Abstract
Background
Esophageal cancer has traditionally been associated with very poor outcomes. A number of therapies are available for the treatment and palliation of esophageal cancer, but little systematic evidence compares the efficacy of different treatment strategies. This meta-analysis aimed to investigate whether treatments in addition to radiotherapy could provide better efficacy and safety.
Material/Methods
We identified a total of 12 eligible studies with 18 study arms by searching PubMed, the Cochrane Library, EMBASE, and Clinical Trials.gov without time or language restrictions. The final search was conducted on 17 August 2016. We calculated mean differences (MD) and risk ratios (RR) with 95% confidence intervals (CI) for continuous and dichotomous data, respectively. Heterogeneity was calculated and reported using Tau2, Chi2, and I2 analyses.
Results
Twelve studies with 18 study arms were included in the analysis. Addition of surgery to chemo-radiotherapy resulted in improved median survival time (p=0.009) compared with chemo-radiotherapy alone, but all other outcomes were unaffected. Strikingly, and in contrast with patients with squamous cell carcinomas, the subset of patients with adenocarcinoma who received therapies in addition to radiotherapy showed a significant improvement in median survival time (p<0.0001), disease-free survival (p=0.007), 2-year survival rates (p=0.002), and 3-year survival rates (p=0.01). The incidence of adverse effects increased substantially with additional therapies.
Conclusions
This meta-analysis reveals stark differences in outcomes in patients depending on the type of carcinoma. Patients with squamous cell carcinoma should be educated about the risks and benefits of undergoing multiple therapies.
MeSH Keywords: Adenocarcinoma; Carcinoma, Squamous Cell; Esophageal Neoplasms; Radiotherapy
Background
The incidence of carcinoma of the esophagus as well as the gastro-esophageal junction is increasing around the world [1]. According to the American Cancer Society (ACS), there are about 17 000 new cases of esophageal cancer diagnosed around the world each year [2]. Among those diagnosed with esophageal cancer, most are men [2], and they accounted for approximately 12 720 of the 15 690 deaths caused by esophageal cancer so far in 2016 [2]. The diagnosis of most esophageal cancers happens at an advanced stage in most patients. This fact makes the best intervention strategy for the advanced cases of esophageal cancer palliative care rather than curative treatment [3,4]. The most predominant symptom found in many patients is dysphagia, which is characterized by pain experienced when swallowing food or beverages.
Squamous cell carcinoma is the most common type of esophageal cancer among African Americans, while Caucasians are affected more by adenocarcinoma [5]. It is notable that although esophageal cancer accounts for only 1% of cancers detected in the US, it is much more common among other countries such as China, India, Africa, Pakistan, and Iran [2].
Radiotherapy has been commonly used in the treatment of esophageal cancer. It results in improvement in 50–85% of the patients diagnosed with esophageal cancer at an advanced stage. However, the rate of recovery and response of the patients can be very slow [4]. The addition, in recent years, adding chemotherapy to radiotherapy has been hailed as a potential cure for esophageal cancers that would previously have been considered fatal [6]. Surgery, mostly esophagectomy, is often carried out in an attempt at curative treatment, especially in early esophageal cancer, but it has a high complication rate, both during and after the procedure [7].
Despite the existence of so many esophageal cancer patients around the world, there is little systematic evidence that highlights the best intervention strategies. Although systematic reviews exist that examine the roles of chemotherapy [8] and surgery [9,10], there is no systematic review that focuses on the role of radiotherapy and whether its combination with other interventions could enhance the efficacy of esophageal cancer treatment. Our study investigated the efficacy and adverse effects of therapies added to radiotherapy, such as radiotherapy+chemotherapy (RT vs. RT+CT), radiotherapy+surgery (RT vs. RT+Surgery), RT+CT+surgery (RT+CT vs. RT+CT+surgery), and RT+immune therapy (RT vs. RT+immune therapy). In order to determine the effect of treatments in addition to radiotherapy, we only included studies that directly compared these 2 treatment modalities. We also performed subgroup meta-analyses by the type of cancer as well as by the type of intervention.
Material and Methods
The current study was carried out in accordance with the 2015 PRISMA guidelines [11].
PICOT
We identified the following PICOT for our study: Population: adults with localized or advanced esophageal cancer; Intervention: radiotherapy plus other interventions; Comparator: radiotherapy without additional interventions or with fewer interventions; Outcomes: median survival time, disease-free survival time, 1-year survival rate, 2-year survival rate, 3-year survival rate, response rate, presence of dysphagia, adverse events; Time: At least 1 year following treatment.
Data sources and search strategies
Searches were carried out using PubMed, the Cochrane Library, EMBASE, and Clinical Trials.gov without time or language restrictions. The final search was conducted on 17 August 2016. The keywords used were (esophageal cancer OR oesophageal cancer) AND (radiotherapy OR chemotherapy OR surgery OR chemo-radiotherapy). Search results were uploaded into Eppi-Reviewer 4 [12] to determine their eligibility.
Selection standards
Studies were included if they met the following criteria: 1) the participants had primary esophageal cancer; 2) the article was a randomized controlled trial (RCT), a cohort study or a retrospective analysis; 3) the study had a control group; 4) the treatment included radiotherapy and at least 1 additional treatment option; 5) the control included radiotherapy; and 6) the study included at least 1 of the outcomes listed in the PICOT above. We excluded studies that did not measure the effect of additional therapy added to radiotherapy (e.g., chemo-radiotherapy plus surgery vs. surgery alone was excluded).
Study selection
The initial search resulted in 387 abstracts (Figure 1). After removal of duplicates, 303 abstracts were subjected to the inclusion criteria. Two authors examined the abstracts, excluding those that did not match the inclusion criteria. This resulted in 28 studies. We obtained the full-text articles and applied the same inclusion criteria. This resulted in 12 studies remaining. Eighteen study arms from these 12 studies were included in the final analysis.
Figure 1.
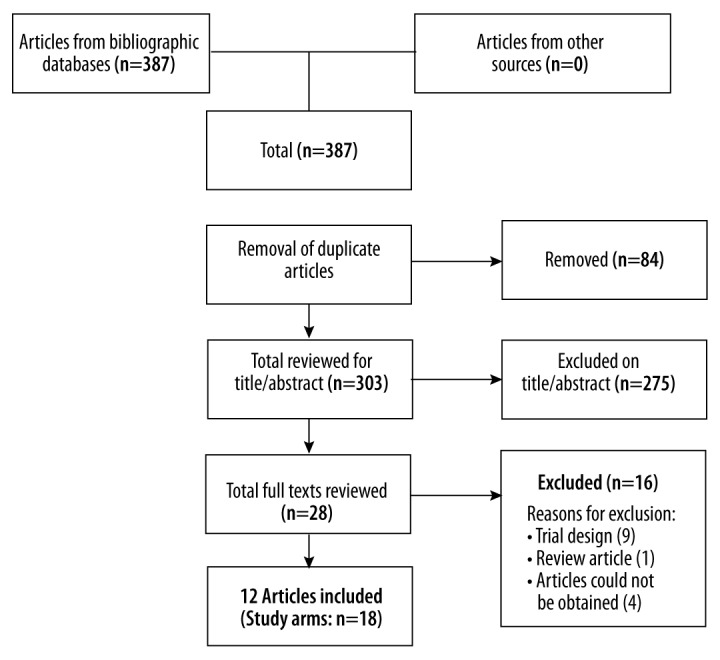
PRISMA diagram of included studies.
Study quality
The quality of the studies was determined using the Cochrane Collaboration risk of bias assessment tool for randomized controlled trials [13]. This tool examines each study for risk of bias in 7 categories: random sequence generation, allocation concealment, blinding or participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The non-randomized studies were assessed using the Newcastle-Ottawa scale for assessing the quality of non-randomized studies in meta-analyses [14]. This scale allows the assessment of the quality of non-RCT studies using the following criteria: selection, comparability, and outcome/exposure. There are separate scales for cohort studies and case-control studies.
Data extraction
Two authors independently extracted the data from studies into electronic forms and crossed-checked them. The extracted data included: the study characteristics (country, type of cancer, stage of cancer, number in intervention/control, type of therapy used, study design); outcome data; and adverse effects data. Depending on the outcome, we either extracted means and standard deviations or rates (as number of events out of total number in the study arm). If standard errors were given, these were converted to standard deviations by multiplying the standard error by the square root of the number in the study arm.
Statistical analysis
Study data were copied into Review Manager 5.3 (Cochrane Collaboration) [15]. Risk ratios with 95% confidence intervals (CI) were used for dichotomous outcomes, and mean differences with 95% CIs were calculated for continuous outcomes. Meta-analysis was carried out with a random effects model, inverse variance calculation, with p<0.05 as the test for statistical significance. Heterogeneity was given as Tau2, Chi2, and I2. A priori subgroup analyses were planned by cancer type and treatment modality. Sensitivity analyses were planned for study design, tumor stage, and type of chemotherapy used.
Results
Search results and study characteristics
Table 1 shows the characteristics of all included study arms. Only 4 of the included 12 studies were RCTs [16–19]. Four studies were prospective but non-randomized trials [20–23], 3 were retrospective case-control studies [24–26], and 1 reported on a sequential, non-randomized phase II trial [27]. In terms of interventions, 4 studies compared radiotherapy plus chemotherapy with radiotherapy alone [16,17,24,25]. Three studies compared radiotherapy plus surgery with radiotherapy alone [17,24,25]. Nine studies compared radiotherapy plus chemotherapy plus surgery with radiotherapy and chemotherapy alone [17,19–24,26,27]. A single study compared radiotherapy plus immunotherapy with radiotherapy alone [18].
Table 1.
Studies included in the meta-analysis.
| Study ID | Country/ethnicity | Type of cancer | Stage of cancer | Number of patients (intervention/ control) | Intervention | Control | Study design | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose of radiation/chemotherapy | Type | Dose of radiation/ chemotherapy | |||||||
| Agronovich 2008-1 | Canada/ Not stated | Any | T1, 2, 3, X | 16 | 77 | RT+ surgery | ≥40 Gy (15 fractions) | RT | ≥40 Gy (15 fractions) | Retrospective case-control |
| Agronovich 2008-2 | Canada/ Not stated | Any | T1, 2, 3, X | 26 | 77 | RT+CT | ≥40 Gy (15 fractions); 5-FU 1000 mg/m2/day + cisplatin 25 mg/m2/day | RT | ≥40 Gy (15 fractions) | Retrospective case-control |
| Agronovich 2008-3 | Canada/ Not stated | Any | T1, 2, 3, X | 12 | 26 | RT+CT+ surgery | ≥40 Gy (15 fractions); 5-FU 1000 mg/m2/day + cisplatin 25 mg/m2/day | RT+CT | ≥40 Gy (15 fractions); 5-FU 1000 mg/m2/day + cisplatin 25 mg/m2/day | Retrospective case-control |
| Algan 1995 | USA/ Not stated | Adenocarcinoma | I, IIA, IIB | 12 | 12 | RT+CT+ surgery | 60 Gy (over 6 wks); 5-FU 1000 mg/m2/day + mitomycin C 10 mg/m2 (single bolus) | RT+CT | 60 Gy (over 6 wks); 5-FU 1000mg/m2/day + mitomycin C 10 mg/m2 (single bolus). | Sequential, non-randomized |
| Burmeister 1995 | Australia/ Not stated | Any | I, IIA, IIB | 78 | 137 | RT+CT+ surgery | 60 Gy (30 fractions); CDDP 80 mg/m2 + 5-FU 800 mg/m2/day | RT+CT | 60 Gy (30 fractions); CDDP 80 mg/m2 + 5-FU 800 mg/m2/day | Prospective, non-randomized |
| Cordice 1990-1 | USA/ Mixed | Any | Not stated | 34 | 52 | RT+ surgery | Not stated | RT | Not stated | Retrospective case-control |
| Cordice 1990-2 | USA/ Mixed | Any | Not stated | 13 | 52 | RT+CT | Not stated | RT | Not stated | Retrospective case-control |
| Hainsworth 2007 | USA/ Not stated | Any | I, II, III | 97 | 50 | RT+CT+ surgery | 45 Gy (25 fractions); 5-FU 225 mg/m2 + carboplatin AUC 6.0 + paclitaxel 200 mg/m2 (PC) | RT+CT | 45 Gy (25 fractions); 5-FU 225 mg/m2 + carboplatin AUC 6.0 + paclitaxel 200 mg/m2 then radiation to 64.8 Gy + 1 additional dose of PC | Prospective, non-randomized |
| Herskovic 1992 | USA/ Mixed | Any | T1, 2, 3, NX, 0, 1 | 61 | 60 | RT+CT | 50 Gy (25 fractions); 5-FU 1000 mg/m2/day + cisplatin 75 mg/m2 | RT | 64 Gy (32 fractions) | RCT |
| Hihara 2014 | Japan/ Japanese | Squamous (26), carcinosarcoma (1) | T4N0M0, T4N1M1, T4N1M1a, T4N1M1b | 17 | 10 | RT+CT+ surgery | 50–66 Gy (25 fractions); CDDP 3–70 mg/m2, 5-FU 250–700 mg/m2 OR docetaxel 7.5 mg/m2 + 5-FU 250 mg/m2 | RT+CT | 50–66 Gy (25 fractions); CDDP 3–70 mg/m2, 5-FU 250–700 mg/m2 OR docetaxel 7.5 mg/m2 + 5-FU 250mg/m2 | Prospective, non-randomized |
| Mukaida 1998 | Japan/ Japanese | Not stated | T1, 2, 3, 4 N0, N1 M0, M1 IIA, IIB, III, IV | 19 | 19 | RT+CT | 40 to 60 Gy; CDDP 50 mg/m2 + 5-FU 500 mg/m2 + VP-16 60 mg/m2 | RT | 40 to 60 Gy | Prospective, non-randomized |
| Shridhar 2014 | USA/ Not stated | Adenoca-rcinoma | T1, 2, 3, 4 N0, N1 M0, M1 IIA, IIB, III, IV | 94 | 60 | RT+CT+ surgery | Mixed protocols | RT+CT | Mixed protocols | Retrospective case-control |
| Smith 1998-1 | USA/ Not stated | Squamous cell carcinoma | I, II | 24 | 32 | RT+ surgery | Maximum 40 Gy | RT | Maximum 60 Gy; | RCT |
| Smith 1998-2 | USA/ Not stated | Squamous cell carcinoma | I, II | 21 | 37 | RT+CT+ surgery | Maximum 40 Gy; 5-FU 1000 mg/m2 + bolus mitomycin C 10 mg/m2 | RT+CT | Maximum 40 Gy; 5-FU 1000 mg/m2 + bolus mitomycin C 10mg/m2 | RCT |
| Smith 1998-3 | USA/ Not stated | Squamous cell carcinoma | I, II | 37 | 32 | RT+CT | Maximum 60 Gy; 5-FU 1000 mg/m2 + bolus mitomycin C 10 mg/m2 | RT | Maximum 60 Gy | RCT |
| Yan 2014 | China/ Chinese | Squamous cell carcinoma | I, II, III, IV | 34 | 34 | RT+ immunotherapy | 60–66 Gy (30–33 fractions); 1×109 CIK cells/day + 1×107 DC cells/day for 5 days | RT | 60–66 Gy (30–33 fractions) | RCT |
| Yoon 2015 | Republic of Korea/ Korean | Squamous (95), adenocarcinoma (2) | II, III, IVa | 47 | 50 | RT+CT+ surgery+ induction CT | Oxaliplatin 130 mg/m2 + S1 40 mg/m2 − 2 cycles followed by 46 Gy (23 fractions) plus concurrent oxaliplatin 130 mg/m2 + S1 30 mg/m2 | RT+CT+ surgery | 46 Gy (23 fractions) plus concurrent oxaliplatin 130 mg/m2 + S1 30 mg/m2 (no induction) | RCT |
RT – radiotherapy; CT – chemotherapy; RCT – randomized controlled trial; FU – fluorouracil, CDDP – cisplatin, S1: combination of tegafur, gimeracil, oteracil potassium. Staging scores: TNM – T1: cancer is growing into tissue under the epithelium; T2: cancer is growing into the muscularis mucosa; T3: cancer is growing into the adventitia; T4: cancer is growing into the pleura, the pericardium, the diaphragm, the trachea, the aorta, the spine, or other crucial structures; TX: primary tumor cannot be assessed. N0: cancer has not spread to lymph nodes; N1: cancer has spread to 1 or 2 nearby lymph nodes; NX: nearby lymph nodes cannot be assessed. M0: no metastasis to distant organs or lymph nodes; M1: cancer has metastasized to distant lymph nodes or other organs. Stage I, II, III, IV – combinations of TNM and cancer grade (46).
Although most studies enrolled patients with any type of esophageal cancer [16,20,21,24,25], 4 studies included only patients with squamous cell carcinoma or had a majority of squamous cell carcinomas [17–19,22], and only 2 studies focused only on patients with adenocarcinomas [26,27].
Quality of studies
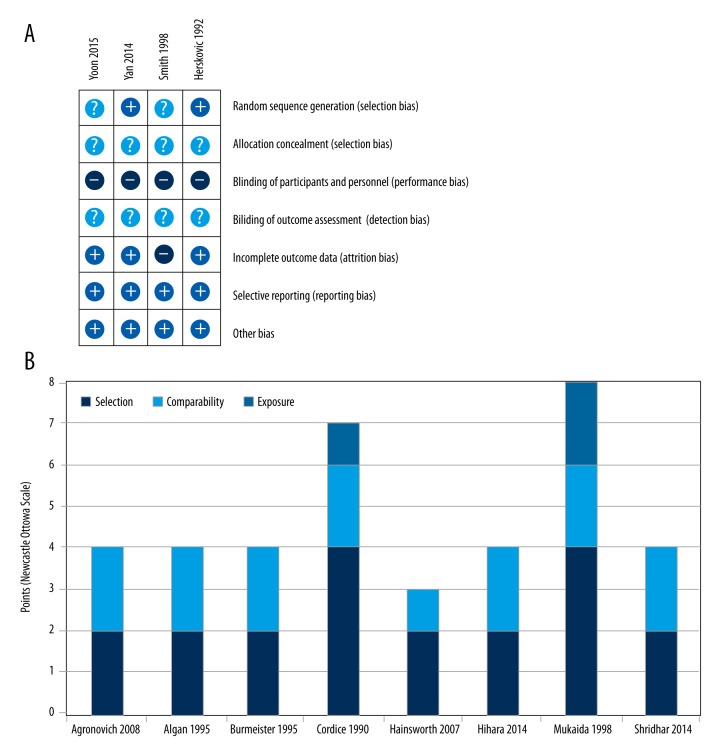
We evaluated the risk of bias in the 4 RCTs using the Cochrane Collaboration tool [13] and the 8 non-randomized studies were assessed using the Newcastle-Ottawa scale [14]. Overall, the risk of bias in the RCTs was mostly acceptable (Figure 2A). None of the studies suffered from reporting bias, and only 1 study [17] lost more than 10% of patients to follow-up. No other obvious bias was present. None of the 4 RCTs undertook blinding of participants or personnel, and blinding of outcome assessors was unclear. Allocation concealment was not reported in any of these studies, and only 2 studies provided a method of randomization.
Figure 2.
Quality of included studies. The randomized controlled trials were subjected to the Cochrane Collaboration’s risk of bias analysis (A). The non-randomized trials were analyzed with the Newcastle-Ottawa Scale (B).
The quality of the non-randomized studies was poor (Figure 2B). Using the scoring methodology as suggested by the authors [14], only 1 study [23] obtained the score of “good” and the other studies were all rated as “poor”, mostly due to their lack of comparability. To investigate whether this had an influence on study outcomes, we undertook a sensitivity analysis of the RCTs.
Median survival times
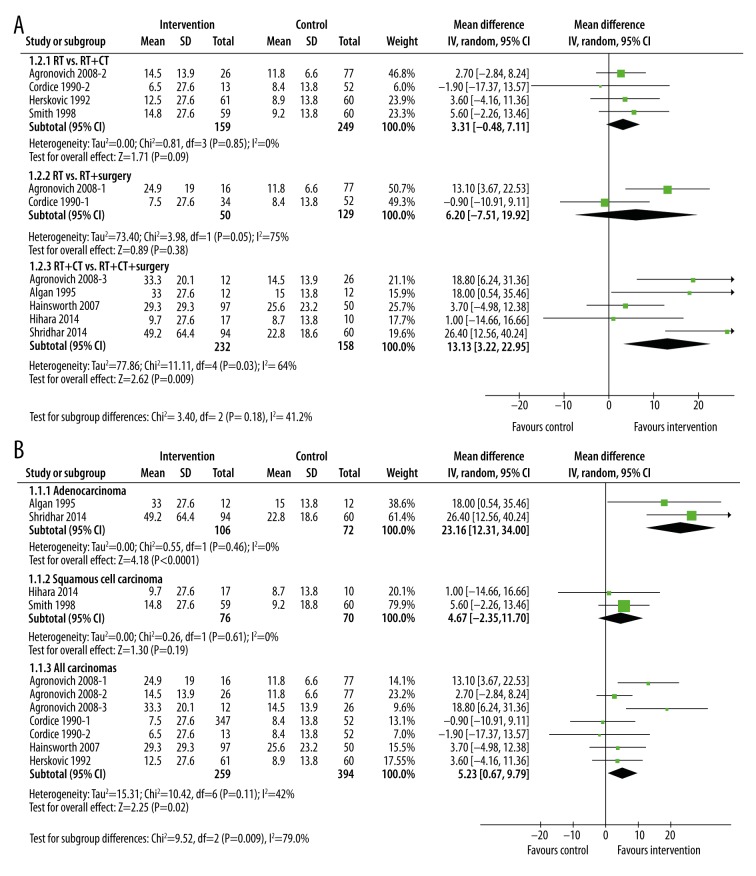
To determine whether additional therapies added to radiotherapy improve survival times, we undertook 2 subgroup meta-analyses (Figure 3). To investigate whether different kinds of interventions produced different results, we did a subgroup analysis of median survival time by treatment type (Figure 3A). Comparing RT with RT+CT showed no significant increase in median survival time (MD 3.31months, 95% CI: −0.48, 7.11, p=0.85, I2=0%); similarly, addition of surgery to RT also showed no significant improvement in median survival time (MD 6.20 months, 95% CI: −7.51, 19.92, p=0.05, I2=75%). In contrast, when surgery was added to RT and CT, a significant increase in median survival times was observed (MD 13.13 months, 95% CI: 3.32, 22.95, p=0.009, I2=64%). However, this did not differ significantly from either of the other 2 interventions (subgroup difference p=0.18).
Figure 3.
Subgroup meta-analysis of median survival times after treatment by type of intervention (A) or type of cancer (B). The interventions included radiotherapy plus chemotherapy, radiotherapy plus surgery, and radiotherapy plus chemotherapy plus surgery. The control groups were radiotherapy alone, or radiotherapy plus chemotherapy.
We also examined differences in survival time by type of cancer (Figure 3B). In the 2 studies in patients with adenocarcinoma, adding further treatments to radiotherapy resulted in a significant increase in median survival time (MD=23.16 months, 95% CI: 12.31, 34.00, p<0.0001). In contrast, the 2 studies in patients with squamous cell carcinoma did not gain any advantage with the addition of further treatments to radiotherapy (MD=4.67 months, 95% CI: −2.35, 11.70, p=0.19). Neither subgroup had any heterogeneity. The studies that did not select patients based on cancer type (subgroup “All carcinomas”) did show an overall increase in median survival time (MD 5.23 months (95% CI: 0.67, 9.79, p=0.02, I2=42%), although this was significantly smaller than in adenocarcinoma (subgroup difference p=0.003).
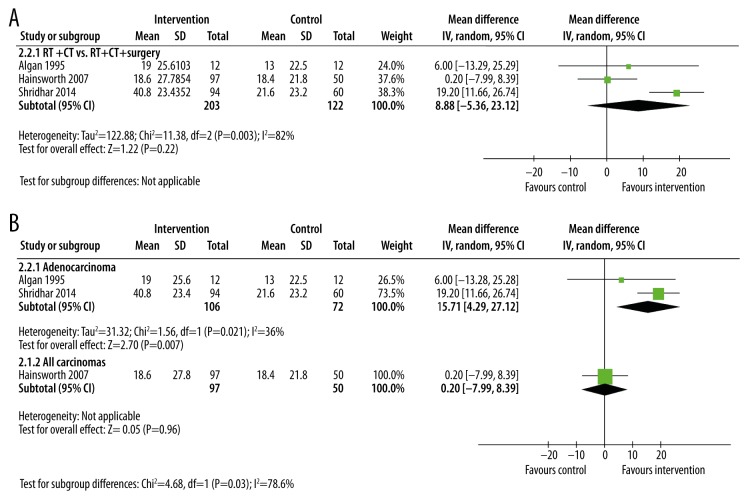
Disease-free survival times
Very few studies reported disease-free survival times [21,26,27] (Figure 4). Whereas the single study reporting this outcome in patients with any type of carcinoma showed no significant difference with additional treatments, those with adenocarcinoma did respond better to adding treatments to radiotherapy (MD=15.71 months, 95% CI: 4.29, 27.12, p=0.007, I2=36%). Coincidentally, all 3 studies reporting this outcome compared chemo-radiotherapy with chemo-radiotherapy plus surgery. Overall, adding surgery to chemo-radiotherapy did not result in any significant difference in disease-free survival times (MD=8.88 months, 95% CI: −5.36, 23.12, p=0.22, I2=82%).
Figure 4.
Subgroup meta-analysis of median disease-free survival times after treatment by type of intervention (A) or of cancer (B). In all 3 studies, the intervention was radiotherapy plus chemotherapy and surgery. The control group was radiotherapy plus chemotherapy without surgery.
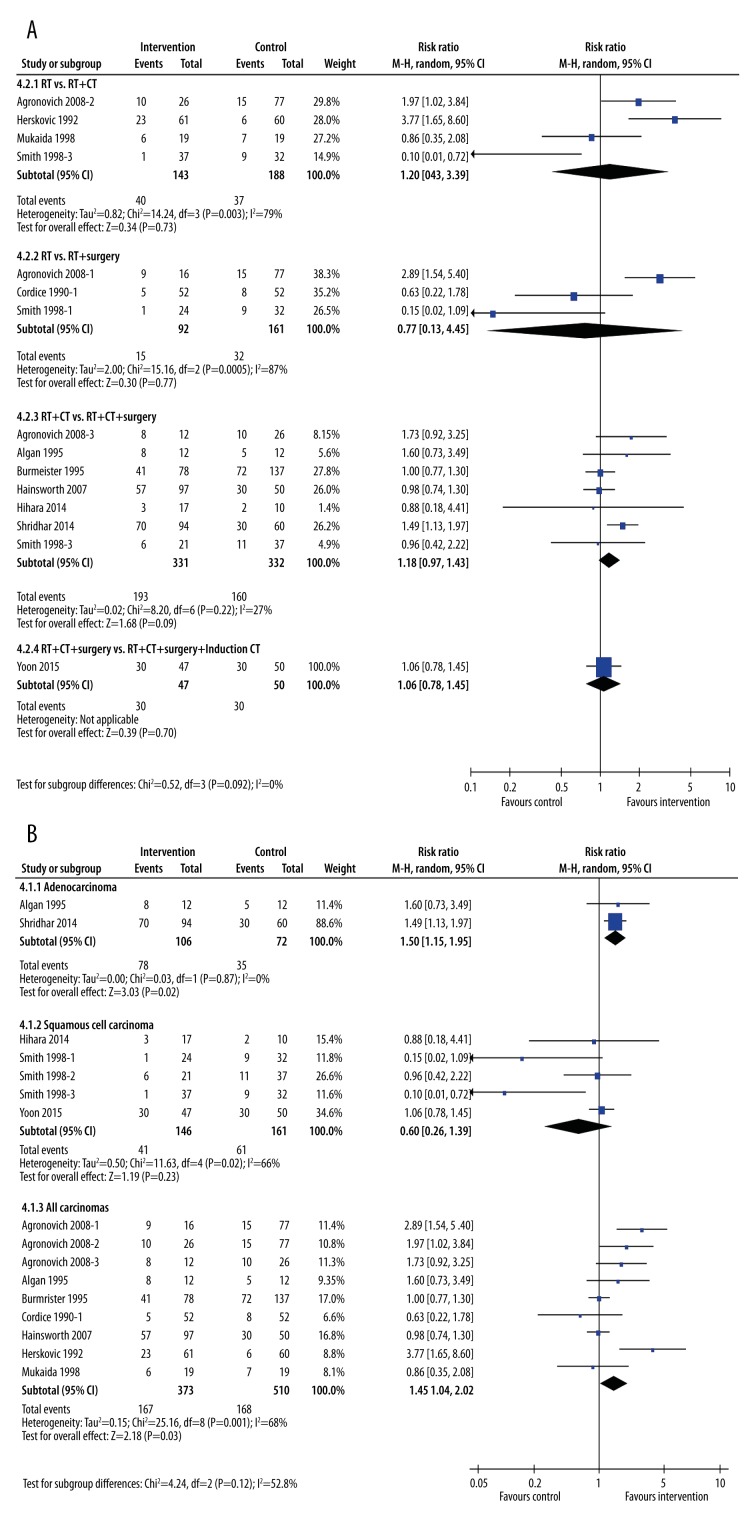
One-year survival rates
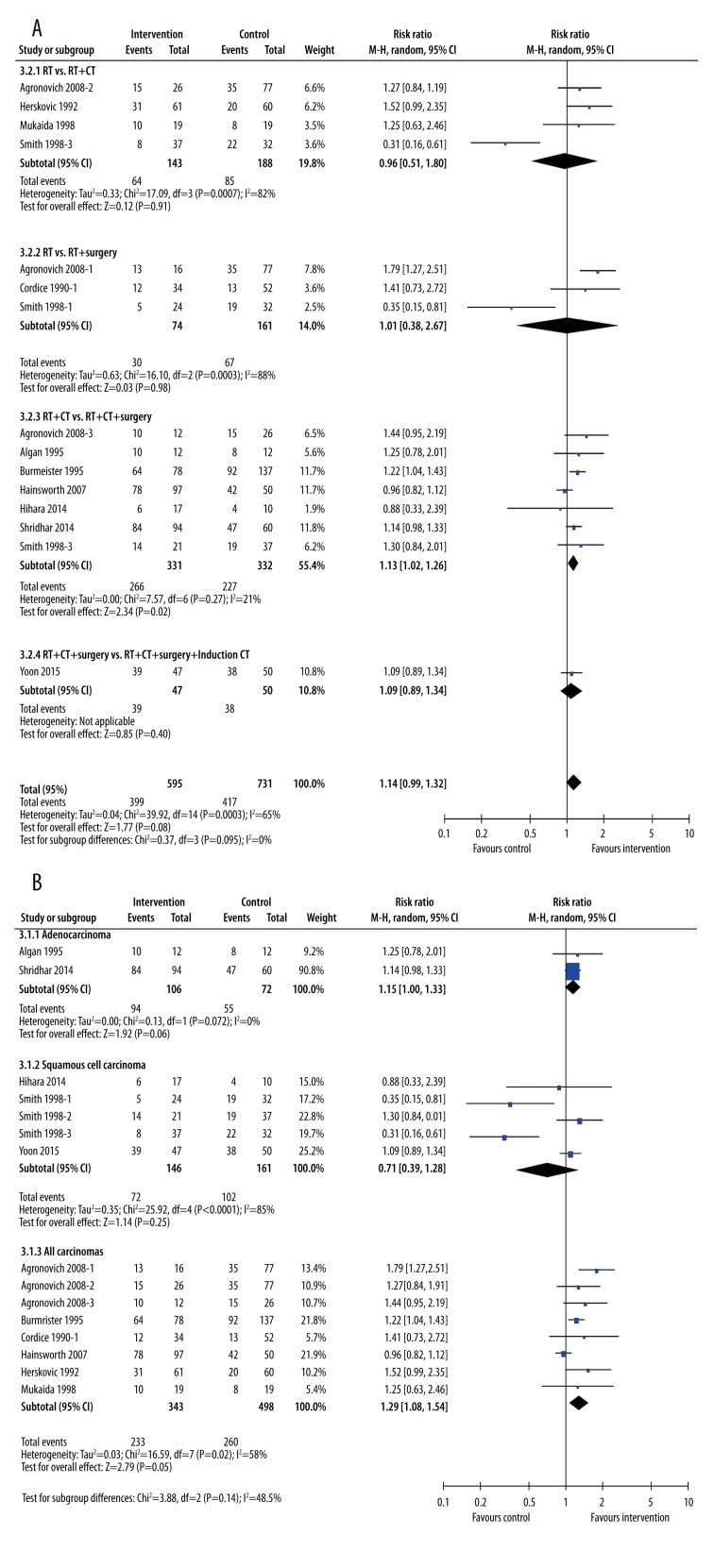
One-year survival rates were largely unaffected by addition of other treatments to radiotherapy (Figure 5). When viewed by type of treatment (Figure 5A), the only improvement in 1-year survival rates was seen in patients undergoing chemo-radiotherapy plus surgery (RR=1.13, 95% CI: 1.02, 1.26, p=0.02, I2=21%), although this was a minimal difference. When grouped by cancer type, the only group that experienced an increased survival rate was the “all carcinomas” group (RR=1.29, 95% CI: 1.08, 1.54, p=0.005). Neither patients with only adenocarcinoma nor only squamous cell carcinoma had a greater chance of surviving to 1 year (Figure 5B).
Figure 5.
Subgroup meta-analysis of 1-year survival rates after treatment by type of intervention (A) or type of cancer (B). The interventions included radiotherapy plus chemotherapy, radiotherapy plus surgery, radiotherapy plus chemotherapy plus surgery, and radiotherapy plus chemotherapy plus surgery plus induction chemotherapy. The control groups were radiotherapy alone, radiotherapy plus chemotherapy, or radiotherapy plus chemotherapy plus surgery.
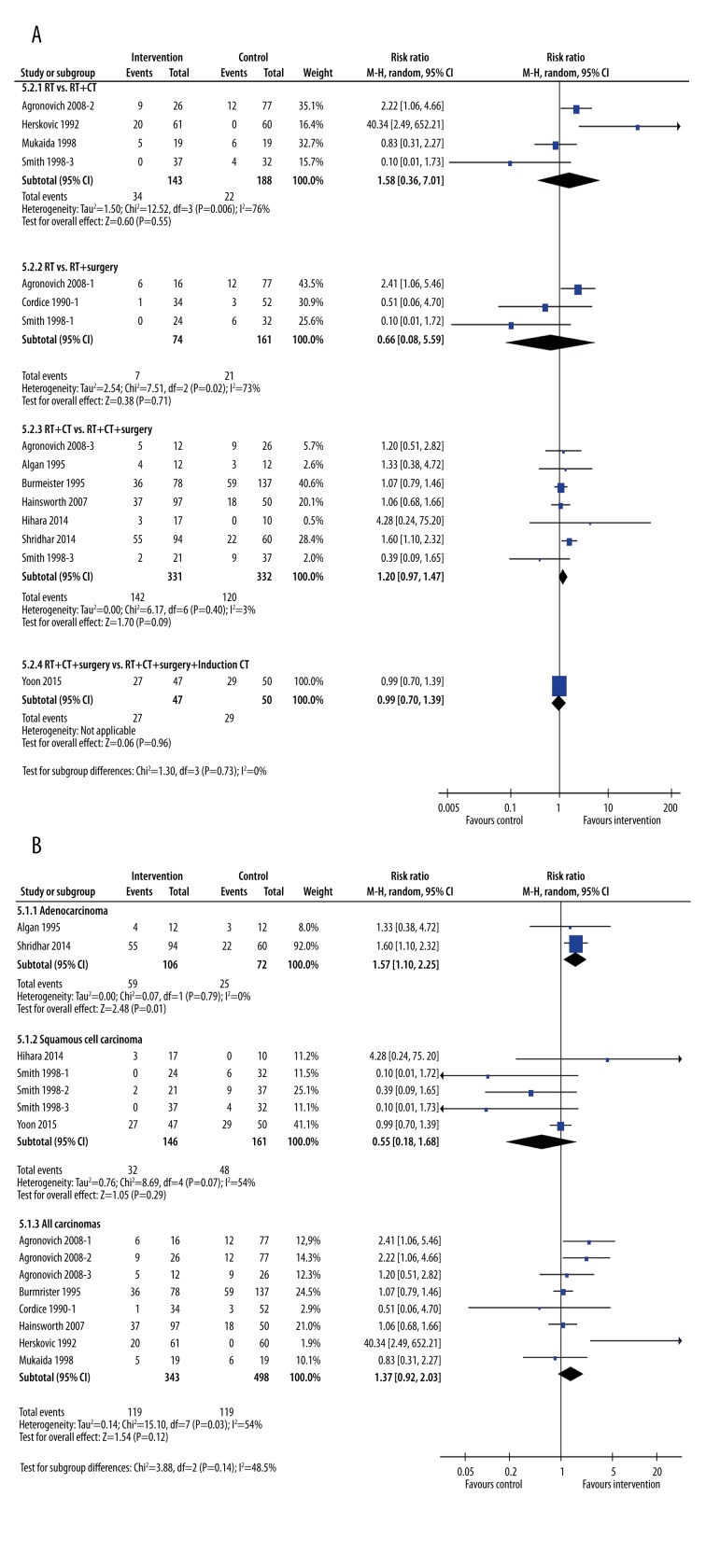
Two-year survival rates
When grouped by the type of intervention (Figure 6A), none of the treatments added to radiotherapy affected survival rates at 2 years. In contrast, when grouped by cancer type, the results for the 2-year survival rates were similar to the 1-year rates (Figure 6B). The exception was that patients with adenocarcinoma improved their chances of surviving to 2 years by adding surgery to chemo-radiotherapy (RR=1.50, 95% CI: 1.15, 1.95, p=0.002, I2=0%). The magnitude of this effect was similar to that seen with the “All carcinomas” group (test for subgroup differences, p=0.86).
Figure 6.
Subgroup meta-analysis of 2-year survival rates after treatment by type of intervention (A) or type of cancer (B). The interventions included radiotherapy plus chemotherapy, radiotherapy plus surgery, radiotherapy plus chemotherapy plus surgery, and radiotherapy plus chemotherapy plus surgery plus induction chemotherapy. The control groups were radiotherapy alone, radiotherapy plus chemotherapy, or radiotherapy plus chemotherapy plus surgery.
Three-year survival rates
At 3 years, when analyzed by type of treatment, none of the treatment subgroups showed any advantage over less invasive treatment (Figure 7A). In contrast, patients with adenocarcinoma again clearly gained an advantage by adding surgery to RT and CT (Figure 7B). Patients with adenocarcinoma who underwent surgery in addition to chemo-radiotherapy had a 57% greater rate of survival compared with those who had chemo-radiotherapy alone (RR=1.57, 95%CI: 1.10, 2.25, p=0.01, I2=0%). Patients with squamous cell carcinoma (p=0.29) and “All carcinomas” (p=0.12) did not see significantly longer rates of survival.
Figure 7.
Subgroup meta-analysis of 3-year survival rates after treatment by type of intervention (A) or type of cancer (B). The interventions included radiotherapy plus chemotherapy, radiotherapy plus surgery, radiotherapy plus chemotherapy plus surgery, and radiotherapy plus chemotherapy plus surgery plus induction chemotherapy. The control groups were radiotherapy alone, radiotherapy plus chemotherapy, or radiotherapy plus chemotherapy plus surgery.
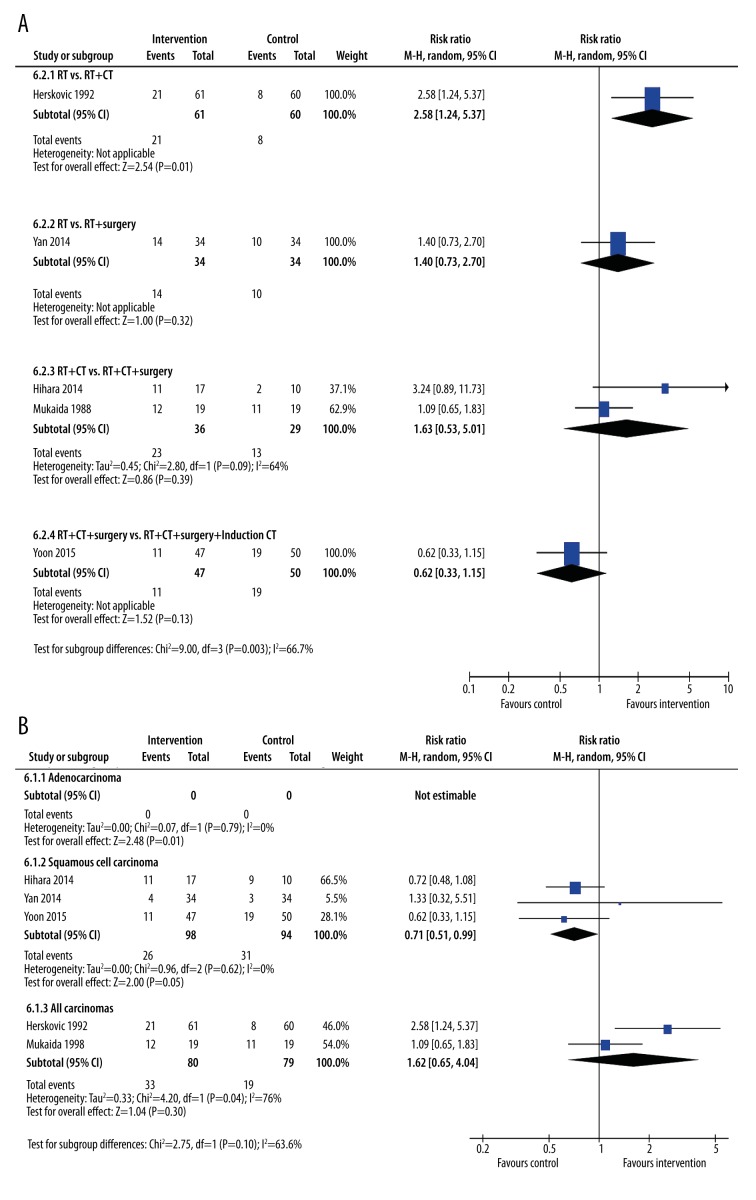
Response rates
Only 5 studies reported response rates [16,18,19,22,23] (Figure 8). Subgroup analysis of response by treatment type was hampered by a lack of studies (Figure 8A). Only 1 treatment type had more than 1 study to analyze (Figure 8A). Of this limited analysis, a study comparing radiotherapy with chemo-radiotherapy [16] showed a significant difference in response (RR 2.5 95% CI: 1.24, 5.37, p=0.01).
Figure 8.
Subgroup meta-analysis of response rates after treatment by type of intervention (A) or type of cancer (B). The interventions included radiotherapy plus chemotherapy, radiotherapy plus immunotherapy, radiotherapy plus chemotherapy plus surgery, and radiotherapy plus chemotherapy plus surgery plus induction chemotherapy. The control groups were radiotherapy alone, radiotherapy plus chemotherapy, or radiotherapy plus chemotherapy plus surgery.
The analysis by the type of cancer was similarly restricted. None of the studies reported response rates for patients with adenocarcinoma. Surprisingly, 3 studies [18,19,22] that reported response rates for patients with squamous cell carcinoma indicated a poor response with additional treatments (Figure 8A), although this was not statistically significant (RR=0.71, 95% CI: 0.51, 0.99, p=0.05, I2=0%). The 2 studies that reported response rates for patients grouped under the “All carcinoma” subgroup also failed to show any significant difference in their response with additional treatments (Figure 8B).
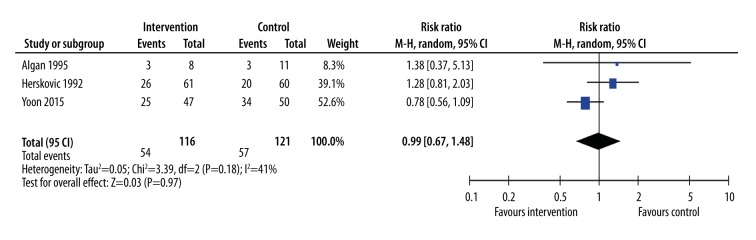
Dysphagia
Although dysphagia is one of the most debilitating symptoms of esophageal cancer, only 3 studies examined differences in this outcome. These studies compared RT+CT+surgery with RT+CT [27], RT+CT with RT alone [16], or adding induction CT to RT+CT+surgery [19] (Figure 9). None of the studies showed a significant improvement in dysphagia with treatments in addition to radiotherapy, and the meta-analysis of these studies was similarly neutral (RR=0.99, 95% CI: 0.67, 1.48, p=0.97, I2=41%).
Figure 9.
Meta-analysis of incidence of dysphagia. The interventions included radiotherapy plus chemotherapy (Herskovich 1992), radiotherapy plus chemotherapy plus surgery (Algan 1995), and radiotherapy plus chemotherapy plus surgery plus induction chemotherapy (Yoon 2015). The control groups were radiotherapy alone (Herskovich 1992), radiotherapy plus chemotherapy (Algan 1995), or radiotherapy plus chemotherapy plus surgery (Yoon 2015).
Adverse effects
Only 3 studies directly compared adverse effects between treatment types [16,18,19] (Table 2). Two of these studies were in people with squamous cell carcinoma [18] or predominately squamous cell carcinoma [19]. One study was in people with any type of carcinoma [16]. Our analysis found that additional therapies resulted in additional adverse effects. This was especially the case when chemotherapy was added to radiotherapy [16], or induction chemotherapy added to chemo-radiotherapy with surgery [19]. However, due to the small number of studies, only 1 adverse effect reached statistical significance – hematological abnormalities (RR=2.59, 95% CI: 1.63, 4.11, p<0.0001). Other adverse effects that did not reach statistical significance, but were substantially increased when additional treatments were added, were over-excitation (35% vs. 15%), upper aerodigestive tract complications (33% vs. 18%), shivering and fever (9% vs. 3%), and tracheitis (32% vs. 26%).
Table 2.
Adverse effects of additional treatment versus control treatment regimens.
| Side effect | Adenocarcinoma | Squamous cell carcinoma | Any carcinoma | Additional treatment (%) | Control treatment (%) | Difference (Int – Cont) (%) | Risk ratio | 95% CIs | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Anorexia/weight loss | +* | + | − | 2 | 2 | 0 | 1.06 | 0.07, 16.53 | 0.96 |
| AST/ALT elevation | +* | + | − | 2 | 0 | 2 | 3.19 | 0.13, 76.36 | 0.47 |
| Dermatological | − | + | − | 5 | 2 | 3 | 2.95 | 0.32, 27.58 | 0.34 |
| Gastrointestinal tract | +* | + | + | 18 | 17 | 1 | 1.01 | 0.61, 1.68 | 0.96 |
| Hematological | +* | + | + | 36 | 14 | 22 | 2.59 | 1.63, 4.11 | <0.0001 |
| Hyperglycemia | +* | + | − | 0 | 2 | −2 | 0.35 | 0.01, 8.48 | 0.52 |
| Infection | +* | + | − | 2 | 0 | 2 | 3.19 | 0.13, 76.36 | 0.47 |
| Insomnia | − | + | − | 12 | 15 | −3 | 0.80 | 0.23, 2.73 | 0.72 |
| Nausea/vomiting | +* | + | − | 0 | 4 | −4 | 0.21 | 0.01, 4.31 | 0.31 |
| Nervous system | − | − | + | 1 | 0 | 1 | 3.06 | 0.13, 74.18 | 0.49 |
| Over-excitation | − | + | − | 35 | 15 | 20 | 2.40 | 0.95, 6.07 | 0.06 |
| Respiratory tract | − | − | + | 3 | 0 | 3 | 4.92 | 0.24, 100.37 | 0.30 |
| Shivering & fever | − | + | − | 9 | 3 | 6 | 3.00 | 0.33, 27.42 | 0.33 |
| Tracheitis | − | + | − | 32 | 26 | 6 | 1.22 | 0.58, 2.57 | 0.60 |
| Upper aerodigestive tract | − | − | + | 33 | 18 | 15 | 1.79 | 0.94, 3.40 | 0.08 |
The treatment modalities in this table included RT+CT vs. RT (any carcinoma) (Herskovich 1992), RT plus immunotherapy vs. RT (squamous cell carcinoma (Yan 2014), and RT+CT+Surgery+Induction CT vs. RT+CT+Surgery (98% squamous cell carcinoma, 2% adenocarcinoma) (Yoon 2015).
Only 2% of cases in this study were adenocarcinoma (Yoon 2015).
Sensitivity analysis – inclusion of RCTs
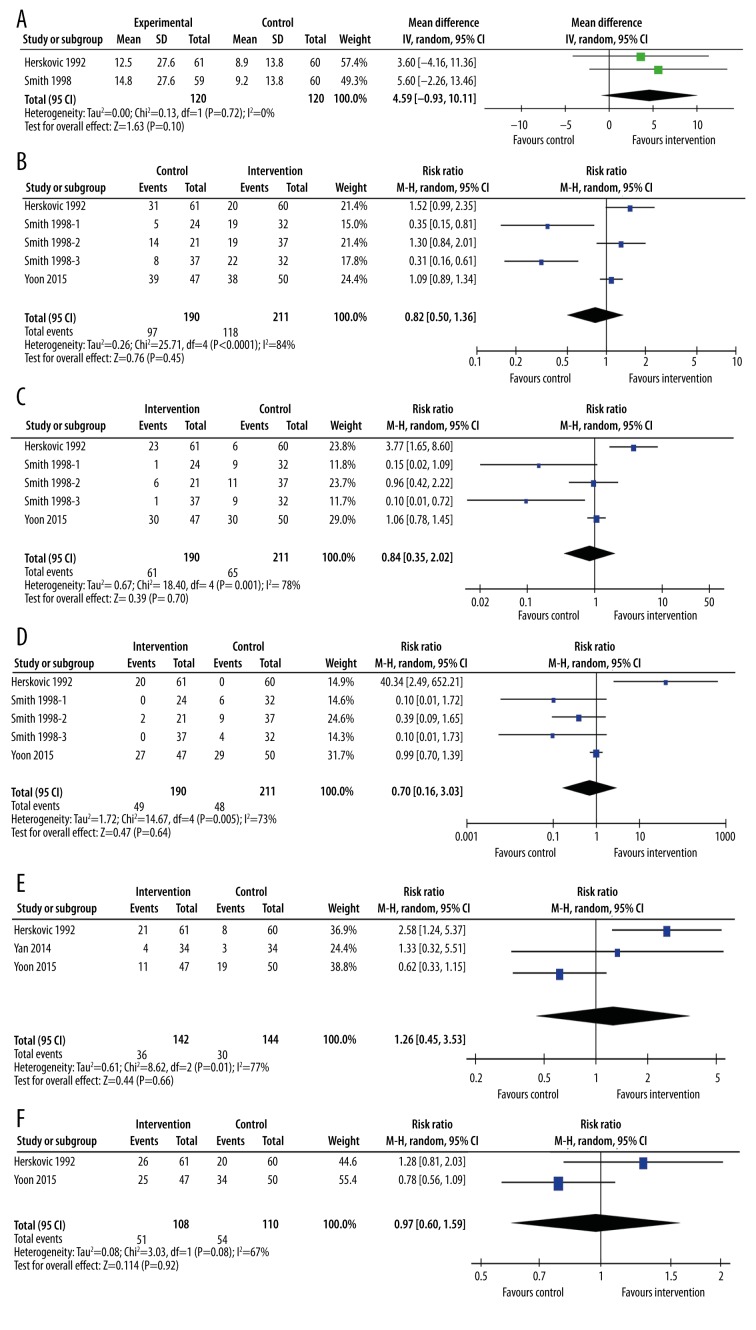
We undertook a sensitivity analysis of RCTs alone where possible (Figure 10). Median survival was reported in only 2 RCTs (Figure 10A), 1 in patients with squamous cell carcinoma [17] and 1 in patients with any carcinoma type [16]. Both studies compared RT with RT+CT. Meta-analysis of the 2 studies yielded similar results to the non-randomized trials (MD=4.59 months, 95% CI: −0.93, 10.11, p=0.10). Similarly, meta-analysis of the 5 study arms looking at 1-year survival (Figure 10B) did not differ substantially from the meta-analysis of all trials (RR=0.82, 95% CI: 0.50, 1.36, p=0.45). The same was true for 2-year survival (RR = 0.84, 95% CI: 0.35, 2.02, p=0.70) (Figure 10C) and 3-year survival (RR=0.70, 95% CI: 0.16, 3.03, p=0.64) (Figure 10D), response rates (RR=1.26, 95% CI: 0.45, 3.53, p=0.66) (Figure 10E), or dysphagia (RR=0.97, 95% CI: 0.60, 1.59, p=0.92) (Figure 10F).
Figure 10.
Meta-analysis of randomized controlled trials. (A) Median survival time; (B) 1-year survival time; (C) 2-year survival time; (D) 3-year survival time; (E) response rate; (F) incidence of dysphagia. The interventions included radiotherapy plus chemotherapy (Herskovich 1992, Smith 1998-3), radiotherapy plus immunotherapy (Yan 2014), radiotherapy plus surgery (Smith 1998-1), radiotherapy plus chemotherapy plus surgery (Smith 1998-2), and radiotherapy plus chemotherapy plus surgery plus induction chemotherapy (Yoon 2015). The control groups were radiotherapy alone (Herskovich 1992, Smith 1998-1, Smith 1998-3 Yan 2014), radiotherapy plus chemotherapy (Smith 1998-2), or radiotherapy plus chemotherapy plus surgery (Yoon 2015).
Sensitivity analysis – tumor stage
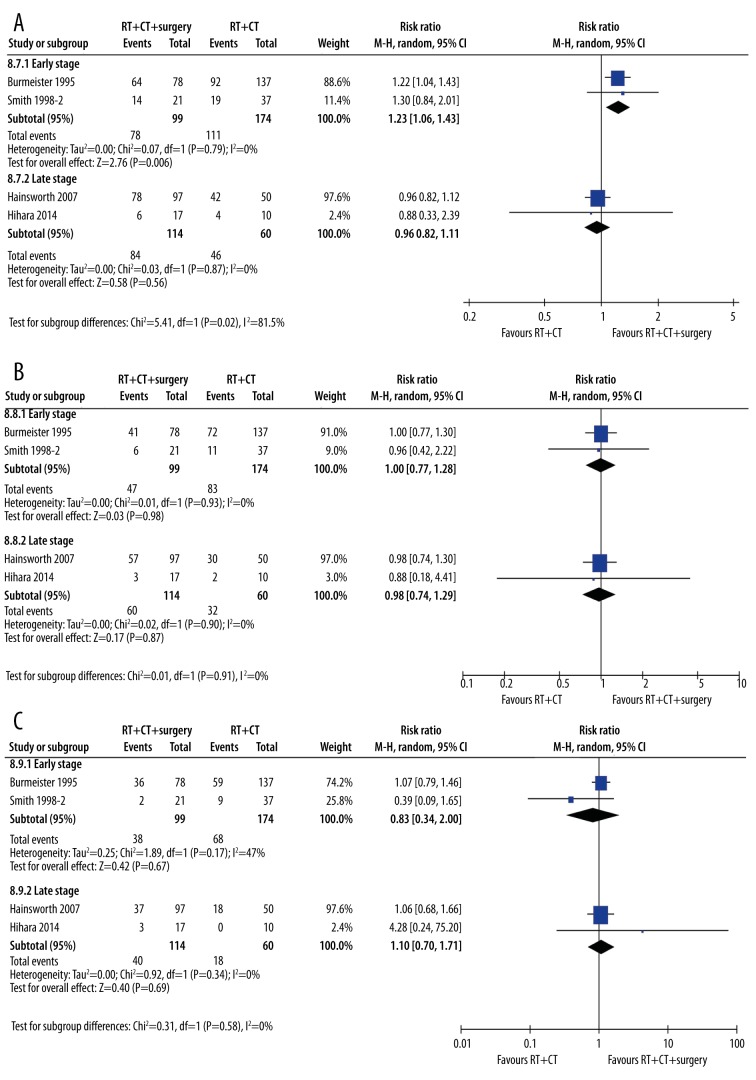
It was possible that the tumor stage of the patients at the beginning of the studies would confound our results. As such, we undertook an a priori sensitivity analysis by tumor stage (Figure 11). As seen in Table 1, many studies included patients of any stage from I to IV. In order to exclude other variables that could influence the results (e.g., tumor type and treatment regimens), we compared studies using the same treatment regimen and excluded studies on adenocarcinoma. We chose 1-year, 2-year, and 3-year survival as the outcomes most likely to be influenced by tumor stage. At 1 year, the studies including only patients with early-stage cancer did demonstrate greater benefit from adding treatments to radiotherapy than studies in people with later-stage cancer (Figure 11A). Early-stage cancer patients had an improvement in survival rates at 1 year (RR=1.23, 95% CI: 1.06, 1.43, p=0.006) compared with late-stage patients (RR=0.96, 95% CI: 0.82, 1.11, p=0.56). The subgroups were statistically different from one another (p=0.02).
Figure 11.
Sensitivity analysis of early versus late-stage tumors. (A) 1-year survival; (B) 2-year survival; (C) 3-year survival. A single treatment type (radiotherapy plus chemotherapy versus radiotherapy plus chemotherapy plus surgery) was chosen, and studies in adenocarcinoma were removed to reduce between-study variability. Two studies in patients with early- to mid-stage cancer (Burmeister 1995, Smith 1998) were compared with 2 studies in patients with mid- to late-stage cancer (Hainsworth 2007, Hihara 2014).
At 2 and 3 years, however, all benefits of adding treatment to radiotherapy in early-stage cancer patients disappeared (Figure 11B, 11C). At 2 years, early-stage cancer patients did not benefit from adding treatments to radiotherapy (RR=1.00, 95% CI: 0.77, 1.28, p=0.98), and neither did late-stage cancer patients (RR=0.98, 95% CI: 0.74, 1.29, p=0.87). There were no subgroup differences (p=0.91). At 3 years, there was no benefit to early-stage cancer patients from adding treatments to radiotherapy (RR=0.78, 95% CI: 0.26, 2.39, p=0.66), nor to late-stage cancer patients (RR=1.18, 95% CI: 0.59, 2.36, p=0.63). There were no subgroup differences (p=053).
Sensitivity analysis – chemotherapy regime
As stated earlier, we had planned a sensitivity analysis by chemotherapy regimen. Unfortunately, the treatment regimens varied widely, and there were insufficient studies to conduct an analysis by chemotherapy type.
Discussion
This meta-analysis investigated the efficacy and safety of using additional treatments with radiotherapy in patients with esophageal carcinoma. Treatment strategies included chemotherapy (RT vs. RT+CT), surgery (RT vs. RT+ surgery, RT+CT vs. RT+CT+surgery), and immune therapy (RT vs. RT+immune therapy).
Median survival time
Figure 3 displays subgroup meta-analyses of the median survival times by type of treatment (A) and cancer type (B). An interesting observation was the startling lack of efficacy of adding extra treatments to radiotherapy. Neither chemotherapy nor surgery improved median survival times (Figure 3A) above that of radiotherapy alone. The addition of surgery to chemo-radiotherapy was significantly better, but this result was driven by the 2 studies in patients with adenocarcinoma. Removal of the 2 adenocarcinoma studies led to a loss of statistical significance for this treatment combination as well.
An imbalance between groups at baseline could explain the lack of efficacy. However, this is unlikely to be the case, as any imbalance, especially towards patients selected for surgery, was more likely to favor additional treatments. That is, patients selected for more aggressive treatment tended to have less advanced cancer, or were physically fitter than those who did not undergo surgery. Thus, it is even more concerning that these groups did not exhibit longer survival times.
A clear finding was that adding treatments to radiotherapy increased the median survival times for patients with adenocarcinomas only. This phenomenon has been observed before in esophageal cancer [28,29] as well as for other cancers such as pulmonary cancers [30–32], but contrasts with survival in cervical cancer [33].
Disease-free survival
Unfortunately, only 3 studies reported on this important outcome. All 3 studies compared chemo-radiotherapy alone with chemo-radiotherapy plus surgery. Two of the 3 studies were in patients with adenocarcinoma [26,27] and the third was in patients with any carcinoma [21]. Similarly to median survival times, disease-free survival was only significantly better in patients with adenocarcinoma. This suggests that in these patients, undertaking surgery will improve outcomes, but that in patients with squamous cell carcinoma, surgery will not improve survival. A similar lack of efficacy was seen in cervical squamous cell carcinomas [34], where disease-free survival times were not significantly extended by the addition of chemotherapy to radiotherapy. Given that surgery necessarily involves risk, it may be that the risks outweigh the benefits for squamous cell carcinoma patients.
Survival rates
Comparison of 1-year, 2-year, and 3-year survival rates underlies the superior prognosis of patients with adenocarcinomas. When looking at treatment types, initial inspection suggests that adding surgery to chemo-radiotherapy benefited patients at 1-year post-treatment. However, removal of the 2 studies in patients with adenocarcinoma shifted the treatment effect from significant (p=0.02) to non-significant (p=0.13). Thus, oncologists should take care to explain to their patients with non-adenocarcinoma the risks versus benefits of potential treatment options.
Although at 1 year there was little difference between the different cancer types, patients with adenocarcinoma benefited increasingly from additional treatments as time went on. In contrast, other carcinoma types failed to show any benefit from adding treatments. Study reports evaluating therapeutic strategies in different cancers, such as lung cancer [31,32], rectal cancer [35,36], and cervical cancer [34] similarly failed to find any benefit in terms of survival rates from adding multiple therapies to radiotherapy.
Response rates
As we have seen with all other outcomes, adding additional treatments to radiotherapy saw no improvement in response rates. Only 5 studies [16,18,19,22] reported on this outcome, and each study defined “response” in a different way; however, overall, no significant difference was seen in any study except Herskovic (1992) [16]. A close examination of this study reveals that 20% of the combined therapy group had adenocarcinoma as opposed to 10% of the radiation therapy group. This additional set of patients with adenocarcinoma may account for the observed increase in effectiveness of combined therapy seen in the study [16].
Dysphagia
Dysphagia is a very common and highly debilitating symptom in esophageal cancer. Indeed, dysphagia, and later treatment for it, can result in malnutrition [37]. Therefore, treatments that reduce dysphagia, even if they do not increase survival times, could be regarded as important for quality of life [38]. It was surprising, then, that only 3 of the included 18 study arms reported on dysphagia incidence between treatment types. One study added surgery to chemo-radiotherapy [27], another study added chemotherapy to radiotherapy [16], and the third study added induction chemotherapy to chemo-radiotherapy [19]. Regardless of the treatment type, no decrease in the incidence of dysphagia was seen in any study. This was a surprising finding, until one looks at the effectiveness of radiotherapy alone for dysphagia. A recent clinical trial determined that radiotherapy alone is as effective as radiotherapy with chemotherapy in reducing dysphagia [39]. Thus, adding chemotherapy, surgery, or induction chemotherapy to radiation would perhaps do more harm than good, compared with the effect of radiation alone.
Adverse effects
With additional cancer treatments comes the risk of increased adverse effects [40–43]. Unfortunately, few studies directly compared adverse effects between different treatment types. As a result, most adverse effects in our meta-analysis are based on between 68 and 120 patients. Only 2 adverse effects (gastrointestinal and hematological) were sufficiently powered (286 patients) to capture incidence of adverse effects with reasonable confidence intervals. Of these, no significant difference was seen for gastrointestinal adverse effects. In contrast, hematological adverse effects were significantly increased. This is not surprising, given that the 2 studies that showed a significant increase both involved either adding or increasing chemotherapy [16,19]. A recent meta-analysis on doublet versus triplet chemotherapy concluded that triplet therapy was more effective, but came at the expense of significant hematological damage [44]. Overall, 12 of the 15 reported that adverse effects were worse when treatments were added to the control therapy. Traditionally, little difference occurs in approaches to therapy based on type of cancer (adenocarcinoma vs. squamous cell carcinoma) [45]. Given this, much thought should be given to the use of chemotherapy in patients with squamous cell carcinoma, and how much patients will benefit compared with the harm done to their health and quality of life.
Limitations
Only 4 of the studies in the meta-analysis were RCTs, and none of these was blinded. However, a sensitivity analysis of RCTs did not reveal any differences between the RCTs alone compared with all studies together.
Conclusions
This meta-analysis has demonstrated the importance of cancer type on response to multiple esophageal cancer treatment. We found that in almost all cases of squamous cell carcinoma, additional treatments did not increase patient survival, but did increase the incidence of adverse effects. In stark contrast to this finding, patients with adenocarcinoma clearly responded to adding treatments such as chemotherapy and surgery to radiotherapy. Given the fact that, at present, little difference occurs in the treatment of the 2 forms of cancer, we believe that this review is of vital importance. Patients with squamous cell carcinoma may experience a significantly better quality of life by forgoing futile interventions. We believe that this evidence is of great value to oncologists discussing treatment options with their patients.
Footnotes
Source of support: Departmental sources
References
- 1.Cheng X, Tian X, Wu X, et al. Relationship between LAPTM4B gene polymorphism and prognosis of patients following tumor resection for colorectal and esophageal cancers. PLoS One. 2016;11(7):e0158715. doi: 10.1371/journal.pone.0158715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Society AC. Find support and treatment. American Cancer Society; 2016. [Available from: http://www.cancer.org] [Google Scholar]
- 3.Society AC. Palliative Care Research. American Cancer Society; 2016. [Available from: http://www.cancer.org/research/survivaltreatmentresearch/palliative-care-research] [Google Scholar]
- 4.Sreedharan A, Harris K, Crellin A, et al. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev. 2009;(4):CD005048. doi: 10.1002/14651858.CD005048.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Wang C, Guan S, Cheng Y. Impacts of physically active and under-active on clinical outcomes of esophageal cancer patients undergoing esophagectomy. Am J Cancer Res. 2016;6(7):1572–81. [PMC free article] [PubMed] [Google Scholar]
- 6.Hulshof MC, van Laarhoven HW. Chemoradiotherapy in tumours of the oesophagus and gastro-oesophageal junction. Best Pract Res Clin Gastroenterol. 2016;30(4):551–63. doi: 10.1016/j.bpg.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Flanagan JC, Batz R, Saboo SS, et al. Esophagectomy and gastric pull-through procedures: Surgical techniques, imaging features, and potential complications. Radiographics. 2016;36(1):107–21. doi: 10.1148/rg.2016150126. [DOI] [PubMed] [Google Scholar]
- 8.Kidane B, Coughlin S, Vogt K, Malthaner R. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev. 2015;(5):CD001556. doi: 10.1002/14651858.CD001556.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Best LM, Mughal M, Gurusamy KS. Non-surgical versus surgical treatment for oesophageal cancer. Cochrane Database Syst Rev. 2016;(3):CD011498. doi: 10.1002/14651858.CD011498.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnott SJ, Duncan W, Gignoux M, et al. Preoperative radiotherapy for esophageal carcinoma. Cochrane Database Syst Rev. 2005;(4):CD001799. doi: 10.1002/14651858.CD001799.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas J, Brunton J, Graziosi S. EPPI-Reviewer 4: Software for research synthesis. London: EPPI-Centre; 2010. [Google Scholar]
- 13.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells GSB, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital; [Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp] [Google Scholar]
- 15.The Nordic Cochrane Centre. Review Manager (RevMan) 5.3 The Cochrane Collaboration. 2014. [Google Scholar]
- 16.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326(24):1593–98. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 17.Smith TJ, Ryan LM, Douglass HO, Jr, et al. Combined chemoradiotherapy vs. radiotherapy alone for early stage squamous cell carcinoma of the esophagus: A study of the Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42(2):269–76. doi: 10.1016/s0360-3016(98)00232-6. [DOI] [PubMed] [Google Scholar]
- 18.Yan L, Wu M, Ba N, et al. Efficacy of dendritic cell-cytokine-induced killer immunotherapy plus intensity-modulated radiation therapy in treating elderly patients with esophageal carcinoma. Genet Mol Res. 2015;14(1):898–905. doi: 10.4238/2015.February.2.13. [DOI] [PubMed] [Google Scholar]
- 19.Yoon DH, Jang G, Kim JH, et al. Randomized phase 2 trial of S1 and oxaliplatin-based chemoradiotherapy with or without induction chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2015;91(3):489–96. doi: 10.1016/j.ijrobp.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Burmeister BH, Denham JW, O’Brien M, et al. Combined modality therapy for esophageal carcinoma: Preliminary results from a large Australasian multicenter study. Int J Radiat Oncol Biol Phys. 1995;32(4):997–1006. doi: 10.1016/0360-3016(94)00449-u. [DOI] [PubMed] [Google Scholar]
- 21.Hainsworth JD, Meluch AA, Gray JR, et al. Concurrent chemoradiation followed by esophageal resection vs. chemoradiation alone for localized esophageal cancer. Community Oncology. 2007;4(7):431–39. [Google Scholar]
- 22.Hihara J, Hamai Y, Emi M, et al. Esophageal bypass operation prior to definitive chemoradiotherapy in advanced esophageal cancer with tracheobronchial invasion. Ann Thorac Surg. 2014;97(1):290–95. doi: 10.1016/j.athoracsur.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 23.Mukaida H, Hirai T, Yamashita Y, et al. Clinical evaluation of adjuvant chemoradiotherapy with CDDP, 5-FU, and VP-16 for advanced esophageal cancer. Jpn J Thorac Cardiovasc Surg. 1998;46(1):11–17. doi: 10.1007/BF03217716. [DOI] [PubMed] [Google Scholar]
- 24.Agranovich A, McGahan CE, Gurjal A. Long-term survivorship of esophageal cancer patients treated with radical intent. Can J Gastroenterol. 2008;22(4):393–98. doi: 10.1155/2008/231878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordice JW., Jr Carcinoma of the esophagus seen in a 12-year period at Queens Hospital Center. J Natl Med Assoc. 1990;82(4):273–79. [PMC free article] [PubMed] [Google Scholar]
- 26.Shridhar R, Freilich J, Hoffe SE, et al. Single-institution retrospective comparison of preoperative versus definitive chemoradiotherapy for adenocarcinoma of the esophagus. Ann Surg Oncol. 2014;21(12):3744–50. doi: 10.1245/s10434-014-3795-2. [DOI] [PubMed] [Google Scholar]
- 27.Algan O, Coia LR, Keller SM, et al. Management of adenocarcinoma of the esophagus with chemoradiation alone or chemoradiation followed by esophagectomy: Results of sequential nonrandomized phase II studies. Int J Radiat Oncol Biol Phys. 1995;32(3):753–61. doi: 10.1016/0360-3016(94)00592-9. [DOI] [PubMed] [Google Scholar]
- 28.Holscher AH, Bollschweiler E, Schroder W, et al. Prognostic differences between early squamous-cell and adenocarcinoma of the esophagus. Dis Esophagus. 1997;10(3):179–84. doi: 10.1093/dote/10.3.179. [DOI] [PubMed] [Google Scholar]
- 29.Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: Pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242(4):566–73. doi: 10.1097/01.sla.0000184211.75970.85. discussion 573–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawase A, Yoshida J, Ishii G, et al. Differences between squamous cell carcinoma and adenocarcinoma of the lung: Are adenocarcinoma and squamous cell carcinoma prognostically equal? Jpn J Clin Oncol. 2012;42(3):189–95. doi: 10.1093/jjco/hyr188. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd FA, Johnston MR, Payne D, et al. Randomized study of chemotherapy and surgery versus radiotherapy for stage IIIA non-small-cell lung cancer: A National Cancer Institute of Canada Clinical Trials Group Study. Br J Cancer. 1998;78(5):683–85. doi: 10.1038/bjc.1998.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephens RJ, Girling DJ, Hopwood P, Thatcher N Medical Research Council Lung Cancer Working Party. A randomised controlled trial of pre-operative chemotherapy followed, if feasible, by resection versus radiotherapy in patients with inoperable stage T3, N1, M0 or T1-3, N2, M0 non-small cell lung cancer. Lung Cancer. 2005;49(3):395–400. doi: 10.1016/j.lungcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Shimada M, Nishimura R, Nogawa T, et al. Comparison of the outcome between cervical adenocarcinoma and squamous cell carcinoma patients with adjuvant radiotherapy following radical surgery: SGSG/TGCU Intergroup Surveillance. Mol Clin Oncol. 2013;1(4):780–84. doi: 10.3892/mco.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorvidhaya V, Chitapanarux I, Sangruchi S, et al. Concurrent mitomycin C, 5-fluorouracil, and radiotherapy in the treatment of locally advanced carcinoma of the cervix: a randomized trial. Int J Radiat Oncol Biol Phys. 2003;55(5):1226–32. doi: 10.1016/s0360-3016(02)04405-x. [DOI] [PubMed] [Google Scholar]
- 35.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 36.Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–25. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 37.Bozzetti F. Nutritional support in patients with oesophageal cancer. Support Care Cancer. 2010;18(Suppl 2):S41–50. doi: 10.1007/s00520-009-0664-9. [DOI] [PubMed] [Google Scholar]
- 38.Vesey S. Dysphagia and quality of life. Br J Community Nurs. 2013;(Suppl):S14, S16, S18–19. doi: 10.12968/bjcn.2013.18.sup5.s14. [DOI] [PubMed] [Google Scholar]
- 39.Penniment M. Radiation vs. CRT: Equally effective for reducing dysphagia. Gastrointestinal Cancers Symposium; January 15, 2015. [Google Scholar]
- 40.Bilek K, Ebeling K, Leitsmann H, Seidel G. Radical pelvic surgery versus radical surgery plus radiotherapy for stage Ib carcinoma of the cervix uteri. Preliminary results of a prospective randomized clinical study. Arch Geschwulstforsch. 1982;52(3):223–29. [PubMed] [Google Scholar]
- 41.Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65(1):169–76. doi: 10.1016/j.ijrobp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Thompson IM, Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: A randomized clinical trial. JAMA. 2006;296(19):2329–35. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 43.Machtay M, Pajak TF, Suntharalingam M, et al. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: A randomized trial of the Radiation Therapy Oncology Group (RTOG 99-03) Int J Radiat Oncol Biol Phys. 2007;69(4):1008–17. doi: 10.1016/j.ijrobp.2007.04.063. [DOI] [PubMed] [Google Scholar]
- 44.Mohammad NH, ter Veer E, Ngai L, et al. Optimal first-line chemotherapeutic treatment in patients with locally advanced or metastatic esophagogastric carcinoma: triplet versus doublet chemotherapy: A systematic literature review and meta-analysis. Cancer Metastasis Rev. 2015;34(3):429–41. doi: 10.1007/s10555-015-9576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berry MF. Esophageal cancer: Staging system and guidelines for staging and treatment. J Thorac Dis. 2014;6(Suppl 3):S289–97. doi: 10.3978/j.issn.2072-1439.2014.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Cancer Society. How is cancer of the esophagus staged? 2016. [Available from: http://www.cancer.org/cancer/esophaguscancer/detailedguide/esophagus-cancer-staging]