Abstract
There is a need for improved treatment of acute vascular inflammation in conditions such as ischemia-reperfusion injury, acute lung injury, sepsis, and stroke. The vascular endothelium represents an important therapeutic target in these conditions. Furthermore, some anti-inflammatory agents (AIAs) (e.g., biotherapeutics) require precise delivery into subcellular compartments. In theory, optimized delivery to the desired site of action may improve the effects and enable new mechanisms of action of these AIAs. Diverse nanocarriers (NCs) and strategies for targeting them to endothelial cells have been designed and explored for this purpose. Studies in animal models suggest that delivery of AIAs using NCs may provide potent and specific molecular interventions in inflammatory pathways. However, the industrial development and clinical translation of complex NC-AIA formulations are challenging. Rigorous analysis of therapeutic/side effect and benefit/cost ratios is necessary to identify and optimize the approaches that may find clinical utility in the management of acute inflammation.
Keywords: vascular immunotargeting, intracellular delivery, liposomes, polymeric nanocarriers, endothelium, cell adhesion molecules
INTRODUCTION
Inflammation is a key contributor to numerous health maladies, including acute and dangerous cardiovascular, pulmonary, and cerebrovascular conditions such as myocardial infarction, acute lung injury, and stroke. Pharmacotherapy of inflammation is a globally important and challenging goal. Since the discovery of acetylsalicylic acid, a long roster of anti-inflammatory agents (AIAs) has been discovered, developed, and approved for clinical use. These include both established drugs, such as steroids and nonsteroidal anti-inflammatory drugs (NSAIDs), as well as newer agents, such as nucleic acids and proteins. These biotherapeutics offer efficacy, potency, and specificity that may exceed those of small molecules. Yet, such novel therapeutics can be costly, can be labile, can have unfavorable pharmacokinetics (PK), and may require delivery to specific compartments in the cells they target.
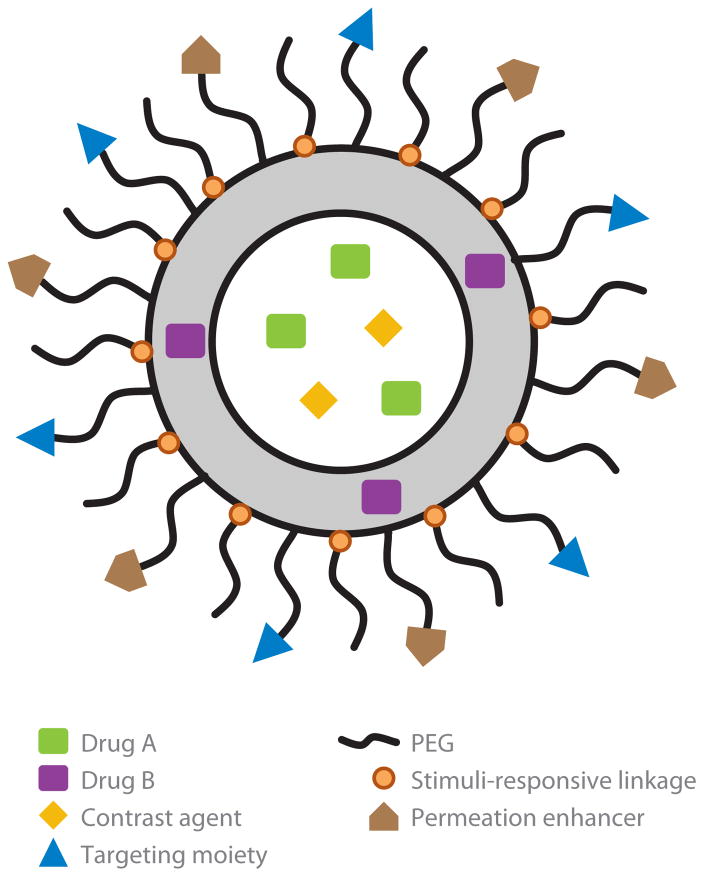
The use of nanocarriers (NCs) may help overcome some of these challenges. A prototypical bare-bones NC is a vesicle or solid core delivery unit that improves the PK of loaded cargo and limits its interactions with the body en route to the therapeutic target. Advanced multifunctional NCs contain additional elements designed to provide targeting, tracing, local release of drug, and/or enhanced intracellular delivery (Figure 1). Overall, the goal of using NCs is to enhance the benefit/risk ratio and enable novel therapeutic mechanisms via optimizing the localization and timing of the drug action at the desired site in the body (1).
Figure 1.
Schematic outline of a prototypic multifunctional nanocarrier (NC). Multifunctional NCs for vascular drug delivery usually vary in size from 30 to 300 nm and may consist of either a solid core or vesicle (depicted here) unit. On the basis of their hydrophobicity, drugs and tracing agents (optical and isotope probes) can be included in either the inner liquid phase or outer structural matrix (lipids, polymers, or biomaterials). Poly(ethylene glycol) (PEG) chains conjugated to the carrier’s surface provide stealth features and additional steric freedom for targeted moieties (e.g., antibodies or peptides with affinity to target determinants) and, if intracellular delivery is needed, peptides facilitating transfer via the cell membranes. High PEG density is preferable en route but may impede cellular uptake of carriers anchoring on the target cell. Removal of excess PEG chains from carriers at the target site is accomplished when the chains are conjugated to the carrier via moieties sensitive to the target microenvironment. Examples of these triggers include specific proteases or the acidic pH found at sites of ischemia, at sites of inflammation, and within endocytic vesicles. “Stimuli-responsive” linkages and other carrier-transforming moieties responsive to such microenvironmental triggers help control local drug release and cytosolic delivery.
AIAs that require significantly improved delivery—e.g., biological agents, steroids, and antioxidants—represent preferable NC cargoes (Table 1). For reasons discussed below, the most realistically anticipated utility of NCs is associated with the transient treatment of serious acute conditions. Owing to their size (up to hundreds of nanometers) and to the need for expediency in these conditions, vascular injection is the most suitable route for NC administration. The vascular route also offers direct access to endothelial cells. These cells regulate key functions in the vasculature including blood fluidity, vascular tone and permeability, and leukocyte recruitment and trafficking. All these functions are involved in and/or affected by inflammation. Therefore, endothelial cells represent an important target for anti-inflammatory interventions.
Table 1.
Anti-inflammatory agents (AIAs): preferable cargoes for delivery by nanocarriers
| AIA class | Mechanism(s) of action | Required localization | Limitations | Examples |
|---|---|---|---|---|
| GCs | Transcriptional regulation of target genes through ligation of the steroid receptor Inhibition of synthesis of lipid mediators |
Cytosol Nucleus |
Systemic side effects (hypertension, hyperglycemia, osteoporosis, adrenal insufficiency, cataract, susceptibility to infection) | Dexamethasone Prednisone Prednisolone Betamethasone |
| Antioxidants | Protection from ROS released by phagocytes Inhibition of inflammatory signaling by intracellular ROS |
Extracellular space, plasma Endosomes, cytosol, mitochondria |
AOEs: costly, labile, insufficient PK, immunogenicity | Nonenzymatic: NAC, GSH, vitamin E Enzymatic (AOE): SOD, catalase |
| NO donors | Inhibition of:
|
Plasma Cytosol |
Hypotension, syncope, nitrate headache | NONOates NO/prednisolone NO/salicylic acid |
| siRNAs | Temporal silencing of proinflammatory protein synthesis | Cytosol | Labile, insufficient PK, immunogenicity | siRNA for: FCγIII/CD16 COX-2 NOV/CCN3 ICAM-1 |
| NSAIDs | COX inhibition | Cytosol | Gastrointestinal complications with long-term administration | Aspirin Indomethacin Ibuprofen |
Spectrum of AIAs explored for nanocarrier delivery in animal models of acute inflammatory pathological conditions. Abbreviations: AOE, antioxidant enzyme; GC, glucocorticoid; GSH, glutathione; ICAM, intercellular adhesion molecule; NAC, N-acetylcysteine; NO, nitric oxide; NONOate, diazeniumdiolate; NSAID, nonsteroidal anti-inflammatory drug; PK, pharmacokinetics; ROS, reactive oxygen species; siRNA, small interfering RNA; SOD, superoxide dismutase.
NANOCARRIERS FOR VASCULAR DELIVERY OF ANTI-INFLAMMATORY AGENTS
The list of NCs employed to date for vascular delivery of AIAs consists of several main types such as antibody- and polymer-drug conjugates; solid lipid, magnetic, and polymeric nanoparticles; liposomes; and dendrimers (Figure 2). Within each platform, specific examples feature diverse geometry, functional moieties, sizes, charges, stability, and kinetics of drug release. Nevertheless, to avoid retention in the microvasculature, the diameter of spherical carriers for vascular delivery is generally <300 nm, and the surface charge is close to neutral.
Figure 2.
Nanocarriers (NCs) for vascular delivery of anti-inflammatory agents (AIAs). Schematics of the common classes of NCs used for AIA delivery along with structural information and AIA cargoes loaded to date. Abbreviations: AOE, antioxidant enzyme; GC, glucocorticoid; NO, nitric oxide; NSAID, nonsteroidal anti-inflammatory drug; PEG, poly(ethylene glycol); siRNA, small interfering RNA.
On one hand, conjugation of drugs to antibodies or other proteins enables targeted delivery and controlled cellular uptake (2). On the other hand, conjugation to hydrophilic polymers, such as poly(ethylene glycol) (PEG), can limit renal filtration, cellular uptake, proteolytic degradation, and immunogenicity, thereby providing the PEGylated molecules and particles with an ability to elude these elimination mechanisms (“stealth” technology) (3). However, the uniformity of chemically produced protein conjugates may be suboptimal for safe, widespread clinical use. Inactivation of labile biotherapeutics during conjugation, in circulation, and in the target cells represents an additional challenge.
Liposomes are phospholipid bilayer vesicles surrounding an aqueous space (4, 5). Drugs can be entrapped in this inner space, conjugated to the surface, or intercalated in the bilayer. Inclusion of PEGylated phospholipids inhibits the destabilizing interaction of liposomes with plasma and cells, thereby prolonging circulation and reducing premature drug leakage and side effects (6). This “stealth” effect is generally proportional to the PEG surface density, although the amount of PEG that can be added to the liposomes is limited (7). Liposomes are used clinically to reduce the toxicity of chemotherapeutic agents (8) and are now being explored for the delivery of diverse AIAs, including steroids, antioxidants, and small interfering RNA (siRNA).
Solid lipid nanoparticles (SLNs) contain a lipid core that is stabilized by emulsifiers and, if needed, decorated by PEG. When prepared with physiological lipids and FDA-approved emulsifiers, SLNs are biocompatible. Their properties (size, drug loading and release, stability) are tightly controlled by their composition (9). For instance, SLNs based on highly crystalline lipids may show reduced drug loading but also a more sustained drug release. Depending on the cargo properties and medical goal, using a combination of lipids or even introducing a liquid lipid (to make what are known as nanostructured lipid carriers) may be desirable (10).
Magnetic nanoparticles are solid particles or polymorphous complexes formed using diverse materials (e.g., polymers, proteins, PEG-containing stabilizers, and stealth moieties) and methods (e.g., controlled precipitation of drugs in multiphase suspensions). Inclusion of magnetically responsive metal nanoparticles (generally, iron grain that is 5–10 nm in diameter) endows formed NCs with the ability to accumulate in certain areas when an external magnetic field or magnetized stent is present (11).
Polymer-based NCs include dendrimers, polymeric micelles and nanoparticles, and filomicelles. Their structural materials include both synthetic polymers [e.g., poly(lactic-co-glycolic acid), PEG] and biological polymers [e.g., chitosan, poly(nucleic acids)] as well as blends of different polymers conjugated into diblocks, triblocks, and higher-order copolymers. Such versatility helps individualize the NC properties and may even allow for the design of NCs with several compartments, multiple layers, or controlled surface heterogeneity (12–15).
Dendrimers, which are repetitively branched multimolecular spherical complexes in the 10–100-nm range and are characterized by a high level of precision in synthesis and structure, have been adopted and tested for AIAs (16). Although drugs can be coupled to the active end groups or encapsulated into the meshwork, the loading capacity is generally lower than for other NCs.
Polymeric micelles and nanoparticles are typically based on amphiphilic block copolymers, which in solution form a distinct structure: The hydrophobic and hydrophilic portions of the polymer form the core and the corona, respectively (17). Technically, micelles have “dynamic” cores, meaning that there is an exchange of polymers among the various aggregates, whereas nanoparticles have solid or “frozen” cores (18). Yet, because the core state may change depending on the conditions (e.g., temperature, pH, solution), this distinction is more conceptual than practical. Owing to higher material stability and stealth features (polymeric particles can be virtually 100% PEGylated), these carriers may exhibit longer PK and residence time in tissues than do lipid-based carriers. These materials degrade via hydrolysis, the rate and mechanism (e.g., surface erosion versus bulk dissolution) of which depend on the carrier size, content, structure, and environment. Challenges in using these carriers include inactivation of labile AIAs loaded into the polymeric core and uncoupled kinetics of drug release and carrier degradation (the former is usually faster).
Recent studies have revealed that vascular delivery and cellular uptake are modulated by hydrodynamic factors and carrier geometry. Nonspherical carriers that align with blood flow display prolonged circulation in the bloodstream. For example, filomicelles are a distinctive subset of polymeric micelles that assemble in dynamic, flow-responsive filamentous morphologies (19, 20). Their ability to align with blood flow minimizes their interactions with cells and prolongs their circulation lifetime for several days—up to 10 times longer than their spherical counterparts (19). Nonspherical flexible polymeric carriers that imitate erythrocytes and platelets also display prolonged circulation but have not been evaluated for AIA delivery (21). Uptake of nonspherical NCs depends on the angle of anchoring on the plasmalemma (22).
Each NC has advantages and/or disadvantages with respect to AIA delivery in a given situation. In theory, a long-circulating nonspherical polymeric carrier that has a sustained circulation and drug release profile may find utility in subacute conditions, whereas a short-lived carrier may be preferable in acute settings. Furthermore, NCs provide a platform that enables targeted delivery of AIAs to selected compartments.
TARGETED DELIVERY OF ANTI-INFLAMMATORY AGENTS
Conjugation of affinity ligands that bind to molecules exposed in pathological sites to a NC provides a mechanism to concentrate drugs in these sites and optimize cellular delivery. Cells and tissue components that play a key role in inflammation and are accessible to submicrometer particles circulating in the blood represent preferable targets for AIA delivery by NCs. For example, AIA delivery to macrophages in the mononuclear phagocyte system may inhibit their inflammatory activities (23, 24). Macrophages naturally take up foreign particles from the blood, favoring delivery of drugs encapsulated in particles to these cells; the majority of injected NCs of any type (including stealth NCs) are taken up by the mononuclear phagocytes.
Endothelial cells are another important target for AIA delivery. First, they play key functions in inflammation, including the recruitment of leukocytes to the inflamed tissue (25) (Figure 3). Cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) act on endothelial cells to expose adhesion molecules and mediators for increased perfusion and permeability of the vascular barrier (26). Second, injurious inflammatory factors, such as reactive oxygen species (ROS), damage the endothelium. This damage compromises vitally important functions of this tissue, including control of vascular tone, permeability, and blood fluidity. Ensuing endothelial dysfunction and damage further propagate the cycle of tissue injury, thrombosis, and ischemia.
Figure 3.
Vascular oxidative stress and inflammation. Vascular inflammation and oxidative stress are intertwined, mutually propagating processes implicated in the pathogenesis and clinical manifestations of pneumonia, hyperoxia, acute lung injury (ALI), pulmonary inflammation, and ischemia-reperfusion injury. These conditions are difficult to treat (e.g., ALI has no effective treatment) and gravely contribute to human morbidity and mortality. ROS (e.g., O2− and H2O2) are implicated as both injurious agents and signaling mediators in these processes. Activated leukocytes and macrophages release ROS, causing tissue damage. Primary insults and cytokines (e.g., TNF, IL) cause endothelial exposure of CAMs, facilitating WBC adhesion and transmigration. These agonists also activate endothelial enzymes (including NOX) to produce ROS, which quench NO, mediate inflammatory signaling, and cause oxidative stress. Endothelial ROS produced in response to pathological factors cause abnormal endothelial activation manifested by, among other signs, proinflammatory changes; loss of thrombomodulin, which aggravates thrombosis and inflammation; and endothelial barrier disruption, which leads to vascular leakage and edema. Activated WBCs bind to endothelium via CAMs and release ROS and other molecules that damage endothelial cells, thereby propagating the vicious cycle of inflammation, oxidative stress, thrombosis, and ischemia. Reprinted from Reference 25 with permission. Abbreviations: CAM, cell adhesion molecule; ICAM, intercellular adhesion molecule; IL, interleukin; NO, nitric oxide; PMN, polymorphonuclear neutrophil; ROS, reactive oxygen species; TM, thrombomodulin; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule; WBC, white blood cell.
Endothelial delivery of AIAs may help alleviate this vicious cycle. In contrast to phagocytes, endothelial cells normally do not bind nanoparticles, despite direct access to the blood. Molecules that bind to endothelial determinants—natural ligands, as well as the common integrin-binding peptide sequence RGD and other peptides, antibodies, and their fragments—serve as affinity moieties for targeting. Some endothelial determinants cannot be used for AIA delivery, as interference with their functions may aggravate pathology. Selection of ligands and epitope(s) dictates targeting efficacy, selectivity, intracellular trafficking, and effects of the NCs (Table 2) (27).
Table 2.
Endothelial targets for delivery of anti-inflammatory agents (AIAs) using affinity nanocarriers
| Target molecule | Advantages | Challenges and limitations | Potential utility validated in animal studies |
|---|---|---|---|
| ACE | ACE is constitutively expressed by endothelium with variable density throughout the vessels Enriched expression and AIA delivery is seen in the pulmonary vasculature ACE is constitutively internalized, promoting a pathway for intracellular delivery Anti-inflammatory and hypotensive effects of ACE inhibition by targeted NC may be beneficial in some cases |
ACE inhibition may be dangerous in hypotensive patients ACE inhibition may aggravate effects of bradykinin ACE level is suppressed in pathologically altered endothelium Mechanisms of the uptake and intracellular trafficking of anti-ACE/NC are not well understood |
Delivery of AIAs to the pulmonary and potentially other areas can help manage acute oxidative stress and inflammation Viral gene therapy can be retargeted to express therapeutic transgenes in the pulmonary vasculature |
| PECAM | PECAM has stable expression at high levels, promoting effective delivery of large doses of drugs PECAM allows both surface anchoring and intracellular uptake of NCs Cargoes internalized via PECAM and ICAM slowly traffic to lysosomes (within hours) PECAM bound NCs inhibit WBC adhesion |
PECAM is a normal pan-endothelial marker, and drugs are likely to be delivered to all endothelial cells in the vasculature; PECAM cannot be used for delivery of agents toxic to or pathologically activating endothelium | AIAs can be delivered prophylactically to endothelium in an organ downstream of injection site, e.g., for preconditioning to subsequent I/R AIAs can be delivered therapeutically to endothelium throughout the vasculature in systemic inflammation (e.g., sepsis, multiorgan failure) |
| ICAM | ICAM has low basal-level expression that is upregulated in inflammation ICAM allows both surface anchoring and intracellular uptake of NCs Cargoes internalized via PECAM and ICAM slowly traffic to lysosomes (within hours) ICAM promotes enriched anchoring of AIAs in inflamed EC Bound NC inhibits WBC adhesion |
ICAM has limitations similar to those of PECAM | ICAM has utilities similar to PECAM, and in addition can be used for detection of inflammation sites and selective delivery of AIA to these sites |
| αVβ3 integrin | α Vβ3 integrin is expressed on the luminal side of endothelial cells in the sites of angiogenesis including tumors, wound healing, and inflammation α Vβ3 integrin offers a multifaceted delivery system to diverse, pathologically altered vascular areas |
There are potential off-target effects in areas of physiological angiogenesis Peptide ligands such as RGD and other cationic peptides also have other targets in the vasculature |
Long-term anti-inflammatory interventions are possible because the target is typical of chronic or subacute conditions (e.g., arthritis, tumors) |
| E-selectin and VCAM | Within hours of insult, E-selectin and VCAM are synthesized and exposed selectively in pathological endothelium including inflammation sites They are constitutively internalized, promoting a pathway for intracellular delivery |
Transient target exposure at relatively low density limits efficacy Internalized materials are rapidly (within minutes) trafficked to and degraded in lysosomes |
E-selectin and VCAM allow selective detection of endothelial pathology Selective AIAs can be targeted to sites of vascular inflammation |
| P-selectin | Within minutes of insult, P-selectin is exposed from intracellular stores by activated endothelium and platelets | Transient target exposure at relatively low density limits efficacy P-selectin is less specific for endothelium when compared with VCAM-1 and E-selectin (platelet binding) |
Vascular pathology and thrombosis can be detected Drugs can be targeted to sites of vascular inflammation |
| APP | APP is an enzyme constitutively expressed in the endothelial caveolae APP is differentially expressed through the vasculature, allowing preferential targeting to the pulmonary and coronary capillaries APP ligands rapidly enter caveolae and transfer across endothelium |
Size of a NC for caveolae is limited to <40 nm Unintentional inhibition of APP may lead to elevation of bradykinin |
Drugs can be targeted across the vascular barrier in selected vascular areas |
Abbreviations: ACE, angiotensin-converting enzyme; APP, aminopeptidase P; E-selectin, endothelial selectin; EC, endothelial cell; I/R, ischemia-reperfusion injury; ICAM, intercellular adhesion molecule; NC, nanocarrier; PECAM, platelet endothelial cell adhesion molecule; P-selectin, platelet selectin; VCAM, vascular cell adhesion molecule; WBC, white blood cell.
Constitutive determinants, such as platelet endothelial cell adhesion molecule 1 (PECAM-1) and angiotensin-converting enzyme (ACE), transmembrane glycoproteins normally anchored in the luminal plasmalemma of endothelial cells, are good targets for prophylactic delivery of AIAs to the vascular areas predisposed to inflammation. For example, systemic injection of NCs targeted to these determinants boosts antioxidant effects in the pulmonary vasculature, which may be helpful in acute settings, including iatrogenic ischemia (e.g., cardiopulmonary bypass or organ transplantation) and acute lung injury (ALI) (28). Arterial infusion provides targeting to the vascular bed downstream of the injection site—to cerebral, coronary, and mesenterial areas, for example (29, 30).
Pathologically activated endothelial cells expose epitopes for preferential delivery to inflamed vasculature. For example, endothelial cells involved in active angiogenesis typical of tumors, chronic inflammation, and ischemia expose elevated levels of integrins that can be recognized by affinity peptides and antibodies (31). Inflamed endothelium exposes abnormally high levels of intercellular adhesion molecule 1 (ICAM-1), a target that is also widely explored for both prophylactic and therapeutic drug delivery (32). Furthermore, endothelial selectin (E-selectin), platelet selectin (P-selectin), and vascular cell adhesion molecule 1 (VCAM-1), which are normally absent in the lumen, represent interesting targets for detection of inflamed sites (33, 34).
Parameters of hemodynamics and binding govern endothelial targeting of NCs. The effect of flow rate (i.e., shear stress) and character (e.g., laminar versus turbulent) on endothelial targeting of NCs has so far been addressed mostly in cell culture models (35). These studies revealed that targeting is inhibited at shear stress higher than the empiric optimal level. The effects of binding parameters, i.e., strength and valence of interaction with target determinants, have received more attention. Results of studies in vitro need to be validated in vivo. Factors that control binding of the NC to the cells include ligand affinity, surface density, spatial organization, and orientation of ligand molecules on the carrier surface, as well as surface density, accessibility, and spatial organization of determinants on the target.
Generally, enhancing ligand density boosts targeting (36). However, an excessive ligand density may be problematic (1). Ligand density on stealth NCs should be minimized in order to avoid interfering with the effects of PEG (37). In many cases, only empirical adjustments yield information on the ligand surface density that affords optimal intermolecular congruency with target determinants (38). Reduction of ligand density on NCs also increases the selectivity of targeting to the inflamed endothelium via suppression of basal binding to quiescent endothelial cells that express a lower level of the target determinant, ICAM-1 (39).
NC size and geometry also modulate targeting. Researchers have shown this effect in animal models by measuring pulmonary accumulation of targeted versus nontargeted carriers. The ratio of the tissue uptake of these two types of NCs, which characterizes the specificity of targeting, increased with enlargement of carriers from diameters of <50 nm to diameters of ~300 nm, likely owing to higher avidity. However, exceeding this optimal size led to a sharp increase in nonspecific uptake, presumably due to entrapment in small vessels (40). Nonspherical carriers, disks, and filomicelles have a higher specificity of ICAM-1-directed endothelial targeting in mice compared with their spherical counterparts (41, 42).
Binding to specific epitopes mediates intracellular delivery. Endothelial cells exhibit numerous vesicular uptake mechanisms, including clathrin-mediated, caveolae-mediated, and noncanonical endocytosis, as well as pinocytosis, providing distinct intracellular trafficking and destinations for internalized ligands. The conventional wisdom is that the binding of NCs to determinants constitutively involved in a given endocytic pathway follows this entry mechanism. Thus, NCs targeted to E-selectin and VCAM-1, cell adhesion molecules internalized via clathrin endocytosis, are internalized via this pathway (33). However, ligands coupled to NCs do not necessarily follow the fate of “free” ligands. For example, antibodies to caveolar aminopeptidase P (APP) rapidly enter and cross endothelium, but NCs coated with APP antibodies do not enter cells because the size of the caveolar aperture is too small to accommodate particles larger than ~50 nm in diameter (43). In contrast, endothelial cells do not internalize antibodies to cell adhesion molecules PECAM-1 and ICAM-1, yet they internalize NCs coated with these antibodies in vitro and in vivo (44). This vesicular mechanism is distinct from known pathways and results in relatively slow trafficking from endosomes to lysosomes, allowing time for the protective effects of AIAs (45).
Similar to binding, internalization is also modulated by carrier geometry, selection of epitopes on the target determinant, hydrodynamic conditions, and the functional status of endothelial cells. For example, ICAM-1-targeted disks enter endothelial cells more slowly than do spherical carriers (42). Whereas spherical NCs directed to certain PECAM-1 epitopes do not enter the endothelium, binding to adjacent epitopes results in rapid uptake and lysosomal delivery (46). Uptake of ICAM-targeted NCs is upregulated in cytokine-activated endothelium in comparison with that in the quiescent cells (47). Finally, exposure to chronic flow leading to reorganization of the cytoskeleton decelerates endocytosis of NCs targeted to ICAM-1 and PECAM-1 (47, 48). Results obtained in cell culture correlated well with in vivo results showing that targeted NCs were internalized significantly better in capillaries than in arterioles (48). However, exposure to acute shear stress (which occurs during reperfusion of ischemic tissues) accelerates endocytosis of PECAM-targeted NCs (48).
In summary, anchoring NCs to endothelial determinants provides a wide range of targeting scenarios, which can be further modulated by elements of NC design, endothelial status, and the microenvironment. These factors play an important role in devising NCs for vascular delivery of AIAs, especially those requiring intracellular delivery.
NANOCARRIER-MEDIATED DELIVERY OF ANTI-INFLAMMATORY AGENTS
Below we briefly review examples of the vascular delivery of AIAs by NCs. We focus on the types of AIAs whose effects either require such a delivery vehicle or can be drastically improved by the use of one. These studies are in the early development phase, with promising prototypes transitioning from in vitro proof of concept to animal studies on the PK, delivery, and effects of the interventions.
Glucocorticoids
Glucocorticoids (GCs) have serious side effects: hypertension, hyperglycemia, osteoporosis, adrenocortical suppression, cataract, and susceptibility to infection. Delivery of small targeted doses using NCs may reduce their systemic exposure (49). Dexamethasone (Dex) is one of the most extensively studied AIAs in this context: It is an inexpensive, readily available drug that can be formulated in its native form, in water-soluble phosphate form, or in lipid-soluble palmitate form. NCs have also been explored for the delivery of other steroids that are more hydrophobic and in need of assisted delivery.
Dex-liposomes, reported as early as the late 1980s (50), have been evaluated in numerous acute and chronic conditions. In models of ALI, pretreatment of animals with liposomal Dex inhibited granulocyte influx and inflammatory mediator expression as well as (51) or even better than (52) treatment with the free drug. In a model of arthritis, a single administration of 4 mg/kg liposomal Dex provided the same clinical benefit as did daily administrations of 1.6 mg/kg free drug for a week (53). With the reduction of dose, drug-induced side effects were also reduced (53).
Steroids have been delivered to the endothelium using targeted liposomes. Dexliposomes conjugated with RGD peptide accumulated in lipopolysaccharide-induced inflammatory sites in rats and provided protective effects superior to those of control Dex-liposomes in a rat adjuvant-induced arthritis model (54). Targeting to E-selectin improved delivery of Dex-liposomes to activated dermal and renal endothelium in animal models of inflammation of skin (55) and kidneys (34). In the latter model, E-selectin-targeted Dex-liposomes reduced glomerular expression of proinflammatory genes and proteins and alleviated renal injury without affecting blood glucose level (34).
Liposomal betamethasone hemisuccinate and methylprednisolone hemisuccinate alleviated the severity of adjuvant arthritis in rats (56). Joint inflammation (even provoked acutely in animal models by adjuvant or antibodies) is a more chronic condition than ischemia-reperfusion injury or ALI. Therefore, alternative NCs more stable than liposomes have been used for sustained delivery of steroids. These include Dex-loaded SLNs, polymeric nanoparticles [monomethoxy poly(ethylene glycol)-block-poly(trimethylene carbonate), poly(glycerol adipate)], and dendrimers (57–60). A single dose of betamethasone phosphate loaded into PEG-poly(lactic acid)/poly(lactic-co-glycolic acid) nanoparticles attenuated the inflammatory response in an arthritic rat model for one week (61, 62).
To induce local drug release at the acidic pH typical of inflamed sites, Dex was conjugated via degradable linkers to an HPMA [N-(2-hydroxypropyl)methacrylamide] copolymer at either its ketone (63) or its hydroxyl moieties (64); alternatively, it was conjugated to PEG-poly(aspartate) micelles via a hydrazone-ester linker (65). Through the use of SLNs loaded with the ester-containing Dex-palmitate, Dex could also be released by carboxylesterases present in the inflamed tissues (66).
Currently, steroidal AIAs are used mainly as a transient bridging therapy for the acute phase of chronic conditions such as rheumatoid arthritis. No decisive therapeutic benefits in the management of ALI and other types of acute severe inflammation have been observed. However, as these glucocorticoid AIAs have a complex mechanism of action involving interaction with diverse targets in both the cytosol and nucleus, improving their delivery to the intracellular compartments of key target cells may lead to more potent and specific effects.
Nonsteroidal Anti-Inflammatory Drugs
The potential benefits of using NCs for the delivery of NSAIDs—AIAs that are more soluble, more cell permeable, and more benign than GCs—are less certain (49). However, some of the more potent, and toxic, NSAIDs (in particular, indomethacin) have been loaded into NCs. For example, loading indomethacin into dendrimers targeted to the folate receptor on activated macrophages provided a delivery to the arthritic sites that was four times greater than the delivery of free drug (67). Indomethacin-loaded polymeric nanocapsules decreased cardiac, renal, and gastrointestinal toxicity versus treatment with a free drug in models of subacute and chronic inflammation (68). These particles also provided a protective effect against cell damage and neuroinflammation induced by amyloid-beta 1–42 in an Alzheimer’s disease model (69). Other examples of NSAID-loaded NCs include piroxicam dendrimers, ketoprofen SLNs, and meloxicam polymeric nanocapsules (9, 70, 71). It remains to be seen whether the improved delivery and effects of NSAIDs afforded by NCs will be sufficient to support clinical translation of this approach.
Nitric Oxide Donors
Endogenous nitric oxide (NO) has numerous functions in the body, including relaxation of vascular smooth muscle cells, inhibition of platelet aggregation and leukocyte adhesion, stabilization of the endothelial monolayer, and assistance in the killing of parasites by leukocytes. Organic nitrates and other compounds that produce this short-lived free radical (72) via enzymatic pathways in the endothelium have been used as vasodilating and antiangina drugs for a century (73). Newer agents that release NO directly, such as diazeniumdiolates (commonly known as NONOates), may have anti-inflammatory effects. However, vasodilation induced by systemic NO donors causes hypotension, which is frequently asymptomatic but can result in syncope. Hemodynamic effects are also thought to underlie the nitrate headache that affects most patients and is frequently dose limiting.
In theory, NCs that control the rate of NO release while decelerating clearance may help optimize the anti-inflammatory effects of NO on the endothelium and blood elements with less accentuated vasodilating activity. In support of this notion, NO donors conjugated to poly(propyleneimine) dendrimers released NO for >16 h (74). Two NO donors, Double JS-K and PABA/NO, have been incorporated into both polystyrene-b-PEG and PLA-b-PEG nanoparticles with sizes ranging from 220 to 450 nm, delaying the drug decomposition time in the presence of glutathione from 15 min to 5 h for PABA/NO and from 4.5 min to 40 min for Double JS-K (75).
Hydrogel nanoparticles with either conjugated S-nitrosothiol (NO-np) or encapsulated S-nitroso-N-acetylcysteine (NAC-SNO-np) produced hypotension and vasodilation in hamsters (73). Interestingly, the effect was prolonged with the NAC-SNO-np formulation, correlating with its ability to maintain higher levels of S-nitrosoglutathione. However, empty nanoparticles induced a detectable inflammatory response apparently masked by the drug (76). NO-np conjugated to generation-4 polyamidoamine dendrimers (G4-SNAP) released NO in the presence of glutathione. In a rat heart ischemia-reperfusion model, perfusion of glutathione (500 μM) mixed with G4-SNAP (15 nM SNAP) reduced infarct size by ~80%, whereas a ~150-fold higher dose of free SNAP was required to achieve a similar effect (77).
The continued transition of NCs for NO donors from in vitro to in vivo models will clarify the potential utility of this approach. Currently it is uncertain, in part owing to the multifaceted effects of NO in the context of inflammation. However, localization of NO release in given vascular areas, theoretically achievable using NCs, may provide paradigm-shifting experimental and therapeutic approaches (78).
Antioxidants
Inflammation is inseparable from the oxidative stress caused by injurious ROS that are released in large amounts by activated phagocytes and are produced at a lower rate by vascular cells—endothelial and smooth muscle cells—in response to cytokines, abnormal flow, and other pathological stimuli. Antioxidant interventions could provide an important anti-inflammatory strategy but have not yet reached clinically significant therapeutic efficacy. Devising carriers for antioxidants aimed at achieving this goal has been an active area of research for several decades.
Small nonenzymatic antioxidants
Prolonged use of megadoses of antioxidants may alleviate mild oxidative stress, but these agents generally fail in tests of severe oxidative stress management (79, 80). Inadequate delivery has long been suspected, and numerous NCs have been proposed. For example, vitamin E, glutathione (GSH), ascorbic acid, and other antioxidants have been conjugated to carriers based on synthetic polymers of poly(acrylic acid) or PEG-poly(methyl methacrylate) as well as natural protein carriers (e.g., gelatin), and these formulations protected cells against oxidative stress in vitro (81, 82). More recently, NCs composed of condensed or polymerized antioxidant materials (e.g., tocopherol) have also been reported (83).
However, as with the other drug classes, liposomes dominate this area of research. Hydrophobic antioxidants including vitamin E, ubiquinones, retinoids, carotenoids, flavonoids, and butylated hydroxytoluene have been incorporated within the liposomal bilayer to improve their solubility and provide protection for the drug (84, 85). The hydrophilic antioxidant, reduced GSH, was encapsulated in the aqueous volume of liposomes and liposome-like spherical self-assembled particles and was shown to protect cells from oxidative stress in vitro (82). N-acetylcysteine (NAC), a hydrophilic derivative of cysteine, has antioxidant activities and replenishes GSH in tissues. Liposomal formulations of NAC devised to enhance its bioavailability and cellular uptake showed superior protection over free NAC after injection in septic liver injury in rats (86) and following tracheal administration in a rat model of ALI (87). Liposomal delivery of resveratrol reduced vascular thickening after endothelial injury in rats (88). Although some positive results have been observed, the mechanism, significance, and utility of NC delivery of antioxidants must be better understood in order for researchers to estimate whether this approach can provide decisive therapeutic benefits.
Antioxidant enzymes
In contrast to nonenzymatic agents, enzymes such as superoxide dismutase (SOD) and catalase are not consumed in their reactions and do not use reducing cofactors, thus representing good candidates for alleviation of acute oxidative stress. Since early studies revealed quick antioxidant enzyme (AOE) elimination from the blood, diverse delivery systems have been developed (80). Both PEGylation and loading in liposomes led to improved results in numerous studies, one of which involved a hyperoxia model in newborn rats (89) and one of which investigated angiotensin II–induced activation of NADPH oxidase and hypertension in rats (90). Refinement of liposomal encapsulation of AOE (91) optimized the cargo activity (92), bioavailability, and protective effects (93) in animal models.
Alternatively, polymerization of SOD modified with vinyl groups resulted in SOD encapsulation in biodegradable 5-nm polymer capsules that attenuated cell death caused by intracellular generation of O2− (94). Electrostatic AOE interaction with PEG-containing cationic block copolymers yielded a series of condensed AOE particles (60–100 nm in diameter) termed nanozymes, which alleviated symptoms in mouse models of Parkinson’s disease (95) and neuronal oxidative stress (96). SOD-pluronic conjugates were reported to deliver active SOD to neuronal cells more effectively than did naked SOD or PEG-SOD (97).
Endothelial targeting of AOEs has also been devised. Conjugation with antibodies and antibody fragments that bind to endothelial surface determinants—such as PECAM-1, ICAM-1, and ACE—significantly boosts AOE binding, uptake, and protective effects in vitro and in animal models (e.g., models of endotoxin-induced inflammation, ischemia-reperfusion injury, and angiotensin II–induced vasoconstriction) (28, 98). Endocytosis of ICAM- and PECAM-targeted AOEs allows quenching of proinflammatory signaling ROS in the endosomes, which are otherwise inaccessible to antioxidants in the milieu (98).
Internalized AOE conjugates undergo lysosomal degradation, which limits the duration of protection (99). Protection can be prolonged through the encapsulation of catalase in polymeric NCs permeable to ROS but not to proteases. A novel formulation scheme that introduced a freezing step during the primary emulsification produced carriers with sizes permissive of intravascular injection (200–300 nm) and with acceptable loading efficiencies of active AOE cargo (100–102). Hydrogen peroxide diffusing through the polymer shell was degraded by encapsulated catalase (102) (Figure 4). Using PEGylated AOEs further enhanced encapsulation efficacy, whereas modulating the mass ratio between the copolymer blocks controlled the rate of carrier degradation and shape, providing filamentous NCs for AOEs (20). Catalase and SOD have also been loaded without inactivation into proteolytically protective magnetic nanoparticles (MNPs) formed using controlled precipitation of oleate-coated magnetite (103). AOEs encapsulated in MNPs were delivered to endothelial cells in vitro through the use of a magnetic field and were shown to achieve a protective effect (104). PECAM antibody conjugated to AOE-loaded polymeric NCs provided targeting to endothelial cells, quenched ROS, and prolonged the antioxidant protection in vitro and in animal models (102).
Figure 4.
Endothelial protection by AOEs delivered via CAM-targeted polymeric NCs. Polymeric NCs targeted to CAMs constitutively expressed (e.g., PECAM) and/or induced by pathological factors (e.g., ICAM) deliver AOE payloads prophylactically and/or therapeutically, respectively. For example, polymeric NCs with affinity to ICAM-1 preferentially bind to and enter inflamed endothelium. Semipermeable polymeric NCs protect AOEs from proteases yet permit quenching of diffusible ROS, including those attacking the endothelium from the bloodstream (e.g., released by activated WBCs), proinflammatory signaling ROS produced by the endothelium within endocytic vesicles, and H2O2 diffusing into the vacuoles from the cytosol and other compartments (e.g., mitochondria). Binding of NCs to CAMs also inhibits WBC adhesion. Abbreviations: AOE, antioxidant enzyme; CAM, cell adhesion molecule; EC, endothelial cell; ICAM, intercellular adhesion molecule; IL, interleukin; LPS, lipopolysaccharide; NC, nanocarrier; PECAM, platelet endothelial cell adhesion molecule; ROS, reactive oxygen species; TNF, tumor necrosis factor; WBC, white blood cell.
Catalase and SOD are examples of candidate biotherapeutics that may find utility in the management of acute vascular oxidative stress, and NCs provide a viable approach for enhanced delivery. Targeting to intracellular compartments of selected cells (e.g., endothelium) is an important additional advantage of this strategy, enabling anti-inflammatory mechanisms that are unavailable to nontargeted AOEs (e.g., interception of endosomal ROS).
Anti-Inflammatory siRNA
Double-stranded siRNA silences gene expression via sequence-specific destruction of complementary message RNA (105). Yet, if therapeutic knockout of proinflammatory proteins in vivo is to be achieved, effective siRNA delivery into the cytosol of target cells is necessary (106, 107). Early strategies employed for this purpose included conjugation of chemically modified siRNA with folate and antibodies, as well as with RGD, cell-penetrating, or fusogenic peptides (106, 108). Almost every type of NC has now also been explored for siRNA (106, 108). Several siRNA-loaded lipid and cyclodextrin-based NCs have reached clinical trials, mostly for oncological purposes (108, 109).
Use of siRNA for anti-inflammatory silencing is an active research area (24, 110). For example, a monoclonal antibody to DC205, a glycoprotein expressed on the surfaces of antigen-presenting cells, facilitated liposomal delivery of siRNA for the CD40/TNF receptor to target cells and suppressed the immune response in mice (111). Targeting 70–80-nm lipid-based siRNA nanoparticles that inhibit the chemokine receptor in monocytes suppressed vascular recruitment of these inflammatory cells and provided protective effects in animal models of myocardial infarction and atherosclerosis (24).
Gene silencing of E-selectin in TNF-activated endothelial cells inhibited adhesion of activated leukocytes in cell culture (112). RGD-targeted liposomal delivery of anti-inflammatory siRNA to the endothelium was also studied in mice (113). E-selectin- and ICAM-targeted nanoparticles carrying siRNA silencing inflammatory mediators suppressed expression of target mediator molecules in cell culture (114). Adenovirus targeted to E-selectin homed to the glomerular microvasculature and suppressed expression of adhesion molecules in a mouse model of glomerulonephritis (114). Cationic lipid-based formulations of siRNA targeted to E-selectin silenced vascular endothelial cadherin in activated endothelial cells in vitro (115).
Targeting to selectins and other endothelial determinants favors NC endocytosis, but transfer from endocytic vacuoles to the cytosol is the major challenge for siRNA delivery. NCs featuring membrane-permeating moieties and pH-dependent disruption of intracellular vacuoles may enhance the efficacy of siRNA delivery. However, whereas the toxic effects of endosomal disruption may be viewed as a bonus in cancer eradication using siRNA, it may create safety issues in the management of inflammation. Design of NCs for safe and effective delivery of siRNA and other nucleic acid agents is a rapidly evolving area and the focus of large investments, providing hope for their utility not only in inflammatory conditions but also in other areas of biomedicine (116, 117).
CONCLUSION: CHALLENGES AND PERSPECTIVES
NCs may be used to optimize the localization and timing of AIA action. Solving delivery issues will enable novel anti-inflammatory interventions of potent AIAs—biotherapeutics, steroids, and antioxidants—and potentially change the treatment of patients. Both rational engineering of carrier parameters (fitting the pathophysiology of the condition and features of AIA cargo) and optimal target selection (capitalizing on its functions, phenotype, and milieu) are needed to achieve this goal.
The medical utility of NCs depends on a balancing act between the advantages and disadvantages. On one hand, NCs offer AIA targeting, milieu sensing, intracellular delivery, and local activation. On the other hand, NCs may cause side effects that include harmful tissue deposition (e.g., in the kidneys, reticuloendothelial system, and microvasculature), unintended activation of host defense (complement, immune response), and damage to target cells. Some of these issues were recognized a long time ago, and contingencies evolved (e.g., PEG stealth technology). Yet, nanotoxicology has a long way to go before clinical utility of NCs for the treatment of non-oncological diseases becomes a reality. For example, biodegradability into nontoxic components does not equate to NC biocompatibility. Also, size does matter; owing to a higher surface/mass ratio, NCs made of materials safely used as implants have kinetics of drug release and surface absorption of molecules that differ from those of their smaller counterparts. Furthermore, in contrast to implants, NCs have features such as circulation in the bloodstream, accessibility to diverse tissues and cells, and cellular uptake—the very features enabling their delivery functions—that potentiate their ability to harm, which may be particularly worrisome in the treatment of inflammation.
The complexity of NCs aggravates these concerns, and translational challenges include difficulties of industrial production and control of size, structure, activity, and homogeneity as well as regulatory complications and costs. These considerations should motivate analysis of the problem’s multiple aspects—drugs, carriers, and conditions to treat—to focus on scenarios with the highest probability of success.
In this context, NCs that enable new AIAs or novel mechanisms for currently used AIAs have a better chance of qualitatively improving the outcomes than approaches that only incrementally improve the efficacy of established interventions. Chronic repetitive treatment is more likely to cause adverse effects; hence, utility of NCs in anti-inflammatory interventions lies primarily in the realms of the treatment of acute conditions, at least initially. In conclusion, it is tempting to postulate that NCs targeting vascular delivery of AIAs to locations critically important for desired effects may ultimately shift the current paradigms of the pharmacological management of these and, perhaps, other forms of inflammation.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–10. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casi G, Neri D. Antibody–drug conjugates: basic concepts, examples and future perspectives. J Control Release. 2012;161:422–28. doi: 10.1016/j.jconrel.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 3.White CW, Jackson JH, Abuchowski A, Kazo GM, Mimmack RF, et al. Polyethylene glycol–attached antioxidant enzymes decrease pulmonary oxygen toxicity in rats. J Appl Physiol. 1989;66:584–90. doi: 10.1152/jappl.1989.66.2.584. [DOI] [PubMed] [Google Scholar]
- 4.Kirby C, Gregoriadis G. Dehydration-rehydration vesicles: a simple method for high yield drug entrapment in liposomes. Nat Biotechnol. 1984;2:979–84. [Google Scholar]
- 5.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–37. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 6.Howard MD, Jay M, Dziubla TD, Lu X. PEGylation of nanocarrier drug delivery systems: state of the art. J Biomed Nanotechnol. 2008;4:133–48. [Google Scholar]
- 7.Hristova K, Kenworthy A, McIntosh TJ. Effect of bilayer composition on the phase behavior of liposomal suspensions containing poly(ethylene glycol)-lipids. Macromolecules. 1995;28:7693–99. [Google Scholar]
- 8.Barenholz Y. Doxil® —The first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Kheradmandnia S, Vasheghani-Farahani E, Nosrati M, Atyabi F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomed: Nanotechnol Biol Med. 2010;6:753–59. doi: 10.1016/j.nano.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–72. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Chorny M, Fishbein I, Yellen BB, Alferiev IS, Bakay M, et al. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc Natl Acad Sci USA. 2010;107:8346–51. doi: 10.1073/pnas.0909506107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misra AC, Bhaskar S, Clay N, Lahann J. Multicompartmental particles for combined imaging and siRNA delivery. Adv Mater. 2012;24:3850–56. doi: 10.1002/adma.201200372. [DOI] [PubMed] [Google Scholar]
- 13.Pochan DJ, Zhu J, Zhang K, Wooley KL, Miesch C, Emrick T. Multicompartment and multigeometry nanoparticle assembly. Soft Matter. 2011;7:2500–6. [Google Scholar]
- 14.Labouta HI, Schneider M. Tailor-made biofunctionalized nanoparticles using layer-by-layer technology. Int J Pharm. 2010;395:236–42. doi: 10.1016/j.ijpharm.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Kohler D, Madaboosi N, Delcea M, Schmidt S, De Geest BG, et al. Patchiness of embedded particles and film stiffness control through concentration of gold nanoparticles. Adv Mater. 2012;24:1095–100. doi: 10.1002/adma.201103958. [DOI] [PubMed] [Google Scholar]
- 16.Guillaudeu SJ, Fox ME, Haidar YM, Dy EE, Szoka FC, Fréchet JMJ. PEGylated dendrimers with core functionality for biological applications. Bioconjug Chem. 2008;19:461–69. doi: 10.1021/bc700264g. [DOI] [PubMed] [Google Scholar]
- 17.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–31. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 18.Nicolai T, Colombani O, Chassenieux C. Dynamic polymeric micelles versus frozen nanoparticles formed by block copolymers. Soft Matter. 2010;6:3111–18. [Google Scholar]
- 19.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–55. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simone EA, Dziubla TD, Colon-Gonzalez F, Discher DE, Muzykantov VR. Effect of polymer amphiphilicity on loading of a therapeutic enzyme into protective filamentous and spherical polymer nanocarriers. Biomacromolecules. 2007;8:3914–21. doi: 10.1021/bm700888h. [DOI] [PubMed] [Google Scholar]
- 21.Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci USA. 2011;108:586–91. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–34. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henne WA, Rothenbuhler R, Ayala-Lopez W, Xia W, Varghese B, Low PS. Imaging sites of infection using a 99mTc-labeled folate conjugate targeted to folate receptor positive macrophages. Mol Pharm. 2012;9:1435–40. doi: 10.1021/mp3000138. [DOI] [PubMed] [Google Scholar]
- 24.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–10. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuvaev VV, Muzykantov VR. Targeted modulation of reactive oxygen species in the vascular endothelium. J Control Release. 2011;153:56–63. doi: 10.1016/j.jconrel.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–15. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 27.Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009;335:283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, et al. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther. 2009;331:404–11. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danielyan K, Ding B-S, Gottstein C, Cines DB, Muzykantov VR. Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema. J Pharmacol Exp Ther. 2007;321:947–52. doi: 10.1124/jpet.107.120535. [DOI] [PubMed] [Google Scholar]
- 30.Scherpereel A, Rome JJ, Wiewrodt R, Watkins SC, Harshaw DW, et al. Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J Pharmacol Exp Ther. 2002;300:777–86. doi: 10.1124/jpet.300.3.777. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H-f, Hu G, Wickline SA, Lanza GM, Pham CTN. Synergistic effect of antiangiogenic nanotherapy combined with methotrexate in the treatment of experimental inflammatory arthritis. Nanomedicine. 2010;5:1065–7. doi: 10.2217/nnm.10.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Deliv. 2005;2:909–26. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 33.Spragg DD, Alford DR, Greferath R, Larsen CE, Lee K-D, et al. Immunotargeting of liposomes to activated vascular endothelial cells: a strategy for site-selective delivery in the cardiovascular system. Proc Natl Acad Sci USA. 1997;94:8795–800. doi: 10.1073/pnas.94.16.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ásgeirsdóttir SA, Kamps JAAM, Bakker HI, Zwiers PJ, Heeringa P, et al. Site-specific inhibition of glomerulonephritis progression by targeted delivery of dexamethasone to glomerular endothelium. Mol Pharmacol. 2007;72:121–31. doi: 10.1124/mol.107.034140. [DOI] [PubMed] [Google Scholar]
- 35.Calderon AJ, Muzykantov V, Muro S, Eckmann DM. Flow dynamics, binding and detachment of spherical carriers targeted to ICAM-1 on endothelial cells. Biorheology. 2009;46:323–41. doi: 10.3233/BIR-2009-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calderon AJ, Bhowmick T, Leferovich J, Burman B, Pichette B, et al. Optimizing endothelial targeting by modulating the antibody density and particle concentration of anti-ICAM coated carriers. J Control Release. 2011;150:37–44. doi: 10.1016/j.jconrel.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hak S, Helgesen E, Hektoen HH, Huuse EM, Jarzyna PA, et al. The effect of nanoparticle polyethylene glycol surface density on ligand-directed tumor targeting studied in vivo by dual modality imaging. ACS Nano. 2012;6:5648–58. doi: 10.1021/nn301630n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fakhari A, Baoum A, Siahaan TJ, Le KB, Berkland C. Controlling ligand surface density optimizes nanoparticle binding to ICAM-1. J Pharm Sci. 2011;100:1045–56. doi: 10.1002/jps.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zern BJ, Chacko A-M, Liu J, Greineder CF, Blankemeyer ER, et al. Reduction of nanoparticle avidity enhances the selectivity of vascular targeting and PET detection of pulmonary inflammation. ACS Nano. 2013;7:2461–69. doi: 10.1021/nn305773f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shuvaev VV, Tliba S, Pick J, Arguiri E, Christofidou-Solomidou M, et al. Modulation of endothelial targeting by size of antibody-antioxidant enzyme conjugates. J Control Release. 2011;149:236–41. doi: 10.1016/j.jconrel.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shuvaev VV, Ilies MA, Simone E, Zaitsev S, Kim Y, et al. Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano. 2011;5:6991–99. doi: 10.1021/nn2015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16:1450–58. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, et al. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol. 2007;25:327–37. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, et al. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116:1599–609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 45.Muro S, Cui X, Gajewski C, Murciano J-C, Muzykantov VR, Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am J Physiol Cell Physiol. 2003;285:C1339–47. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 46.Garnacho C, Albelda SM, Muzykantov VR, Muro S. Differential intra-endothelial delivery of polymer nanocarriers targeted to distinct PECAM-1 epitopes. J Control Release. 2008;130:226–33. doi: 10.1016/j.jconrel.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhowmick T, Berk E, Cui X, Muzykantov VR, Muro S. Effect of flow on endothelial endocytosis of nanocarriers targeted to ICAM-1. J Control Release. 2012;157:485–92. doi: 10.1016/j.jconrel.2011.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han J, Zern BJ, Shuvaev VV, Davies PF, Muro S, Muzykantov V. Acute and chronic shear stress differently regulate endothelial internalization of nanocarriers targeted to platelet-endothelial cell adhesion molecule-1. ACS Nano. 2012;6:8824–36. doi: 10.1021/nn302687n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quan LD, Thiele GM, Tian J, Wang D. The development of novel therapies for rheumatoid arthritis. Expert Opin Ther Pat. 2008;18:723–38. doi: 10.1517/13543776.18.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonanomi MH, Velvart M, Stimpel M, Roos KM, Fehr K, Weder HG. Studies of pharmacokinetics and therapeutic effects of glucocorticoids entrapped in liposomes after intraarticular application in healthy rabbits and in rabbits with antigen-induced arthritis. Rheumatol Int. 1987;7:203–12. doi: 10.1007/BF00541378. [DOI] [PubMed] [Google Scholar]
- 51.Hegeman MA, Cobelens PM, Kamps JAAM, Hennus MP, Jansen NJG, et al. Liposome-encapsulated dexamethasone attenuates ventilator-induced lung inflammation. Br J Pharmacol. 2011;163:1048–58. doi: 10.1111/j.1476-5381.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suntres ZE, Shek PN. Prophylaxis against lipopolysaccharide-induced lung injuries by liposome-entrapped dexamethasone in rats. Biochem Pharmacol. 2000;59:1155–61. doi: 10.1016/s0006-2952(99)00411-6. [DOI] [PubMed] [Google Scholar]
- 53.Rauchhaus U, Schwaiger FW, Panzner S. Separating therapeutic efficacy from glucocorticoid side-effects in rodent arthritis using novel, liposomal delivery of dexamethasone phosphate: long-term suppression of arthritis facilitates interval treatment. Arthritis Res Ther. 2009;11:R190. doi: 10.1186/ar2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koning GA, Schiffelers RM, Wauben MHM, Kok RJ, Mastrobattista E, et al. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate–containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006;54:1198–208. doi: 10.1002/art.21719. [DOI] [PubMed] [Google Scholar]
- 55.Everts M, Koning GA, Kok RJ, Ásgeirsdóttir SA, Vestweber D, et al. In vitro cellular handling and in vivo targeting of E-selectin-directed immunoconjugates and immunoliposomes used for drug delivery to inflamed endothelium. Pharm Res. 2003;20:64–72. doi: 10.1023/a:1022298725165. [DOI] [PubMed] [Google Scholar]
- 56.Ulmansky R, Turjeman K, Baru M, Katzavian G, Harel M, et al. Glucocorticoids in nano-liposomes administered intravenously and subcutaneously to adjuvant arthritis rats are superior to the free drugs in suppressing arthritis and inflammatory cytokines. J Control Release. 2012;160:299–305. doi: 10.1016/j.jconrel.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 57.Xiang QY, Wang MT, Chen F, Gong T, Jian YL, et al. Lung-targeting delivery of dexamethasone acetate loaded solid lipid nanoparticles. Arch Pharm Res. 2007;30:519–25. doi: 10.1007/BF02980228. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Grijpma DW, Feijen J. Poly(trimethylene carbonate) and monomethoxy poly(ethylene glycol)-block-poly(trimethylene carbonate) nanoparticles for the controlled release of dexamethasone. J Control Release. 2006;111:263–70. doi: 10.1016/j.jconrel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Puri S, Kallinteri P, Higgins S, Hutcheon GA, Garnett MC. Drug incorporation and release of water soluble drugs from novel functionalized poly(glycerol adipate) nanoparticles. J Control Release. 2008;125:59–67. doi: 10.1016/j.jconrel.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Kim JY, Ryu JH, Hyun H, Kim HA, Sig Choi J, et al. Dexamethasone conjugation to polyamidoamine dendrimers G1 and G2 for enhanced transfection efficiency with an anti-inflammatory effect. J Drug Target. 2012;20:667–77. doi: 10.3109/1061186X.2012.712127. [DOI] [PubMed] [Google Scholar]
- 61.Ishihara T, Kubota T, Choi T, Higaki M. Treatment of experimental arthritis with stealth-type polymeric nanoparticles encapsulating betamethasone phosphate. J Pharmacol Exp Ther. 2009;329:412–17. doi: 10.1124/jpet.108.150276. [DOI] [PubMed] [Google Scholar]
- 62.Ishihara T, Kubota T, Choi T, Takahashi M, Ayano E, et al. Polymeric nanoparticles encapsulating betamethasone phosphate with different release profiles and stealthiness. Int J Pharm. 2009;375:148–54. doi: 10.1016/j.ijpharm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Wang D, Miller S, Liu X-M, Anderson B, Wang XS, Goldring S. Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res Ther. 2007;9:R2. doi: 10.1186/ar2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krakovicová H, Etrych T, Ulbrich K. HPMA-based polymer conjugates with drug combination. Eur J Pharm Sci. 2009;37:405–12. doi: 10.1016/j.ejps.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 65.Howard M, Ponta A, Eckman A, Jay M, Bae Y. Polymer micelles with hydrazoneester dual linkers for tunable release of dexamethasone. Pharm Res. 2011;28:2435–46. doi: 10.1007/s11095-011-0470-1. [DOI] [PubMed] [Google Scholar]
- 66.Lu X, Howard MD, Talbert DR, Rinehart JJ, Potter PM, et al. Nanoparticles containing anti-inflammatory agents as chemotherapy adjuvants II: role of plasma esterases in drug release. AAPS J. 2009;11:120–22. doi: 10.1208/s12248-009-9086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chandrasekar D, Sistla R, Ahmad FJ, Khar RK, Diwan PV. The development of folate-PAMAM dendrimer conjugates for targeted delivery of anti-arthritic drugs and their pharmacokinetics and biodistribution in arthritic rats. Biomaterials. 2007;28:504–12. doi: 10.1016/j.biomaterials.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 68.Bernardi A, Zilberstein A, Jäger E, Campos MM, Morrone FB, et al. Effects of indomethacin- loaded nanocapsules in experimental models of inflammation in rats. Br J Pharmacol. 2009;158:1104–11. doi: 10.1111/j.1476-5381.2009.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bernardi A, Frozza RL, Meneghetti A, Hoppe JB, Battastini AMO, et al. Indomethacin-loaded lipid-core nanocapsules reduce the damage triggered by Aβ1-42 in Alzheimer’s disease models. Int J Nanomed. 2012;7:4927–42. doi: 10.2147/IJN.S35333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prajapati RN, Tekade RK, Gupta U, Gajbhiye V, Jain NK. Dendrimer-mediated solubilization, formulation development and in vitro–in vivo assessment of piroxicam. Mol Pharm. 2009;6:940–50. doi: 10.1021/mp8002489. [DOI] [PubMed] [Google Scholar]
- 71.Ianiski FR, Alves CB, Souza ACG, Pinton S, Roman SS, et al. Protective effect of meloxicam-loaded nanocapsules against amyloid-βpeptide-induced damage in mice. Behav Brain Res. 2012;230:100–7. doi: 10.1016/j.bbr.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 72.Liu X, Miller MJS, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR. Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–13. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 73.Nacharaju P, Tuckman-Vernon C, Maier KE, Chouake J, Friedman A, et al. A nanoparticle delivery vehicle for S-nitroso-N-acetyl cysteine: sustained vascular response. Nitric Oxide. 2012;27:150–60. doi: 10.1016/j.niox.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stasko NA, Schoenfisch MH. Dendrimers as a scaffold for nitric oxide release. J Am Chem Soc. 2006;128:8265–71. doi: 10.1021/ja060875z. [DOI] [PubMed] [Google Scholar]
- 75.Kumar V, Hong SY, Maciag AE, Saavedra JE, Adamson DH, et al. Stabilization of the nitric oxide (NO) prodrugs and anticancer leads, PABA/NO and Double JS-K, through incorporation into PEG-protected nanoparticles. Mol Pharm. 2009;7:291–98. doi: 10.1021/mp900245h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med. 2010;49:530–38. doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson TA, Stasko NA, Matthews JL, Cascio WE, Holmuhamedov EL, et al. Reduced ischemia/reperfusion injury via glutathione-initiated nitric oxide–releasing dendrimers. Nitric Oxide. 2010;22:30–36. doi: 10.1016/j.niox.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Muzykantov VR. NO gets a test ride on high-tech transporting nanodevices: a commentary on “Sustained-release nitric oxide from long-lived circulating nanoparticles”. Free Radic Biol Med. 2010;49:528–29. doi: 10.1016/j.freeradbiomed.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 79.Christofidou-Solomidou M, Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med. 2006;5:47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 80.Muzykantov VR. Delivery of antioxidant enzyme proteins to the lung. Antioxid Redox Signal. 2001;3:39–62. doi: 10.1089/152308601750100489. [DOI] [PubMed] [Google Scholar]
- 81.Fleming C, Maldjian A, Da Costa D, Rullay AK, Haddleton DM, et al. A carbohydrate-antioxidant hybrid polymer reduces oxidative damage in spermatozoa and enhances fertility. Nat Chem Biol. 2005;1:270–74. doi: 10.1038/nchembio730. [DOI] [PubMed] [Google Scholar]
- 82.Williams SR, Lepene BS, Thatcher CD, Long TE. Synthesis and characterization of poly(ethylene glycol)-glutathione conjugate self-assembled nanoparticles for antioxidant delivery. Biomacromolecules. 2009;10:155–61. doi: 10.1021/bm801058j. [DOI] [PubMed] [Google Scholar]
- 83.Astete CE, Dolliver D, Whaley M, Khachatryan L, Sabliov C. Antioxidant poly(lactic-co-glycolic) acid nanoparticles made with α-tocopherol-ascorbic acid surfactant. ACS Nano. 2011;5:9313–25. doi: 10.1021/nn102845t. [DOI] [PubMed] [Google Scholar]
- 84.Hood E, Simone E, Wattamwar P, Dziubla T, Muzykantov V. Nanocarriers for vascular delivery of antioxidants. Nanomedicine. 2011;6:1257–72. doi: 10.2217/nnm.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stone WL, Smith M. Therapeutic uses of antioxidant liposomes. Mol Biotechnol. 2004;27:217–30. doi: 10.1385/MB:27:3:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitsopoulos P, Omri A, Alipour M, Vermeulen N, Smith MG, Suntres ZE. Effectiveness of liposomal-N-acetylcysteine against LPS-induced lung injuries in rodents. Int J Pharm. 2008;363:106–11. doi: 10.1016/j.ijpharm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 87.Fan J, Shek PN, Suntres ZE, Li YH, Oreopoulos GD, Rotstein OD. Liposomal antioxidants provide prolonged protection against acute respiratory distress syndrome. Surgery. 2000;128:332–38. doi: 10.1067/msy.2000.108060. [DOI] [PubMed] [Google Scholar]
- 88.Hung CF, Chen JK, Liao MH, Lo HM, Fang JY. Development and evaluation of emulsion-liposome blends for resveratrol delivery. J Nanosci Nanotechnol. 2006;6:2950–58. doi: 10.1166/jnn.2006.420. [DOI] [PubMed] [Google Scholar]
- 89.Tanswell AK, Freeman BA. Liposome-entrapped antioxidant enzymes prevent lethal O2 toxicity in the newborn rat. J Appl Physiol. 1987;63:347–52. doi: 10.1152/jappl.1987.63.1.347. [DOI] [PubMed] [Google Scholar]
- 90.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II–induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–93. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 91.Corvo LM, Jorge JCS, van’t Hof R, Cruz MEM, Crommelin DJA, Storm G. Superoxide dismutase entrapped in long-circulating liposomes: formulation design and therapeutic activity in rat adjuvant arthritis. Biochim Biophys Acta (BBA): Biomembr. 2002;1564:227–36. doi: 10.1016/s0005-2736(02)00457-1. [DOI] [PubMed] [Google Scholar]
- 92.Xu X, Costa A, Burgess DJ. Protein encapsulation in unilamellar liposomes: high encapsulation efficiency and a novel technique to assess lipid-protein interaction. Pharm Res. 2012;29:1919–31. doi: 10.1007/s11095-012-0720-x. [DOI] [PubMed] [Google Scholar]
- 93.Gaspar MM, Boerman OC, Laverman P, Corvo ML, Storm G, Cruz ME. Enzymosomes with surface-exposed superoxide dismutase: in vivo behaviour and therapeutic activity in a model of adjuvant arthritis. J Control Release. 2007;117:186–95. doi: 10.1016/j.jconrel.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 94.Yan M, Du JJ, Gu Z, Liang M, Hu YF, et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat Nanotechnol. 2010;5:48–53. doi: 10.1038/nnano.2009.341. [DOI] [PubMed] [Google Scholar]
- 95.Brynskikh AM, Zhao Y, Mosley RL, Li S, Boska MD, et al. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine. 2010;5:379–96. doi: 10.2217/nnm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenbaugh EG, Roat JW, Gao L, Yang RF, Manickam DS, et al. The attenuation of central angiotensin II–dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials. 2010;31:5218–26. doi: 10.1016/j.biomaterials.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yi X, Zimmerman MC, Yang R, Tong J, Vinogradov S, Kabanov AV. Pluronic-modified superoxide dismutase 1 attenuates angiotensin II–induced increase in intracellular superoxide in neurons. Free Radic Biol Med. 2010;49:548–58. doi: 10.1016/j.freeradbiomed.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, et al. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J. 2011;25:348–57. doi: 10.1096/fj.10-169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muro S, Mateescu M, Gajewski C, Robinson M, Muzykantov VR, Koval M. Control of intracellular trafficking of ICAM-1-targeted nanocarriers by endothelial Na+/H+ exchanger proteins. Am J Physiol Lung Cell Mol Physiol. 2006;290:L809–17. doi: 10.1152/ajplung.00311.2005. [DOI] [PubMed] [Google Scholar]
- 100.Giovagnoli S, Luca G, Casaburi I, Blasi P, Macchiarulo G, et al. Long-term delivery of superoxide dismutase and catalase entrapped in poly(lactide-co-glycolide) microspheres: in vitro effects on isolated neonatal porcine pancreatic cell clusters. J Control Release. 2005;107:65–77. doi: 10.1016/j.jconrel.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 101.Dziubla TD, Karim A, Muzykantov VR. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J Control Release. 2005;102:427–39. doi: 10.1016/j.jconrel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 102.Dziubla TD, Shuvaev VV, Hong NK, Hawkins BJ, Madesh M, et al. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials. 2008;29:215–27. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chorny M, Fishbein I, Alferiev I, Levy RJ. Magnetically responsive biodegradable nanoparticles enhance adenoviral gene transfer in cultured smooth muscle and endothelial cells. Mol Pharm. 2009;6:1380–87. doi: 10.1021/mp900017m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chorny M, Hood E, Levy RJ, Muzykantov VR. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J Control Release. 2010;146:144–51. doi: 10.1016/j.jconrel.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 106.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–38. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–57. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 108.Yuan X, Naguib S, Wu Z. Recent advances of siRNA delivery by nanoparticles. Expert Opin Drug Deliv. 2011;8:521–36. doi: 10.1517/17425247.2011.559223. [DOI] [PubMed] [Google Scholar]
- 109.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6:659–68. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 110.Kuldo JM, Ogawara KI, Werner N, Ásgeirsdóttir SA, Kamps JAAM, et al. Molecular pathways of endothelial cell activation for (targeted) pharmacological intervention of chronic inflammatory diseases. Curr Vasc Pharmacol. 2005;3:11–39. doi: 10.2174/1570161052773898. [DOI] [PubMed] [Google Scholar]
- 111.Zheng X, Vladau C, Shunner A, Min WP. siRNA specific delivery system for targeting dendritic cells. Methods Mol Biol. 2010;623:173–88. doi: 10.1007/978-1-60761-588-0_11. [DOI] [PubMed] [Google Scholar]
- 112.Nishiwaki Y, Yokota T, Hiraoka M, Miyagishi M, Taira K, et al. Introduction of short interfering RNA to silence endogenous E-selectin in vascular endothelium leads to successful inhibition of leukocyte adhesion. Biochem Biophys Res Commun. 2003;310:1062–66. doi: 10.1016/j.bbrc.2003.09.125. [DOI] [PubMed] [Google Scholar]
- 113.Vader P, Crielaard BJ, van Dommelen SM, van der Meel R, Storm G, Schiffelers RM. Targeted delivery of small interfering RNA to angiogenic endothelial cells with liposome-polycation-DNA particles. J Control Release. 2012;160:211–16. doi: 10.1016/j.jconrel.2011.09.080. [DOI] [PubMed] [Google Scholar]
- 114.Kuldo JM, Ásgeirsdóttir SA, Zwiers PJ, Bellu AR, Rots MG, et al. Targeted adenovirus mediated inhibition of NF-κB-dependent inflammatory gene expression in endothelial cells in vitro and in vivo. J Control Release. 2013;166:57–65. doi: 10.1016/j.jconrel.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 115.Ásgeirsdóttir SA, Talman EG, de Graaf IA, Kamps JAAM, Satchell SC, et al. Targeted transfection increases siRNA uptake and gene silencing of primary endothelial cells in vitro—a quantitative study. J Control Release. 2010;141:241–51. doi: 10.1016/j.jconrel.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 116.Zuckerman JE, Choi CHJ, Han H, Davis ME. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc Natl Acad Sci USA. 2012;109:3137–42. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee JB, Zhang K, Tam YYC, Tam YK, Belliveau NM, et al. Lipid nanoparticle siRNA systems for silencing the androgen receptor in human prostate cancer in vivo. Int J Cancer. 2012;131:E781–90. doi: 10.1002/ijc.27361. [DOI] [PubMed] [Google Scholar]