sensitivity differences (0°–8°) plotted as a function of foveal MP density or age. Open and closed symbols as in left panel. From [53].
A second analysis examined the log S-cone sensitivity difference between 0° and 8°, plotted as a function of MP density. This is shown in the middle panel of Fig. 8; the linear regression is statistically significant. This relationship is expected from the MP protection hypothesis, but also if there is simply a gain change due to filtering by the MP pigment. However, an age dependency is only expected from the MP protection hypothesis. When these differences are plotted as a function of age, as in the right panel of Fig. 8, there is no statistically significant correlation. Although this does not entirely refute the possible role of MP in retinal protection, any role does not seem to be age dependent and could be explained by gain adjustments in the S cones or their postreceptoral pathways. Hue cancellation [100], increment thresholds [101], and single-unit recordings from macaque ganglion cells [22,102] across retinal eccentricity are consistent with gain changes in an S-cone pathway that could compensate for reduced photon capture associated with variations in MP density across retinal eccentricity.
B. Gain Control in S-Cone Detection and Discrimination
Because of the limited dynamic range of visual mechanisms, photoreceptors and/or their postreceptoral pathways must adjust their steady-state sensitivity to accommodate a range of photopic illuminance covering at least 6 log units [103]. This is especially critical for S-cone pathways because their stimulation is reduced more by lenticular senescence than is that for M and L cones, as seen in Fig. 5. Their sensitivity range must also be adjusted more than M and L cones to maintain a balance in cone signals across changes in daylight illumination. To analyze age-related changes in S-cone detection in terms of a quantitative model, tvr functions were measured with two chromatic adapting backgrounds, a fixed long-wave adapting background (570 nm) and a series of superposed short-wave fields (470 nm) of increasing radiance [95]. The radiance of the 570 nm adapting background was selected so that it would be centered on the plateau of the tvr function. Test stimuli were then presented to the fovea as 1°, 250 msec, 440 nm flashes.
The data shown in Fig. 9 (left) represent averages for younger (24 years) and older (71 years) observers. Both of these mean functions and the data of individual observers were well described by Stiles’s ζ (x) template, except for upward deviations at high levels of S-cone excitation. This is consistent with previous work showing that for combinations of short-wave test and adapting fields, the tvr functions may exhibit saturating rather than Weber-like behavior [104]. Under conditions of natural viewing, reductions in pupillary diameter with increasing light level will provide some gain control through a multiplicative scaling of the light, but this can only adjust the incoming light by a maximum of about 1 log unit. In Fig. 9, none of the sensitivity adjustments are due to changes in the area of the pupil because tests were conducted in Maxwellian view. Sensitivity adjustments at a neural level must be responsible for determining these threshold elevations as light level increases, but the results show clearly that age-related changes in sensitivity are intensity dependent.
Fig. 9.
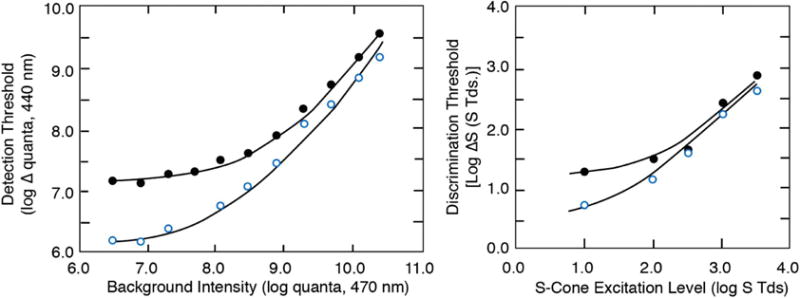
S-cone detection (left) [95] and S-cone chromatic discrimination (right) [96] plotted for groups of younger (open symbols) and older (filled symbols) observers. Curves are based on model fits as described in the text. Left: Foveal detection thresholds for a 440 nm flash plotted as a function of a 470 nm background (superimposed on a 570 nm auxiliary field). Right: Foveal discrimination thresholds plotted as a function of log S-cone Tds.
The tvr functions fitted to the data were analyzed in terms of the model of Pugh and Mollon [105] of the π1 mechanism. According to this model, S-cone signals are attenuated by two gain sites in series. The first site is related to photon capture by the S cones, while the second site of adaptation is dependent on the net imbalance between inputs from S cones and M and L cones. Details of the modeling are provided elsewhere [95]. The model fits shown in Fig. 9 indicated that sensitivity differences between younger and older observers at lower adapting levels are due to a loss in efficiency of S cones that is mathematically equivalent to a loss in the ability to either capture photons or a loss in the photoreceptor efficiency following photon absorption. Specifically, 68% of the age-related sensitivity losses could be attributed to changes in ocular media density and an adaptation site where gain depends on photon capture. The remaining sensitivity loss in terms of the model was at a postreceptoral site where gain is controlled by a net imbalance between S versus M and L cones. Further evidence of second-site losses was demonstrated by Werner et al. [106], who measured the magnitude of transient tritanopia as a function of age.
Similar results were obtained with measurements of chromatic discrimination mediated by S cones, as shown in Fig. 9 (right). For this experiment, individual tritan pairs were obtained for 30 subjects (20–77 years), defined by matches in a 2° bipartite field under strong (420 nm) S-cone adaptation. The resulting tritan metamers were consistent with the color-matching data obtained by Wright [33] for a group of congenital tritanopes. Discrimination was determined using two isoluminant chromatic patches presented in a bipartite field separated by a 10′ gap. Stimuli fell either along a tritan axis or a constant S-cone stimulation axis. The results in Fig. 9 show that the differences between younger and older observers are greatest at lower levels of S-cone excitation (S Td), as was also the case for detection of stimuli through an L-cone pathway (not shown). The fitted functions shown in Fig. 9 were based on the Boynton and Kambe [107] model of chromatic discrimination. This exercise indicated that there is no significant age-related change in Weber fractions, but the significant age-related loss in chromatic discrimination was due, in part, to age-related increases in neural noise and/or gain changes that multiplicatively scale all incident light.
The results of these experiments are consistent with the FM-100 hue tests of Knoblauch et al. [45] and Verriest [37], showing intensity and age-related dependencies in errors along a tritan axis, as discussed above. In general, gain control mechanisms cannot compensate for conditions near the threshold where performance is limited by photon capture and noise. However, when light level increases and sensitivity is within the “Weber region” of the operating range, differences between young and older observers decrease.
Classical wavelength discrimination functions (Δλ versus λ) were measured for four younger and four older observers using isoluminant stimuli and a forced-choice method [108]. Contrary to Ruddock [109], small but consistent elevations in discrimination were found for older compared with younger observers. On theoretical grounds [110], from wavelength discrimination functions obtained from congenital tritanopes [33,111] and conditions in which small-field tritanopia may be expected [112], it is clear that contributions of an S-cone pathway dominate discrimination over the spectral band from 450 to 510 nm. These data were analyzed in terms of S-cone stimulation [113], which refers to the number of photons arriving at the retina after adjustment for lens and macular pigment absorption. There was no significant difference between young and old in their implied Weber fractions. A plot of log ΔS versus log S closely resembles the functions shown in Fig. 9, with differences between younger and older observers at low levels of S-cone stimulation and convergence of the functions at higher levels of S-cone stimulation.
The results from these experiments of S-cone mediated detection and discrimination reveal an interesting similarity in that age-related changes decrease at increasing levels of S-cone excitation. However, the same pattern was observed for tests of M- and L-cone mechanisms. Selective losses in sensitivity of S-cone pathways were not observed in these studies of normal aging.
5. S-CONE SPATIAL CONTRAST SENSITIVITY IN THE AGING VISUAL SYSTEM
Chromatic vision is carried by signals that are multiplexed in pathways to provide information about space and time. Multiple pathways carry these signals, albeit at different spatial scales due to a sparse S-cone mosaic and postreceptoral convergence. Those channels carrying signals from S cones have low spatial and temporal resolution and, in the frequency domain, are low pass with low frequency cutoffs [114–116].
Several studies have attempted to measure age-related changes using chromatically varying patterns modulated along nominal cardinal axes [117–120]. However, none of these prior studies have measured contrast sensitivity with stimuli that were purely chromatic, with controls for chromatic aberration, and with correction for individual differences in the luminosity function.
Spatial chromatic contrast sensitivity functions [121] were measured for groups of younger (18–30 years) and older (65–77 years) observers for temporally ramped Gabor patches of constant spatial bandwidth that were modulated along S-varying (tritan) and (L–M)-varying axes in color space [122]. For one set of measurements, stimuli were equated at the cornea (i.e., the same for all observers) while for a second set of measurements with the same observers, stimuli were generated from color spaces based on cone fundamentals adjusted by separate measurements of ocular media density for each individual. Thresholds were measured with a temporal two-alternative, forced-choice (2AFC) procedure. A Maxwellian-view optical system permitted superimposition of monochromatic stimuli with stimuli generated by a cathode-ray tube (CRT) viewed through a Keplerian telescope. Chromatic aberration was minimized by use of an achromatizing lens and adjustment for each individual’s chromatic axis determined in situ. Stimuli were presented as Gabor patches with spatial frequencies from 0.5 to 4 cycles per degree (cpd) and having a constant number of cycles (i.e., stimulus area decreased with increasing spatial frequency). Because of the relatively sparse retinal sampling by the S-cone mosaic, 4 cpd approaches the resolution limit [123].
Age-related losses in contrast sensitivity were found for all spatial frequencies and for both the S-varying and (L–M)-varying chromatic axes. These losses were evident both when stimuli were equated at the cornea and when equated at the retina. The latter demonstrates losses due to neural factors. As shown in Fig. 10 (left), with stimuli equated at the cornea, the differences for S-varying stimuli were greater, and this demonstrates combined contributions from both optical and neural factors. For stimuli equated at the cornea, some subjects were unable to detect the S-varying stimuli for higher spatial frequencies even at the maximum contrast available on the monitor. Importantly, in the conditions equating stimuli at the retina, differences between older and younger subjects for S-varying stimuli were less than the corresponding differences for (L–M)-varying stimuli. This finding is inconsistent with the hypothesis that S-cone spatial pathways are more vulnerable to aging than L–M cone spatial pathways. Parallel results were reported by Page and Crognale [124], who showed similar rates of aging measured with the visually evoked cortical potential for S−(L+M) and L–M isoluminant gratings.
Fig. 10.
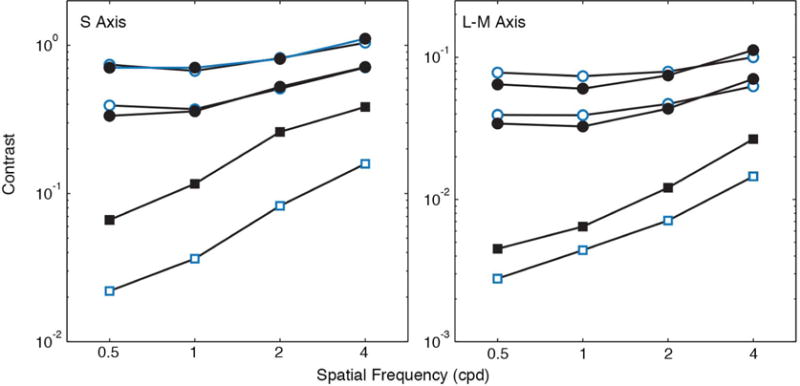
Spatial contrast sensitivity functions (squares) [121] and contrast matching functions at two luminance levels of the standard (circles) [125] for modulation on S and L–M varying axes. Contrast sensitivity (normalized cone contrast vector length) is plotted as a function of spatial frequency for younger (open symbols) and older (closed symbols) groups of observers.
To probe spatial mechanisms at suprathreshold levels, and to simulate more naturalistic viewing conditions [125], contrast matching functions (CMFs) for sinusoidal gratings were tested with modulation along S, L–M, and luminance axes. Hetero-chromatic flicker photometry was used to ensure isoluminance of the chromatic stimuli for each observer, and subjects were refracted for the test distance, but no other adjustments were made to compensate for age-related changes in ocular media density or pupil area. A 2AFC procedure was used to find a match of the perceived contrast of various spatial frequencies (0.5–4 cpd for chromatic stimuli plus 8 cpd for luminance varying stimuli) to a standard pattern (matched to an S-axis standard at 2 cpd) at two different luminances (5 and 30 cd∕m2). Results for S-varying and (L–M)-varying stimuli were compared for younger and older groups by using stimuli that were perceptually anchored to the same physical contrasts. The shapes of the CMFs were similar for the two age groups across spatial frequencies and conditions. There is no evidence for selective age-related losses in spatial pathways mediated by S cones. There is evidence for gain control and perhaps compensation at suprathreshold levels to maintain similarly perceived contrast at different spatial scales.
6. S-CONE TEMPORAL CONTRAST SENSITIVITY IN THE AGING VISUAL SYSTEM
To compare the temporal dynamics of S-cone pathways for younger and older observers, an impulse response function (IRF) was derived from psychophysical thresholds. The IRF is the theoretical response to a flash of infinitely short duration and can be converted to the temporal contrast sensitivity function (tCSF) using the Fourier transform. In several studies, the IRF was obtained using the two-pulse method in which contrast was measured as a function of stimulus-onset asynchrony [126]. This has an advantage over direct measures of the tCSF because the latter does not preserve phase information [127].
In all experiments the stimuli were either luminance modulated or modulated along individually determined tritan lines. A Gaussian patch was presented in one of four quadrants relative to a fixation cross and modulated at constant luminance on a background equivalent to equal-energy white. Because the tCSF depends on retinal illuminance [128], stimuli were equated by HFP, and pupil senescence was controlled using a Maxwellian-view optical system that combined monochromatic lights with a CRT.
The model of Burr and Morrone [129] was used to calculate the IRF from the two-pulse data on the assumption that the underlying IRF can be characterized by an exponentially damped, frequency modulated sine wave. This model fit earlier measurements of young and older observers using pulses varying in luminance [130]. The IRF for S-cone pathways might be expected to differ from luminance modulation, although the IRFs of isolated S cones do not appear to differ from those of single M and L cones [12]. S-cone postreceptoral pathways are, however, more sluggish [13,15]. Nevertheless, differences in temporal tuning among chromatic channels have not been found for isoluminant stimuli in some studies [131–133].
The IRFs obtained for individually determined tritan modulation were monophasic, and the duration was protracted compared to luminance modulation [134]. As with luminance modulation, the amplitude of the S-cone IRF, but not the duration, decreased with age. Further comparisons with the red–green chromatic modulation of Burr and Morrone suggest that the latter are carried by a different pathway from luminance modulation (i.e., parvocellular pathway) and S-cone modulation (i.e., a slower koniocellular pathway). Physiological evidence supports this interpretation. Cottaris and De Valois [135] estimate that S-cone signals from chromatic pathways arrive at cortical area V1 of the macaque monkey about 30–40 msec after M–L chromatic signals. The loss of S-cone IRF amplitude is consistent with losses in sensitivity observed with two-color increment thresholds (Section 4).
A. S-Cone ON and OFF Pathways in the Aging Visual System
There have been few previous attempts to psychophysically separate the temporal ON and OFF responses of S-cone pathways [136], perhaps because OFF cells receiving S-cone input are so rare [27,28], and their very existence has even been questioned [137]. S-cone ON and OFF temporal channels can be separated psychophysically using sawtooth adaptation methods [138,139] and modulation along a tritan line [140]. Modulation along this line from the white point at constant luminance toward short and middle wavelengths selectively stimulates putative S-cone ON and OFF pathways, respectively [141].
S-cone increments and decrements were measured for groups of younger and older observers [142]. Representative results are shown in Fig. 11 for ∼27- and 80-year-old observers. Every observer, whether younger or older, was less sensitive to S-cone decrements than S-cone increments. The speed of response was also slower for decrements than increments, but more so in the elderly. That is, the mean S-cone OFF response was 37 ms later than ON in young observers, but 96 ms later in older observers. The timing for young observers is consistent with ON and OFF S-cone recordings in macaque LGN and cortex [143]. The dissociation in the rate of aging provides compelling support for separate S-cone circuitry for ON and OFF pathways.
Fig. 11.
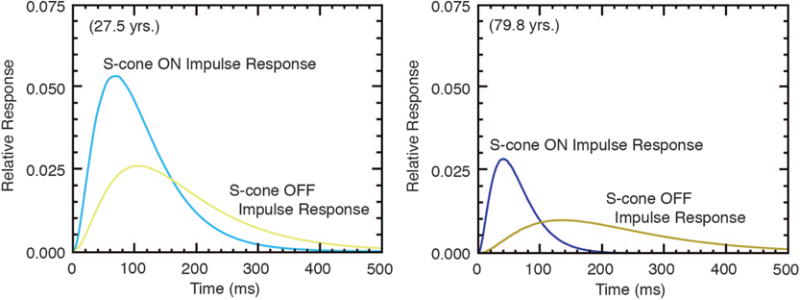
Impulse response functions showing relative response as a function of time for putative S-cone ON and OFF channels of a younger and older observer. Blue and yellow show responses to S-cone increments and decrements, respectively. From [142].
The small bistratified ganglion cell is well established as a carrier of S-cone ON signals via S-cone bipolars [20]. The search for an S-cone OFF bipolar has been more controversial [144–146], although the existence of ganglion and LGN cells with S-OFF input is certain [28,147]. The amplitudes of the S-cone IRFs for putative ON and OFF channels were highly correlated independently of age, but the timing was not. The high correlation between the amplitudes of S-ON and S-OFF was interpreted as meaning that ON and OFF amplitude is controlled by neural circuitry that is common to the two detection systems (the photoreceptors). The uncorrelated timing may be due to different retinal circuitry, and this view is buttressed by the greater age-related decrease for OFF than ON speed, possibly due to a shared S-cone bipolar cell [148] but a sign-inverting (or inhibitory) amacrine cell intermediary [149,150] and/or a slow melanopsin-containing ganglion cell [151]. This additional synaptic connection may explain why the S-cone OFF pathway is slower than the ON. If this interpretation is correct, then these studies may point to a site of GABAergic loss in the elderly. Age-related losses in retinal inhibitory synapses have not been found in the retina, but age-related loss of GABA—an inhibitory neurotransmitter—has been found in the primate visual cortex [152].
These results demonstrate that putative S-cone ON IRFs have higher amplitude and speed than S-cone OFF IRFs. Related results were obtained by measuring reaction times for isoluminant stimuli varied along L–M and S−(L+M) cone axes [153,154]. As shown in Fig. 12, the tCSFs for S-cone modulation and luminance modulation are also consistent with previous measurements showing that they are low [155] and high pass [15], respectively.
Fig. 12.
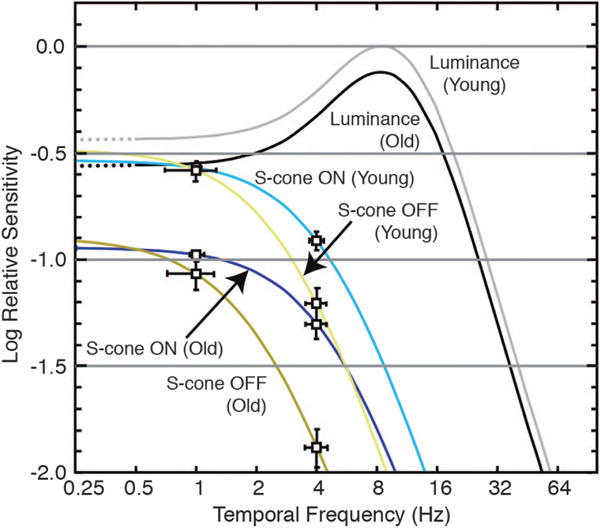
Temporal contrast sensitivity derived from the inverse Fourier transform of impulse response functions. Luminance and chromatic modulation were based on the amplitude of the sine wave divided by the mean luminance or chromaticity. Sensitivity of the luminance maximum (young) was normalized to unity, and the minimum sensitivity was taken as −2 log unit, corresponding to flicker modulation from 0.01 to 1 [156]. Error bars denote the possible range of curves based on ±1 SEM. From [142].
7. ESSENTIAL CONTRIBUTIONS OF S CONES IN MAINTAINING STABILITY OF HUE AND SATURATION ACROSS THE LIFE SPAN
How are changes in ocular media density, receptor sensitivity, and age-related losses in ganglion cell density [157] manifested in color appearance? There are numerous assertions in the literature based on what is known about lenticular senescence that have led to the conclusion that the elderly should have a loss in the ability to see blue [158,159], a model assuming a doubtful direct link between cone stimulation and color appearance. While earlier sections of this paper established a loss in the ability to discriminate tritan stimuli with age, at higher levels some of this loss is mitigated by gain control mechanisms.
The assertion that there is an age-related loss in color appearance was questioned by Wright [160], who, as an octogenarian, asked: “Why do the colors of familiar objects look exactly the same to me now as they did when I was a boy? That was a long, long time ago and the more I think about it, the more remarkable it seems to be” (p. 138).
Changes in color perception with aging provide a test not only of early stage losses in S-cone pathways, but potentially also in subsequent stages where their activity is remapped [161,162]. To that end, a number of studies of color appearance, each using somewhat different methods and exploring different regions of color space, were conducted to study saturation [163,164], hue [165], and brightness [166]. The results of these studies indicated substantial stability of color appearance across the life span, which implies that S-cone signals maintain a balance with M- and L-cone signals to high-level processes.
To more specifically probe S-cone contributions to color appearance, pairs of stimuli from the Optical Society of America Uniform Color Scales [167] were identified at three lightness levels (L = −4, 0, and +4) that plotted approximately on a tritan axis in MacLeod–Boynton receptor excitation space or on an axis of constant S-cone stimulation [122]. Fifteen younger (mean = 21 years) and 15 older (mean = 72 years) observers scaled the chromatic and achromatic components of stimuli that were presented as 2° test surfaces that were freely viewed against a gray field (L = 0) in a ganzfeld-like hemisphere with an illuminant having a correlated temperature of 6200 K [168]. Following Gordon and Abramov [169], the appearance of each sample was described using the hue terms red, green, yellow, and blue, and then in terms of the proportion of achromatic content. The results showed that hue-naming percentages did not differ by more than about 5% for the two age groups. The achromatic content, however, did differ for the two groups. In general, older observers reported less chromatic content, and the differences from younger observers increased with decreasing lightness and luminance. The two age groups differed most in the chromatic content of darker colors, possibly because of the lower effective luminance of such colors. Similar results were reported by Okajima et al. [170]. Most importantly from the perspective of this review, there was no age-related difference between stimuli that were modulated on a tritan axis or on an axis of constant S-cone stimulation.
In previous studies of color appearance, stimuli were identical physically for young and old, or age-related changes in ocular media density were simulated with a filter based on average values in the literature. Hardy et al. [171] used psychophysical procedures to measure ocular media density individually for 10 younger (18–35 years) and 10 older (65–85 years) observers. This made it possible to present the same set of stimuli for the two groups (i.e., equated at the cornea) as well as for the same retinal stimulation (i.e., equated at the retina). The stimuli were based on procedures described by Lindsey and Brown [159], who compared young observers tested with normal viewing and with simulated aging using a yellow filter. Forty simulated Munsell chips were presented as 4.4° disks on a background that was metameric to CIE Illuminant C. Subjects identified each stimulus by choosing one monolexemic word from a set of 11 basic color terms [172]. The condition in which the younger group viewed stimuli filtered by the simulated aging lens yielded results that were essentially identical to those of Lindsey and Brown [159]. That is, with the yellow filter there was a significant reduction in the number of “blue” responses. However, not tested by Lindsey and Brown was the simple comparison of young and old with stimuli equated at the cornea. In this case, color naming was virtually identical for the two groups, implying that the same names were applied by young and old despite different short-wave retinal stimulation. Specifically, at 400 nm, the young observer with the clearest ocular media received 41 times more light at short wavelengths than the older observer with the highest ocular media density. More telling, however, is to plot the proportion of responses in which the term “blue” was used as a function of ocular media density or, as shown in Fig. 13, the S/(L+M) stimulation (calculated from ocular media density on an individual basis). There is no correlation between the blue responses and cone stimulation, presumably subsequent to compensation by postreceptoral pathways for reduced photon capture across the life span.
Fig. 13.
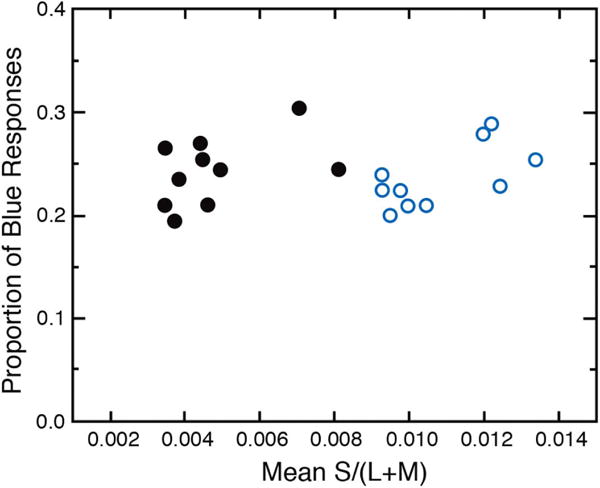
The proportion of “blue” responses to 40 simulated Munsell chips on a CRT is plotted as a function of relative S-cone stimulation. Open and closed symbols represent younger and older groups, respectively. Mean S/(L+M) was calculated from psychophysical measures of ocular media density for each participant. Data from [171].
8. ACHROMATIC LOCUS: COMPENSATION FOR REDUCED S-CONE STIMULATION
The achromatic point, or white point, occupies a special place in colorimetry and vision science [173,174]. Walraven and Werner [175] have shown that it is invariant over a range of about 4 log units of retinal illuminance, which considering the nonlinear compression of photoreceptors would require that the white point stimulates all receptor classes equally and may thus be used as the normalization point for the cone action spectra. The achromatic locus is also the equilibrium point for chromatic mechanisms [174]. Color theories are united at this point, and any disturbances in its mean position in chromaticity space with advancing age may signal changes at one or more stages of color processing. The achromatic point can thus be considered a probe for all directions in color space simultaneously. Fortunately, the white point can be measured reliably because discrimination is excellent in this region of chromaticity space [176–178].
The achromatic locus was measured by a hue cancellation task in which observers adjusted the mixture of their unique blue and unique yellow so that it appeared neither blue nor yellow but achromatic [179]. These spectral unique hues vary among observers, but their wavelength is not correlated with age [165]. Because spectral unique blue and yellow are intensity-invariant, they will be unaffected by age-related changes in the ocular media density when measured with monochromatic lights; however, additive mixtures of these hues would be strongly affected by ocular media density. That is, increasing radiance of unique blue in an achromatic stimulus would be required to compensate for age-related increases in the density of the ocular media, thereby shifting the plotted chromaticity toward the short-wave spectrum locus with age, as illustrated by Fig. 14 (left). Different arrows show the expected shifts for different pairs of complementary lights. If an age-related loss in the sensitivity of one cone type occurred, it would further shift the achromatic locus in the chromaticity diagram toward the copunctal point of the affected cone type. As an example, if there were selective losses in S-cone sensitivity and/or S-cone postreceptoral pathways, this, too, would have to be compensated by increasing the radiance of the unique blue in the mixture. Figure 14 (left) shows predicted shifts in the achromatic point from age 10 to 80 years, plotted in CIE u‘v’ chromaticity coordinates. Clearly, this does not occur as seen in Fig. 14 (left), showing the mean achromatic point for observers in various age groups indicated by the key.
Fig. 14.
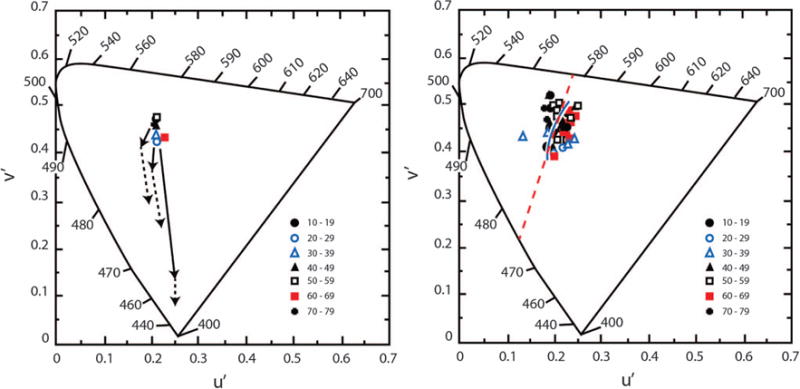
Left: The achromatic locus for a theoretical observer is plotted in CIE 1976 u‘v’ chromaticity coordinates calculated for pairs of spectral complementary lights. Predicted shifts in the achromatic locus from age 10 (the central point) to 80 years based on age-related change in ocular media density (solid arrows) and additional age-related losses in S-cone sensitivity of 0.07 log unit/decade (dashed arrows). These shifts were calculated on the basis of transformation equations from receptor space to CIE space [175,180]. The cluster of data points denote the measured achromatic loci (additive mixtures of each observer’s spectral unique blue and yellow) for different age groups indicated by the key. Right: Achromatic loci for individual observers stratified by ages indicated in the key. The solid contour passing through the points represents various phases of daylight illumination [181]. The red dashed line connects 475 and 577 nm, the mean unique blue and yellow for these observers, which is the definition of the Cerulean line [182].
Achromatic points for 50 observers (11–78 years) are shown in Fig. 14 (right). They were measured at three different retinal illuminances (1–3 log Td.) using the method of constant stimuli. While the measurements are based on an additive mixture of individually determined unique blue and yellow wavelengths, additional verification of the achromatic locus was obtained for 23 subjects using a mixture composed of 600 nm light and its individually determined spectral complement. Contrary to predictions from Fig. 14 (left), there was no significant change in the achromatic locus as a function of age. This does not mean there are no changes in the visual system with age; on the contrary, some compensation mechanisms are required to keep the achromatic locus stable with age. S cones are modulated by more than an order of magnitude than L cones along a Cerulean line, connecting unique blue and yellow [183], and thus must play an essential role in the stability of the achromatic locus.
While compensation seems to work remarkably well, it cannot be perfect for all directions of color space [184,185] because the spectral signatures of the ocular media and macular pigment do not match the spectral sensitivity of the cones or their postreceptoral combinations. Figure 14 (right) shows that one axis that is preserved is that between spectral unique blue and yellow. Why this axis is privileged is unclear, but a possibility is that natural sources of illumination serve as reference points for normalization of color mechanisms. This may explain why the achromatic points in this study, and others [175,186–188], fall along the curve in the middle of Fig. 14 (right), which represents the chromaticity of the various phases of daylight illumination. Werner and Schefrin [179] modeled these data by assuming that after lenticular senescence, there is long-term renormalization of cone sensitivities—a multiplicative scaling, or von Kries adaptation [189], of the receptor sensitivities to an arbitrary white. That is, the sensitivity of each cone type was assumed to be reduced by a factor that is proportional to its excitation by natural sources of daylight illumination. Such rescaling would support stable unique blue and yellow loci and an invariant achromatic locus across the life span. A similar proposal was put forth in a different context by Pokorny and Smith [190] and Mollon [81]. This type of scaling works well with short-term chromatic adaptation [191,192], but the results in Fig. 14 require adjustments in chromatic response systems over a protracted time scale.
The same problem occurs in maintaining constant color appearance within an observer across retinal eccentricity, owing to variations in macular pigment optical density, in photoreceptor sampling and photopigment optical density [193], and in the chromatic organization of ganglion cell receptive fields [194]. Yet the achromatic point also does not differ between the fovea and the near periphery [195,196]. The compensation with retinal eccentricity measured for the white point again appears to be due to changes primarily along a yellow–blue axis, and not along other chromatic axes [100]. Webster et al. [196] found that much but not all of this stability can be explained by a long-term von Kries–type adaptation.
9. TEST OF RENORMALIZATION OF THE ACHROMATIC LOCUS
To test the hypothesis that the visual system continuously renormalizes itself to compensate for changes in cone stimulation, the achromatic point was measured for four observers (63–84 years) before and up to one year after removal of their cataractous lens [197]. Their task was to adjust a 9.5° diameter disk (30 cd · m−2) on a computer screen in a dark room until it appeared achromatic. Chromaticity was adjusted with a game controller in CIE L*a*b* color space. All tests were conducted monocularly.
Figure 15 shows the locus of the achromatic point in CIE x, y chromaticity space for different times before and after cataract surgery. One day after surgery, there is a large shift in the white point toward the middle-wave spectrum locus, much of which may be due simply to the change in the retinal stimulus. The open circle shows the location of the white point expected simply from removal of the cataractous lens and consequent increased short-wave light reaching the retina. Note, however, that the white point drifts back in the direction of the presurgery chromaticity as shown by points with postsurgery days denoted. It follows an exponential time course and appears to approach an asymptotic value at about three months. Tests on three other subjects show a similar pattern of results.
Fig. 15.
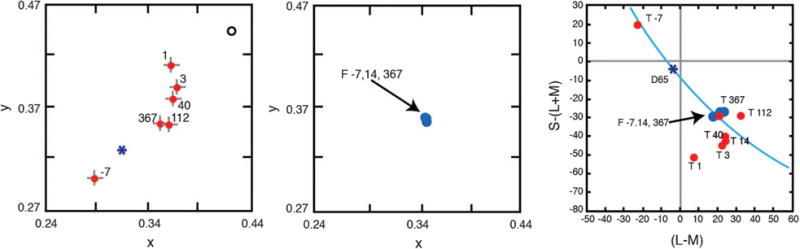
Left: Loci of the achromatic point of a 71-year-old observer before and after cataract extraction plotted in CIE x, y coordinates. Each datum is labeled according to the day the measurement was made relative to the surgery date. The open symbol represents the presurgery setting specified at the retina, calculated from measurements of ocular media density. The asterisk is the chromaticity of CIE Illuminant D65. Some settings have been omitted for clarity, but all follow the pattern shown. Error bars denote ±1 SEM. Center: Achromatic point of the same observer’s fellow eye with measurement dates referring to the test eye. Data from [197]. Right: Data from the two left panels (same color coding) plotted in cone excitation space following the axis normalization of Webster and Mollon [198]. Blue contour shows phases of daylight illumination.
Data from the same observer’s fellow eye are shown in the middle panel of Fig. 15. This eye had a cataract removed eight months before the test eye. The locus of the achromatic point was constant within the period when the other eye’s achromatic point was shifting. The measurements after one year show that the two eyes now have the same achromatic point. The long time course of changes in the achromatic locus differs dramatically from the changes observed in typical color constancy experiments [199,200] and may imply involvement of monocular cortical mechanisms.
Color discrimination measured with the Cambridge Colour Test [201] indicated a tritan-like deficiency before surgery but normal color vision by one day after surgery. Performance did not change during the period in which the achromatic locus was changing. This implies different sites of mediation for color discrimination and color appearance. Shinomori et al. [108] found that postreceptoral mechanisms, presumably in the retina and LGN, can account for many aspects of hue discrimination and their age-related changes. It is well known that cortical adaptation contributes to changes in color appearance [106,202], and these processes, involving chromatic contrast, are robust in the elderly visual system [203].
The white point settings were converted to cone excitation values, and it can be seen that most of the changes in the achromatic point can be accounted for initially by changes in S-cone response, as is expected from normal lens aging having the greatest effect on S-cone excitation [77]. However, the subsequent slow migration of the achromatic point involves shifts along both the S- and (L–M)-axes. S cones thus provide a spectral ballast to M and L cones, and without their stability and long-term adaptation across the life span, color appearance would not be stable.
10. CONCLUSIONS
The S cones and their postreceptoral pathways are thought to be responsible for losses in color discrimination with age that are prominent along a tritan axis. Examination of the aging visual system shows that all three cone types are functional in infancy, and losses in sensitivity occur from adolescence or early adulthood and continue throughout the life span. These losses are made worse at short wavelengths by ocular media density changes with age. Nevertheless, several studies show that sensitivity losses in S-cone pathways are not substantially different from those of M- and L-cone pathways at a neural level. What is critical to the performance of S-cone pathways is the light level or their adaptation level, but this is also true of M- and L-cone pathways.
Stimulation of S cones is dramatically reduced by lenticular senescence across the life span, and discovering how the visual system nevertheless accomplishes stability in color appearance may lend insight that goes beyond the study of aging to reveal principles of visual coding that may be difficult to capture by studying only one age.
While color appearance data indicate that the visual system is capable of adaptive modifications that compensate, in large part, for age-related changes in the intensity and spectral distribution of the stimulus, as well as sensitivity losses beginning in the photoreceptors and their retinal pathways, it is doubtful that these processes could ever be perfect due to nonlinearities in visual processing and the mismatches between the spectral signatures of color mechanisms and the senescent ocular media. M and L cones can only support perceptual stability in concert since their absorption spectra are highly correlated [204], but S cones are spectrally isolated, resulting in much greater variance in S-cone stimulation than for M or L cones for changes in illuminants, reflectances, and ocular media density. Two studies of the spectral reflectance of large numbers of natural stimuli demonstrated correlations in L:M cone activity >0.98 [205,206]. Robust S cones to balance M- and L-cone stimulation are key to color stability across the life span, necessitated further by the majority of natural colors and illuminants varying along yellow or blue hue axes [183]. Hence, most white points vary along a yellow–blue axis, but the large variation may reflect adaptation mechanisms.
To this it may be added that without these natural processes of compensation, our color lexicon would be without meaning, for the blue of the young would be the yellow of the old. Yet to be useful in communication, color appearance needs to be stable across the life span. For natural viewing, this compensation is apparently satisfactory, save for conditions of low illumination or advanced cataract where compensation processes apparently reach the physiological limit [207].
Acknowledgments
The helpful comments of valued collaborators are gratefully acknowledged, including Peter Delahunt, Sarah L. Elliott, Susan Garcia, Joseph L. Hardy, Kyle McDermott, John Mollon, Athanasios Panorgias, Lothar Spillmann, Vicki J. Volbrecht, and Michael Webster. This paper was written during a sabbatical in the laboratory of Mark Greenlee at the University of Regensburg.
Funding. Alexander von Humboldt-Stiftung (Senior Scientist Award); National Institute on Aging (NIA) (R01 AG 04058); Research to Prevent Blindness (RPB).
Footnotes
OCIS codes: (330.0330) Vision, color, and visual optics, (330.5510) Psychophysics, (330.4300) Vision system - noninvasive assessment.
References
- 1.Mollon JD. ‘Tho’ she kneel’d in that place where they grew.’ The uses and origins of primate colour vision. J Exp Biol. 1989;146:21–38. doi: 10.1242/jeb.146.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Curcio CA, Allen KA, Sloan KR, Lerea CL, Hurley JB, Klock IB, Milam AH. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J Comp Neurol. 1991;312:610–624. doi: 10.1002/cne.903120411. [DOI] [PubMed] [Google Scholar]
- 3.Marc RE, Sperling HG. Chromatic organization of primate cones. Science. 1977;196:454–456. doi: 10.1126/science.403607. [DOI] [PubMed] [Google Scholar]
- 4.Williams DR, MacLeod D, Hayhoe MM. Foveal tritanopia. Vis Res. 1981;21:1341–1356. doi: 10.1016/0042-6989(81)90241-8. [DOI] [PubMed] [Google Scholar]
- 5.König A. Über den menschlichen Sehpurpur und seine Bedeutung für das Sehen. Sitzungsber K Preuss Adak Wiss. 1895;30:577–598. [Google Scholar]
- 6.Willmer EN, Wright WD. Colour sensitivity of the fovea centralis. Nature. 1945;156:119–121. [Google Scholar]
- 7.Wald G. Blue-blindness in the normal fovea. J Opt Soc Am. 1967;57:1289–1303. doi: 10.1364/josa.57.001289. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell JC. On the theory of compound colours, and the relations of the colours of the spectrum. Philos Trans R Soc London. 1860;150:57–84. [Google Scholar]
- 9.Magnussen S, Spillmann L, Stürzel F, Werner JS. Unveiling the foveal blue scotoma through an afterimage. Vis Res. 2004;44:377–383. doi: 10.1016/j.visres.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 10.DeMonasterio FM, Schein SJ, McCrane EP. Staining of blue-sensitive cones of the macaque retina by a fluorescent dye. Science. 1981;213:1278–1281. doi: 10.1126/science.7268439. [DOI] [PubMed] [Google Scholar]
- 11.Ahnelt PK, Kolb H, Pflug R. Identification of a subtype of cone photoreceptor, likely to be blue sensitive, in the human retina. J Comp Neurol. 1987;255:18–34. doi: 10.1002/cne.902550103. [DOI] [PubMed] [Google Scholar]
- 12.Schnapf JL, Nunn BJ, Meister M, Baylor DA. Visual transduction in cones of the monkey Macaca fascicularis. J Physiol. 1990;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brindley GS, Du Croz JJ, Rushton WAH. The flicker fusion frequency of the blue-sensitive mechanism of colour vision. J Physiol. 1966;183:497–500. doi: 10.1113/jphysiol.1966.sp007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DG. The contrast sensitivity of the colour mechanisms of the human eye. J Physiol. 1968;196:415–429. doi: 10.1113/jphysiol.1968.sp008515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly DH. Spatio-temporal frequency characteristics of color-vision mechanisms. J Opt Soc Am. 1974;64:983–990. doi: 10.1364/josa.64.000983. [DOI] [PubMed] [Google Scholar]
- 16.Tansley BW, Boynton RM. A line, not a space, represents visual distinctness of borders formed by different colors. Science. 1976;191:954–957. doi: 10.1126/science.1082644. [DOI] [PubMed] [Google Scholar]
- 17.Eisner A, MacLeod DI. Blue-sensitive cones do not contribute to luminance. J Opt Soc Am. 1980;70:121–123. doi: 10.1364/josa.70.000121. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Stromeyer CF., III Contribution of human short-wave cones to luminance and motion detection. J Physiol. 1989;413:563–593. doi: 10.1113/jphysiol.1989.sp017669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ripamonti C, Woo WL, Crowther E, Stockman A. The S-cone contribution to luminance depends on the M- and L-cone adaptation levels: silent surrounds? J Vis. 2009;9(3):101–16. doi: 10.1167/9.3.10. [DOI] [PubMed] [Google Scholar]
- 20.Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- 21.Martin PR, White AJR, Goodchild AK, Wilder HD, Sefton AE. Evidence that blue-on cells are part of the third geniculocortical pathway in primates. Eur J Neurosci. 1997;9:1536–1541. doi: 10.1111/j.1460-9568.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 22.Zrenner E, Gouras P. Characteristics of the blue sensitive cone mechanism in primate retinal ganglion cells. Vis Res. 1981;21:1605–1609. doi: 10.1016/0042-6989(81)90042-0. [DOI] [PubMed] [Google Scholar]
- 23.Brindley GS. The summation areas of human colour-receptive mechanisms at increment threshold. J Physiol. 1954;124:400–408. doi: 10.1113/jphysiol.1954.sp005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volbrecht VJ, Shrago EE, Schefrin BE, Werner JS. Spatial summation in human cone mechanisms from 0° to 20° in the superior retina. J Opt Soc Am A. 2000;17:641–650. doi: 10.1364/josaa.17.000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- 26.Wässle H, Boycott BB, Illing RB. Morphology and mosaic of on- and off-beta cells in the cat retina and some functional considerations. Proc R Soc London Ser B. 1981;212:177–195. doi: 10.1098/rspb.1981.0033. [DOI] [PubMed] [Google Scholar]
- 27.Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J Physiol. 1984;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valberg A, Lee BB, Tigwell DA. Neurones with strong inhibitory S-cone inputs in the macaque lateral geniculate nucleus. Vis Res. 1986;26:1061–1064. doi: 10.1016/0042-6989(86)90040-4. [DOI] [PubMed] [Google Scholar]
- 29.Harwerth RS, Sperling HG. Prolonged color blindness induced by intense spectral lights in rhesus monkeys. Science. 1971;174:520–523. doi: 10.1126/science.174.4008.520. [DOI] [PubMed] [Google Scholar]
- 30.Sperling HG, Johnson C, Harwerth RS. Differential spectral photic damage to primate cones. Vis Res. 1980;20:1117–1125. doi: 10.1016/0042-6989(80)90049-8. [DOI] [PubMed] [Google Scholar]
- 31.Köllner H. Die Störungen des Farbensinnes: Ihre Klinische Bedeutung und Ihre Diagnose. S Karger; 1912. [Google Scholar]
- 32.Pinckers A, Pokorny J, Smith VC, Verriest G. Congenital and Acquired Color Vision Defects. Grune & Stratton; 1979. [Google Scholar]
- 33.Wright WD. The characteristics of tritanopia. J Opt Soc Am. 1952;42:509–521. doi: 10.1364/josa.42.000509. [DOI] [PubMed] [Google Scholar]
- 34.Mollon JD. A taxonomy of tritanopia. Doc Ophthalmol Proc Ser. 1982;33:87–101. [Google Scholar]
- 35.Smithson HE. S-cone psychophysics. Vis Neurosci. 2014;31:211–225. doi: 10.1017/S0952523814000030. [DOI] [PubMed] [Google Scholar]
- 36.Stockman A, Sharpe LT. The spectral sensitivities of the middle-and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vis Res. 2000;40:1711–1737. doi: 10.1016/s0042-6989(00)00021-3. [DOI] [PubMed] [Google Scholar]
- 37.Verriest G. Further studies on acquired deficiency of color discrimination. J Opt Soc Am. 1963;53:185–197. doi: 10.1364/josa.53.000185. [DOI] [PubMed] [Google Scholar]
- 38.Verriest G, Laethem JV, Uvijls A. A new assessment of the normal ranges of the Farnsworth–Munsell 100-Hue test scores. Am J Ophthalmol. 1982;93:635–642. doi: 10.1016/s0002-9394(14)77380-5. [DOI] [PubMed] [Google Scholar]
- 39.Smith VC, Pokorny J, Pass AS. Color-axis determination on the Farnsworth–Munsell 100-Hue test. Am J Ophthalmol. 1985;100:176–182. doi: 10.1016/s0002-9394(14)75002-0. [DOI] [PubMed] [Google Scholar]
- 40.Ventura DF, Silveira LCL, Rodrigues AR, DeSouza JM, Gualtieri M, Bonci D, Costa MF. Preliminary norms for the Cambridge Colour Test. In: Mollon JD, Pokorny J, Knoblauch K, editors. Normal and Defective Colour Vision. Oxford University; 2003. pp. 331–339. [Google Scholar]
- 41.Paramei GV, Oakley B. Variation of color discrimination across the life span. J Opt Soc Am A. 2014;31:A375–A384. doi: 10.1364/JOSAA.31.00A375. [DOI] [PubMed] [Google Scholar]
- 42.Shinomori K, Panorgias A, Werner JS. Discrimination thresholds of normal and anomalous trichromats by the Cambridge Colour Test: implications of senescent changes in lens optical density. J Opt Soc Am A. 2016;33:A65–A76. doi: 10.1364/JOSAA.33.000A65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbur JL, Rodriguez-Carmona M. Color vision changes in normal aging. In: Elliot AJ, Fairchild MD, Franklin A, editors. Handbook of Color Psychology. Cambridge University; 2015. pp. 180–196. [Google Scholar]
- 44.Verriest G, van de Velde R, Van der Donck R. Étude quantitative de l’effet qu’exerce sur la discrimination chromatique une absorption sélective de la partie froide du spectre visible. Rev Opt. 1962;41:3–118. [Google Scholar]
- 45.Knoblauch K, Saunders F, Kusuda M, Hynes R, Podgor M, Higgins KE, de Monasterio FM. Age and illuminance effects in the Farnsworth–Munsell 100-hue test. Appl Opt. 1987;26:1441–1448. doi: 10.1364/AO.26.001441. [DOI] [PubMed] [Google Scholar]
- 46.Hood DC, Benimoff NI, Greenstein VC. The response range of the blue-cone pathways: a source of vulnerability to disease. Invest Ophthalmol Visual Sci. 1984;25:864–867. [PubMed] [Google Scholar]
- 47.Dalton J. Extraordinary facts relating to the vision of colours: with observations. Mem Proc Manchester Lit Philos Soc. 1798;5:28–45. [Google Scholar]
- 48.Hering E. Über individuelle Verschiedenheiten des Farbensinnes. Lotos. 1885;6:142–198. [Google Scholar]
- 49.Norren DV, Vos JJ. Spectral transmission of the human ocular media. Vis Res. 1974;14:1237–1244. doi: 10.1016/0042-6989(74)90222-3. [DOI] [PubMed] [Google Scholar]
- 50.Wald G, Brown PK. Human rhodopsin. Science. 1958;127:222–249. doi: 10.1126/science.127.3292.222. [DOI] [PubMed] [Google Scholar]
- 51.Alpern M, Pugh EN. The density and photosensitivity of human rhodopsin in the living retina. J Physiol. 1974;237:341–370. doi: 10.1113/jphysiol.1974.sp010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Werner JS. Development of scotopic sensitivity and the absorption spectrum of the human ocular media. J Opt Soc Am. 1982;72:247–258. doi: 10.1364/josa.72.000247. [DOI] [PubMed] [Google Scholar]
- 53.Werner JS, Bieber ML, Schefrin BE. Senescence of foveal and parafoveal cone sensitivities and their relations to macular pigment density. J Opt Soc Am A. 2000;17:1918–1932. doi: 10.1364/josaa.17.001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pokorny J, Smith VC, Lutze M. Aging of the human lens. Appl Opt. 1987;26:1437–1440. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- 55.Weale RA. Age and the transmittance of the human crystalline lens. J Physiol. 1988;395:577–587. doi: 10.1113/jphysiol.1988.sp016935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stiles WS, Burch JM. N.P.L. colour-matching investigation: final report (1958) Opt Acta. 1959;6:1–26. [Google Scholar]
- 57.Soemmerring ST. De foramine centrali limbo luteo cincto retinae humanae. Soc Reg Sci Goetting. 1799;13:3–13. [Google Scholar]
- 58.Nussbaum JJ, Pruett RC, Delori FC. Historic perspectives. macular yellow pigment. The first 200 years. Retina. 1981;1:296–310. [PubMed] [Google Scholar]
- 59.Wald G. Human vision and the spectrum. Science. 1945;101:653–658. doi: 10.1126/science.101.2635.653. [DOI] [PubMed] [Google Scholar]
- 60.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–849. [PubMed] [Google Scholar]
- 61.Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:660–673. [PubMed] [Google Scholar]
- 62.Schultze M. Über den Gelben Fleck der Retina, Seinen Einfluss auf Normales Sehen und auf Farbenblindheit. von Max Cohen & Sohn; 1866. [Google Scholar]
- 63.Haidinger W. Ueber das directe Erkennen des polarisirten Lichts und der Lage der Polarisationsebene. Ann Phys Chem. 1844;139:29–39. [Google Scholar]
- 64.Vos JJ. Literature review of human macular absorption in the visible and its consequences for the cone receptor primaries. Technical Report TNO No IZF (Institute for Perception. 1972 [Google Scholar]
- 65.Murray IJ. Macular pigment: Characteristics and role in the older eye. In: Werner JS, Chalupa LM, editors. The New Visual Neurosciences. MIT; 2014. pp. 1547–1561. [Google Scholar]
- 66.Werner JS, Wooten BR. Opponent chromatic mechanisms: relation to photopigments and hue naming. J Opt Soc Am. 1979;69:422–434. doi: 10.1364/josa.69.000422. [DOI] [PubMed] [Google Scholar]
- 67.Berendschot TT, van Norren D. On the age dependency of the macular pigment optical density. Exp Eye Res. 2005;81:602–609. doi: 10.1016/j.exer.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 68.Wyszecki G, Stiles WS. Color Science: Concepts and Methods, Quantitative Data and Formulae. 2nd. Wiley; 1982. [Google Scholar]
- 69.Werner JS, Donnelly SK, Kliegl R. Aging and human macular pigment density; appended with translations from the work of Max Schultze and Ewald Hering. Vis Res. 1987;27:257–268. doi: 10.1016/0042-6989(87)90188-x. [DOI] [PubMed] [Google Scholar]
- 70.Wright WD. A re-determination of the trichromatic coefficients of the spectral colours. Trans Opt Soc. 2002;30:141–164. [Google Scholar]
- 71.Wright WD. Researches on Normal and Defective Colour Vision. CV Mosby; 1947. [Google Scholar]
- 72.Ruddock KH. The effect of age upon colour vision—II. changes with age in light transmission of the ocular media. Vis Res. 1965;5:47–58. doi: 10.1016/0042-6989(65)90074-x. [DOI] [PubMed] [Google Scholar]
- 73.Judd DB, Plaza L, Farnsworth D. Tritanopia with abnormally heavy ocular pigmentation. J Opt Soc Am. 1950;40:833–841. [Google Scholar]
- 74.Moreland JD. Matching range and age in a blue–green equation. In: Drum B, editor. Colour Vision Deficiencies XI. Kluwer Academic; 1993. pp. 129–134. [Google Scholar]
- 75.Kliegl R, Volbrecht VJ, Werner JS. Influences of variation in lenticular and macular pigmentation in dichromatic neutral points. In: Verriest G, editor. Colour Vision Deficiencies VII. Dr W Junk; 1984. pp. 155–163. [Google Scholar]
- 76.Werner JS, Wooten BR. Age changes in ocular media density and consequences for colour vision. In: Verriest G, editor. Colour Vision Deficiencies V. Adam Hilger; 1980. pp. 355–359. [Google Scholar]
- 77.Werner JS. Visual problems of the retina during ageing: compensation mechanisms and colour constancy across the life span. Prog Retinal Eye Res. 1996;15:621–645. [Google Scholar]
- 78.Teller DY, Peeples DR, Sekel M. Discrimination of chromatic from white light by two-month-old human infants. Vis Res. 1978;18:41–48. doi: 10.1016/0042-6989(78)90075-5. [DOI] [PubMed] [Google Scholar]
- 79.Werner JS, Wooten BR. Human infant color vision and color perception. Infant Behav Dev. 1979;2:241–273. [Google Scholar]
- 80.Pulos E, Teller DY, Buck SL. Infant color vision: a search for short-wavelength-sensitive mechanisms by means of chromatic adaptation. Vis Res. 1980;20:485–493. doi: 10.1016/0042-6989(80)90123-6. [DOI] [PubMed] [Google Scholar]
- 81.Mollon JD. Color vision. Ann Rev Psychol. 1982;33:41–85. doi: 10.1146/annurev.ps.33.020182.000353. [DOI] [PubMed] [Google Scholar]
- 82.Volbrecht VJ, Werner JS. Isolation of short-wavelength-sensitive cone photoreceptors in 4–6-week-old human infants. Vis Res. 1987;27:469–478. doi: 10.1016/0042-6989(87)90094-0. [DOI] [PubMed] [Google Scholar]
- 83.Bieber ML, Knoblauch K, Werner JS. M- and L-cones in early infancy: II. action spectra at 8 weeks of age. Vis Res. 1998;38:1765–1773. doi: 10.1016/s0042-6989(97)00384-2. [DOI] [PubMed] [Google Scholar]
- 84.Bieber ML, Werner JS, Knoblauch K, Neitz J, Neitz M. M- and L-cones in early infancy: III. comparison of genotypic and phenotypic markers of color vision in infants and adults. Vis Res. 1998;38:3293–3297. doi: 10.1016/s0042-6989(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 85.De Vries H. On the basic sensation curves of the three-color theory. J Opt Soc Am. 1946;36:121–127. doi: 10.1364/josa.36.000121. [DOI] [PubMed] [Google Scholar]
- 86.Stiles WS. Further studies of visual mechanisms by the two-colour increment threshold method. Coloq Probl Opt Visual Union Int Phys Pure Appl. 1953;1:65–103. [Google Scholar]
- 87.Estévez O, Spekreijse H, Van den Berg TJTP, Cavonius CR. The spectral sensitivities of isolated human color mechanisms determined from contrast evoked potential measurements. Vis Res. 1975;15:1205–1212. doi: 10.1016/0042-6989(75)90163-7. [DOI] [PubMed] [Google Scholar]
- 88.Mitchell DE, Rushton WA. The red–green pigments of normal vision. Vis Res. 1971;11:1045–1056. doi: 10.1016/0042-6989(71)90111-8. [DOI] [PubMed] [Google Scholar]
- 89.Eisner A, Fleming SA, Klein ML, Mauldin WM. Sensitivities in older eyes with good acuity: eyes whose fellow eye has exudative AMD. Invest Ophthalmol Vis Sci. 1987;28:1832–1837. [PubMed] [Google Scholar]
- 90.Haegerstrsom-Portnoy G, Hewlett SE, Barr SAN. S cone loss with aging. Mod Probl Ophthalmol. 1989;52:345–352. [Google Scholar]
- 91.Johnson CA, Adams AJ, Twelker JD, Quigg JM. Age-related changes in the central visual field for short-wavelength-sensitive pathways. J Opt Soc Am A. 1988;5:2131–2139. doi: 10.1364/josaa.5.002131. [DOI] [PubMed] [Google Scholar]
- 92.Werner JS, Steele VG. Sensitivity of human foveal color mechanisms throughout the life span. J Opt Soc Am A. 1988;5:2122–2130. doi: 10.1364/josaa.5.002122. [DOI] [PubMed] [Google Scholar]
- 93.Knoblauch K, Vital-Durand F, Barbur JL. Variation of chromatic sensitivity across the life span. Vis Res. 2001;41:23–36. doi: 10.1016/s0042-6989(00)00205-4. [DOI] [PubMed] [Google Scholar]
- 94.Stiles WS. Color vision: the approach through increment-threshold sensitivity. Proc Natl Acad Sci USA. 1959;45:100–114. [Google Scholar]
- 95.Schefrin BE, Werner JS, Plach M, Utlaut N, Switkes E. Sites of age-related sensitivity loss in a short-wave cone pathway. J Opt Soc Am A. 1992;9:355–363. doi: 10.1364/josaa.9.000355. [DOI] [PubMed] [Google Scholar]
- 96.Schefrin BE, Shinomori K, Werner JS. Contributions of neural pathways to age-related losses in chromatic discrimination. J Opt Soc Am A. 1995;12:1233–1241. doi: 10.1364/josaa.12.001233. [DOI] [PubMed] [Google Scholar]
- 97.Kadlecová V, Peleška M, Vaško A. Dependence on age of the diameter of the pupil in the dark. Nature. 1958;182:1520–1521. doi: 10.1038/1821520a0. [DOI] [PubMed] [Google Scholar]
- 98.Haegerstrom-Portnoy G. Short wavelength-sensitive-cone sensitivity loss with aging: a protective role for macular pigment? J Opt Soc Am A. 1988;5:2140–2144. doi: 10.1364/josaa.5.002140. [DOI] [PubMed] [Google Scholar]
- 99.Hammond BR, Wooten BR, Snodderly DM. Preservation of visual sensitivity of older subjects: association with macular pigment density. Invest Ophthalmol Vis Sci. 1998;39:397–406. [PubMed] [Google Scholar]
- 100.Hibino H. Red–green and yellow–blue opponent-color responses as a function of retinal eccentricity. Vis Res. 1992;32:1955–1964. doi: 10.1016/0042-6989(92)90055-n. [DOI] [PubMed] [Google Scholar]
- 101.Stringham JM, Hammond BR, Wooten BR, Snodderly DM. Compensation for light loss resulting from filtering by macular pigment: relation to the S-cone pathway. Optom Vis Sci. 2006;83:887–894. doi: 10.1097/01.opx.0000249976.00534.2d. [DOI] [PubMed] [Google Scholar]
- 102.de Monasterio FM. Macular pigmentation and the spectral sensitivity of retinal ganglion cells of macaques. Vis Res. 1978;18:1273–1277. doi: 10.1016/0042-6989(78)90217-1. [DOI] [PubMed] [Google Scholar]
- 103.Walraven J, Enroth-Cugell C, Hood DC, MacLeod DIA, Schnapf J. The control of visual sensitivity: receptoral and postreceptoral processes. In: Spillmann L, Werner JS, editors. Visual Perception the Neurophysiological Foundations. Academic Press; 1990. pp. 53–101. [Google Scholar]
- 104.Pugh EN, Jr, Larimer J. Test of the identity of the site of blue/yellow hue cancellation and the site of chromatic antagonism in the π1 pathway. Vis Res. 1980;20:779–788. doi: 10.1016/0042-6989(80)90009-7. [DOI] [PubMed] [Google Scholar]
- 105.Pugh EN, Mollon JD. A theory of the π1 and π3 color mechanisms of Stiles. Vis Res. 1979;19:293–312. doi: 10.1016/0042-6989(79)90175-5. [DOI] [PubMed] [Google Scholar]
- 106.Werner A, Sharpe LT, Zrenner E. Asymmetries in the time-course of chromatic adaptation and the significance of contrast. Vis Res. 2000;40:1101–1113. doi: 10.1016/s0042-6989(00)00012-2. [DOI] [PubMed] [Google Scholar]
- 107.Boynton RM, Kambe N. Chromatic difference steps of moderate size measured along theoretically critical axes. Color Res Appl. 1980;5:13–23. [Google Scholar]
- 108.Shinomori K, Schefrin BE, Werner JS. Age-related changes in wavelength discrimination. J Opt Soc Am A. 2001;18:310–318. doi: 10.1364/josaa.18.000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruddock KH. The effect of age upon colour vision—I. response in the receptoral system of the human eye. Vis Res. 1965;5:37–45. doi: 10.1016/0042-6989(65)90073-8. [DOI] [PubMed] [Google Scholar]
- 110.Mollon JD, Astell S, Cavonius CR. A reduction in stimulus duration can improve wavelength discriminations mediated by shortwave cones. Vis Res. 1992;32:745–755. doi: 10.1016/0042-6989(92)90189-p. [DOI] [PubMed] [Google Scholar]
- 111.Kaiser PK, Boynton RM. Role of the blue mechanism in wavelength discrimination. Vis Res. 1985;25:523–529. doi: 10.1016/0042-6989(85)90155-5. [DOI] [PubMed] [Google Scholar]
- 112.Bedford RE, Wyszecki GW. Wavelength discrimination for point sources. J Opt Soc Am. 1958;48:129–135. doi: 10.1364/josa.48.000129. [DOI] [PubMed] [Google Scholar]
- 113.Yeh T, Pokorny J, Smith VC. Colour Vision Deficiencies XI. Vol. 56. Springer; 1993. S-cone discrimination sensitivity and performance on arrangement tests; pp. 293–302. (Documenta Ophthalmologica Proceedings Series). [Google Scholar]
- 114.Bradley A, Switkes E, De Valois K. Orientation and spatial frequency selectivity of adaptation to color and luminance gratings. Vis Res. 1988;28:841–856. doi: 10.1016/0042-6989(88)90031-4. [DOI] [PubMed] [Google Scholar]
- 115.DeValois KK. The role of color in spatial vision. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. MIT; 2004. pp. 924–935. [Google Scholar]
- 116.Mullen KT. The contrast sensitivity of human colour vision to red–green and blue–yellow chromatic gratings. J Physiol. 1985;359:381–400. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Werner A, Schwarz G, Paulus W. Ageing and chromatic contrast sensitivity. In: Drum B, editor. Colour Vision Deficiencies XII. Kluwer Academic; 1995. pp. 235–241. [Google Scholar]
- 118.Steen R, Whitaker D, Elliott DB, Wild JM. Age-related effects of glare on luminance and color contrast sensitivity. Optom Vis Sci. 1994;71:792–796. doi: 10.1097/00006324-199412000-00010. [DOI] [PubMed] [Google Scholar]
- 119.Fiorentini A, Porciatti V, Morrone MC, Burr DC. Visual ageing: unspecific decline of the responses to luminance and colour. Vis Res. 1996;36:3557–3566. doi: 10.1016/0042-6989(96)00032-6. [DOI] [PubMed] [Google Scholar]
- 120.Crognale MA. Development, maturation, and aging of chromatic visual pathways: VEP results. J Vis. 2002;2(6):438–450. doi: 10.1167/2.6.2. [DOI] [PubMed] [Google Scholar]
- 121.Hardy JL, Delahunt PB, Okajima K, Werner JS. Senescence of spatial chromatic contrast sensitivity. Detection I. under conditions controlling for optical factors. J Opt Soc Am A. 2005;22:49–59. doi: 10.1364/josaa.22.000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Macleod DIA, Boynton RM. Chromaticity diagram showing cone excitation by stimuli of equal luminance. J Opt Soc Am. 1979;69:1183–1186. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- 123.Humanski RA, Wilson HR. Spatial frequency mechanisms with short-wavelength-sensitive cone inputs. Vis Res. 1992;32:549–560. doi: 10.1016/0042-6989(92)90247-g. [DOI] [PubMed] [Google Scholar]
- 124.Page JW, Crognale MA. Differential aging of chromatic and achromatic visual pathways: behavior and electrophysiology. Vis Res. 2005;45:1481–1489. doi: 10.1016/j.visres.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 125.Delahunt PB, Hardy JL, Okajima K, Werner JS. Senescence of spatial chromatic contrast sensitivity. II. Matching under natural viewing conditions. J Opt Soc Am A. 2005;22:60–67. doi: 10.1364/josaa.22.000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ikeda M. Temporal summation of positive and negative flashes in the visual system. J Opt Soc Am. 1965;55:1527–1533. [Google Scholar]
- 127.Victor JD. Temporal impulse responses from flicker sensitivities: causality, linearity, and amplitude data do not determine phase. J Opt Soc Am A. 1989;6:1302–1303. doi: 10.1364/josaa.6.001302. [DOI] [PubMed] [Google Scholar]
- 128.DeLange H. Research into the dynamic nature of the human fovea–cortex systems with intermittent and modulated light. I. attenuation characteristics with white and colored light. J Opt Soc Am. 1958;48:777–783. doi: 10.1364/josa.48.000777. [DOI] [PubMed] [Google Scholar]
- 129.Burr DC, Morrone MC. Impulse-response functions for chromatic and achromatic stimuli. J Opt Soc Am A. 1993;10:1706–1713. [Google Scholar]
- 130.Shinomori K, Werner JS. Senescence of the temporal impulse response to a luminous pulse. Vis Res. 2003;43:617–627. doi: 10.1016/S0042-6989(03)00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith VC, Bowen RW, Pokorny J. Threshold temporal integration of chromatic stimuli. Vis Res. 1984;24:653–660. doi: 10.1016/0042-6989(84)90206-2. [DOI] [PubMed] [Google Scholar]
- 132.Uchikawa K, Ikeda M. Temporal integration of chromatic double pulses for detection of equal-luminance wavelength changes. J Opt Soc Am A. 1986;3:2109–2115. doi: 10.1364/josaa.3.002109. [DOI] [PubMed] [Google Scholar]
- 133.Yoshizawa T, Uchikawa K. Temporal integration characteristics of chromatic response as determined by use of the isoluminant double-pulse method. J Opt Soc Am A. 1997;14:2069–2080. [Google Scholar]
- 134.Shinomori K, Werner JS. Impulse response of an S-cone pathway in the aging visual system. J Opt Soc Am A. 2006;23:1570–1577. doi: 10.1364/josaa.23.001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cottaris NP, De Valois RL. Temporal dynamics of chromatic tuning in macaque primary visual cortex. Nature. 1998;395:896–900. doi: 10.1038/27666. [DOI] [PubMed] [Google Scholar]
- 136.McLellan JS, Eskew RT., Jr ON and OFF S-cone pathways have different long-wave cone inputs. Vis Res. 2000;40:2449–2465. doi: 10.1016/s0042-6989(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 137.Malpeli JG, Schiller PH. Lack of blue OFF-center cells in the visual system of the monkey. Brain Res. 1978;141:385–389. doi: 10.1016/0006-8993(78)90211-1. [DOI] [PubMed] [Google Scholar]
- 138.Krauskopf J. Discrimination and detection of changes in luminance. Vis Res. 1980;20:671–677. doi: 10.1016/0042-6989(80)90091-7. [DOI] [PubMed] [Google Scholar]
- 139.Kremers J, Lee BB, Pokorny J, Smith VC. Responses of macaque ganglion cells and human observers to compound periodic waveforms. Vis Res. 1993;33:1997–2011. doi: 10.1016/0042-6989(93)90023-p. [DOI] [PubMed] [Google Scholar]
- 140.Shinomori K, Spillmann L, Werner JS. S-cone signals to temporal OFF-channels: asymmetrical connections to postreceptoral chromatic mechanisms. Vis Res. 1999;39:39–49. doi: 10.1016/s0042-6989(97)00460-4. [DOI] [PubMed] [Google Scholar]
- 141.Shinomori K, Werner JS. The impulse response of S-cone pathways in detection of increments and decrements. Vis Neurosci. 2008;25:341–347. doi: 10.1017/S0952523808080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shinomori K, Werner JS. Aging of human short-wave cone pathways. Proc Natl Acad Sci USA. 2012;109:13422–13427. doi: 10.1073/pnas.1119770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tailby C, Solomon SG, Lennie P. Functional asymmetries in visual pathways carrying S-cone signals in macaque. J Neurosci. 2008;28:4078–4087. doi: 10.1523/JNEUROSCI.5338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dacey DM, Peterson BB, Robinson FR. Identification of an S-cone opponent OFF pathway in the macaque monkey retina: morphology, physiology and possible circuitry. Invest Ophthalmol Visual Sci Suppl. 2002;43:2983. [Google Scholar]
- 145.Klug K, Herr S, Ngo IT, Sterling P, Schein S. Macaque retina contains an S-cone OFF midget pathway. J Neurosci. 2003;23:9881–9887. doi: 10.1523/JNEUROSCI.23-30-09881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee SCS, Telkes I, Grünert U. S-cones do not contribute to the OFF-midget pathway in the retina of the marmoset, Callithrix jacchus. Eur J Neurosci. 2005;22:437–447. doi: 10.1111/j.1460-9568.2005.04231.x. [DOI] [PubMed] [Google Scholar]
- 147.Szmajda BA, Buzas P, FitzGibbon T, Martin PR. Geniculocortical relay of blue-off signals in the primate visual system. Proc Natl Acad Sci USA. 2006;103:19512–19517. doi: 10.1073/pnas.0606970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mariani AP. Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature. 1984;308:184–186. doi: 10.1038/308184a0. [DOI] [PubMed] [Google Scholar]
- 149.Sher A, DeVries SH. A non-canonical pathway for mammalian blue-green color vision. Nat Neurosci. 2012;15:952–953. doi: 10.1038/nn.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen S, Li W. A color-coding amacrine cell may provide a blue-off signal in a mammalian retina. Nat Neurosci. 2012;15:954–956. doi: 10.1038/nn.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dacey DM, Liao H-W, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 152.Leventhal AG, Yang Y, Pu M, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- 153.McKeefry DJ, Parry NRA, Murray IJ. Simple reaction times in color space: the influence of chromaticity, contrast, and cone opponency. Invest Ophthalmol Vis Sci. 2003;44:2267–2276. doi: 10.1167/iovs.02-0772. [DOI] [PubMed] [Google Scholar]
- 154.Smithson HE, Mollon JD. Is the S-opponent chromatic subsystem sluggish? Vis Res. 2004;44:2919–2929. doi: 10.1016/j.visres.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 155.Wisowaty JJ, Boynton RM. Temporal modulation sensitivity of the blue mechanism: measurements made without chromatic adaptation. Vis Res. 1980;20:895–909. doi: 10.1016/0042-6989(80)90071-1. [DOI] [PubMed] [Google Scholar]
- 156.Kelly DH. Visual responses to time-dependent stimuli. I. Amplitude sensitivity measurements. J Opt Soc Am. 1961;51:422–429. doi: 10.1364/josa.51.000422. [DOI] [PubMed] [Google Scholar]
- 157.Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer’s disease and aging. Ann Neurol. 1993;33:248–257. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- 158.Weale RA. The Aging Eye. Lewis; 1963. [Google Scholar]
- 159.Lindsey DT, Brown AM. Color naming and the phototoxic effects of sunlight on the eye. Psychol Sci. 2002;13:506–512. doi: 10.1111/1467-9280.00489. [DOI] [PubMed] [Google Scholar]
- 160.Wright WD. Talking about color. Color Res Appl. 1988;13:138–139. [Google Scholar]
- 161.Müller GE. Uber die Farbenempfindungen. Zeitschr Psychol Physiol Sinnesorg. 1930;17:1–430. [Google Scholar]
- 162.De Valois RL, De Valois KK. A multi-stage color model. Vis Res. 1993;33:1053–1065. doi: 10.1016/0042-6989(93)90240-w. [DOI] [PubMed] [Google Scholar]
- 163.Kraft JM, Werner JS. Aging and the saturation of colors. 2. Scaling of color appearance. J Opt Soc Am A. 1999;16:231–235. doi: 10.1364/josaa.16.000231. [DOI] [PubMed] [Google Scholar]
- 164.Knau H, Werner JS. Senescent changes in parafoveal color appearance: saturation as a function of stimulus area. J Opt Soc Am A. 2002;19:208–214. doi: 10.1364/josaa.19.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schefrin BE, Werner JS. Loci of spectral unique hues throughout the life span. J Opt Soc Am A. 1990;7:305–311. doi: 10.1364/josaa.7.000305. [DOI] [PubMed] [Google Scholar]
- 166.Kraft JM, Werner JS. Spectral efficiency across the life span: flicker photometry and brightness matching. J Opt Soc Am. 1994;11:1213–1221. doi: 10.1364/josaa.11.001213. [DOI] [PubMed] [Google Scholar]
- 167.MacAdam DL. Uniform color scales. J Opt Soc Am. 1974;64:1691–1702. doi: 10.1364/josa.64.001691. [DOI] [PubMed] [Google Scholar]
- 168.Schefrin BE, Werner JS. Age-related changes in the color appearance of broadband surfaces. Color Res Appl. 1993;18:380–389. [Google Scholar]
- 169.Gordon J, Abramov I. Scaling procedures for specifying color appearance. Color Res Appl. 1988;13:146–152. [Google Scholar]
- 170.Okajima K, Tsuchiya N, Yamashita K. Age-related changes in color appearance depend on unique-hue components. Proc SPIE. 2002;4421:259–262. [Google Scholar]
- 171.Hardy JL, Frederick CM, Kay P, Werner JS. Color naming, lens aging, and grue: what the optics of the aging eye can teach us about color language. Psychol Sci. 2005;16:321–327. doi: 10.1111/j.0956-7976.2005.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Berlin B, Kay P. Basic Color Terms: Their Universality and Evolution. University of California; 1969. [Google Scholar]
- 173.Judd DB. Response functions for types of vision according to the Müller theory. J Res Natl Bur Stan. 1949;42:1. doi: 10.6028/jres.042.001. [DOI] [PubMed] [Google Scholar]
- 174.Jameson D, Hurvich LM. Use of spectral hue-invariant loci for the specification of white stimuli. J Exp Psychol. 1951;41:455–463. doi: 10.1037/h0063029. [DOI] [PubMed] [Google Scholar]
- 175.Walraven J, Werner JS. The invariance of unique white: a possible implication for normalizing cone action spectra. Vis Res. 1991;31:2185–2193. doi: 10.1016/0042-6989(91)90171-z. [DOI] [PubMed] [Google Scholar]
- 176.Krauskopf J, Gegenfurtner K. Color discrimination and adaptation. Vis Res. 1992;32:2165–2175. doi: 10.1016/0042-6989(92)90077-v. [DOI] [PubMed] [Google Scholar]
- 177.MacLeod DIA. Colour discrimination, colour constancy and natural scene statistics. In: Mollon JD, Pokorny J, Knoblauch K, editors. Normal and Defective Colour Vision. Oxford University; 2003. pp. 189–217. [Google Scholar]
- 178.Danilova MV, Mollon JD. Parafoveal color discrimination: a chromaticity locus of enhanced discrimination. J Vis. 2010;10(1):4, 1–9. doi: 10.1167/10.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Werner JS, Schefrin BE. Loci of achromatic points throughout the life span. J Opt Soc Am A. 1993;10:1509–1516. doi: 10.1364/josaa.10.001509. [DOI] [PubMed] [Google Scholar]
- 180.Vos JJ. Colorimetric and photometric properties of a 2° fundamental observer. Color Res Appl. 1978;3:125–128. [Google Scholar]
- 181.Judd DB, MacAdam DL, Wyszecki G, Budde HW, Condit HR, Henderson ST, Simonds JL. Spectral distribution of typical daylight as a function of correlated color temperature. J Opt Soc Am. 1964;54:1031–1040. [Google Scholar]
- 182.Mollon JD. Monge. Vis Neuro. 2006;23:297–309. doi: 10.1017/S0952523806233479. [DOI] [PubMed] [Google Scholar]
- 183.Macleod DIA, Von Der Twer T. The Pleistochrome: optimal opponent codes for natural colours. In: Mausfeld RA, Heyer D, editors. Colour Perception: Mind and the Physical World. Oxford University; 2003. pp. 155–185. [Google Scholar]
- 184.Worthey JA, Brill MH. Heuristic analysis of von Kries color constancy. J Opt Soc Am A. 1986;3:1708–1712. doi: 10.1364/josaa.3.001708. [DOI] [PubMed] [Google Scholar]
- 185.Mizokami Y, Werner JS, Crognale MA, Webster MA. Nonlinearities in color coding: compensating color appearance for the eye’s spectral sensitivity. J Vis. 2006;6(9):996–1007. doi: 10.1167/6.9.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Priest IG. The spectral distribution of energy required to evoke the gray sensation. Sci Pap US Bur Stand. 1921;17:231–265. [Google Scholar]
- 187.Hurvich LM, Jameson D. A psychophysical study of white. I. Neutral adaptation. J Opt Soc Am. 1951;41:521–527. doi: 10.1364/josa.41.000521. [DOI] [PubMed] [Google Scholar]
- 188.Honjyo K, Monaka M. Perception of white in a 10° field. J Opt Soc Am. 1970;60:1690–1694. doi: 10.1364/josa.60.001690. [DOI] [PubMed] [Google Scholar]
- 189.von Kries J. Influence of adaptation on the effects produced by luminous stimuli. Handbuch der Physiologie des Menschen (1905) 3:109–282. [Reprinted in Sources of Color Science, MacAdam D. L., ed./trans. (MIT, 1970), 120–126] [Google Scholar]
- 190.Pokorny J, Smith VC. Evaluation of single-pigment shift model of anomalous trichromacy. J Opt Soc Am. 1977;67:1196–1209. doi: 10.1364/josa.67.001196. [DOI] [PubMed] [Google Scholar]
- 191.Werner JS, Walraven J. Effect of chromatic adaptation on the achromatic locus: the role of contrast, luminance and background color. Vis Res. 1982;22:929–943. doi: 10.1016/0042-6989(82)90029-3. [DOI] [PubMed] [Google Scholar]
- 192.Brainard DH, Wandell BA. Asymmetric color matching: how color appearance depends on the illuminant. J Opt Soc Am A. 1992;9:1433–1448. doi: 10.1364/josaa.9.001433. [DOI] [PubMed] [Google Scholar]
- 193.Renner AB, Knau H, Neitz M, Neitz J, Werner JS. Photopigment optical density of the human foveola and a paradoxical senescent increase outside the fovea. Vis Neurosci. 2004;21:827–834. doi: 10.1017/S0952523804216030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Solomon SG. Chromatic organization of ganglion cell receptive fields in the peripheral retina. J Neurosci. 2005;25:4527–4539. doi: 10.1523/JNEUROSCI.3921-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Webster MA, Leonard D. Adaptation and perceptual norms in color vision. J Opt Soc Am A. 2008;25:2817–2825. doi: 10.1364/josaa.25.002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Webster MA, Halen K, Meyers AJ, Winkler P, Werner JS. Colour appearance and compensation in the near periphery. Proc R Soc London Ser B. 2010;277:1817–1825. doi: 10.1098/rspb.2009.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Delahunt PB, Webster MA, Ma L, Werner JS. Long-term renormalization of chromatic mechanisms following cataract surgery. Vis Neurosci. 2004;21:301–307. doi: 10.1017/S0952523804213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Webster MA, Mollon JD. The influence of contrast adaptation on color appearance. Vis Res. 1994;34:1993–2020. doi: 10.1016/0042-6989(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 199.Arend L, Reeves A. Simultaneous color constancy. J Opt Soc Am A. 1986;3:1743–1751. doi: 10.1364/josaa.3.001743. [DOI] [PubMed] [Google Scholar]
- 200.Rinner O, Gegenfurtner KR. Time course of chromatic adaptation for color appearance and discrimination. Vis Res. 2000;40:1813–1826. doi: 10.1016/s0042-6989(00)00050-x. [DOI] [PubMed] [Google Scholar]
- 201.Mollon JD, Reffin JP. Handbook of the Cambridge Colour Test. 2000 https://sites.oxy.edu/clint/physio/article/CAMBRIDGECOLOURTESTHandbook.pdf.
- 202.Webster MA, Mollon JD. Colour constancy influenced by contrast adaptation. Nature. 1995;373:694–698. doi: 10.1038/373694a0. [DOI] [PubMed] [Google Scholar]
- 203.Elliott SL, Werner JS, Webster MA. Individual and age-related variation in chromatic contrast adaptation. J Vis. 2012;12(8):11. doi: 10.1167/12.8.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Buchsbaum G, Gottschalk A. Trichromacy, opponent colours coding and optimum colour information transmission in the retina. Proc R Soc London Ser B. 1983;220:89–113. doi: 10.1098/rspb.1983.0090. [DOI] [PubMed] [Google Scholar]
- 205.Brown RO. The world is not grey. Invest Ophthalmol Visual Sci Suppl. 1994;35 [Google Scholar]
- 206.Ruderman DL, Cronin TW, Chiao C-C. Statistics of cone responses to natural images: implications for visual coding. J Opt Soc Am A. 1998;15:2036–2045. [Google Scholar]
- 207.Werner JS. Aging through the eyes of Monet. In: Backhaus WG, Kliegl R, Werner JS, editors. Color Vision: Perspectives From Different Disciplines. De Gruyter; 1998. pp. 3–42. [Google Scholar]


