Abstract
Single autologous hematopoietic cell transplant (AHCT) with high-dose melphalan prolongs survival in patients with multiple myeloma but is not curative. We conducted a study of intensive single AHCT using tandem chemomobilization with CY and etoposide followed by high-dose conditioning with melphalan 200 mg/m2 plus carmustine 15 mg/kg. One hundred and eighteen patients in first consolidation (CON1) and 58 patients in relapse (REL) were transplanted using this intensified approach. Disease response improved from 32% very good PR (VGPR) + CR pre-mobilization to 76% VGPR + CR post transplant in CON1. With a median follow-up of 4.7 years, the median EFS was 2.8 years, and the median OS was 5.1 years in CON1. OS from time of transplant was significantly shorter for REL (3.4 years) compared with CON1 (5.1 years; P = 0.02). However, OS from time of diagnosis was similar in REL (6.1 years) and CON1 (6.0 years; P = 0.80). The 100-day non-relapse mortality in the CON1 and REL groups was 0% and 7%, respectively. In summary, intensified single AHCT with tandem chemo-mobilization and augmented high-dose therapy is feasible in multiple myeloma and leads to high-quality response rates.
Keywords: myeloma, transplant, autologous
Introduction
High-dose chemotherapy with autologous hematopoietic cell transplantation (AHCT) improves response rates, EFS and OS in patients with multiple myeloma.1,2 The most commonly used preparative regimen for single AHCT is high-dose melphalan dosed at 200 mg/m2.3 Tandem AHCT has been shown to improve EFS and OS, particularly in patients who do not achieve an optimal response after the first transplant.4 However, 22–35% of patients in randomized studies did not receive a planned second transplant,4,5 and many patients in clinical practice cannot tolerate or decline a second transplant. Dose intensification with chemotherapy during induction and consolidation plus tandem autotransplant has shown improved disease response in the ‘Total Therapy’ program.6 However, previous attempts to intensify the transplant preparative regimen with cytotoxic chemotherapy have demonstrated increased toxicity without survival benefit.7–9
Following a pilot study for dosing and safety,10 patients undergoing single AHCT for multiple myeloma at Stanford from 1996–2006 were treated with an intensified protocol. Patients in first consolidation (CON1) or relapse (REL) were eligible. This intensified regimen consisted of tandem chemo-mobilization with CY then etoposide followed by single AHCT using high-dose melphalan and carmustine (BCNU). This intensive alkylator based program was developed before the advent of immunomodulatory agents or proteasome inhibitors. The goal of tandem chemomobilization was to reduce disease burden before transplant. The addition of BCNU to standard melphalan at transplant was intended to intensify the ablative regimen without radiation. Nearly all patients undergoing single AHCT for myeloma at Stanford in this time period were treated with this intensified protocol. We present an analysis of 176 patients treated by this intensified approach with nearly 5-year median follow-up.
Materials and methods
Patients
Patients aged 18–75 years with multiple myeloma with Stage II–III Durie–Salmon disease were eligible for this protocol as consolidation following frontline induction or as salvage therapy for relapsed disease. Patients were required to have Karnofsky performance status > 70%, diffusion lung capacity (DLCO) ≥ 60% of predicted, alanine aminotransferase and aspartate aminotransferase ≤ 2 × normal, total bilirubin < 2 mg/dL and serum creatinine < 2.0 mg/dL or 24 h creatinine clearance ≥ 60 mL/min. Pathology review at Stanford University Medical Center was required. Exclusion criteria were pregnancy, active lactation, HIV positivity and previous hematopoietic cell transplant. This protocol was approved by the Stanford Institutional Review Board and Scientific Review Committee. All patients provided written informed consent.
Tandem chemo-mobilization, high-dose therapy and autologous transplant
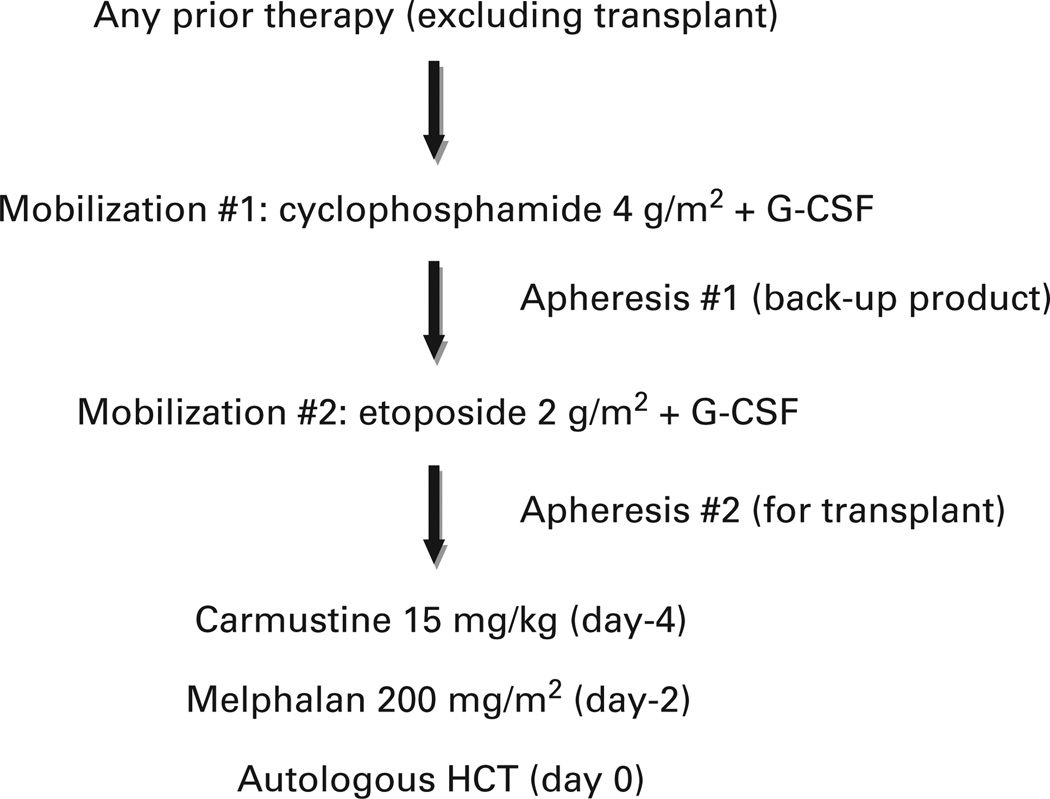
The overall treatment plan is outlined in Figure 1. The first chemo-mobilization consisted of i.v. CY dosed at 4 g/m2. Filgrastim 10 mcg/kg/day s.c. was administered beginning the day following CY. Leukapheresis of PBPCs was performed daily following leukocyte recovery with a goal of ≥ 2 × 106 CD34 + cells/kg. This product was cryopreserved and stored as a backup product.
Figure 1.
Treatment plan. Patients were allowed any previous therapy excluding hematopoietic cell transplant. Patients underwent a first chemomobilization with CY and G-CSF, and PBSCs were collected. This first collection was cryopreserved and stored as a backup product. Three weeks later, a second chemo-mobilization was performed with etoposide and G-CSF, and PBSCs were collected and cryopreserved to be used after the planned preparative regimen. Five weeks later, the preparative regimen was given consisting of high-dose BCNU and melphalan followed by autologous PBSC rescue.
The second chemo-mobilization consisted of i.v. etoposide dosed at 2 g/m2 to be administered approximately 2 weeks after completing leukapheresis from the first mobilization. Filgrastim 10 mcg/kg/day s.c. was administered beginning the day following etoposide. Leukapheresis of PBPCs was performed daily following leukocyte recovery with a goal of ≥ 3 × 106 CD34 + cells/kg. This product was cryopreserved and used for AHCT.
Approximately 5 weeks (minimum 28 days) following etoposide, patients began the preparative regimen. BCNU 15 mg/kg, capped at 550 mg/m2, was administered i.v. on day −4, and melphalan 200 mg/m2 was administered i.v. on day −2. Patients received their autologous graft on day 0 and were discharged from the hospital on day + 1. Filgrastim 5 mcg/kg/day was administered beginning on day + 6 until neutrophil recovery. Standard supportive care was provided, and patients were readmitted to the hospital as necessary.
Response assessment
Disease response was assessed before first mobilization, before transplant, then 3 months and 6 months post transplant, and annually post transplant or sooner as clinically indicated. Response was categorized as CR, very good PR (VGPR), PR, stable disease, or progressive disease, according to the International Uniform Response Criteria for Multiple Myeloma,11 with the following clarification: patients who had a negative urine analysis at diagnosis and REL for Bence-Jones and monoclonal protein did not have repeat urine studies at each assessment. Free light-chain analysis was not routinely performed.
Statistical methods
EFS and OS were estimated by the Kaplan–Meier method and compared by the log-rank test. Fisher’s exact test was used to compare pneumonitis and non-relapse mortality (NRM). A Cox proportional hazards model was used for univariate and multivariate analysis, and the significance of variables determined by the log-rank test.
Results
Patient characteristics
One hundred and seventy-six patients transplanted from January 1996 to September 2006 are included in this analysis. Patient characteristics are summarized in Table 1. Of the 176 patients, 118 were transplanted in CON1, and 58 were transplanted in REL. The median number of previous regimens was 1 (range 1–2) for CON1 and 2 (range 1–4) for REL. The median time from diagnosis to transplant was 0.9 years (range 0.3–2.8) for CON1 and 1.7 years (range 0.5–16.8) for REL. No patients transplanted in CON1 received bortezomib before transplant, and no patients in either group received lenalidomide before transplant. Nearly all patients were diagnosed by their local physician and received induction therapy locally. Cytogenetic, FISH, lactate dehydrogenase, albumin, β-2 microglobulin and International Staging System data from diagnosis were not available for most patients. Also, information on the total number of induction cycles and usage of bisphosphonates was not routinely available. Post transplant maintenance therapy was not included in this protocol, and information regarding salvage therapy for post transplant REL was not available.
Table 1.
Patient characteristics
| First consolidation |
Relapsed | |
|---|---|---|
| N | 118 | 58 |
| Median age (range) | 58 (37–74) | 56 (37–68) |
| Male | 58% | 64% |
| Durie–Salmon stage at diagnosis | ||
| 1A | 0 | 12% |
| 1B | 0 | 0 |
| 2A | 36% | 33% |
| 2B | 3% | 0 |
| 3A | 46% | 43% |
| 3B | 13% | 12% |
| Unknown | 2% | 0 |
| End organ damage at diagnosis | ||
| Calcium > 12 mg/dL | 9% | NA |
| Creatinine ≥ 2mg/dL | 14% | NA |
| Hb 8.5–10 g/dL | 18% | NA |
| Hb <8.5 g/dL | 19% | NA |
| Lytic lesions (not extensive) | 44% | NA |
| Advanced lytic lesions | 26% | NA |
| Isotype | ||
| IgA | 29% | 14% |
| IgG κ | 32% | 50% |
| IgG λ | 17% | 21% |
| Light chain only | 18% | 10% |
| IgD | 1% | 0 |
| Non-secretory | 3% | 5% |
| Prior regimens | ||
| Median no. (range) | 1 (1–2) | 2 (1–4) |
| VAD | 79% | 79% |
| Alkylator based | 10% | 50% |
| Thalidomide | 34% | 64% |
| Bortezomib | 0 | 31% |
| Lenalidomide | 0 | 0 |
| Median years diagnosis to BMT (range) |
0.86 (0.33–2.8) | 1.71 (0.45–16.8) |
Abbreviations: NA = not applicable; VAD = VCR, doxorubicin, dexamethasone.
Characteristics of patients transplanted in first consolidation or relapse.
Tandem mobilization
Most patients were able to mobilize stem cells successfully after the two planned sequential chemo-mobilization regimens of CY followed by etoposide. The median cell dose collected during the first (CY) chemo-mobilization was 14.8 × 106 CD34 + /kg (range 1.8–268). The median cell dose collected during the second chemo-mobilization was 7 × 106 CD34 + /kg (range 0.3–107), and 93% of patients used stem cells from only the second mobilization for transplant. Seven percent of patients were not able to yield at least 2 × 106 CD34 + /kg during the second chemo-mobilization, so backup cells from the first (CY) chemo-mobilization were utilized for transplant instead. Two patients did not complete tandem mobilization and were excluded from this analysis (one patient for disease progression and one patient for infectious toxicity complicated by deconditioning).
Outcome
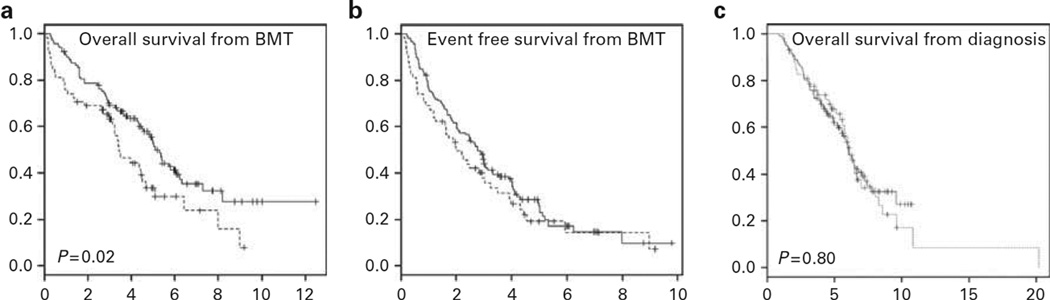
At a median follow-up of 4.7 years (range 0.9–12.5) for all living patients, the median OS from time of transplant was significantly longer for patients in CON1 at 5.1 years compared with those in REL at 3.4 years (P = 0.02; Figure 2a). The median EFS from time of transplant was not significantly different between CON1 (2.8 years) and relapsed (2.0 years) patients (P = 0.26; Figure 2b). However, when measured from the time of diagnosis, the median OS did not significantly differ between the patients in CON1 at 6.0 years vs REL at 6.1 years (P = 0.80; Figure 2c).
Figure 2.
Survival. (a) OS in years from time of transplant for patients in CON1 vs REL. (b) EFS in years from time of transplant for patients in CON1 vs REL. (c) OS in years from time of diagnosis for patients in CON1 vs REL. Solid line: first consolidation (N = 118). Dashed line: relapse (N = 58).
Response assessment for patients transplanted in CON1 is summarized in Table 2. Tandem chemo-mobilization improved response in evaluable patients from 32% VGPR + CR pre-mobilization to 53% VGPR + CR pretransplant. Response rate further increased with augmented high-dose therapy to 76% VGPR + CR at day 90 post transplant. Patients who attained VGPR + CR status had an improved EFS compared with those who did not (median 2.5 vs 3.0 years; P = .02), but there was no improvement in OS (3.9 vs 4.4 years; P = 0.58).
Table 2.
Efficacy
| Category | Pre-mobilization | Pre-transplant | Post transplant |
|---|---|---|---|
| No. evaluable | 108 | 91 | 93 |
| Progressive disease | 0 | 0 | 3% |
| Stable disease | 4% | 2% | 1% |
| Partial response | 64% | 45% | 20% |
| VGPR | 20% | 33% | 47% |
| CR | 12% | 20% | 29% |
| VGPR + CR | 32% | 53% | 76% |
Abbreviation: VGPR = very good PR.
Percentage of evaluable first consolidation patients in each response category pre-mobilization, pre-transplant and 90 days post-transplant. Not all patients had evaluable data for each time point (total N = 118).
Toxicity
Early 100 day NRM was significantly higher in REL at 7% compared with 0 in CON1 (P = 0.01). There was no significant difference in total NRM between REL (17%) and CON1 (11%; P = 0.35). The causes of late NRM in CON1 were unknown,4 MDS/AML,3 pneumonitis,2 infection,2 cerebral hemorrhage1 and lung cancer.1 The interstitial pneumonitis rate was 33% for all patients, and interstitial pneumonitis did not correlate with smoking history, CMV serology or REL status at transplant.
The median time to neutrophil engraftment (≥ 500/µL) was 11 days, and the median time to platelet engraftment (≥ 20 000/µL) was 14 days. Patients were discharged on day + 1 following transplant, and 76% of all patients required rehospitalization within the first 30 days, typically for neutropenic fever and/or mucositis. In all, 24% of patients were able to complete this intensified protocol as outpatients.
Univariate and multivariate analysis of CON1 patients
Univariate analyses for EFS and OS from time of transplant in CON1 examined pneumonitis, previous thalidomide, age ≥ 65 years and Durie–Salmon Stage 3 disease at diagnosis. No variables were significant for EFS in univariate or multivariate analysis. Only Durie–Salmon Stage 3 disease was significant for OS in univariate analysis, and it remained significant for OS in multivariate analysis (hazard ratio 1.5; P = 0.04).
Discussion
Single AHCT with high-dose melphalan at 200 mg/m2 is the most commonly used preparative regimen for multiple myeloma, but nearly all patients eventually develop progressive disease. To improve outcomes, intensified treatment protocols have been explored, such as additional cytotoxic chemotherapy, radiation therapy, maintenance therapy, tandem autografts and tandem auto-allografts.3,4,6,12,13 Our protocol attempted to improve on single agent melphalan 200 mg/m2 by intensifying the mobilization and preparative regimen without subjecting patients to a second transplant or radiation. Our intensified protocol was able to achieve high-quality response rates and favorable EFS and OS with manageable toxicity. Patients in CON1 had a high VGPR + CR rate of 76% post transplant, especially as only 34% of patients received frontline thalidomide, and none received lenalidomide or bortezomib.
We utilized a novel strategy of tandem chemo-mobilization followed by augmented high-dose therapy. Restaging studies demonstrated an improvement in response premobilization vs pre-transplant (post-mobilization) to tandem chemo-mobilization with CY and etoposide. Tandem chemo-mobilization improved response rates, and may have conferred an in vivo purging effect from the harvested graft.
BCNU 15 mg/kg was added to melphalan 200 mg/m2 to intensify the high-dose therapy in this protocol in an attempt to improve outcomes compared with single agent melphalan. One third of patients developed interstitial pneumonitis requiring medical intervention (at least CTCAE grade 2). Pneumonitis is a known toxicity of high-dose BCNU and typically presented as dyspnea, malaise and fever 6–10 weeks post transplant.14 Pneumonitis was confirmed by a decrease in DLCO by pulmonary function testing, and this complication was managed as an outpatient with corticosteroids in most cases. There was no 100-day NRM in CON1 patients, and only 2 late deaths from pneumonitis in CON1 patients. A smaller frontline study of 49 patients from Memorial Sloan-Kettering Cancer Center reported a 10% rate of grade ≥ 2 pulmonary toxicity using a lower dose of BCNU (300 mg/m2) with melphalan.15 We are now conducting a modified protocol using a lower dose of BCNU to reduce the incidence of pneumonitis.
Despite our more intensive preparative regimen, engraftment was not delayed, and one quarter of patients were able to complete the transplant as an outpatient. In contrast to BCNU, the addition of idarubicin and CY to standard melphalan resulted in 20% early NRM,8 and the addition of busulfan to melphalan was associated with an increased incidence of veno-occlusive disease of the liver.9 Our intensive cytotoxic treatment plan was associated with a 1.7% rate of MDS/AML, and all cases were fatal.
The most noteworthy observation with our intensified regimen was the marked improvement in quality response rate from 32% VGPR + CR pre-mobilization to 76% VGPR + CR post transplant in CON1 patients. The IFM9502, Bologna96 and MRC7 studies are the largest randomized trials of 200 mg/m2 melphalan.2,3,5 The major benefit of our protocol in response rate appeared to be a higher rate of VGPR, rather than CR. Our VGPR + CR rate in CON1 of 76% compares favorably with 55% in IFM9502 (not reported in Bologna96 and MRC7). The CR rate in CON1 was 29%, compared with 35% in IFM9502 and 44% in MRC7 (not reported in Bologna96). The median EFS in CON1 in our study was 2.8 years, which compares favorably with the median EFS of 1.7, 1.9 and 2.6 years reported in the IFM9502, Bologna96 and MRC7 studies, respectively. With regard to OS, the median OS in CON1 in our study was 5.1 years, and it was not reported, 5.4 years and 4.5 years in the IFM9502, Bologna96 and MRC7 studies, respectively.
Patients in CON1 attaining VGPR or better had improved EFS but not OS. The time period of our study overlapped the introduction of immunomodulatory agents and proteasome inhibitors, and these newer agents have been associated with improved OS following REL post transplant.16 Salvage therapy, particularly novel agents, given to our patients for post transplant REL likely confounded OS. There was not a uniform approach, and data on post transplant therapy were not available. No patients in our study received maintenance therapy.
When measured from time of diagnosis, OS did not differ between CON1 and REL, but when measured from time of transplant OS was significantly longer in the CON1 group. EFS did not significantly differ between CON1 and REL when measured from time of transplant. There was higher early (100 day) NRM in REL, but there was no difference in overall NRM. As most relapsed patients were transplanted in first REL, our results suggest that delaying transplant to first REL is a reasonable option, as OS from diagnosis was similar for CON1 and REL. These results concur with the Myelome Autogreffe randomized study that reported no difference in OS from time of diagnosis for upfront vs delayed transplant.17
Recently, newer agents have been incorporated with transplant. The addition of novel agents in induction therapy has significantly improved quality response rates,18 and the survival results of several ongoing studies are pending.19 Bortezomib has been added to the preparative regimen of high-dose melphalan resulting in a CR rate of 35% and a VGPR + CR rate of 70%, similar to our VGPR + CR rate of 76%.20 Maintenance therapy with thalidomide post transplant has improved survival, particularly in those with detectable myeloma post-AHCT,21 but this has not been widely adopted due to significant side effects. Early results with lenalidomide maintenance after auto-hematopoietic cell transplant show a significant improvement in EFS, although there is no difference in OS with short follow-up.22,23
In summary, our study aimed to intensify the efficacy of high-dose melphalan by incorporating tandem chemomobilization and adding BCNU to the conditioning regimen. Our intensified cytotoxic protocol achieved a high VGPR + CR rate of 76%, although OS was comparable to historical controls. Incorporating novel agents for maintenance post transplant with our intensified transplant protocol is being considered to improve survival.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 4.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 5.Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–2441. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 6.Barlogie B, Jagannath S, Desikan KR, Mattox S, Vesole D, Siegel D, et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999;93:55–65. [PubMed] [Google Scholar]
- 7.Anagnostopoulos A, Aleman A, Ayers G, Donato M, Champlin R, Weber D, et al. Comparison of high-dose melphalan with a more intensive regimen of thiotepa, busulfan, and cyclophosphamide for patients with multiple myeloma. Cancer. 2004;100:2607–2612. doi: 10.1002/cncr.20294. [DOI] [PubMed] [Google Scholar]
- 8.Fenk R, Schneider P, Kropff M, Huenerlituerkoglu AN, Steidl U, Aul C, et al. High-dose idarubicin, cyclophosphamide and melphalan as conditioning for autologous stem cell transplantation increases treatment-related mortality in patients with multiple myeloma: results of a randomised study. Br J Haematol. 2005;130:588–594. doi: 10.1111/j.1365-2141.2005.05641.x. [DOI] [PubMed] [Google Scholar]
- 9.Carreras E, Rosinol L, Terol MJ, Alegre A, de Arriba F, Garcia-Larana J, et al. Veno-occlusive disease of the liver after high-dose cytoreductive therapy with busulfan and melphalan for autologous blood stem cell transplantation in multiple myeloma patients. Biol Blood Marrow Transplant. 2007;13:1448–1454. doi: 10.1016/j.bbmt.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Taylor TL, Hu WW, Johnston LJ, Shizuru JA, Negrin RS, Chao NJ, et al. Total body irradiation does not affect outcome in patients autografted for multiple myeloma. Biol Blood Marrow Transplant. 2000;6:160. [Google Scholar]
- 11.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 12.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 13.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 14.Jones RB, Matthes S, Shpall EJ, Fisher JH, Stemmer SM, Dufton C, et al. Acute lung injury following treatment with high-dose cyclophosphamide, cisplatin, and carmustine: pharmacodynamic evaluation of carmustine. J Natl Cancer Inst. 1993;85:640–647. doi: 10.1093/jnci/85.8.640. [DOI] [PubMed] [Google Scholar]
- 15.Comenzo RL, Hassoun H, Kewalramani T, Klimek V, Dhodapkar M, Reich L, et al. Results of a phase I/II trial adding carmustine (300 mg/m2) to melphalan (200 mg/m2) in multiple myeloma patients undergoing autologous stem cell transplantation. Leukemia. 2006;20:345–349. doi: 10.1038/sj.leu.2404003. [DOI] [PubMed] [Google Scholar]
- 16.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 18.Barlogie B, Anaissie E, van Rhee F, Haessler J, Hollmig K, Pineda-Roman M, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138:176–185. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 19.Bensinger W. Stem-cell transplantation for multiple myeloma in the era of novel drugs. J Clin Oncol. 2008;26:480–492. doi: 10.1200/JCO.2007.11.6863. [DOI] [PubMed] [Google Scholar]
- 20.Roussel M, Moreau P, Huynh A, Mary JY, Danho C, Caillot D, et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM) Blood. 2010;115:32–37. doi: 10.1182/blood-2009-06-229658. [DOI] [PubMed] [Google Scholar]
- 21.Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy PL, Owzar K, Stadtmauer EA, Giralt S, Hurd DD, Hassoun H, et al. Phase III intergroup study of lenalidomide (CC-5013) versus placebo maintenance therapy following single autologous stem cell transplant for multiple myeloma: CALGB 100104. J Clin Oncol. 2010;28(15 s) Abstract no. 8017. [Google Scholar]
- 23.Attal M, Cristini C, Marit G, Caillot D, Facon T, Hullin C, et al. Lenalidomide maintenance after transplantation for myeloma. J Clin Oncol. 2010;28(15 s) Abstract no. 8018. [Google Scholar]