Abstract
Transient receptor potential melastatin 8 (TRPM8) is activated by innocuous cool and noxious cold and plays a crucial role in cold-induced acute pain and pain hypersensitivity. To help understand the mechanism of TRPM8-mediated cold perception under normal and pathologic conditions, we used light microscopic immunohistochemistry and Western blot analysis in mice expressing a genetically encoded axonal tracer in TRPM8-positive (+) neurons. We investigated the coexpression of TRPM8 and vesicular glutamate transporter 1 (VGLUT1) and VGLUT2 in the trigeminal ganglion (TG) and the dental pulp before and after inducing pulpal inflammation. Many TRPM8+ neurons in the TG and axons in the dental pulp expressed VGLUT2, while none expressed VGLUT1. TRPM8+ axons were dense in the pulp horn and peripheral pulp and also frequently observed in the dentinal tubules. Following pulpal inflammation, the proportion of VGLUT2+ and of VGLUT2+/TRPM8+ neurons increased significantly, whereas that of TRPM8+ neurons remained unchanged. Our findings suggest the existence of VGLUT2 (but not VGLUT1)-mediated glutamate signaling in TRPM8+ neurons possibly underlying the cold-induced acute pain and hypersensitivity to cold following pulpal inflammation.
Keywords: TRPM8, vesicular glutamate transporter, inflammation, dental pulp, cold pain
INTRODUCTION
Transient receptor potential melastatin 8 (TRPM8), a member of TRP channel superfamily, is activated by innocuous cool and noxious cold, as well as by menthol (Dhaka et al., 2007; Knowlton and McKemy, 2011). It plays a crucial role in mediating cold-induced acute pain and hypersensitivity following inflammation and nerve injury (McKemy et al., 2002; Bautista et al., 2007; Knowlton et al., 2010). Stimulation of TRPM8 in the central afferents of dorsal root ganglion (DRG) neurons by cold or menthol increases the frequency of miniature excitatory postsynaptic currents in the postsynaptic dorsal horn neurons, suggesting that transmission of TRPM8-mediated cold in the 1st relay nucleus in the CNS is mediated by glutamate (Baccei et al., 2003; Tsuzuki et al., 2004; Wrigley et al., 2009). Studies with electrical and chemical stimulation of peripheral axons, and administration of glutamate into peripheral tissues, also showed that glutamate released from peripheral axons plays a crucial role in the signaling of acute pain and hyperalgesia (Du et al., 2001; Svensson et al., 2003; Miller et al., 2011). However, the type of vesicular glutamate transporters (VGLUTs) associated with the signal transduction of TRPM8-meidated cold remains unknown.
VGLUTs are involved in the packing of glutamate into synaptic vesicles prior to exocytotic release and thus play a crucial role in the glutamatergic transmission (Fremeau et al., 2004). Of the three VGLUTs, VGLUT1 and VGLUT2 are expressed by largely non-overlapping and functionally distinct populations of primary sensory neurons (Fremeau et al., 2004; Brumovsky et al., 2007). Recent reports, including studies using VGLUT2-deficient mice, suggest that VGLUT2-dependant glutamate release from primary sensory afferents plays an important role in normal acute nociception and pathologic pain (Moechars et al., 2006; Leo et al., 2009; Liu et al., 2010; Scherrer et al., 2010; Lagerstrom et al., 2011; Rogoz et al., 2012). Specifically, VGLUT2 increases in the trigeminal ganglion (TG) neurons following Complete Freund’s Adjuvant (CFA)-induced pulpal inflammation, suggesting that VGLUT2-dependant glutamate signaling may mediate pulpal inflammatory pain (Yang et al., 2014). However, little is known about the alteration in expression of TRPM8 and the associated VGLUTs in TG neurons following dental pulp inflammation.
To help understand the role of neuronal TRPM8 and VGLUT in the dental hypersensitivity to cold, we investigated the coexpression of TRPM8 and VGLUTs in the TG and dental pulp before and after CFA-induced pulpal inflammation, using immunohistochemistry and Western blot analysis in transgenic mice expressing a genetically encoded axonal tracer in TRPM8-positive neurons.
EXPERIMENTAL PROCEDURES
Animals and tissue preparation
All experimental procedures and animal care were conducted in accordance with the National Institutes of Health guidelines and were approved by the Kyungpook National University Intramural Animal Care and Use Committee (permit number, KNU 2011-4). Experiments were designed to minimize the number of animals used and their discomfort. Transgenic (TRPM8GFP) mice, expressing enhanced green fluorescent protein (GFP) by the TRPM8 transcriptional promoter were generated, mated to C57BL/6 mice, and their offspring were genotyped as described previously (Takashima et al., 2007). Twenty-nine male TRPM8GFP mice (weight 25–30 g), aged 6–10 weeks, were used for this study: Nineteen for light microscopic (LM) immunohistochemistry (three for normal group, five each for CFA treatment with 3 days of survival and their control, and six for retrograde labeling of tooth pulp afferents), and 10 for Western blotting.
Tooth pulp inflammation model and retrograde labeling
To induce tooth pulp inflammation, mice were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and the occlusal enamel and dentin of the right maxillary 1st (M1) and 2nd (M2) molars were filed off to just before exposing the pulp using a low-speed dental drill with a round bur under water-cooling. A fine paper point soaked in 50% CFA solution in saline (experimental) or saline for control (sham) was applied to the exposed dentinal surfaces for 5 min. For retrograde labeling of pulpal afferents, the dental pulp of M1 and M2 was exposed and crystals of tetramethylrhoda mine-conjugated dextran amine (TMR-DA, 3000 MW, D3308, Invitrogen, Carlsbad, CA, USA) were applied for 5 min. After that, the dentinal or pulpal surfaces were sealed with dental cement. Mice were euthanized 3 days after the application of CFA, saline or TMR-DA.
LM immunohistochemistry
For immunofluorescence, mice were deeply anesthetized with sodium pentobarbital (40 mg/kg, i.p.), transcardially perfused with heparinized normal saline, followed by freshly prepared fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The right TGs and maxillae including M1 and M2 were dissected out and postfixed in the same fixative for 2 h at 4 °C. For immunofluorescence of the dental pulp, the fixed maxillae were decalcified in 10% ethylenediaminetetraacetic acid (EDTA, pH 7.4) at 4 °C for 6 days, changing the EDTA solution daily, and then were rinsed in PB for 30 min. Samples were cryoprotected in 30% sucrose in PB overnight at 4 °C. The next day, 40-µm-thick sections were cut on a freezing microtome.
Sections of TGs and dental pulp were permeabilized with 50% ethanol for 30 min, blocked with 10% normal donkey serum (NDS, Jackson ImmunoResearch, West Groove, PA, USA) for 30 min, and incubated overnight in a mixture of rabbit anti-GFP antibody and VGLUT1, VGLUT2, protein gene product 9.5 (PGP9.5), Calcitonin Gene-Related Peptide (CGRP) or anti-neurofilament 200 (NF200) antibodies. The primary antibodies were rabbit anti-GFP (1:3000, A11122, Invitrogen, Carlsbad, CA, USA), guinea-pig anti-VGLUT1 (1:2000, 135304, Synaptic Systems, Gottingen, Germany), guinea-pig anti-VGLUT2 (1:1000, Af670, Frontier Institute Co. Ltd., Hokkaido, Japan), mouse anti-PGP 9.5 (1:5000, YM8104, Accurate Chemical and Scientific Corp., Westbury, NY, USA), mouse anti-CGRP (1:1000, ab81887, Abcam, Cambridge, MA, USA) or mouse anti-NF200 (1:100,000, N0142, Sigma–Aldrich, St. Louis, MO, USA). On the next day, the sections were rinsed with phosphate-buffered saline (PBS; 0.01 M, pH 7.4) several times and incubated for 3 h in a mixture of fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit and Cyanine 3 (Cy3)-conjugated donkey anti-guinea-pig or anti-mouse antibody or in a mixture of FITC-conjugated donkey anti-rabbit and Cy5-conjugated donkey anti-guinea-pig or donkey anti-mouse antibodies (1:200, Jackson ImmunoResearch). The sections were then rinsed, mounted on slides, and coverslipped with Vectashield (Vector Lab, Burlingame, CA, USA). Micrographs were obtained with an Exi digital camera (Q-imaging Inc., Surrey, CA, USA), attached to a Zeiss Axioplan 2 fluorescence microscope (Carl Zeiss Inc., Jena, Germany) or with a confocal microscope (LSM 510 META, Carl Zeiss, confocal images were acquired at a same optical slice thickness for all channels).
To confirm that the inflammation is confined to the dental pulp, sections containing dental pulp were processed, as described above, using goat anti-CD64 antibody (1:500, sc-7642, Santa Cruz Biotechnology, Dallas, TX, USA), and FITC-conjugated donkey anti-goat antibody (1:200, Jackson ImmunoResearch), as primary and secondary antibodies, respectively.
To control for the specificity of the primary antibodies, sections were processed as described above, except that primary or secondary antibodies were omitted or blocking peptides were added: Omission of primary or secondary antibodies or preadsorption with blocking peptides for VGLUT1 (15 µg/ml, 135-3P, Synaptic Systems) and VGLUT2 (10 µg/ml, G07B, Frontier Institute Co. Ltd.) abolished the respective staining. The specificity of the anti-GFP antibody was confirmed in a non-GFP-expressing mouse line (Moldrich et al., 2010): GFP was not detected in the TG and dental pulp in the wild-type C57BL/6 mice using a rabbit anti-GFP antibody.
Quantitative analysis
To quantify the proportion of TRPM8+, VGLUT1+, and VGLUT2+ somata in the TG, a total of 36–48 images from the maxillary portion of the TG, which contains many TRPM8+, VGLUT1+ or VGLUT2+ somata, from 4 tissue sections each of TG on each side of normal, control, and CFA-3 day mice were collected using 20× objective (857.14 × 652.94 µm, 1360 × 1036 pixels). Images were converted to grayscale and brightness and contrast were enhanced using identical adjustment steps for all images. The threshold level for defining a neuron as immunopositive was set at 100–120 (out of 255) gray levels using Image J software (NIH, Bethesda, MD, USA). The fraction of TRPM8+, VGLUT1+, and VGLUT2+ neurons of all TG neurons and of TRPM8+ neurons coexpressing VGLUT1 or VGLUT2 were calculated and the differences between CFA- and control groups were analyzed with Student’s t-test; statistical significance was set at p < 0.05.
Western blot
Mice were perfused with saline and maxillary portion of the TGs were dissected out in the same way as described for immunohistochemistry. All chemicals, unless stated otherwise, were purchased from Sigma–Aldrich. The samples were homogenized in 150 µl of ice-cold lysis buffer (20 mM Tris–HCl pH 7.4, 5 mM EDTA, 140 mM NaCl, 1% Triton X-100, 1 mM Na3VO4, 1 mM PMSF, 10 mM NaF, and 1 µg/ml aprotinin). The particulate debris was removed by centrifugation (12,000g for 20 min at 4 °C) and the protein in supernatants was adjusted for equal protein concentration with Rad Protein Assay kit (Bio-Rad, Hercules, CA, USA). After boiling in 5× sodium dodecyl sulfate (SDS)-loading buffer for 5 min, the samples were subjected to 12% SDS–polyacrylamide gel and the resolved proteins were electrotransferred to Immobilon-P membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with blocking solution (1× Tris-buffered saline (TBS), 5% nonfat milk, 0.02% NaN3) for 2 h and incubated overnight at 4 °C with primary antibodies; mouse anti-β-actin (1:2000, sc81178, Santa Cruz Biotechnology), VGLUT1 (Synaptic Systems), VGLUT2 (Frontier Institute Co. Ltd.) or GFP (Invitrogen) antisera were used at the same dilutions as for immunohistochemistry. After incubation, the membranes were washed with TBS-tween 20 and incubated with horseradish peroxidase-conjugated goat anti-rabbit (1:2000, sc2004, Santa Cruz Biotechnology) or goat anti-guinea-pig immunoglobulin G antibody (1:2000, sc2438, Santa Cruz Biotechnology) for 1 h at room temperature. The membranes were treated with ECL solution (EMD Millipore) and visualized by ChemiDoc (BioRad, Hercules, CA, USA). Protein concentrations were compared using Student’s t-test, statistical significance was set at p < 0.05.
RESULTS
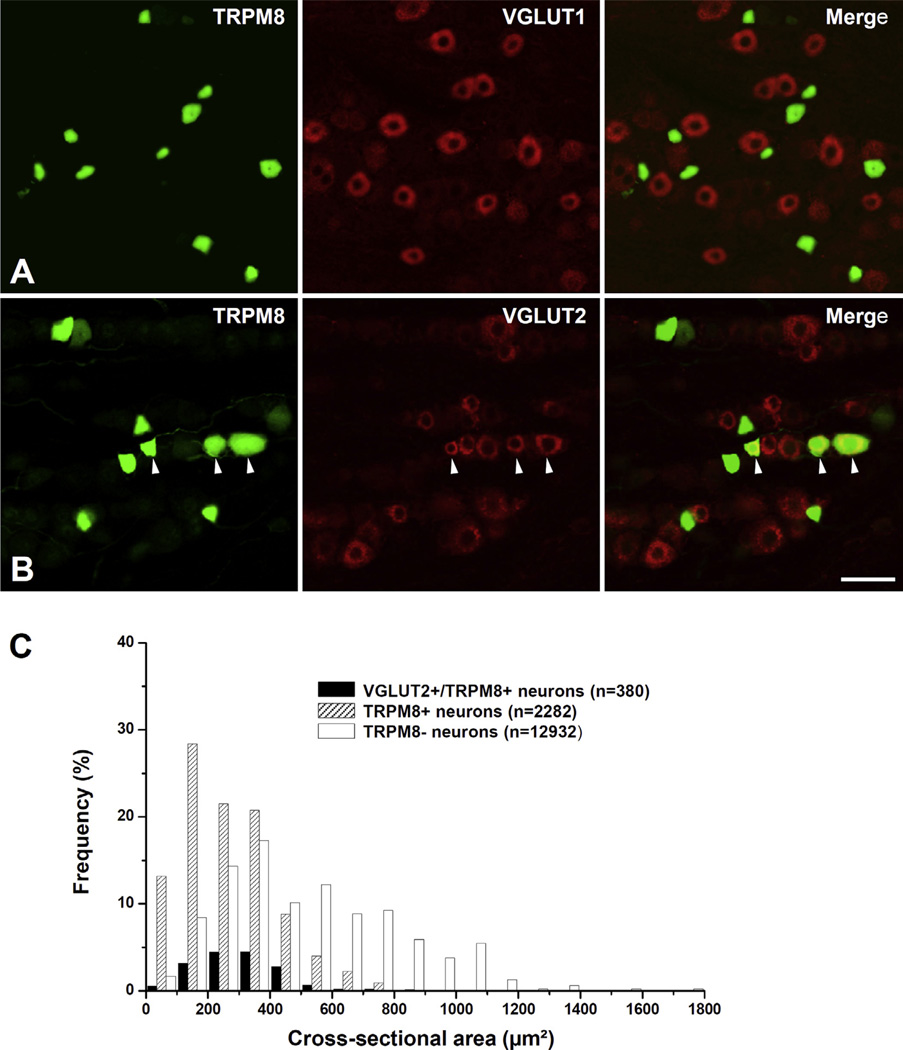
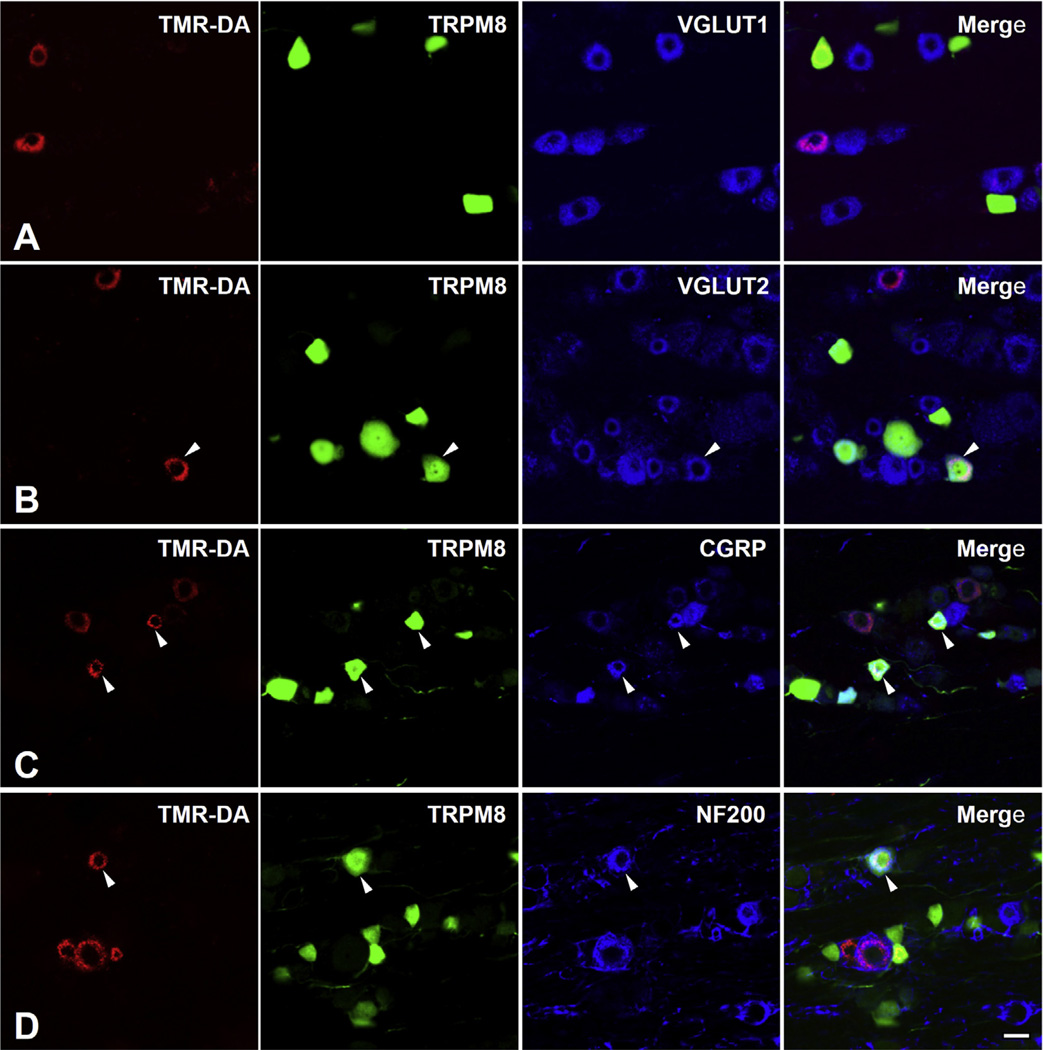
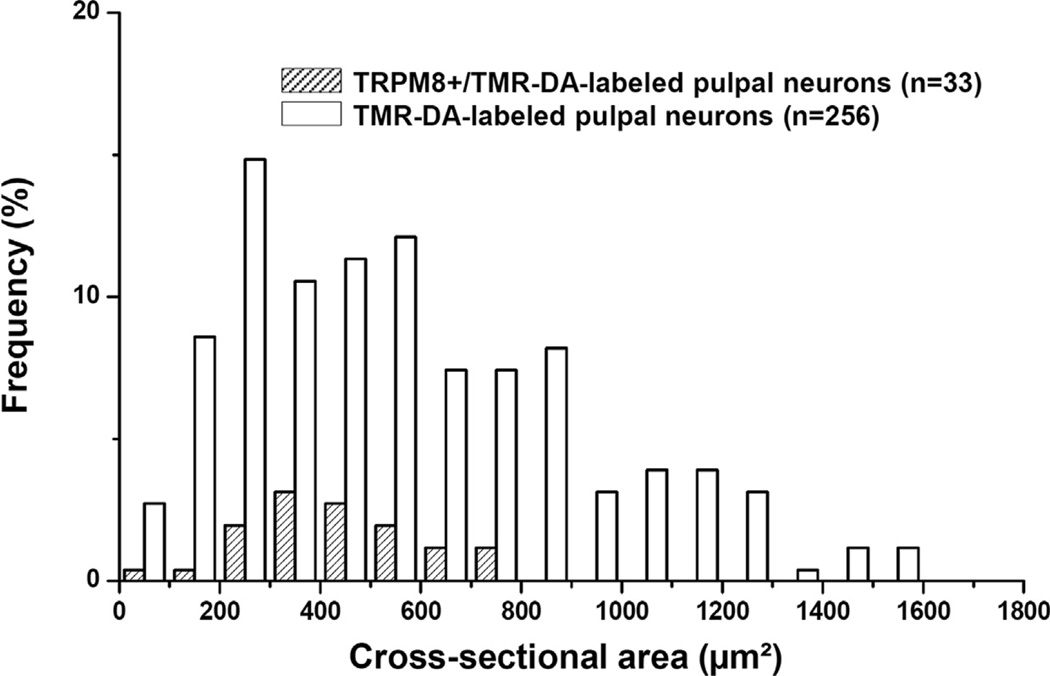
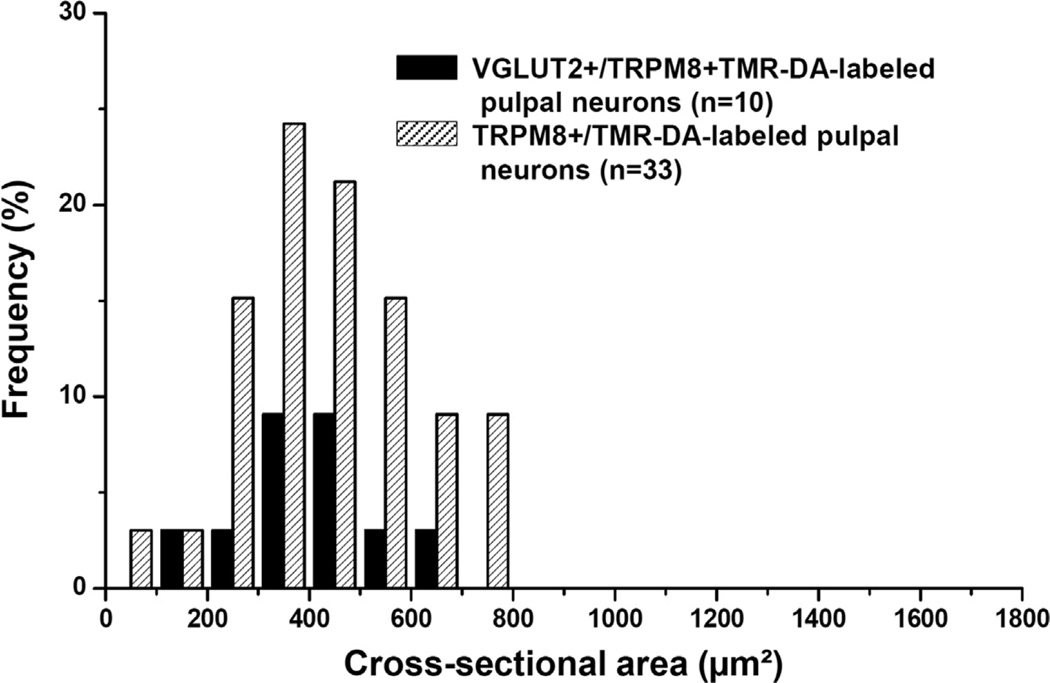
In the TG, immunoreactivity for the reporter GFP was confined to the cytoplasm of predominantly small- and medium-sized neurons (size range: 58–893 µm2 in cross-sectional area). Double immunofluorescence staining for TRPM8 and VGLUT1 or VGLUT2 showed that a minor proportion of TRPM8+ neurons coexpressed VGLUT2 (18.0 ± 1.0%) whereas none coexpressed VGLUT1. VGLUT2 was expressed mostly in small- and medium-sized TRPM8+ neurons (Fig. 1). In addition, neurons of various size were retrogradely labeled by TMR-DA injection into the maxillary molars pulp (size range: 68–1568 µm2, Figs. 2 and 3), consistent with previous reports (Fried et al., 1989; Stenholm et al., 2002; Paik et al., 2009). Among the TMR-DA-labeled pulpal neurons, TRPM8 was expressed mostly in small- and medium-sized neurons (size range: 81–797 µm2, Figs. 3 and 4). In addition, the TRPM8+ pulpal neurons frequently coexpressed CGRP or NF200, suggesting that TRPM8 is expressed in pulpal nociceptive neurons, as well as in A myelinated fiber neurons (Fig. 2C, D). Many TRPM8+ pulpal neurons coexpressed VGLUT2, but not VGLUT1: The VGLUT2+/TRPM8+ pulpal neurons were also mostly small- and medium-sized (size range: 136–690 µm2, Figs. 2 and 4).
Fig. 1.
Double immunofluorescent staining for TRPM8 and VGLUT1 (A) or VGLUT2 (B), and size distribution (C) of TRPM8+ neurons and VGLUT2+/TRPM8+ neurons in the trigeminal ganglion. Many TRPM8+ neurons coexpress VGLUT2 (B, arrowheads). Note that the TRPM8+ neurons and VGLUT2+/TRPM8+ neurons are mostly small- and medium-sized. n = number of neurons. Scale bar = 50 µm.
Fig. 2.
Triple immunofluorescent staining for TMR-DA, TRPM8 and VGLUT1 (A), VGLUT2 (B), CGRP (C) or NF200 (D) in the TG, 3 days after TMR-DA injection into the maxillary 1st and 2nd molar pulps. A TMR-DA-labeled neuron that innervates dental pulp coexpresses TRPM8 and VGLUT2 (B, arrowheads). TRPM8+/TMR-DA-labeled pulpal neurons frequently coexpress CGRP (C, arrowheads) and NF200 (D, arrowhead). Scale bar = 20 µm.
Fig. 3.
Size distribution of TMR-DA-labeled and TRPM8+/TMR-DA-labeled neurons in the TG. TRPM8+ neurons that innervate the dental pulp are mostly small- and medium-sized.
Fig. 4.
Size distribution of TRPM8+ TMR-DA-labeled pulpal neurons coexpressing VGLUT2 in the TG. VGLUT2+/TRPM8+ neurons that innervate the dental pulp are small- and medium-sized.
In the dental pulp, the immunoreactivity for the reporter GFP colocalized with PGP9.5, indicating TRPM8 expression in axons (Fig. 5). TRPM8+ axons were rare within the axon bundles in the radicular pulp, but dense in the peripheral pulp under the odontoblast layer and pulp horn, where they branched extensively and formed networks. TRPM8+ axons were also common in the dentinal tubules “ascending” toward the enamel (Fig. 6). Many TRPM8+ pulpal axons coexpressed VGLUT2, but not VGLUT1; immunostaining for VGLUT2 was concentrated in the beaded portion of the TRPM8+ axons in the coronal pulp (Fig. 7).
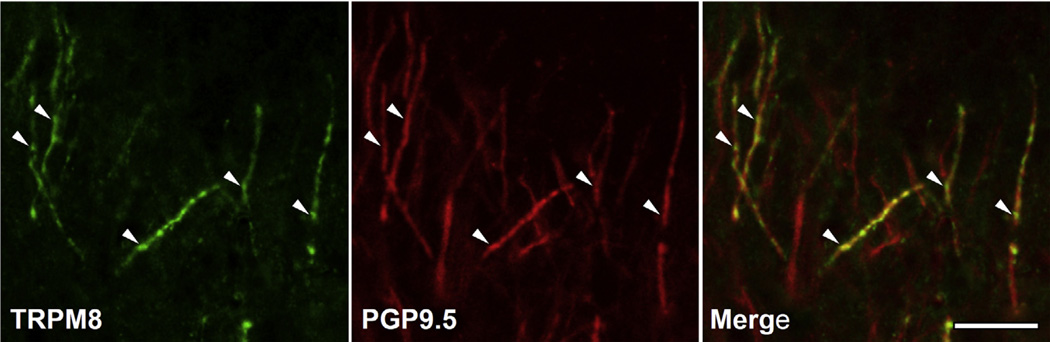
Fig. 5.
Double immunofluorescent staining for TRPM8 and PGP9.5 in the coronal portion of the dental pulp. Co-staining for TRPM8 and PGP9.5 (arrowheads) indicates expression of TRPM8 in pulpal axons. Scale bar = 20 µm.
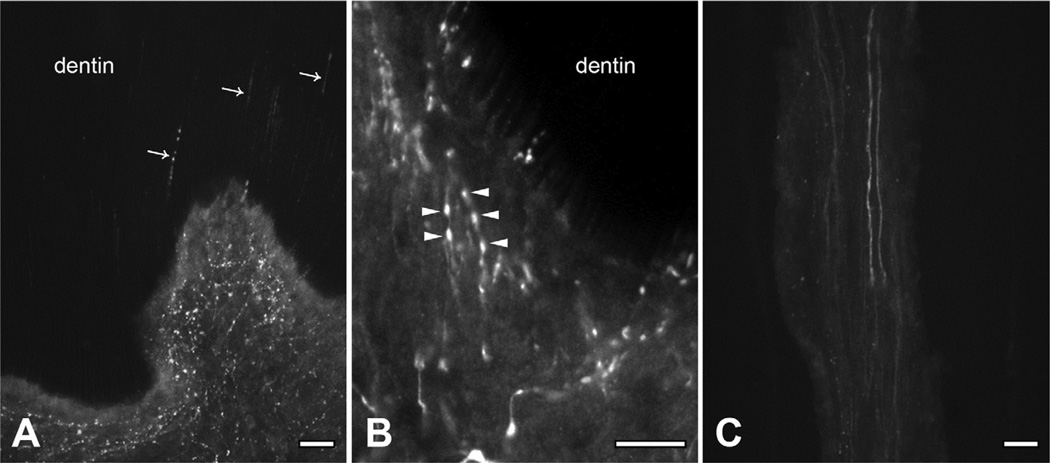
Fig. 6.
Immunofluorescent staining for TRPM8 in the pulp horn (A), the peripheral portion of the coronal pulp (B), and in the radicular pulp (C). (A, B) TRPM8+ axons are dense and branch extensively in the pulp horn and peripheral pulp. Some TRPM8+ axons “ascend” toward the enamel within the dentinal tubules (arrows in A). TRPM8 is densely expressed in axonal beads (arrowheads in B). (C) TRPM8+ axons are sparse and rarely have collaterals in the radicular pulp. Scale bar = 20 µm.
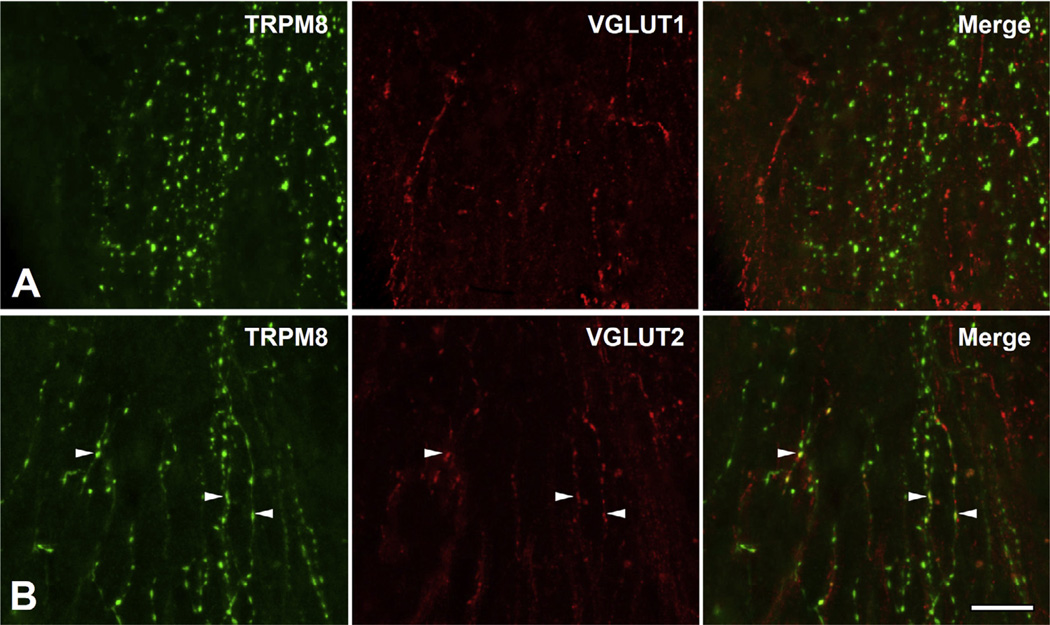
Fig. 7.
Double immunofluorescent staining for TRPM8 and VGLUT1 (A) or VGLUT2 (B) in the dental pulp. Many TRPM8+ pulpal axons express VGLUT2 (arrowheads), but not VGLUT1. Scale bar = 20 µm.
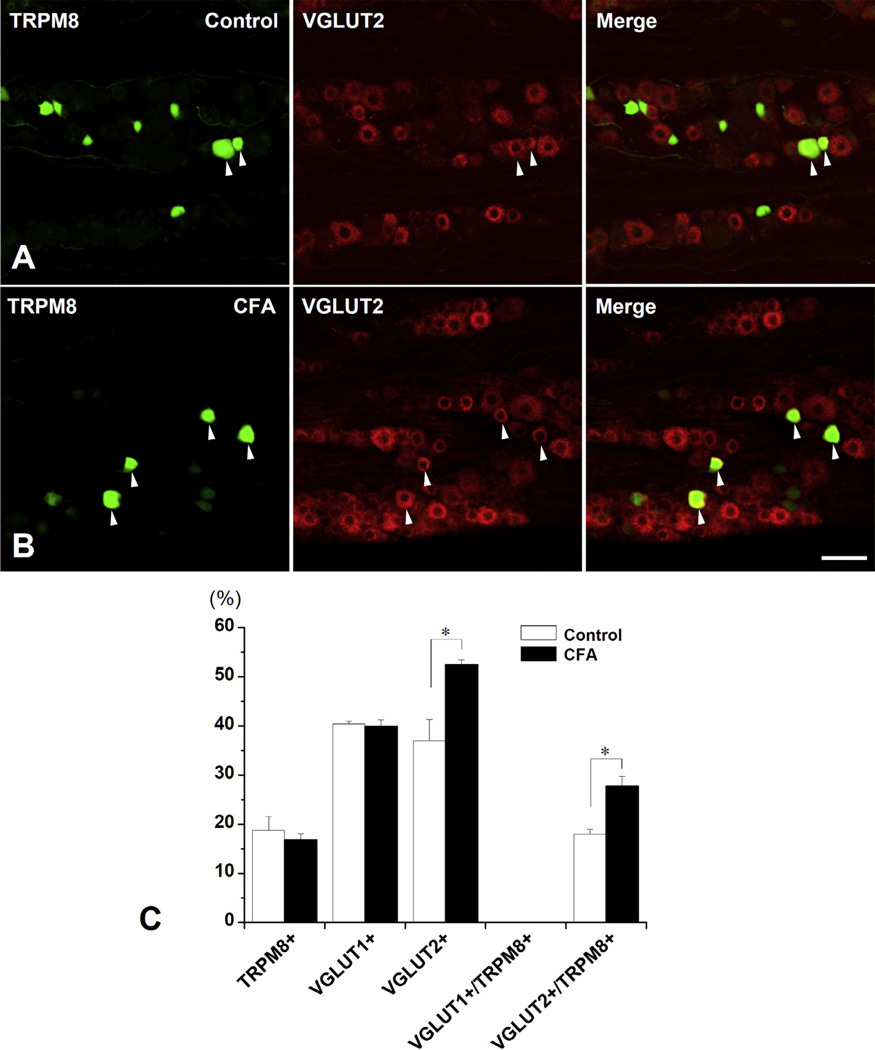
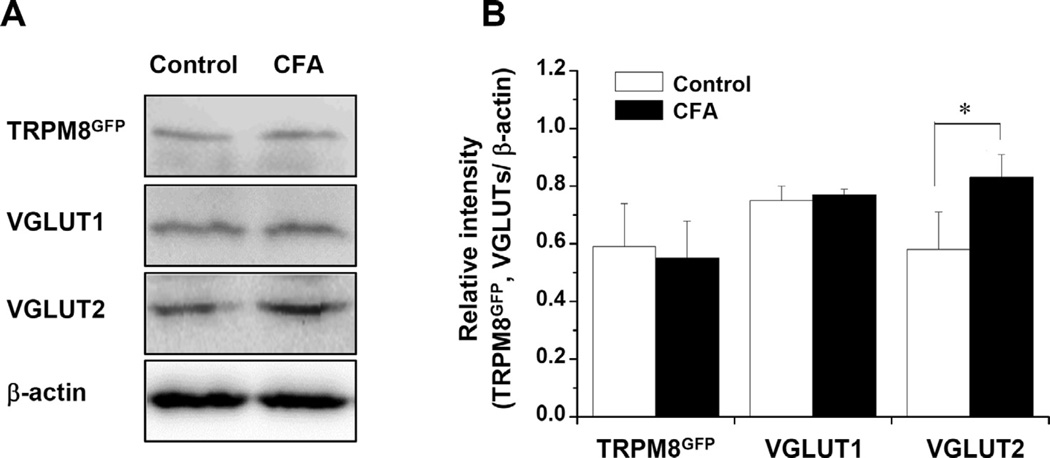
CD64+ cells were exclusively within the pulp in the CFA-treated group, indicating that inflammation had not spread to periapical tissues. The proportion (fraction of all neurons) of TRPM8+ and VGLUT1+ neurons in the TG did not differ significantly between the CFA-treated and control groups (TRPM8+ neurons: 16.8 ± 1.2% vs. 18.7 ± 2.3%, VGLUT1+ neurons: 39.9 ± 1.4% vs. 40.4 ± 0.5%). However, the proportion of VGLUT2+ neurons and of VGLUT2-expressing TRPM8+ neurons (VGLUT2+/TRPM8+ neurons) increased significantly in the CFA-treated group, compared to control (VGLUT2+ neurons: 52.5 ± 1.0% vs. 37.0 ± 4.4%, VGLUT2+/TRPM8+ neurons: 27.8 ± 2.0% vs. 18.0 ± 1.0%, p < 0.05, Fig. 8). The significant increase in the proportion of VGLUT2+/TRPM8+ neurons was observed in small- and medium-sized TRPM8+ neurons (Fig. 9), suggesting that both small- and medium-sized TRPM8+ neurons express VGLUT2 or increase their VGLUT2 expression following inflammation. The protein levels of TRPM8GFP and VGLUT1 were not significantly different between the CFA-treated and control groups, whereas the protein level of VGLUT2 increased significantly in the CFA-treated group, compared to control (Fig. 10).
Fig. 8.
Double immunofluorescent staining for TRPM8 and VGLUT2 (A, B) and proportion (%) of TRPM8+, VGLUT1+, VGLUT2+ and VGLUT2+/TRPM8+ neurons (C) in the TG in the control vs. the CFA-treated mice. (A, B) The number of VGLUT2+/TRPM8+ neurons (arrowheads) increases significantly in the CFA-treated group compared to control. (C) The proportion (Mean ± SE) of VGLUT2+/TRPM8+ neurons, but not of VGLUT1+/TRPM8+ neurons, is significantly higher in the CFA-treated group compared to control. N = 5 animals per group. *p < 0.05. Scale bar = 50 µm.
Fig. 9.
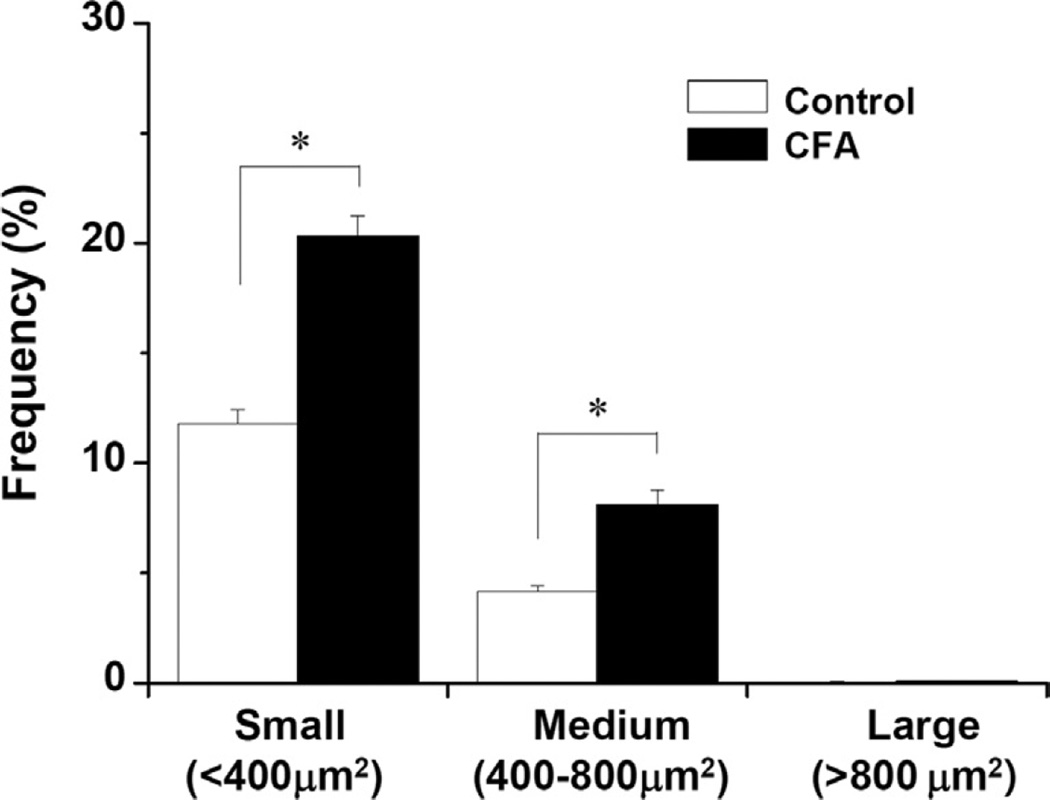
Proportion (%) of small-, medium-, and large-sized VGLUT2+/TRPM8+ neurons in the TG in control vs. CFA-treated mice. The proportion (Mean ± SE) of both small- and medium-sized VGLUT2+/TRPM8+ neurons are significantly higher in the CFA-treated group than in the control. N = 5 animals per group. *p < 0.05.
Fig. 10.
Protein levels of TRPM8GFP, VGLUT1 and VGLUT2 in the TG in the control group and CFA-treated group. (A) Western blot assay. (B) Protein levels (Mean ± SE) of TRPM8GFP and VGLUT1 are not significantly different between the control group and the CFA-treated group. However, the protein level of VGLUT2 is significantly higher in the CFA-treated group than in the control group. N = 5 animals per group. *p < 0.05.
DISCUSSION
The main findings of the present study are that (1) a minor proportion of TRPM8+ neurons in the TG and axons in the pulp express VGLUT2, but not VGLUT1, (2) TRPM8+ axons are dense in the pulp horn and peripheral pulp and frequently observed in the dentinal tubules, and (3) the number of TRPM8+ neurons in TG is unaltered, but the number of VGLUT2+/TRPM8+ neurons increases significantly following CFA-induced pulpal inflammation. These findings suggest that VGLUT2-mediated, but not VGLUT1-mediated, glutamate signaling from TRPM8+ neurons may mediate the transmission of pain and hypersensitivity to cold following pulpal inflammation.
Expression of VGLUT2 in TRPM8+ neurons
TRPM8−/− mice show significantly decreased pain response to noxious cold, implicating TRPM8 in the signaling of noxious cold (Bautista et al., 2007; Dhaka et al., 2007; Knowlton et al., 2010; McCoy et al., 2011). VGLUT2 is expressed in primary sensory neurons and plays a crucial role in acute nociceptive, inflammatory, and neuropathic pain (Moechars et al., 2006; Leo et al., 2009; Rogoz et al., 2012). That role depends on the type of nociceptive neuron, such that VGLUT2 in TRPV1+ neurons is involved in thermal nociception, whereas in NaV1.8+ neurons, it is involved in mechanical but not in thermal nociception (Lagerstrom et al., 2010, 2011; Rogoz et al., 2014). Applying the same reasoning, we surmise that VGLUT2+/TRPM8+ trigeminal neurons are involved in the mediation of noxious cold, whereas TRPM8+ -only neurons are involved in the mediation of innocuous cold. If this is correct, it would be in agreement with the observation that TRPM8 is expressed in two distinct populations of somatosensory neurons, nociceptive and non-nociceptive (Xing et al., 2006; Zanotto et al., 2007).
Activation of TRPM8 expressed by DRG neurons induces glutamate release from their terminals, which underlies the TRPM8-mediated signaling of cold (Baccei et al., 2003; Tsuzuki et al., 2004; Wrigley et al., 2009). In the present study, a minor proportion of TRPM8+ neurons expressed VGLUT2, and none expressed VGLUT1, suggesting that transmission of TRPM8-mediated noxious cold depends on VGLUT2−, not VGLUT1-mediated, glutamate signaling. The majority of TRPM8+ -only neurons may use neurotransmission mechanism not dependent on VGLUT1 or VGLUT2, but possibly on VGLUT3 (this may contribute a little, if it is present, because VGLUT3 is found in a small percentage of primary sensory neurons, Malet et al., 2013) and/or mechanism other than regular glutamatergic neurotransmission (Nicholls and Attwell, 1990) or non-glutamatergic neurotransmission (Tracey et al., 1991; Stoyanova and Lazarov, 2005).
The proportion of VGLUT2+ neurons in the mouse TG in the present study (37%) was lower than previously reported: from only a few neurons or almost all neurons in rat DRG (Oliveira et al., 2003; Landry et al., 2004), 65–69% in mouse DRG (Brumovsky et al., 2007; Malet et al., 2013), 15% or 85% in rat TG (Li et al., 2003; Yang et al., 2014). This variability may be due to differences in sensitivity of the antibodies used, choice of threshold levels for VGLUT2-immunoreactivity, species, or DRG vs. TG.
In the dental pulp, TRPM8+ axons formed a dense plexus in the peripheral pulp and pulp horn, and were also observed in the dentinal tubule “ascending” toward the enamel, suggesting that TRPM8-mediated cold may be perceived primarily at the peripheral pulp and in the dentinal tubules. Both TRPM8 and VGLUT2 were densely expressed primarily in the bead portion of pulpal axons, suggesting that the TRPM8-mediated cold transduction and VGLUT2-dependent glutamate release occurs preferentially in the varicosities of the pulpal axons rather than in the inter-varicose strand portions. The release of glutamate from nociceptive pulpal axons may be analogous to the inflammation-induced glutamate release from peripheral axons, which induces pain (Omote et al., 1998; deGroot et al., 2000). The expression of VGLUT1 and VGLUT2 in the mouse pulpal axons in the present study is at variance with our previous report (Paik et al., 2012), showing a small number of VGLUT2+ axons in the human dental pulp, possibly due to differences in species or antibodies.
Upregulation of VGLUT2 in TRPM8+ neurons following pulpal inflammation
In the present study, the number of TRPM8+ neurons in the TG did not change following pulpal inflammation, consistent with studies in the DRG following CFA-induced paw inflammation and spinal nerve injury (Obata et al., 2005; Dhaka et al., 2008), but at odds with findings of down-regulation of TRPM8 in the radicular pulp of human patients with irreversible pulpitis (Alvarado et al., 2007). The latter can be due to differences in species or regions examined (TG vs. radicular pulp). The amount of TRPM8GFP, though which may not be directly related to TRPM8 protein level, also did not alter following pulp inflammation. The number of VGLUT2+ and VGLUT2+/TRPM8+ neurons, and the VGLUT2 protein level in the TG increased significantly following pulpal inflammation, the latter possibly inducing a phenotypic switch of VGLUT2−/TRPM8+ neurons to VGLUT2+/TRPM8+ neurons. The increase in VGLUT2 expression in the TG and peripheral tissues following inflammation has been also reported previously (Yang et al., 2014). Considering that both TRPM8 and VGLUT2 are involved in mediating the cold hypersensitivity during pathologic conditions (Moechars et al., 2006; Colburn et al., 2007; Leo et al., 2009; Rogoz et al., 2012; Knowlton et al., 2013), we interpret our results to suggest that cold hypersensitivity in the dental pulp following inflammation is more closely related to the increased number of TRPM8+ neurons that express VGLUT2 through phenotypic alterations by inflammatory mediators and neurotrophic factors (Woolf and Ma, 2007) than to a direct effect of upregulation of TRPM8. Thus, activation of TRPM8 by cold stimuli during inflammation can stimulate VGLUT2 to induce an increase in the VGLUT2-dependant glutamate release from the TRPM8+ central and peripheral afferents (Daniels et al., 2011). In the dental pulp, the released glutamate can further activate and/or sensitize glutamate receptor-expressing nociceptive axons in a paracrine or autocrine manner (Lam et al., 2009; Miller et al., 2011), thereby contributing to peripheral sensitization to cold. If this is so, local application of selective VGLUT2 and/or TRPM8 blockers to the peripheral pulp or the dentinal tubules can be effective for relieving dental cold hypersensitivity while avoiding the side effects of systemic application.
In the present study, inflammation of the maxillary 1st and 2nd molars led to a significant increase of the proportion of VGLUT2+ neurons in the maxillary portion of the TG, even though these two teeth are innervated by only about 250 TG neurons (Pan et al., 2003). Such a strong response may be explained by interactions between TG neurons innervating the inflamed pulps and neurons that innervate non-inflamed tissues: Recent studies showed that the excitation of TG neurons that innervate non-inflamed tissues following inflammation, is mediated by substances such as nerve growth factor and substance P, released from neurons innervating inflamed tissue (Shinoda et al., 2011; Yasuda et al., 2012; Matsuura et al., 2013).
Acknowledgments
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP, 2008-0062282). The authors sincerely thank Dr. Juli Valtschanoff for helpful discussion and careful reading of the manuscript.
Abbreviations
- CGRP
Calcitonin Gene-Related Peptide
- CFA
Complete Freund’s Adjuvant
- Cy
Cyanine
- DRG
dorsal root ganglion
- EDTA
ethylenediaminetetraacetic acid
- FITC
fluorescein isothiocyanate
- GFP
green fluorescent protein
- LM
light microscopy
- NDS
normal donkey serum
- NF200
neurofilament 200
- PB
phosphate buffer
- PGP9.5
protein gene product 9.5
- TG
trigeminal ganglion
- SDS
sodium dodecyl sulfate
- TBS
Tris-buffered saline
- TMR-DA
tetrame thylrhodamine-conjugated dextran amine
- TRPM8
transient receptor potential melastatin 8
- VGLUT
vesicular glutamate transporter.
Footnotes
The authors declare that they have no conflict of interest.
REFERENCES
- Alvarado LT, Perry GM, Hargreaves KM, Henry MA. TRPM8 axonal expression is decreased in painful human teeth with irreversible pulpitis and cold hyperalgesia. J Endod. 2007;33:1167–1171. doi: 10.1016/j.joen.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccei ML, Bardoni R, Fitzgerald M. Development of nociceptive synaptic inputs to the neonatal rat dorsal horn: glutamate release by capsaicin and menthol. J Physiol. 2003;549:231–242. doi: 10.1113/jphysiol.2003.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Watanabe M, Hokfelt T. Expression of the vesicular glutamate transporters-1 and-2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147:469–490. doi: 10.1016/j.neuroscience.2007.02.068. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Daniels RW, Miller BR, DiAntonio A. Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol Dis. 2011;41:415–420. doi: 10.1016/j.nbd.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deGroot J, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. NeuroReport. 2000;11:497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001;89:187–198. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Fried K, Arvidsson J, Robertson B, Brodin E, Theodorsson E. Combined retrograde tracing and enzyme/immunohistochemistry of trigeminal ganglion cell bodies innervating tooth pulps in the rat. Neuroscience. 1989;33:101–109. doi: 10.1016/0306-4522(89)90314-x. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68–77. doi: 10.2174/138920111793937961. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, Wood JN, Wallen-Mackenzie A, Kullander K. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerstrom MC, Rogoz K, Abrahamsen B, Lind AL, Olund C, Smith C, Mendez JA, Wallen-Mackenzie A, Wood JN, Kullander K. A sensory subpopulation depends on vesicular glutamate transporter 2 for mechanical pain, and together with substance P, inflammatory pain. Proc Natl Acad Sci U S A. 2011;108:5789–5794. doi: 10.1073/pnas.1013602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin effects on trigeminal nociception I: activation and peripheral sensitization of deep craniofacial nociceptive afferents. Brain Res. 2009;1251:130–139. doi: 10.1016/j.brainres.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M, Bouali-Benazzouz R, El Mestikawy S, Ravassard P, Nagy F. Expression of vesicular glutamate transporters in rat lumbar spinal cord, with a note on dorsal root ganglia. J Comp Neurol. 2004;468:380–394. doi: 10.1002/cne.10988. [DOI] [PubMed] [Google Scholar]
- Leo S, Moechars D, Callaerts-Vegh Z, D’Hooge R, Meert T. Impairment of VGLUT2 but not VGLUT1 signaling reduces neuropathy-induced hypersensitivity. Eur J Pain. 2009;13:1008–1017. doi: 10.1016/j.ejpain.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Li JL, Xiong KH, Dong YL, Fujiyama F, Kaneko T, Mizuno N. Vesicular glutamate transporters, VGluT1 and VGluT2, in the trigeminal ganglion neurons of the rat, with special reference to coexpression. J Comp Neurol. 2003;463:212–220. doi: 10.1002/cne.10755. [DOI] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malet M, Vieytes CA, Lundgren KH, Seal RP, Tomasella E, Seroogy KB, Hokfelt T, Gebhart GF, Brumovsky PR. Transcript expression of vesicular glutamate transporters in lumbar dorsal root ganglia and the spinal cord of mice – effects of peripheral axotomy or hindpaw inflammation. Neuroscience. 2013;248:95–111. doi: 10.1016/j.neuroscience.2013.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura S, Shimizu K, Shinoda M, Ohara K, Ogiso B, Honda K, Katagiri A, Sessle BJ, Urata K, Iwata K. Mechanisms underlying ectopic persistent tooth-pulp pain following pulpal inflammation. PLoS One. 2013;8:e52840. doi: 10.1371/journal.pone.0052840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy DD, Knowlton WM, McKemy DD. Scraping through the ice: uncovering the role of TRPM8 in cold transduction. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1278–R1287. doi: 10.1152/ajpregu.00631.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Miller KE, Hoffman EM, Sutharshan M, Schechter R. Glutamate pharmacology and metabolism in peripheral primary afferents: physiological and pathophysiological mechanisms. Pharmacol Ther. 2011;130:283–309. doi: 10.1016/j.pharmthera.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, van der Spek P, Kass S, Meert T, D’Hooge R, Rosenmund C, Hampson RM. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci. 2006;26:12055–12066. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich RX, Gobius I, Pollak T, Zhang J, Ren T, Brown L, Mori S, De Juan Romero C, Britanova O, Tarabykin V, Richards LJ. Molecular regulation of the developing commissural plate. J Comp Neurol. 2010;518:3645–3661. doi: 10.1002/cne.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res. 1998;787:161–164. doi: 10.1016/s0006-8993(97)01568-0. [DOI] [PubMed] [Google Scholar]
- Paik SK, Park KP, Lee SK, Ma SK, Cho YS, Kim YK, Rhyu IJ, Ahn DK, Yoshida A, Bae YC. Light and electron microscopic analysis of the somata and parent axons innervating the rat upper molar and lower incisor pulp. Neuroscience. 2009;162:1279–1286. doi: 10.1016/j.neuroscience.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Paik SK, Kim SK, Choi SJ, Yang ES, Ahn SH, Bae YC. Vesicular glutamate transporters in axons that innervate the human dental pulp. J Endod. 2012;38:470–474. doi: 10.1016/j.joen.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wheeler EF, Bernanke JM, Yang H, Naftel JP. A model experimental system for monitoring changes in sensory neuron phenotype evoked by tooth injury. J Neurosci Methods. 2003;126:99–109. doi: 10.1016/s0165-0270(03)00071-2. [DOI] [PubMed] [Google Scholar]
- Rogoz K, Lagerstrom MC, Dufour S, Kullander K. VGLUT2-dependent glutamatergic transmission in primary afferents is required for intact nociception in both acute and persistent pain modalities. Pain. 2012;153:1525–1536. doi: 10.1016/j.pain.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Rogoz K, Andersen HH, Lagerstrom MC, Kullander K. Multimodal use of calcitonin gene-related peptide and substance P in itch and acute pain uncovered by the elimination of vesicular glutamate transporter 2 from transient receptor potential cation channel subfamily V member 1 neurons. J Neurosci. 2014;34:14055–14068. doi: 10.1523/JNEUROSCI.1722-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci U S A. 2010;107:22296–22301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda M, Asano M, Omagari D, Honda K, Hitomi S, Katagiri A, Iwata K. Nerve growth factor contribution via transient receptor potential vanilloid 1 to ectopic orofacial pain. J Neurosci. 2011;31:7145–7155. doi: 10.1523/JNEUROSCI.0481-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm E, Bongenhielm U, Ahlquist M, Fried K. VRl- and VRL-l-like immunoreactivity in normal and injured trigeminal dental primary sensory neurons of the rat. Acta Odontol Scand. 2002;60:72–79. doi: 10.1080/000163502753509455. [DOI] [PubMed] [Google Scholar]
- Stoyanova II, Lazarov NE. Localization of nitric oxide synthase in rat trigeminal primary afferent neurons using NADPH-diaphorase histochemistry. J Mol Histol. 2005;36:187–193. doi: 10.1007/s10735-005-1694-3. [DOI] [PubMed] [Google Scholar]
- Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, Sessle BJ. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain. 2003;101:221–227. doi: 10.1016/S0304-3959(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey DJ, De Biasi S, Phend K, Rustioni A. Aspartate-like immunoreactivity in primary afferent neurons. Neuroscience. 1991;40:673–686. doi: 10.1016/0306-4522(91)90004-8. [DOI] [PubMed] [Google Scholar]
- Tsuzuki K, Xing H, Ling J, Gu JG. Menthol-induced Ca2+ release from presynaptic Ca2+ stores potentiates sensory synaptic transmission. J Neurosci. 2004;24:762–771. doi: 10.1523/JNEUROSCI.4658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors–noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Jeong HJ, Vaughan CW. Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones. Br J Pharmacol. 2009;157:371–380. doi: 10.1111/j.1476-5381.2009.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H, Ling J, Chen M, Gu JG. Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J Neurophysiol. 2006;95:1221–1230. doi: 10.1152/jn.01035.2005. [DOI] [PubMed] [Google Scholar]
- Yang ES, Jin MU, Hong JH, Kim YS, Choi SY, Kim TH, Cho YS, Bae YC. Expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in the rat dental pulp and trigeminal ganglion following inflammation. PLoS One. 2014;9:e109723. doi: 10.1371/journal.pone.0109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Shinoda M, Kiyomoto M, Honda K, Suzuki A, Tamagawa T, Kaji K, Kimoto S, Iwata K. P2X3 receptor mediates ectopic mechanical allodynia with inflamed lower lip in mice. Neurosci Lett. 2012;528:67–72. doi: 10.1016/j.neulet.2012.08.067. [DOI] [PubMed] [Google Scholar]
- Zanotto KL, Merrill AW, Carstens MI, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. J Neurophysiol. 2007;97:966–978. doi: 10.1152/jn.00996.2006. [DOI] [PubMed] [Google Scholar]