Abstract
Background
Colorectal cancer (CRC) remains one of the most common lethal malignant tumors worldwide. The correlation between lncRNAs expression and CRC development has not been well identified in the recent literature. This study focused on the role of lncRNA-ROR on CRC progression and development.
Material/Methods
Quantitative real-time PCR (qRT-PCR) assay was conducted to identify the expression level of lncRNA-ROR. Cell proliferation and viability were examined by MTT assay and colony formation assay. Cell cycle distribution and apoptosis were detected by flow cytometry. Expressions of p53, p21, and FAS protein levels were assessed by Western blotting. CRC cells transfected with lncRNA-shRNA were injection into nude mice to identify the function of lncRNA-ROR on tumorigenesis in vivo.
Results
The expression level of lncRNA-ROR was elevated in CRC tissues when compared to adjacent tissues (n=78). lncRNA-ROR knockdown significantly suppressed cell proliferation and viability, while lncRNA-ROR overexpression had the opposite effect. Decreased lncRNA-ROR expression enhanced cell apoptosis and triggered cell cycle arrest in G0/G1 phase, while elevated lncRNA-ROR expression presented the opposite effect. Protein levels of p53 and p53 target genes were affected by lncRNA-ROR in vitro, and downregulation of lncRNA-ROR impeded tumorigenesis in vivo.
Conclusions
Our study demonstrates that lncRNA-ROR participates in controlling CRC proliferation, viability, and apoptosis, partially by modulating p53, which provides potential and prospective therapeutic targets for CRC.
MeSH Keywords: Apoptosis; Cell Proliferation; Colorectal Neoplasms; Genes, p53; RNA, Long Noncoding
Background
Colorectal cancer (CRC), the most common type of malignant tumor, has a high degree of malignancy and poor prognosis [1]. It ranks third in the number of cancer-related deaths worldwide, accounting for more than approximately 690 000 deaths per year [2]. Its onset is primarily in situ, and can also be transferred from a distance, or caused by infringement of adjacent tissue lesions [3]. Unfortunately, although advances in diagnosis and treatment of CRC have been achieved recently, the recurrence and mortality still remain high, with 5-year survival rate less than 65% [4]. CRC cell proliferation and metastasis contribute to the high mortality of CRC patients [5–7]. Therefore, further understanding of the molecular mechanisms of CRC occurrence and development is needed for developing new therapeutic targets for CRC patients.
Long non-coding RNAs (lncRNAs), a type of non-coding RNA transcript, lack protein-coding capacity longer than 200 nucleotides in length [8]. Many studies have shown that aberrant expression of lncRNAs plays a vital role in various cell processes [8], including epigenetic regulation, genomic imprinting, alternative splicing, inflammatory pathologies, breast cancer [9], lung cancer [10], gastric cancer [11], and colorectal cancer [12]. Therefore, understanding lncRNAs may be of great value in explaining the occurrence and development of tumors.
The lncRNA-regulator of reprogramming (ROR), a newly-discovered lncRNA located at chromosome 18q21.31, was first discovered in induced pluripotent stem cells (iPSCs) and can be directly regulated by SOX2, OCT4, and NANOG [13]. It has been demonstrated to be upregulated and plays a tumor-promoting role in breast cancer [14], gallbladder cancer [15], nasopharyngeal carcinoma [16], and pancreatic cancer [13]. However, until now, no research had shown the role of lncRNA-ROR in progression and development of CRC.
In this study, we explored the expression level of lncRNA-ROR in CRC cell tissues and lines, and investigated the functioning mode of lncRNA-ROR in CRC tumor viability and proliferation. We also identified the molecular regulatory mechanisms of lncRNA-ROR in CRC, aiming to find a potential and innovative therapeutic target for CRC.
Material and Methods
Clinical samples
We obtained the 78 pairs of CRC tissues and matched paracarcinoma normal tissues used in this study from patients undergoing routine surgery at the Central Hospital of Weihai from 2015 to 2016. All surgical specimens were collected and then frozen immediately in liquid nitrogen until use. The tumor tissues were diagnosed and confirmed by pathological examination. The present research was approved by the Ethics Committee of the Central Hospital of Weihai. Written informed consent was signed by all participants before the study began.
Cell culture
The CRC cell lines M5, DLD1, HCT116, SW480, SW620, and HT29 and normal human intestinal epithelial cells (HIECs) were all purchased from the Shanghai Model Cell Bank. These cells were cultured in RPMI1640 medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 ug/mL streptomycin at 37°C with 5% CO2.
Plasmid and transfection
For lncRNA-ROR overexpression in M5 cells, the lncRNA-ROR gene was cloned into the lentiviral-vector pcDNA3.1 (System Biosciences, USA) and primer sequences are as follows: lncRNA-ROR: forward, 5′-GGG GTA CCG TTC TCA TTT TTC TAC TGC TCG TG-3′ and reverse, 5′-CGG GAT CCA TGT AAT CAA TCA TTT TAT TAT TTT CAT C-3′. M5 cells were transfected with LV-Vector or LV-ROR.
For knockdown of lncRNA-ROR in HT29 cells, 2 shRNA sequences that target for lncRNA-ROR were designed as follows: shROR1: 5′-CCT GAG AGT TGG CAT GAA T-3′; shROR2: 5′-GGT TAA AGA CAC AGG GGA A-3′, and the NC-shRNA was purchased from Genechem (Shanghai, China). HT29 cells were transfected with NC-shRNA or lncRNA-shRNA.
RNA extraction and qRT-PCR
Total RNA was extracted from collected frozen tissue samples and cell lines by using TRIzol reagent (Invitrogen, USA) following the manufacturer’s protocols. The Reverse Transcription Kit (Takara, China) was used to synthesize cDNAs. lncRNA-ROR mRNA level was quantified by SYBR Green real-time PCR and normalized to GAPDH using the following primers: forward, 5′-TCC AAA CAC ATC GCC ACT CT-3′ and reverse, 5′-TCC TAG GCC ATG AGG AGT CA-3′; and for GAPDH, forward, 5′-CGG AGT TGT TCG TAT TCG G-3′ and reverse, 5′-TAC TAG CCG ATG ATG GCA TT-3′. QRT-PCR was performed using the ABI 7500 system (Applied Biosystems, USA).
MTT assay
CRC cells in good status were seeded onto a 96-well plate and incubated overnight at 37°C with 5% CO2. Then, 20 L of MTT solution was added into the 96-well plate per well and cultured for 4h at 37°C. Then 150 L DMSO was added into the 96-well plate and incubated for 10 min. The absorbance was detected by an enzyme-linked immunosorbent assay (ELISA) reader at 490 nm.
Colony formation assay
To further investigate cell proliferation of CRC cells, cells were plated in 6-well plates at a density of 5×102 per well and cultured for 2 weeks. The colonies were fixed in ice-cold 70% methanol for 10 min and stained with 0.5% crystal violet for 10 min, then we washed each well 3 times with phosphate-buffered saline (PBS).
Cell cycle analysis
Cells for cell cycle analysis were prepared with a final concentration of 2×105/ml, treated with RNase A, fixed with 70% ice-cold ethanol overnight, washed twice in PBS and then stained with 50 mg/ml PI, 100 μg/ml RNase A, and 0.2% Triton X-100 for 30 min at 4°C in the dark. The percentages of the cell population in G0/G1, S, or G2/M phase were detected with a FACSCalibur flow cytometer (BD Bioscience, USA).
Cell apoptosis analysis
Cells were cultured in 6-well plates with the final concentration of 1×105/ml and then treated with 0.25% trypsin and fixed in 70% ice-cold ethanol. Cell suspension was prepared and washed twice with PBS. Afterwards, cells were double-stained with 5 μl Annexin V-FITC and 1 μl propidium iodide (PI, 50 μg/ml). After being incubated in the dark for 15 min, the cells were analyzed by a flow cytometer equipped with CellQuest software (BD Bioscience, USA).
Western blotting analysis
To investigate relative protein expression level, cells were washed with ice-cold PBS and lysed using lysis buffer. Then, we measured the concentration of collected protein using a protein assay kit purchased from Beyotime. The extracted protein (sum of 20 μg) was degenerated and chilled on ice. We used 10% SDS-PAGE to separate protein, then it was shifted to PVDF membranes purchased from Millipore. We used 5% fat-free milk to block non-specific protein interactions in TBST buffer containing Tris-HCl (50 mM), NaCl (150 mM), and Tween 20 (0.05%) at 4°C for 1 h. The membranes loaded with proteins were incubated at 4ºC within the fat-free milk overnight with the following primary antibody against p53 (AbSci, Nanjing, China), p21(Abnova, Taipei, Taiwan), FAS (Abnova, Taipei, Taiwan). TBST buffer was used to wash the unbound antibody (10 min each time for 3 times). The membranes were then incubated at room temperature with secondary antibody conjugated with horseradish peroxide (1 h). After washing these membranes 3 times in TBST buffer, we developed the membranes using ECL (Millipore, NY, USA) following the manufacturer’s instructions.
Xenograft model
The research was approved by the Animal Ethics Committee of Qingdao University Animal Center. HT29 cells (6×105/mL) transfected with NC-shRNA or LncRNA-shRNA were implanted into both axillae of NOD/SCID mice (4–5 weeks old) subcutaneously. Tumor diameters were detected every 3 days. Tumor volume was calculated as the formula (volume=length×width2×1/2). Mice were sacrificed and the grafts were removed after 4 weeks. After fixing with 4% paraformaldehyde, these tumors were embedded with paraffin for immunohistochemical (IHC) staining.
Statistical analysis
SPSS 18.0 software (IBM, USA) was used for statistical analysis; statistical data are presented with Graph PAD prism software and quantitative data are presented as mean ±SD. The independent samples t-test (SPSS, USA) was used to perform statistical analysis. The regression and correlation analysis was analyzed using the Spearman chi-squared test. The relative expression of mRNA was measured using the method of 2−ΔΔCT. Results were deemed statistically significant at P<0.05.
Results
Ectopic expression of lncRNA-ROR in CRC cell lines and tissues
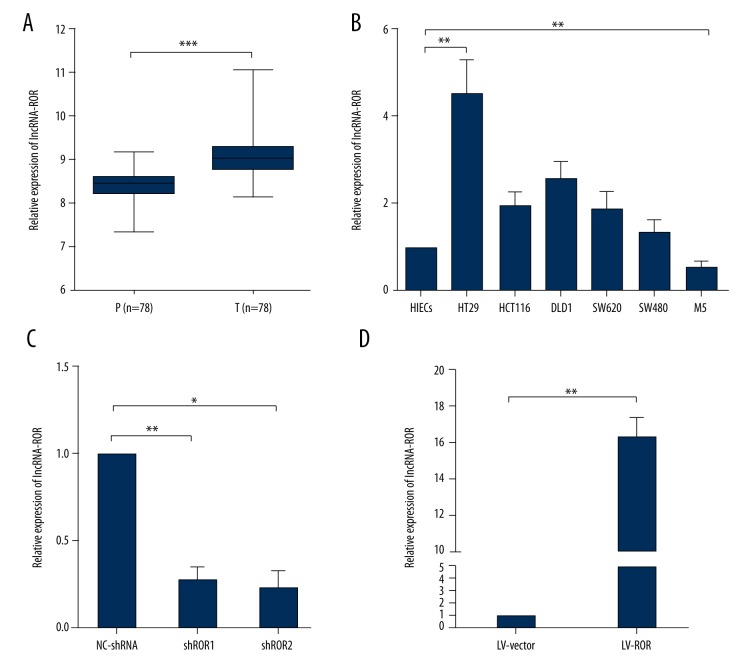
By means of qRT-PCR, we measured lncRNA-ROR expression level in both CRC tissues and adjacent normal tissues. The expression level of lncRNA-ROR was remarkably higher in CRC tissues than the paraneoplastic (Figure 1A), which was consistent with other previous research, implying that the aberrant lncRNA-ROR expression might be involved in CRC progression and development.
Figure 1.
Ectopic expression of lncRNA-ROR in CRC cell lines and tissues. (A) Analysis of lncRNA-ROR expression level in paracarcinoma tissues (P) and tumor tissues (T). LncRNA-ROR was significantly elevated in CRC tumor tissues compared with the paracarcinoma tissues; (B) Analysis of lncRNA-ROR expression level in 8 cell lines; (C) Analysis of transfection efficiency in HT29 cells and M5 cells. HT29 cells were transfected with NC-shRNA or lncRNA-shRNA and M5 cells were transfected with LV-Vector or LV-ROR. Total RNA was detected by qRT-PCR and GAPDH was used as an internal control. Data are presented as the mean ±SD of 3 independent experiments. * P<0.05; ** P<0.01; *** P<0.001.
Then, we investigated lncRNA-ROR expression level in several CRC and normal cell lines with qRT-RCR. Compared with HIECs cell line, HT29, HCT116, DLD1, SW620, and SW480 expressed a relatively higher level of lncRNA-ROR, while M5 expressed a relatively lower level of lncRNA-ROR (Figure 1B). To identify the functioning mode of lncRNA-ROR in CRC progression and development, we chose HT29 cell line for lncRNA-ROR knockdown and M5 cell line for lncRNA-ROR overexpression, and the transfection efficiency were both subsequently detected via qRT-PCR (Figure 1C). The lncRNA-ROR expression was effectively suppressed in HT29 cells by lncRNA-shRNA and elevated in M5 cells by LV-ROR.
Effects of lncRNA-ROR on CRC cell proliferation
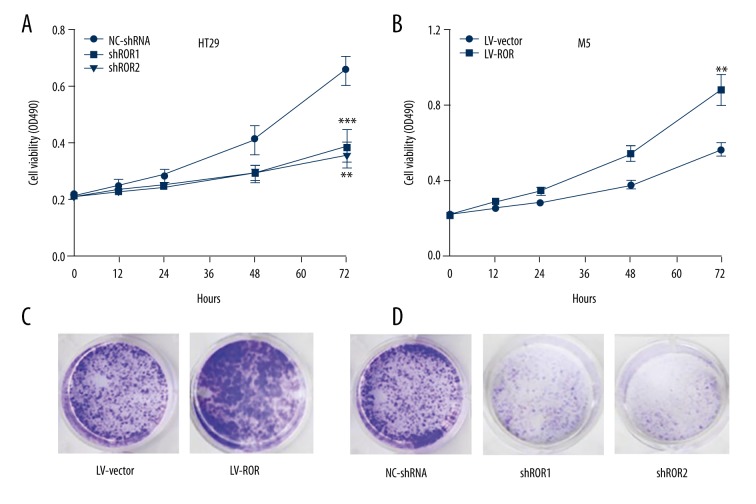
To identify the biological functioning mode and mechanism of lncRNA-ROR on CRC tumorigenesis, we investigated the effect of lncRNA-ROR on CRC cell viability and proliferation. As shown with a MTT assay, cell viability was dramatically impaired in HT29 cells transfected with lncRNA-shRNA compared with NC-shRNA (Figure 2A). In contrast, cell viability was obviously enhanced in M5 cells transfected with LV-ROR compared with LV-vector (Figure 2B).
Figure 2.
Effects of lncRNA-ROR on CRC cell proliferation. (A) MTT assay was performed to determine the viability of HT29 cells transfected with lncRNA-shRNA and NC-shRNA, respectively; (B) MTT assay was performed to determine the viability of M5 cells transfected with LV-ROR and LV-vector, respectively; (C) Colony formation assay was performed to determine the proliferation of M5 cells transfected with LV-ROR and LV-vector, respectively; (D) Colony formation assay was performed to determine the proliferation of HT29 cells transfected with lncRNA-shRNA and NC-shRNA, respectively. Data are presented as the mean ±SD of 3 independent experiments. ** P<0.01; *** P<0.001.
Colony formation assay was also carried out to measure CRC cell proliferation ability, revealing there were more formed colonies in M5 cells transfected with LV-ROR were bigger than with LV-vector (Figure 2C), but there were fewer formed colonies in HT29 cells transfected with lncRNA-shRNA were smaller than NC-shRNA (Figure 2D). Corporately, these results indicated that lncRNA-ROR promoted CRC cells viability and proliferation.
Effects of lncRNA-ROR on CRC cell cycle and cell apoptosis
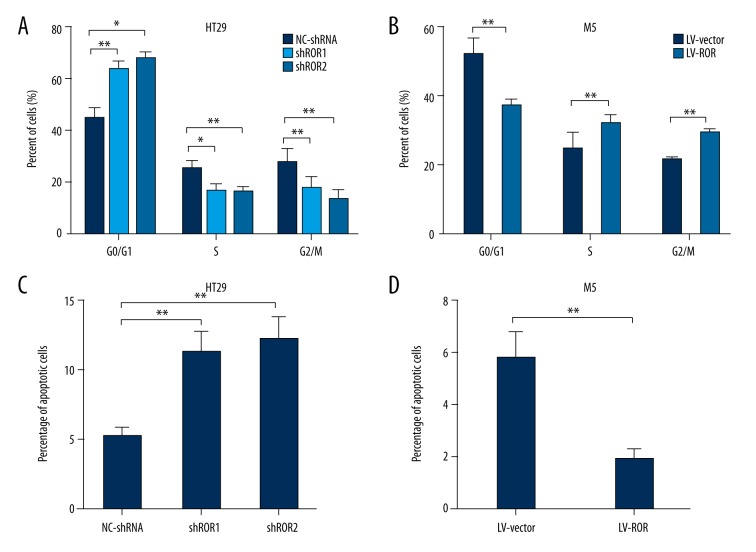
Since knockdown of lncRNA-ROR appeared to down-regulate CRC cell proliferation, we further explored the effects of lncRNA-ROR on CRC cell cycle progression by using flow cytometric analysis. We found that the percentage of HT29 cells transfected with lncRNA-shRNA in G0/G1 phase increased obviously compared with NC-shRNA, while the percentage of M5 cells transfected with LV-ROR in G0/G1 phase decreased remarkably compared with LV-vector (Figure 3A, 3B).
Figure 3.
Effects of lncRNA-ROR on CRC cell cycle and cell apoptosis. (A) Flow cytometric analysis was performed to detect cell cycle progression of HT29 cells transfected with lncRNA-shRNA and NC-shRNA; (B) Flow cytometric analysis was performed to detect cell cycle progression of M5 cells transfected with LV-ROR and LV-vector; (C) Flow cytometric analysis was performed to detect the apoptotic rates of HT29 cells transfected with lncRNA-shRNA and NC-shRNA; (D) Flow cytometric analysis was performed to detect the apoptotic rates of M5 cells transfected with LV-ROR and LV-vector. Data are presented as the mean ±SD of 3 independent experiments. * P<0.05; ** P<0.01.
Next, cell apoptosis was monitored by flow cytometric assay. The results indicated that the apoptotic rate of HT29 cells transfected with lncRNA-shRNA increased remarkably compared with NC-shRNA, whereas the apoptotic rate of M5 cells transfected with LV-ROR clearly decreased compared with LV-vector (Figure 3C, 3D). Collectively, these results demonstrate that lncRNA-ROR knockdown induced G0/G1 phase arrest and promoted CRC cell apoptosis.
LncRNA-ROR inhibited p53 expression
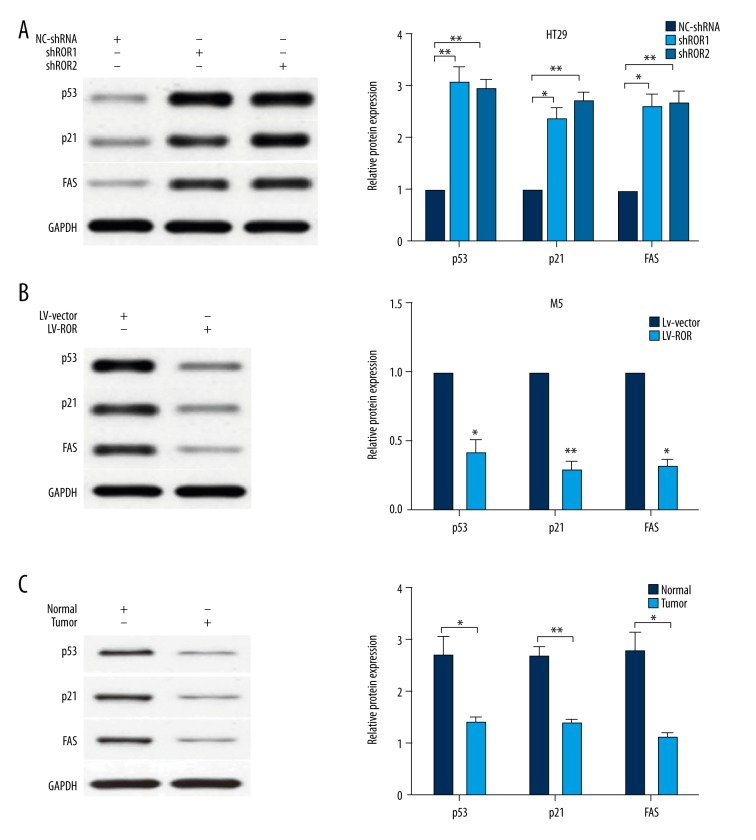
It has been well recognized that p53 can be modulated to regulate cancer cell growth by lncRNAs, and it has been reported that lncRNA-ROR can inactivate p53 protein in HCC. To better understand the underlying mechanisms of lncRNA-ROR-mediated CRC cell apoptosis and cell proliferation, we used Western blotting to assay the expression level of p53. The results demonstrated that p53 and its target genes, p21 and FAS, were significantly upregulated in HT29 cells transfected with lncRNA-shRNA compared with NC-shRNA (Figure 4A). Conversely, p53 and its target genes were remarkably downregulated in M5 cells transfected with LV-ROR compared with LV-vector (Figure 4B).
Figure 4.
LncRNA-ROR inhibited p53 expression. (A) p53, p21, and FAS were significantly upregulated in HT29 cells transfected with lncRNA-shRNA compared with NC-shRNA; (B) p53, p21 and FAS were significantly downregulated in M5 cells transfected with LV-ROR compared with LV-vector; (C) p53, p21 and FAS were significantly upregulated in the paraneoplastic tissues compared with the tumor tissues. Data are presented as the mean ±SD of 3 independent experiments. * P<0.05; ** P<0.01.
To further investigate whether the protein expression level of p53, p21, and FAS in CRC tissues was changed as the same way as that in CRC cell lines, we also used Western blotting to assay the expression level of p53 and its target genes in CRC tissues. The result demonstrated that p53, p21, and FAS were significantly upregulated in the paraneoplastic tissues compared with the tumor tissues (Figure 4C). All these results confirm lncRNA-ROR serves as an oncogene via regulating p53 activation partially in CRC.
LncRNA-ROR knockdown suppressed CRC growth in vivo
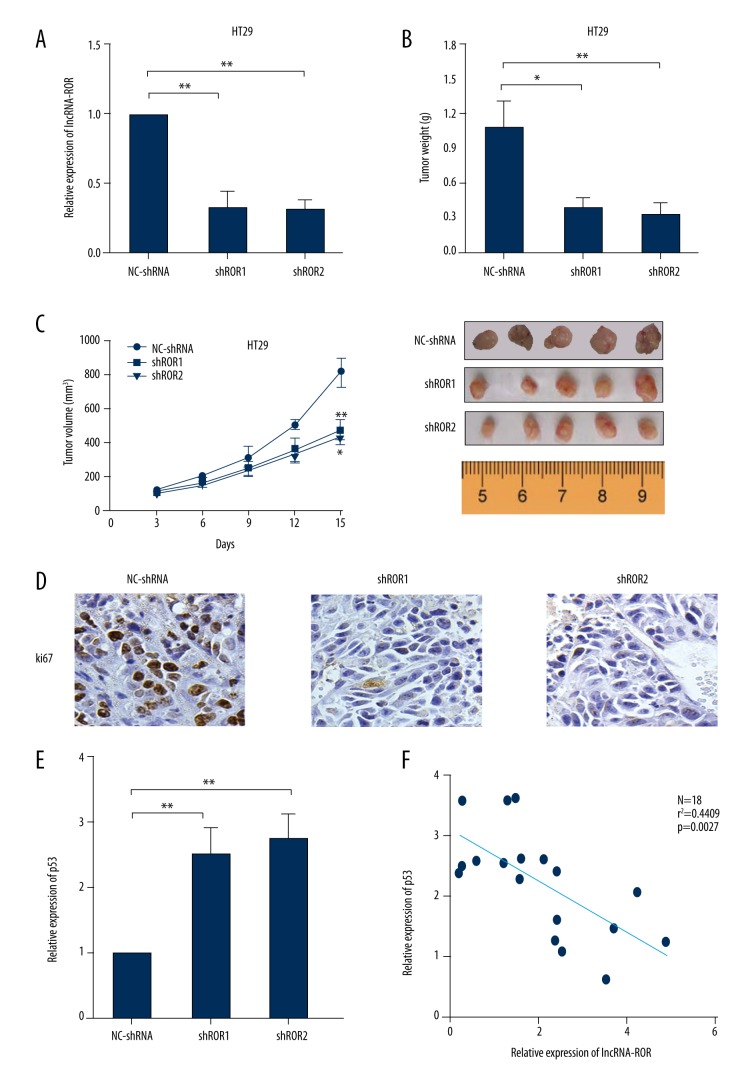
HT29 cells transfected with lncRNA-shRNA or NC-shRNA were injected into mice subcutaneously. LncRNA-ROR expression level in tumor grafts was detected by qRT-PCR and it showed that lncRNA-ROR expression in lncRNA-shRNA groups was decreased compared with the expression in LV-Vector group (Figure 5A). Tumor weight became obviously less in lncRNA-shRNA groups compared to the weight in the NC-shRNA group (Figure 5B). Tumor volume was significantly smaller in lncRNA-shRNA groups compared to the volume in NC-shRNA group (Figure 5C). IHC staining of tumor tissues showed that there was a decrease in proliferation marker (ki67) in lncRNA-shRNA groups versus the NC-shRNA group (Figure 5D).
Figure 5.
LncRNA-ROR knockdown suppressed CRC growth in vivo. (A) Analysis of lncRNA-ROR expression level in tumor tissues was detected by using RT-PCR, GAPDH was used as an internal control; (B) Tumor weights are represented as means of tumor weights ±s.d.; (C) Tumor volume was determined by using calipers; (D) IHC staining was used to show the expression of proliferation marker (ki67); (E) Analysis of p53 expression level in tumor tissues was detected by using RT-PCR, and GAPDH was used as an internal control; (F) Correlation between the expression of protein levels of lncRNA-ROR and p53. * P<0.05; ** P<0.01.
We also detected p53 expression in tumor grafts by qRT-PCR and further investigated the correlation of the expressions between lncRNA-ROR and p53 in xenograft. The results demonstrated that the expression level of p53 in lncRNA-shRNA groups was elevated compared with the expression in the LV-Vector group (Figure 5E), and lncRNA-ROR expression level was negatively correlated with that of p53 (Figure 5F). These data all verify that lncRNA-ROR knockdown suppressed CRC cell growth in vivo.
Discussion
High-throughput technology has demonstrated that about 98% of the human genome are non-coding genes, and lncRNAs make up more than 80% of them [17]. It was initially considered as “transcriptional noise”, while increasingly studies indicate that lncRNAs may be highly involved in the progression and development of various types of tumors. For instance, Ming Sun et al. [18] revealed that MEG3 regulated gastric cancer cell proliferation and apoptosis, and thus can be identified as a poor prognostic biomarker in gastric cancer. Bin Liu et al. [10] found that lncRNA-LET exhibited a tumor-suppressive effect on lung adenocarcinoma progression by inhibiting EMT and Wnt/β-catenin pathway. You J et al. [19] demonstrated that SNHG1 is dysregulated in NSCLC cell lines and may provide a promising therapeutic perspective for NSCLC intervention.
LncRNA-ROR is a newly-discovered gene that participates in the process of tumor development. Jiayan Fan et al. [20] revealed that lncRNA-ROR might serve as a decoy oncoRNA that promotes tumorigenesis by means of a variety of biological function models to specify a new pattern of histone modifications. Han-xiang Zhan et al. [13] indicated that lncRNA-ROR promotes invasion and metastasis in pancreatic cancer by regulating ZEB1. Hou et al. [14] found that lncRNA-ROR might serve as an important regulator of epithelial-to-mesenchymal transition (EMT) and regulated miRNAs expression to promote progression and metastasis in breast cancer. Li Li et al. [16] demonstrated that lncRNA-ROR was associated with metastasis, proliferation, apoptosis, and chemoresistance of nasopharyngeal carcinoma. In our present study, we explored the role of lncRNA-ROR in CRC. We revealed that lncRNA-ROR expression level was obviously elevated in CRC tissues when compared to adjacent tissues, suggesting that lncRNA-ROR might participate in the progression and development of CRC. We found that lncRNA-ROR knockdown significantly attenuated HT29 cell proliferation, triggered cell cycle arrest, and enhanced cell apoptosis, while lncRNA-ROR overexpression had a significant opposite effect on the M5 cell line. Furthermore, we revealed that lncRNA-ROR knockdown suppressed cell growth and proliferation in vivo. Collectively, we documented that lncRNA-ROR might work as a cancer-promoting gene in CRC.
P53, as a well-known tumor suppressor, is involved in tumorigenesis and development of various cancers [21,22]. It can suppress tumor growth and development by attenuating cell proliferation or by activating cell death progression [23,24]. In this study, we revealed that lncRNA-ROR knockdown remarkably increased the protein level of p53 and its target genes, while lncRNA-ROR overexpression had the opposite effect. The p53 protein level was negatively correlated with that of lncRNA-ROR in vivo. These data suggest that the mechanism of lncRNA-ROR on CRC progression and development may be partially through the p53 signaling pathway.
Conclusions
The present study demonstrates that lncRNA-ROR has a tumor-promoting effect on CRC progression and proliferation in vitro and in vivo, and that lncRNA-ROR can partially suppress the p53 pathway. Our findings suggest that lncRNA-ROR can serve as an innovative and prospective therapeutic target for CRC.
Footnotes
Source of support: Departmental sources
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the veterans affairs central cancer registry. Clin Colorectal Cancer. 2016;15:e199–e204. doi: 10.1016/j.clcc.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raine R, Wong W, Scholes S, et al. Social variations in access to hospital care for patients with colorectal, breast, and lung cancer between 1999 and 2006: Retrospective analysis of hospital episode statistics. BMJ. 2010;340:b5479. doi: 10.1136/bmj.b5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Fang M, Song Y, et al. Brother of Regulator of Imprinted Sites (BORIS) suppresses apoptosis in colorectal cancer. Sci Rep. 2017;7:40786. doi: 10.1038/srep40786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han P, Li JW, Zhang BM, et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/beta-catenin signaling. Mol Cancer. 2017;16:9. doi: 10.1186/s12943-017-0583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang LL, Zhan L, Jin YD, et al. SIRT2 mediated antitumor effects of shikonin on metastatic colorectal cancer. Eur J Pharmacol. 2017;797:1–8. doi: 10.1016/j.ejphar.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Harries LW. Long non-coding RNAs and human disease. Biochem Soc Trans. 2012;40:902–6. doi: 10.1042/BST20120020. [DOI] [PubMed] [Google Scholar]
- 9.Qian K, Liu G, Tang Z, et al. The long non-coding RNA NEAT1 interacted with miR-101 modulates breast cancer growth by targeting EZH2. Arch Biochem Biophys. 2016;615:1–9. doi: 10.1016/j.abb.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Pan CF, He ZC, et al. Long non-coding RNA-LET suppresses tumor growth and EMT in lung adenocarcinoma. Biomed Res Int. 2016;2016:4693471. doi: 10.1155/2016/4693471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang K, Shi H, Xi H, et al. Genome-Wide lncRNA microarray profiling identifies novel circulating lncRNAs for detection of gastric cancer. Theranostics. 2017;7:213–27. doi: 10.7150/thno.16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang ZY, Yang F, Zhang YL, et al. LncRNA-ANCR downregulation suppresses invasion and migration of colorectal cancer cells by regulating EZH2 expression. Cancer Biomark. 2017;18(1):95–104. doi: 10.3233/CBM-161715. [DOI] [PubMed] [Google Scholar]
- 13.Zhan HX, Wang Y, Li C, et al. LincRNA-ROR promotes invasion, metastasis and tumor growth in pancreatic cancer through activating ZEB1 pathway. Cancer Lett. 2016;374:261–71. doi: 10.1016/j.canlet.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Hou P, Zhao Y, Li Z, et al. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5:e1287. doi: 10.1038/cddis.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SH, Zhang MD, Wu XC, et al. Overexpression of LncRNA-ROR predicts a poor outcome in gallbladder cancer patients and promotes the tumor cells proliferation, migration, and invasion. Tumour Biol. 2016;37:12867–75. doi: 10.1007/s13277-016-5210-z. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Gu M, You B, et al. Long non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinoma. Cancer Sci. 2016;107:1215–22. doi: 10.1111/cas.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Li X, Wu M, et al. Knockdown of long non-coding RNA GHET1 inhibits cell proliferation and invasion of colorectal cancer. Oncol Res. 2016;23:303–9. doi: 10.3727/096504016X14567549091305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun M, Xia R, Jin F, et al. Downregulated long non-coding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biol. 2014;35:1065–73. doi: 10.1007/s13277-013-1142-z. [DOI] [PubMed] [Google Scholar]
- 19.You J, Fang N, Gu J, et al. Non-coding RNA small nucleolar RNA host gene 1 promote cell proliferation in nonsmall cell lung cancer. Indian J Cancer. 2014;51(Suppl 3):e99–e102. doi: 10.4103/0019-509X.154092. [DOI] [PubMed] [Google Scholar]
- 20.Fan J, Xing Y, Wen X, et al. Long non-coding RNA ROR decoys gene-specific histone methylation to promote tumorigenesis. Genome Biol. 2015;16:139. doi: 10.1186/s13059-015-0705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carra G, Crivellaro S, Taulli R, et al. Mechanisms of p53 functional De-Regulation: Role of the IkappaB-alpha/p53 complex. Int J Mol Sci. 2016;17(12) doi: 10.3390/ijms17121997. pii: E1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamil S, Hojabrpour P, Duronio V. The small molecule 2-phenylethynesulfonamide induces covalent modification of p53. Biochem Biophys Res Commun. 2017;482:154–58. doi: 10.1016/j.bbrc.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Cordani M, Butera G, Pacchiana R, Donadelli M. Molecular interplay between mutant p53 proteins and autophagy in cancer cells. Biochim Biophys Acta. 2016;1867:19–28. doi: 10.1016/j.bbcan.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Gong B, Wang Z, Zhang M, et al. Disturbed P53-MDM2 feedback loop contributes to thoracic aortic dissection formation and may be a result of TRIM-25 overexpression. Ann Vasc Surg. 2016 doi: 10.1016/j.avsg.2016.07.091. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]