Abstract
BACKGROUND
Among various cardiac autoantibodies (AAb), those recognizing the β1 adrenergic receptor (β1AR) demonstrate agonist-like effects and induce myocardial damage that can be reversed by β-blockers and immunoglobulin G3 (IgG3) immunoadsorption.
OBJECTIVES
We investigated the role of β1AR-AAbs belonging to the IgG3 subclass in patients with recent-onset cardiomyopathy.
METHODS
Peripheral blood was drawn at enrollment in subjects with recent-onset cardiomyopathy (left ventricular ejection fraction [LVEF] ≤0.40; <6 months). Presence of IgG and IgG3-β1AR-AAb was determined and echocardiograms assessed at baseline and 6 months. Subjects were followed for up to 4 years.
RESULTS
Among the 353 enrolled subjects, 62 (18%) were positive for IgG3-β1AR-AAb (IgG3), 58 (16%) were positive for IgG but not IgG3 (non-IgG3), and the remaining were negative. There were no significant differences in baseline systolic blood pressure, heart rate, or LVEF among the groups at baseline. LV end-diastolic and end-systolic (LVEDD and LVESD, respectively) diameters were significantly larger in the non-IgG3 group compared to the other groups (LVEDD: p < 0.01; LVESD: p = 0.03). At 6 months, LVEF was significantly higher in the IgG3 group (p = 0.007). Multiple regression analysis demonstrated IgG3-β1AR-AAb was an independent predictor of LVEF at 6 months and change in LVEF over 6 months, even after multivariable adjustment (LVEF at 6 months, β = 0.20, p = 0.01; change in LVEF, β = 0.20, p = 0.008). In the subjects with high New York Heart Association functional class (III or IV) at baseline, the IgG3 group had lower incidence of the composite endpoint of all-cause death, cardiac transplantation, and hospitalization due to heart failure, whereas the non-IgG3 group had the highest incidence of the composite endpoint.
CONCLUSIONS
IgG3-β1AR-AAb was associated with more favorable myocardial recovery in patients with recent-onset cardiomyopathy.
Keywords: autoantibody, β-blocker, IgG3, recent-onset cardiomyopathy
Idiopathic dilated cardiomyopathy (DCM) has been an important cause of systolic heart failure (HF) and is the most common cause of HF in young people referred for cardiac transplantation (1). It has been believed that this diagnosis contains varied etiologies and patients have highly variable presentations. There has been a longstanding belief that dysregulated autoimmune processes might lead to disease progression in HF. Specifically, several cardiac autoantibodies (AAb) against specific cardiac antigens have been detected in sera from patients with DCM (2–4). Among the various anticardiac AAbs, autoantibodies against the β1 adrenergic receptor (β1AR-AAb) have been detected in 30% to 40% of these patients (5–11). Clinical studies conducted in the 1980s and 1990s (prior to the broad adoption [12] of β-adrenergic blockers) demonstrated associations between detectable β1AR-AAbs and increased rates of mortality (9), fatal ventricular arrhythmias, and sudden death (8,13) in patients with DCM. Mechanistic studies have also demonstrated that β1AR-AAb may possess agonist-like properties (14–17), which induce some detrimental effects on the heart (18) including receptor uncoupling (12,19,20), myocyte apoptosis (21), and sustained calcium influx resulting in electric instability of the heart (18,22).
These effects can be abolished by β-blockers based on in vitro (17) and in vivo (12) experiments. Indeed, β1AR-AAb-positive patients with HF have demonstrated more favorable recovery of cardiac performance than β1AR-AAb-negative patients in response to β-adrenergic blocker therapy (10). Furthermore, immunoadsorption (IA) using columns specific for β1AR-AAb was effective in alleviating the cardiac dysfunction of an observational series of patients with DCM (23,24). In addition, the elimination of immunoglobin G subclass 3 (IgG3)-AAbs by IA was associated with beneficial effects in patients with DCM (25–28). These findings suggest the importance of AAbs of the IgG3 subclass in the pathology of DCM.
The IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study was a multicenter trial that enrolled 373 patients with recent-onset cardiomyopathy and examined the myocardial recovery and clinical prognosis for those patients on contemporary therapy including β-blockers (29). Herein, the objective of our study was to determine the clinical significance of specific β1AR-AAbs belonging to the IgG3 subclass in patients in the IMAC-2 study.
METHODS
The IMAC-2 study was a prospective, multicenter investigation of myocardial recovery in subjects with recent-onset nonischemic DCM and myocarditis that enrolled subjects at 16 centers from May 2002 through December 2008 (see the Online Appendix for participating institutions). All subjects had a left ventricular ejection fraction (LVEF) of 0.40 or less by echocardiography and symptoms ≤6 months in duration. Informed consent was obtained from all subjects and the protocol was approved by the institutional review boards of all participating centers. Demographic information included self-designated race (white, black, Asian, or other). Subjects underwent angiography or noninvasive screening to exclude coronary artery disease, which was defined as a single coronary artery stenosis of a major epicardial vessel >50% or a previous history of myocardial infarction. They also underwent transthoracic echocardiography to rule out valvular disease.
Patients with significant diabetes (requiring therapy with insulin or an oral agent for more than 1 year); uncontrolled hypertension (diastolic blood pressure >95 mm Hg or systolic blood pressure >160 mm Hg); suspected alcoholism; tachycardia-induced cardiomyopathy; uncorrected thyroid disease; or systemic disorders with associated cardiomyopathy, such as lupus erythematosus, hemochromatosis, or sarcoidosis, were excluded. Right ventricular endomyocardial biopsy was not required based on current practice guidelines (30). LVEF was assessed by transthoracic echocardiography at entry and at 6 months. Patients were followed for up to 48 months. All deaths and hospitalizations were adjudicated by an independent events committee.
IMAGING AND ASSAYS
Echocardiographic studies were reviewed in a blinded fashion by a core laboratory at the University of Pittsburgh. Digital routine grayscale 2-dimensional cine loops were obtained at frame rates of 40 to 90 Hz (mean: 60 ± 15 Hz) from standard apical 4-chamber, 2-chamber, and long-axis views. Left ventricular (LV) volume and LVEF were assessed by biplane Simpson’s rule using manual tracing of digital images. Left ventricular end-diastolic and end-systolic diameter (LVEDD and LVESD, respectively) were assessed in the parasternal long-axis view.
The presence of β1AR-AAb was determined by enzyme-linked immunoabsorbent assay (ELISA) using a synthetic peptide corresponding to the putative sequence of the second extracellular loop of human β1AR (amino acid sequence number, 197 to 222; H-W-W-R-A-E-S-D-E-A-R-R-C-Y-N-D-P-K-C-C-D-F-V-T-N-R) as an epitope peptide. Anti-human IgG antibody or IgG3 antibody was used as a secondary antibody to detect β1AR-AAb belonging to the IgG or IgG3 subclass. Positivity was defined as 2.5 times the background density as consistent with prior reports (8,10,28,31). IgG β1AR-AAb-positive but IgG3 β1AR-AAb-negative subjects were classified as the non-IgG3 group.
STATISTICAL ANALYSIS
All values were expressed as mean ± SD. Demographic and clinical characteristics were compared by the status of β1AR-AAb (negative/non-IgG3/IgG3) at baseline. Differences among the 3 groups were compared using analysis of variance (ANOVA) or Kruskal-Wallis test. When it was significant, multiple comparisons were performed using the Tukey-Kramer test or Steel-Dwass method. For myocardial recovery, LVEF at 6 months and change in LVEF over 6 months were compared. In multivariate analysis, multiple linear regression was used to identify independent predictors of change in LVEF at 6 months (i.e., verified as approximately normally distributed). In addition to the covariates chosen in the main study (29) by use for stepwise selection (forward) with an entry and retainment p value of 0.05, the status of β1AR-AAb at baseline was included in this study.
Kaplan-Meier survival curves for the composite endpoint of all-cause death, cardiac transplantation, or hospitalization due to the exacerbation of HF were calculated and log-rank test was performed by dividing the study cohort into 3 groups: IgG3 β1AR-AAb-positive, non-IgG3 β1AR-AAb-positive, and β1AR-AAb-negative groups. The differences among groups were analyzed by the log-rank test. A p value < 0.05 was considered statistically significant. All statistical analyses were performed in JMP 10.0.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Among 373 subjects enrolled in IMAC-2, 353 subjects had adequate blood samples for analysis. In this cohort, 120 (34%) patients had detectable β1AR-AAb: 62 (18%) patients were in the IgG3 group; 58 (19%) patients were in the non-IgG3 group; and the remaining 233 (66%) patients were in the negative group.
PATIENT CHARACTERISTICS
According to baseline characteristics of the study population based on the β1AR-AAb status (Table 1), there were no significant differences in demographic characteristics, vital signs, or specified laboratory data (hematocrit, serum creatinine, and serum sodium) among the 3 groups. In addition, there were no significant differences in medication, including β-blockers or the proportion of patients who underwent therapeutic device implantation among the 3 groups. Of note, most patients (82%) were treated with β-blockers at baseline. There were no significant differences in the proportion of subjects treated with β-blockers among the 3 groups (Table 1) or mean dose converted to carvedilol units (negative: 18 ± 16 mg; non-IgG3: 18 ± 14 mg; IgG3: 20 ± 17 mg; p = NS). The proportion of subjects who were administered β-blockers increased up to 94% (294 of 313) at 6 months. There was no significant difference in the rate of β-blocker use (negative: 202 of 211 [96%]; non-IgG3: 45 of 48 [94%]; IgG3: 47 of 54 [87%]; p = NS) or mean dose (negative: 33 ± 21 mg; non-IgG3: 36 ± 25 mg; IgG3: 38 ± 27 mg; p = NS).
TABLE 1.
Baseline Characteristics
| β1AR-AAb | p Value | |||
|---|---|---|---|---|
| Negative (n = 233) | Non-IgG3 (n = 58) | IgG3 (n = 62) | ||
| Age, years | 46 ± 14 | 43 ± 15 | 43 ± 14 | 0.08 |
| Male | 135 (58) | 40 (69) | 41 (66) | NS |
| Black | 49 (21) | 15 (26) | 11 (18) | NS |
| Peripartum cardiomyopathy | 24 (10) | 5 (9) | 7 (11) | NS |
| Months from onset | 2.4 ± 1.7 | 2.1 ± 1.6 | 2.1 ± 1.7 | NS |
| NYHA class (I/II/III/IV) | 39/107/75/12 | 12/30/12/4 | 14/25/15/8 | NS |
| Heart rate, beats/min | 83 ± 16 | 81 ± 18 | 84 ± 20 | NS |
| Systolic BP, mm Hg | 112 ± 19 | 112 ± 20 | 113 ± 18 | NS |
| Diastolic BP, mm Hg | 71 ± 13 | 71 ± 11 | 69 ± 13 | NS |
| Atrial fibrillation | 23 (11) | 2 (4) | 5 (8) | NS |
| Hematocrit, % | 41 ± 6 | 41 ± 6 | 39 ± 6 | NS |
| Serum creatinine, mg/dl | 1.0 ± 0.4 | 1.0 ± 0.3 | 1.1 ± 0.1 | NS |
| Serum sodium level, mEq/l | 138 ± 4 | 139 ± 4 | 139 ± 4 | NS |
| Medication | ||||
| β-blocker | 191 (82) | 49 (84) | 50 (81) | NS |
| β-blocker agent (carvedilol/metoprolol/others) | 146/43/2 (76/23/1) | 40/9/0 (82/18/0) | 35/14/1 (70/28/2) | NS |
| ACE inhibitor | 191 (82) | 50 (86) | 49 (79) | NS |
| ARB | 26 (11) | 4 (7) | 4 (6) | NS |
| Aldosterone antagonist | 67 (29) | 19 (33) | 12 (19) | NS |
| Diuretics | 163 (70) | 43 (74) | 40 (65) | NS |
| Digoxin | 67 (29) | 18 (3) | 17 (27) | NS |
| Hydralazine | 8 (3) | 1 (2) | 1 (2) | NS |
| Nitrate | 8 (3) | 2 (2) | 3 (5) | NS |
| Inotropes | 18 (8) | 2 (3) | 7 (11) | NS |
| IABP | 1 (<1) | 1 (2) | 1 (2) | NS |
| LVAD/BiVAD | 2 (1) | 1 (2) | 2 (3) | NS |
Values are mean ± SD, n (%), or n.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; β1AR-AAb = autoantibody against β1-adrenergic receptor; BiVAD = biventricular assist device; BP = blood pressure; IABP= intra-aortic balloon pumping; IgG3 = immunoglobin G subclass 3; LVAD = left ventricular assist device; NYHA = New York Heart Association functional class.
CHANGE OF LVEF AND LV SIZE
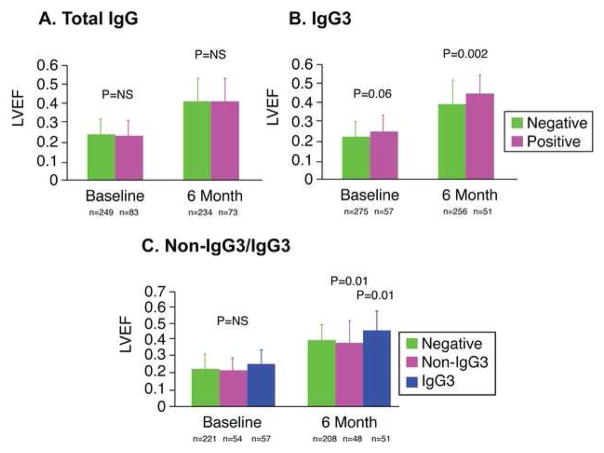
When the population was divided into 2 groups based on total IgG-β1AR-AAb positivity, there was no significant difference in LVEF at baseline (negative: 0.24 ± 0.08; positive: 0.23 ± 0.08; p = 0.92), LVEF at 6 months (negative: 0.40 ± 0.12; positive: 0.41 ± 0.12; p = 0.96) (Central Illustration 1A), or change in LVEF over 6 months (negative: 0.17 ± 0.13; positive: 0.18 ± 0.13; p = 0.97). However, when the population was divided into 2 groups based on IgG3-β1AR-AAb positivity, LVEF at 6 months was significantly higher in the IgG3-positive group (negative: 0.40 ± 0.12; positive 0.46 ± 0.10; p = 0.002) (Central Illustration 1B), whereas there was no significant difference at baseline (negative: 0.23 ± 0.08; positive: 0.26 ± 0.08; p = 0.06) (Central Illustration 1B). LVEF increased in the IgG3- group to a greater degree than the negative group (p < 0.001 by repeated measures ANOVA) (Central Illustration 1B). The absolute change in LVEF was higher in the IgG3-positive group compared to the IgG3-negative group with borderline significance (IgG3 negative: +0.17 ± 0.13 vs. IgG3 positive: +0.20 ± 0.11; p = 0.10).
CENTRAL ILLUSTRATION. Autoantibodies Specifically against β1ARs in Cardiomyopathy.
In investigating the role of β1 adrenergic receptor autoantibodies (β1AR-AAb) belonging to the immunoglobulin G3 (IgG3) subclass in patients with recent-onset cardiomyopathy, we found no significant difference in left ventricular ejection fraction (LVEF) at baseline and 6 months based on presence or absence of total IgG (A). However, when the population was divided based on IgG3 positivity, a significant difference in LVEF emerged at 6 months (B). When the population was further divided into patients who were β1AR-AAb negative, non-IgG3-β1AR-AAb positive and IgG3-β1AR-AAb positive, the IgG3 groups demonstrated significantly higher LVEF compared to each of the other cohorts.
When the population was divided into 3 groups of negative, non-IgG3, and IgG3, there was no significant difference in LVEF among the 3 at baseline (negative: 0.23 ± 0.08; non-IgG3: 0.22 ± 0.08; IgG3: 0.26 ± 0.08, p = NS). However, at 6 months LVEF was significantly higher in the IgG3 group compared to the non-IgG3 or negative groups (negative: 0.40 ± 0.12; non-IgG3: 0.38 ± 0.13; IgG3: 0.46 ± 0.10; p = 0.007 by Kruskal-Wallis; p = 0.01 between negative and IgG3; p = 0.01 between non-IgG3 and IgG3 by the Steel-Dwass method) (Central Illustration 1C). LVEF significantly increased over 6 months in all groups (p < 0.0001) (Online Figure 1), but increased in the IgG3 group to a greater degree than the negative or non-IgG3 group (p < 0.01 by repeated measures ANOVA) (Central Illustration 1C). There was no significant difference in the absolute change in LVEF among the 3 groups (negative: +0.17 ± 0.13; non-IgG3: +0.17 ± 0.14; IgG3 +0.20 ± 0.11; p = 0.25). Baseline echocardiography showed that LVEDD and LVESD were significantly larger in the non-IgG3 group compared to the negative and IgG3 groups (LVEDD p = 0.028; LVESD: p = 0.025) (Table 2). Both LVEDD and LVESD significantly decreased at 6 months in all 3 groups (LVEDD: p < 0.005; LVESD: p < 0.0001), but they remained larger in the non-IgG3 group compared to the negative and IgG3 groups (LVEDD: p = 0.012; LVESD: p = 0.010) (Table 2).
TABLE 2.
Echocardiographic Change of LV and LA Dimension
| At baseline | β1AR-AAb | p Value | ||
|---|---|---|---|---|
| Negative (n = 233) | Non-IgG3 (n = 58) | IgG3 (n = 62) | ||
| LVEDD, cm | 6.2 ± 0.9 | 6.7 ± 1.4 | 6.2 ± 1.0 | 0.028 |
| LVESD, cm | 5.5 ± 1.0 | 5.8 ± 1.2 | 5.3 ± 1.3 | 0.025 |
| IVS, cm | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.52 |
| PW, cm | 1.1 ± 0.6 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.70 |
| LA diameter, cm | 4.5 ± 0.9 | 4.5 ± 1.0 | 4.6 ± 1.0 | 0.97 |
| At 6 months | Negative (n = 195) | Non-IgG3 (n = 46) | IgG3 (n = 47) | p Value |
| LVEDD, cm | 5.7 ± 0.9 | 6.2 ± 1.1 | 5.7 ± 0.9 | 0.012 |
| LVESD, cm | 4.5 ± 1.1 | 5.1 ± 1.4 | 4.2 ± 1.1 | 0.010 |
| IVS, cm | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.58 |
| PW, cm | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.08 |
| LA diameter, cm | 4.1 ± 0.8 | 4.2 ± 1.2 | 4.2 ± 0.9 | 0.91 |
Values are mean ± SD.
IVS = interventricular septum; LA = left atrium; LV = left ventricle; LVEDD = left ventricular end-diastolic diameter; LVESD = left ventricular end-systolic diameter; PW = posterior wall; other abbreviations as in Table 1.
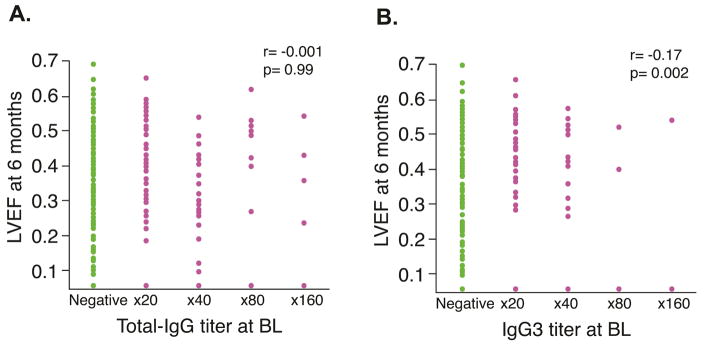
While total IgG titer did not show any significant correlation with LVEF at baseline (r = −0.038; p = 0.94) or at 6 months (r = −0.001; p = 0.99) (Figure 1A), the IgG3 titer at baseline showed a modest positive correlation with LVEF at 6 months (r = 0.17; p = 0.002) (Figure 1B), although there was no significant correlation with LVEF at baseline (r = 0.10; p = 0.06). Multiple regression analysis was performed to identify independent predictors of LVEF at 6 months and change in LVEF during the same period. As shown in Table 3, β1AR-AAb was an independent predictor of LVEF at 6 months as well as for 6-month change in LVEF even after adjusting for covariates that were used for stepwise selection in the main study (30).
FIGURE 1. 6-month LVEF Correlated with Baseline IgG.
At 6 months, there was no significant correlation between left ventricular ejection fraction (LVEF) and the baseline titer of immunoglobulin G3 β1 adrenergic receptor autoantibodies (IgG-β1AR-AAb) (A) but the correlation became significant in the presence of the immunoglobulin G3 subclass β1AR-AAb (IgG3) (B).
TABLE 3.
Independent Predictors for LVEF at 6 Months and Change in LVEF
| LVEF at 6 Months
| |||||
|---|---|---|---|---|---|
| t Ratio | β | Lower 95% | Higher 95% | p Value | |
|
| |||||
| LVEDD | −6.72 | −0.41 | −0.06 | −0.03 | <0.0001 |
| Systolic BP | 3.21 | 0.18 | 0.0005 | 0.002 | 0.002 |
| Black race | −2.34 | −0.12 | −0.07 | −0.006 | 0.02 |
| NYHA | −2.49 | −0.14 | −0.04 | −0.004 | 0.01 |
| Age | −1.14 | −0.06 | −0.001 | 0.0004 | 0.26 |
| LVEF at BL | 0.39 | 0.02 | −0.14 | 0.21 | 0.69 |
| Sex | 0.59 | 0.03 | −0.02 | 0.04 | 0.55 |
| β1AR-AAb at BL IgG3 vs. neg | 2.58 | 0.20 | 0.007 | 0.05 | 0.01 |
| non-IgG3 vs. neg | −0.75 | −0.06 | −0.03 | 0.02 | 0.45 |
| Change in LVEF
| |||||
|---|---|---|---|---|---|
| t Ratio | β | Lower 95% | Upper 95% | p Value | |
|
| |||||
| LVEDD | −6.72 | −0.39 | −0.06 | −0.03 | <0.0001 |
| Systolic BP | 3.21 | 0.17 | 0.0005 | 0.002 | 0.002 |
| Black race | −2.34 | −0.12 | −0.07 | −0.006 | 0.02 |
| NYHA | −2.49 | −0.13 | −0.04 | −0.004 | 0.01 |
| Age | −1.14 | −0.06 | −0.001 | 0.0004 | 0.26 |
| LVEF at BL | −11.00 | −0.61 | −1.14 | −0.79 | <.0001 |
| Sex | 0.59 | 0.03 | −0.02 | 0.04 | 0.55 |
| β1AR-AAb at BL IgG3 vs. neg | 2.58 | 0.19 | 0.007 | 0.05 | 0.01 |
| non-IgG3 vs. neg | −0.75 | −0.06 | −0.03 | 0.02 | 0.45 |
CLINICAL EVENTS BASED ON B1AR-AAB STATUS
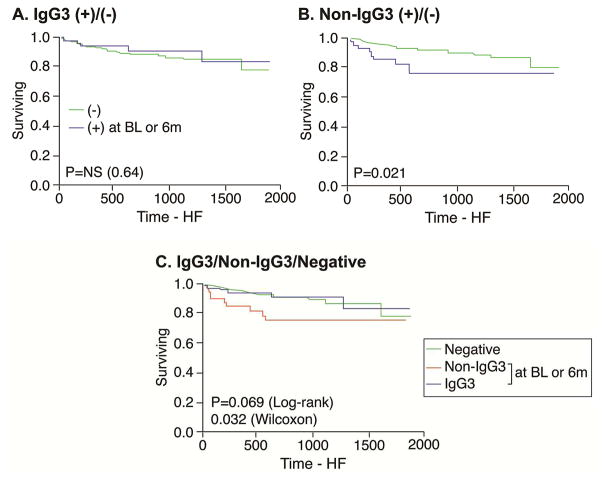
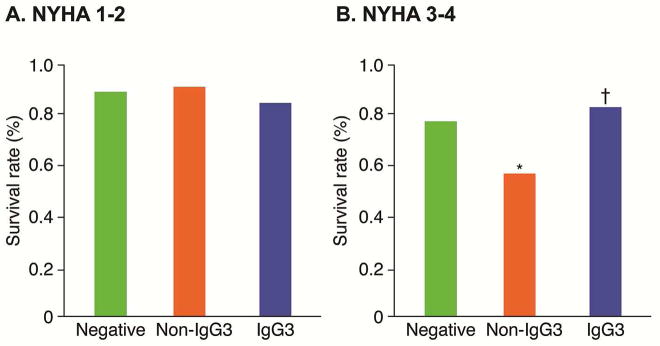
During 3.8 ± 1.5 years of follow-up, there was no significant difference in the composite endpoint of all-cause death, cardiac transplantation, and hospitalization due to HF among based on β1AR-AAb status, a point replicated in the population with low New York Heart Association (NYHA) functional class (I or II) at baseline (Figure 2A). However, in the population with high NYHA functional class (III or IV) at baseline, IgG3 subjects had the lowest rate of adverse clinical events, and non-IgG3 patients had the highest rate (log-rank p = 0.03) (Figure 2B). Specifically, within the β1AR-AAb positive cohort, the difference in adverse event rates between IgG3 versus non-IgG3 groups was statistically significant (log-rank p = 0.02) (Figure 2B). In subjects with β-blocker use at baseline and continued at 6 months or with β-blocker use by 6 months (n = 322), the presence of non-IgG3 was associated with worse overall survival from HF hospitalization (log-rank p = 0.021), whereas no differences were observed between the IgG3 and negative groups (p = 0.64) (Figure 3).
FIGURE 2. Composite Endpoint: NYHA Class.
When divided into populations based on baseline New York Heart Association (NYHA) functional class status (I–II vs. III–IV), there were no significant differences in the 3 groups of β1AR-AAb negative, non-IgG3-β1AR-AAb positive (non-IgG), and IgG3for the composite endpoint of all-cause death, cardiac transplantation, or hospitalization due to exacerbation of heart failure in subjects with low NYHA class (A), but significance was seen in sicker patients (B). *p = 0.02 vs. Negative group; †p = 0.02 vs. non-IgG3. Abbreviations as in Figure 1.
FIGURE 3. HF Hospitalization and β-blocker Use.
In 322 subjects who used β-blockers at baseline and continued at 6 months or were using β-blockers at 6 months, there was no significant difference in overall survival from heart failure (HF) hospitalization in patients whether IgG3 was present (A) but the presence of non-IgG3 was associated with worse overall survival (B). No differences were observed in the negative group (C). Abbreviations as in Figures 1 and 2.
DISCUSSION
In this study, we reported 3 main findings. First, the myocardial recovery represented by LVEF at 6 months after enrollment was more evident in the IgG3 group compared to the negative or non-IgG3 groups. Second, LVEF at 6 months was positively correlated with IgG3-β1AR-AAb titer but not with IgG-β1AR-AAb titer. The IgG3-β1AR-AAb titer was an independent predictor of LVEF at 6 months and increased in LVEF over 6 months even after adjusting for some confounding factors. Third, in subjects with higher NYHA class (III or IV), patients with IgG3-β1AR-AAb had the lowest incidence of the composite endpoint and patients with non-IgG3-β1AR-AAb had the highest. Of note, this finding is consistent with our single-center pilot study, which enrolled a stable HF population (31). Taken together, these findings implied the possibility that β1AR-AAb IgG subclasses might play differential roles in the pathophysiology of cardiomyopathies. Specifically, it is conceivable that the β1AR-AAb IgG3 subclass may exert a more direct pathological effect related to a primary autoimmune process, such as failure of self-tolerance, than other non-IgG3-β1AR-AAbs that are more dependent on secondary autoimmune responses to self-antigens released as a result of cardiac damage.
Previous studies suggested some types of AAbs exert their effect by binding to the Fc receptor as well as its epitope. Often referred to as “cardio-depressant" AAbs, certain types of AAb purified from patients with DCM have been found to induce a negative inotropy in vitro (32) and ex vivo (27,33). Interestingly, patients with cardio-depressant AAbs demonstrated an acute increase in cardiac index and LVEF after IA therapy (27,32). Staudt and colleagues have reported that the cardio-depressant effects of these AAbs are unlikely to be induced by either the F(ab′)2 or Fc fragment alone (34). Therefore, the effects of the AAb may vary depending on the structure of the Fc fragment, the very factor that determines IgG subclasses. IgG subclasses 1 and 3 are most likely to trigger effector function and be involved in immunoregulatory activities and complement activation (33,35,36). The presence of AAbs against β1AR and muscarinic M2 receptors belonging to the IgG3 subclass has been shown to be an independent predictor of the presence of cardio-depressant AAb (27).
The importance of IgG3 AAbs was further supported when IA via antihuman IgG columns (high affinity for all IgG subclasses) resulted in additional improvement of cardiac function compared to using protein A (high affinity for IgG1, 2, and 4, but low affinity for IgG3) (24). Alternatively using the tryptophan column, the IgG3 subclass was eliminated effectively by IA and to a greater extent than other subclasses (28), although these findings need to be confirmed. Furthermore, in a pilot study, direct administration of cyclic peptide against β1AR-AAb improved LVEF (37). The increase in LVEF after IA was better correlated with AAb titers belonging to the IgG3 subclass than total IgG, which also suggested that the removal of IgG3-AAb is important to maximize the effect of IA in patients with DCM (27). Interestingly in the Myocarditis Treatment Trial, an association between cardiac IgG and better LVEF was observed (38), suggesting that the timing of interpretation of AAb data (acute vs. chronic) might also be a factor.
There is emerging appreciation that only a subset of β1AR-AAbs may be functionally active (39,40). Interestingly, in the analysis of weaned DCM patients who tested positive for β1AR-AAb before left ventricular assist device implantation, β1AR-AAb became undetectable after LV unloading by mechanical circulatory assist support (41). This finding suggests that certain β1AR-AAbs can be generated, at least partly, by cardiac loading or damage. The notion that IgG3-β1AR-AAbs might serve as a potential pathogenic factor that can be counteracted with β-blockers raises an exciting possibility that their detection in patients at risk of developing cardiomyopathies might provide a potential indication for preventive β-blocker therapy. Further investigations are warranted into the presence of IgG3-β1AR-AAbs in at-risk patients.
It is also possible that anti-HF therapy including β-blockers can suppress the production of AAbs in patients with recent-onset DCM. A previous report demonstrated that β1AR-AAbs enhanced proliferation of rat CD3+ T lymphocytes in vitro, which was blocked by the selective β1AR antagonist metoprolol (42). β1AR-AAbs also inhibited the secretion of interferon-γ while promoting an increase in interleukin-4 levels. These findings suggested that β1AR-AAbs promote humoral immunity, possibly through the agonistic effect on β1AR expressed on T lymphocytes. It might be the important mechanism by which β-blockers are especially effective for patients with IgG3-β1AR-AAbs. Although we evaluated β1AR-AAb status at baseline and at 6 months by ELISA and examined clinical outcomes in this study, we could not find any significant association between change in β1AR-AAb status and the clinical outcome mechanism that differentiated IgG3- and non-IgG3-β1AR-AAb (data not shown).
STUDY LIMITATIONS
The present study had several limitations. Patients with non-IgG3-β1AR-AAbs had larger LV size at baseline. Previous studies showed that the presence of β1AR-AAb was associated with reduced cardiac function at baseline (7,9,10), but these studies did not examine the IgG subclasses of β1AR-AAb. Although our findings might support these previous findings, this might affect the findings in the present study. We do not have any data that support the proposed mechanisms previously mentioned that differentiate the IgG3- and non-IgG3-β1AR-AAb as the ELISA assay do not include a functional bioassay (e.g., protein kinase A activity). Moreover, we did not further determine the IgG subclasses of β1AR-AAb IgG due to limited sample availability. Meanwhile, targeting of the β1AR first extracellular loop by the IgG3 was not directly tested. In addition, although the administration of β-blockers has been speculated as a mechanism that might have yielded more favorable outcomes in patients with IgG3-β1AR-AAb, the observational nature of our study did not allow further clarification regarding the interrelationship between β-blockers and IgG3-β1AR-AAb.
CONCLUSIONS
The presence of the IgG3 subclass of β1AR-AAbs was associated with favorable myocardial recovery in recent-onset cardiomyopathy. Future investigations will be necessary to better elucidate the detailed mechanisms that differentiate the effects of IgG3- and non-IgG3-β1AR-AAbs.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
In patients with heart failure, autoantibodies against β1 adrenergic receptors may be antagonized by administration of β-blocker drugs. Immunoabsorption studies suggest that antibody subclasses may differ in terms of pathogenicity and potential for recovery of myocardial function in response to β-blocker therapy.
TRANSLATIONAL OUTLOOK
Detection of pathogenetic subclasses of β1-adrenoceptor autoantibodies responsive to β-blocker therapy may have implications for treatment to prevent progression of cardiomyopathy.
Acknowledgments
Funding: The IMAC-2 study was supported by National Heart, Lung, and Blood Institute contracts HL075038, HL086918, HL69912, and the National Institutes of Health, Bethesda, Maryland. See Supplemental Data for list of IMAC-2 investigators. This study was also supported by funding from the Cleveland Clinic Research Programs Committee. Dr. Nagatomo is supported by the Postdoctoral Fellowship award from the Myocarditis Foundation (MYF1401MF). Dr. Tang is supported by a grant from the National Institutes of Health (1R01HL103931).
ABBREVIATIONS AND ACRONYMS
- AAb
autoantibody
- β1AR
β1 adrenergic receptor
- β1AR-AAb
β1 adrenergic receptor autoantibody
- DCM
dilated cardiomyopathy
- IgG3
immunoglobin G subclass 3
- LV
left ventricle/ventricular
- LVEDD
left ventricular end-diastolic diameter
- LVESD
left ventricular end-systolic diameter
- LVEF
left ventricular ejection fraction
Footnotes
Conflict of Interest: All other authors reported no relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor DO, Stehlik J, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report-2009. J Heart Lung Transplant. 2009;28:1007–22. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Caforio AL, Grazzini M, Mann JM, et al. Identification of alpha- and beta-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation. 1992;85:1734–42. doi: 10.1161/01.cir.85.5.1734. [DOI] [PubMed] [Google Scholar]
- 3.Schultheiss HP, Schulze K, Schauer R, Witzenbichler B, Strauer BE. Antibody-mediated imbalance of myocardial energy metabolism. A causal factor of cardiac failure? Circ Res. 1995;76:64–72. doi: 10.1161/01.res.76.1.64. [DOI] [PubMed] [Google Scholar]
- 4.Limas CJ, Goldenberg IF, Limas C. Autoantibodies against beta-adrenoceptors in human idiopathic dilated cardiomyopathy. Circ Res. 1989;64:97–103. doi: 10.1161/01.res.64.1.97. [DOI] [PubMed] [Google Scholar]
- 5.Magnusson Y, Marullo S, Hoyer S, et al. Mapping of a functional autoimmune epitope on the beta 1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1990;86:1658–63. doi: 10.1172/JCI114888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnusson Y, Wallukat G, Waagstein F, Hjalmarson A, Hoebeke J. Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta 1-adrenoceptor with positive chronotropic effect. Circulation. 1994;89:2760–7. doi: 10.1161/01.cir.89.6.2760. [DOI] [PubMed] [Google Scholar]
- 7.Jahns R, Boivin V, Siegmund C, Inselmann G, Lohse MJ, Boege F. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99:649–54. doi: 10.1161/01.cir.99.5.649. [DOI] [PubMed] [Google Scholar]
- 8.Iwata M, Yoshikawa T, Baba A, Anzai T, Mitamura H, Ogawa S. Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:418–24. doi: 10.1016/s0735-1097(00)01109-8. [DOI] [PubMed] [Google Scholar]
- 9.Stork S, Boivin V, Horf R, et al. Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J. 2006;152:697–704. doi: 10.1016/j.ahj.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Nagatomo Y, Yoshikawa T, Kohno T, et al. A pilot study on the role of autoantibody targeting the beta1-adrenergic receptor in the response to beta-blocker therapy for congestive heart failure. J Card Fail. 2009;15:224–32. doi: 10.1016/j.cardfail.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Baba A. Targeted Autoantibodies in Apheresis Treatment against Severe Heart Failure. Japanese Journal of Apheresis. 2010;29:187–93. [Google Scholar]
- 12.Iwata M, Yoshikawa T, Baba A, et al. Autoimmunity against the second extracellular loop of beta(1)-adrenergic receptors induces beta-adrenergic receptor desensitization and myocardial hypertrophy in vivo. Circ Res. 2001;88:578–86. doi: 10.1161/01.res.88.6.578. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda Y, Miyoshi S, Tanimoto K, et al. Autoimmunity against the second extracellular loop of beta(1)-adrenergic receptors induces early afterdepolarization and decreases in K-channel density in rabbits. J Am Coll Cardiol. 2004;43:1090–100. doi: 10.1016/j.jacc.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 14.Wallukat G, Wollenberger A, Morwinski R, Pitschner HF. Anti-beta 1-adrenoceptor autoantibodies with chronotropic activity from the serum of patients with dilated cardiomyopathy: mapping of epitopes in the first and second extracellular loops. J Mol Cell Cardiol. 1995;27:397–406. doi: 10.1016/s0022-2828(08)80036-3. [DOI] [PubMed] [Google Scholar]
- 15.Mobini R, Magnusson Y, Wallukat G, Viguier M, Hjalmarson A, Hoebeke J. Probing the immunological properties of the extracellular domains of the human beta(1)-adrenoceptor. J Autoimmun. 1999;13:179–86. doi: 10.1006/jaut.1999.0310. [DOI] [PubMed] [Google Scholar]
- 16.Mobini R, Fu M, Wallukat G, Magnusson Y, Hjalmarson A, Hoebeke J. A monoclonal antibody directed against an autoimmune epitope on the human beta1-adrenergic receptor recognized in idiopathic dilated cardiomyopathy. Hybridoma. 2000;19:135–42. doi: 10.1089/02724570050031176. [DOI] [PubMed] [Google Scholar]
- 17.Staudt A, Mobini R, Fu M, et al. beta(1)-Adrenoceptor antibodies induce positive inotropic response in isolated cardiomyocytes. Eur J Pharmacol. 2001;423:115–9. doi: 10.1016/s0014-2999(01)01113-x. [DOI] [PubMed] [Google Scholar]
- 18.Jahns R, Boivin V, Hein L, et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–29. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podlowski S, Luther HP, Morwinski R, Muller J, Wallukat G. Agonistic anti-beta1-adrenergic receptor autoantibodies from cardiomyopathy patients reduce the beta1-adrenergic receptor expression in neonatal rat cardiomyocytes. Circulation. 1998;98:2470–6. doi: 10.1161/01.cir.98.22.2470. [DOI] [PubMed] [Google Scholar]
- 20.Jahns R, Boivin V, Krapf T, Wallukat G, Boege F, Lohse MJ. Modulation of beta1-adrenoceptor activity by domain-specific antibodies and heart failure-associated autoantibodies. J Am Coll Cardiol. 2000;36:1280–7. doi: 10.1016/s0735-1097(00)00881-0. [DOI] [PubMed] [Google Scholar]
- 21.Staudt Y, Mobini R, Fu M, Felix SB, Kuhn JP, Staudt A. Beta1-adrenoceptor antibodies induce apoptosis in adult isolated cardiomyocytes. Eur J Pharmacol. 2003;466:1–6. doi: 10.1016/s0014-2999(03)01431-6. [DOI] [PubMed] [Google Scholar]
- 22.Christ T, Wettwer E, Dobrev D, et al. Autoantibodies against the beta1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol. 2001;33:1515–25. doi: 10.1006/jmcc.2001.1414. [DOI] [PubMed] [Google Scholar]
- 23.Wallukat G, Muller J, Hetzer R. Specific removal of beta1-adrenergic autoantibodies from patients with idiopathic dilated cardiomyopathy. N Engl J Med. 2002;347:1806. doi: 10.1056/NEJM200211283472220. [DOI] [PubMed] [Google Scholar]
- 24.Braun N, Gutenberger S, Erley CM, Risler T. Immunoglobulin and circulating immune complex kinetics during immunoadsorption onto protein A sepharose. Transfus Sci. 1998;19(Suppl):25–31. doi: 10.1016/s0955-3886(97)00099-4. [DOI] [PubMed] [Google Scholar]
- 25.Warraich RS, Noutsias M, Kazak I, et al. Immunoglobulin G3 cardiac myosin autoantibodies correlate with left ventricular dysfunction in patients with dilated cardiomyopathy: immunoglobulin G3 and clinical correlates. Am Heart J. 2002;143:1076–84. doi: 10.1067/mhj.2002.124406. [DOI] [PubMed] [Google Scholar]
- 26.Staudt A, Bohm M, Knebel F, et al. Potential role of autoantibodies belonging to the immunoglobulin G-3 subclass in cardiac dysfunction among patients with dilated cardiomyopathy. Circulation. 2002;106:2448–53. doi: 10.1161/01.cir.0000036746.49449.64. [DOI] [PubMed] [Google Scholar]
- 27.Baba A, Akaishi M, Shimada M, et al. Complete elimination of cardiodepressant IgG3 autoantibodies by immunoadsorption in patients with severe heart failure. Circ J. 2010;74:1372–8. doi: 10.1253/circj.cj-09-0748. [DOI] [PubMed] [Google Scholar]
- 28.Nagatomo Y, Baba A, Ito H, et al. Specific immunoadsorption therapy using a tryptophan column in patients with refractory heart failure due to dilated cardiomyopathy. J Clin Apher. 2011;26:1–8. doi: 10.1002/jca.20268. [DOI] [PubMed] [Google Scholar]
- 29.McNamara DM, Starling RC, Cooper LT, et al. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J Am Coll Cardiol. 2011;58:1112–8. doi: 10.1016/j.jacc.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–31. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Nagatomo Y, Li D, Kirsop J, Borowski A, Thakur A, Tang WH. Autoantibodies Specifically Against beta1 Adrenergic Receptors and Adverse Clinical Outcome in Patients With Chronic Systolic Heart Failure in the beta-Blocker Era: The Importance of Immunoglobulin G3 Subclass. J Card Fail. 2016;22:417–22. doi: 10.1016/j.cardfail.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staudt A, Staudt Y, Dorr M, et al. Potential role of humoral immunity in cardiac dysfunction of patients suffering from dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:829–36. doi: 10.1016/j.jacc.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 33.Baba A. Autoantigen estimation and simple screening assay against cardiodepressant autoantibodies in patients with dilated cardiomyopathy. Ther Apher Dial. 2008;12:109–16. doi: 10.1111/j.1744-9987.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- 34.Staudt A, Eichler P, Trimpert C, Felix SB, Greinacher A. Fc(gamma) receptors IIa on cardiomyocytes and their potential functional relevance in dilated cardiomyopathy. J Am Coll Cardiol. 2007;49:1684–92. doi: 10.1016/j.jacc.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 35.Bruggemann M, Williams GT, Bindon CI, et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351–61. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redpath S, Michaelsen T, Sandlie I, Clark MR. Activation of complement by human IgG1 and human IgG3 antibodies against the human leucocyte antigen CD52. Immunology. 1998;93:595–600. doi: 10.1046/j.1365-2567.1998.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munch G, Boivin-Jahns V, Holthoff HP, et al. Administration of the cyclic peptide COR-1 in humans (phase I study): ex vivo measurements of anti-beta1-adrenergic receptor antibody neutralization and of immune parameters. Eur J Heart Fail. 2012;14:1230–9. doi: 10.1093/eurjhf/hfs118. [DOI] [PubMed] [Google Scholar]
- 38.Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–75. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 39.Jahns R, Boivin V, Siegmund C, Boege F, Lohse MJ, Inselmann G. Activating beta-1-adrenoceptor antibodies are not associated with cardiomyopathies secondary to valvular or hypertensive heart disease. J Am Coll Cardiol. 1999;34:1545–51. doi: 10.1016/s0735-1097(99)00381-2. [DOI] [PubMed] [Google Scholar]
- 40.Bornholz B, Weidtkamp-Peters S, Schmitmeier S, et al. Impact of human autoantibodies on beta1-adrenergic receptor conformation, activity, and internalization. Cardiovasc Res. 2013;97:472–80. doi: 10.1093/cvr/cvs350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dandel M, Weng Y, Siniawski H, et al. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation. 2008;118:S94–105. doi: 10.1161/CIRCULATIONAHA.107.755983. [DOI] [PubMed] [Google Scholar]
- 42.Du Y, Yan L, Wang J, et al. beta1-Adrenoceptor autoantibodies from DCM patients enhance the proliferation of T lymphocytes through the beta1-AR/cAMP/PKA and p38 MAPK pathways. PLoS One. 2012;7:e52911. doi: 10.1371/journal.pone.0052911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.