Abstract
Background
Type 2 diabetes is a significant public health concern. With the completion of the Diabetes Prevention Program, there has been a proliferation of studies attempting to translate this evidence base into practice. However, the cost, effectiveness, and cost-effectiveness of these adapted interventions is unknown.
Objective
The purpose of this systematic review was to conduct a comprehensive meta-analysis to synthesize the effectiveness, cost, and cost-effectiveness of lifestyle diabetes prevention interventions and compare effects by intervention delivery agent (dietitian vs non-dietitian) and channel (in-person vs technology-delivered).
Methods
English and full-text research articles published up to July 2015 were identified using the Cochrane Library, PubMed, ERIC, CAB Direct, Science Direct and Google Scholar. Sixty-nine studies met inclusion criteria. Most employed both dietary and physical activity intervention components (four of 69 were diet-only interventions). Changes in weight, fasting and 2-hour blood glucose concentration, and hemoglobin A1c were extracted from each article. Heterogeneity was measured by the I^2 index, and study-specific effect sizes or mean differences were pooled using a random effects model when heterogeneity was confirmed.
Results
Participants receiving intervention with nutrition education experienced a reduction of 2.07 kg (95% CI: 1.52 to 2.62; p<0.001; I2=90.99%, 95% CI: 88.61% to 92.87%) in weight at 12 months with effect sizes over time ranging from small (0.17, 95% CI: 0.04 to 0.30; p=0.012; I2= 86.83%, 95% CI: 80.42% to 91.14%) to medium (0.65, 95% CI: 0.49 to 0.82; p<0.001; I2= 98.75%, 95% CI: 98.52% to 98.94). Effect sizes for 2-h blood glucose and HbA1c changes ranged from small to medium. The meta-regression analysis revealed a larger relative weight loss in dietitian-delivered interventions than in those delivered by non-dietitians (full sample: −1.0 kg; US subsample: −2.4 kg), and did not find statistical evidence that the delivery channel was an important predictor of weight loss. The average cost per kg weight loss ranged from $53.87 over 2 months to $1,005.36 over 12 months. The cost of intervention per participant delivered by dietitians was lower than interventions delivered by non-dietitians, though few studies reported costs.
Conclusions
Lifestyle interventions are effective in reducing body weight and glucose-related outcomes. Dietitian-delivered interventions, compared to those delivered by other personnel, achieved greater weight reduction. No consistent trend was identified across different delivery channels.
Keywords: obesity, Type 2 diabetes, lifestyle intervention, nutrition education, dietitian, technology, meta-analysis
Introduction
Type 2 diabetes (T2D) has increased significantly worldwide.1 Among adults aged 20–79 worldwide, 8.8% were estimated to have diabetes in 2015, and the prevalence of diabetes is estimated to increase to one in ten adults by 2040.1 Among adults in the United States (US), the estimated lifetime risk of developing T2D is 40%.2 Type 2 diabetes is a challenging public health problem, with serious consequences on health and health care costs. 3,4 This condition greatly reduces life expectancy and leads to numerous medical complications, such as renal disease, diabetic neuropathy, and macrovascular disease.5 This condition has contributed to substantial increases in total economic costs in the US- from $174 billion in 2007 to $245 billion in 2012, and shows no signs of slowing down. 6
Since the completion of the Diabetes Prevention Program, there has been a proliferation of studies attempting to translate lifestyle interventions into clinical and community practice in an attempt to halt this growing public health epidemic.7–9 Lifestyle intervention, specifically intensive diet and physical activity behavioral counseling programs, are recommended for the prevention of T2D.10 Dietitians, who are trained to deliver medical nutrition therapy, play an important role in diabetes prevention counseling.8,9,11 However, given limited access to dietitians and possibly higher program costs relative to other types of intervention delivery agents, nutrition education is sometimes provided by other types of delivery agents such as healthcare professionals, community health workers, or others (e.g. average salary for a community health worker is $37,490, vs. the average salary for a dietitian is $56,300).12,13 While understanding the appropriate personnel to deliver diabetes prevention intervention content has significant cost implications for clinical and community organizations, the relative effectiveness and cost-effectiveness of dietitian-delivered nutrition education compared to nutrition education delivered by other agents has yet to be examined.
As reported by Sherwood et.al., consumers desire interventions with less person-to-person contact. Technology-based programs represent an alternative approach to minimize in-person interactions.14 Technology-based lifestyle interventions have been broadly defined as those which utilize web-based platforms, mobile applications, telecommunication technology and phone counseling sessions such as interactive voice response calls, or text messaging. These technologies may be used alone or in combination with in-person intervention contacts. Technology-based approaches also have the potential advantage of reducing personnel resource demand, and can overcome transportation barriers to reach geographically disparate population groups.15 Therefore, there is a need to investigate the relative effectiveness of technology-based interventions, compared to traditional in-person interventions. Having these data available could inform decision-making related to organizational selection, adaptation, and implementation of diabetes preventions programs.2,3
Seven meta-analyses that evaluated nutrition education in diabetes prevention programs were identified. They reported non-standardized mean differences in weight and glucose tolerance or economic evaluation ratio,13,16–21 and four analyses focused on changes in health outcomes at 12 months13,16–18. Two meta-analyses reported the effectiveness of prevention programs at multiple time points.19,21 Those that limited the review to only randomized controlled trials (RCT) reported a reduction in 2-h blood glucose (BG).17 Those focused on clinical care settings reported change in weight,18 and those conducted in “real-world” settings reported the percentage of body weight change.16 Meta-analyses focused on routine clinical settings examined the effectiveness of adherence to guidelines,13 and others did not describe the study setting in their inclusion criteria. Only two meta-analyses included a summary of costs; the reported median program cost per participant was $653 (2013 U.S. dollars) 20 and it was suggested that nonmedical personnel may reduce program costs without sacrificing effectiveness.16 Overall, these meta-analyses have demonstrated that lifestyle-based diabetes intervention programs are effective in reducing risk for developing T2D in adults.
While the findings across these meta-analyses are promising there still is an absence in evidence synthesis that examines the effectiveness, costs, and cost-effectiveness of diabetes prevention interventions across delivery personnel, delivery channel, setting, and populations. The paucity of these data makes it difficult for typical clinical or community organizations to determine if: (1) intervention delivery is affordable, (2) program delivery personnel are available, or (3) the intervention content is adaptable to fit the context.22 The purpose of this systematic review was to conduct a comprehensive meta-analysis to synthesize the effectiveness, cost, and cost-effectiveness reported across studies testing diabetes prevention interventions. We also examined, with subgroup analyses, if differences in these outcomes existed between interventions that were delivered by dietitians compared to non-dietitians, or if differences existed based on technology versus in-person intervention delivery. Non-dietitians included intervention delivery agents such as wellness instructors, lay leaders, community health workers (CHW), health department counselors, lifestyle coaches, healthcare professionals, group leaders, diabetes educators, health educators, community residents, research staff, nutritional scientists, physiotherapists, general practitioners, study physician, nurses, facilitators and pharmacists. Subgroup analyses were performed to explore the average effect size differences in intervention effects across those subgroup dimensions including US versus non-US study locations, RCT versus studies using a quasi-experimental design (QED), and length of study follow-up (3, 6, and 12 months, and up to 60 months).
Materials and Methods
This review was conducted according to the PRISMA guidelines 23 and was registered with the PROSPERO International register of systematic reviews (registration number CRD42014013817).
Eligibility Criteria
Studies that focused on diabetes prevention for high-risk adults through lifestyle interventions, used RCT or QED (with and without control groups), and reported relevant clinical outcomes within five years (e.g., body weight, fasting BG [FBG], or glucose tolerance) were included. Risk criteria for T2D included the following: overweight or obesity status (BMI≥24, BMI ≥ 23 for Asian adults), prediabetes (FBG ranging from 95–125 mg/dl), impaired fasting glucose (Fasting blood glucose measurement of 101– 108 mg/dl [5.6–6.0 mmol/l] or fasting venous plasma glucose measurement of 110 – 124 mg/dl [6.1–6.9 mmol/l] or fasting glucose measurements of 100 – 125 mg/dl [5.55–6.9 mmol/]), impaired glucose tolerance and diabetes risk score reflecting increased risk for T2D (American Diabetes Association (ADA)-score ≥10, Finnish Diabetes Risk score (FINDRISC) score ≥9, Australian diabetes risk (AUSDRISK) score ≥ 12). Only studies with interventions lasting more than four weeks were included, as shorter-term lifestyle programs are not likely to produce sustained changes.24 Studies published before July 2015 in the English language were included. Studies that aimed to improve dietary knowledge among health care workers or medical students were excluded, as were studies focused solely on weight loss which were unrelated to diabetes prevention.
Search Strategy
A systematic literature search was conducted using the following databases: the Cochrane Library, PubMed, ERIC, CAB Direct, Science Direct and Google Scholar. The search terms used combinations of ‘nutrition education’, ‘lifestyle intervention’, ‘behavioral intervention’, ‘nutritionist’, ‘registered dietitian/dietician’, ‘dietary intake’, ‘nutrition’, ‘prediabetes’, ‘dietary education’, ‘diabetes prevention’, ‘glucose tolerance’, ‘glucose homeostasis’, ‘fasting glucose’, ‘cost-effectiveness’. Citations and abstracts of all retrieved studies were imported into ENDNOTE X7 citation management software. Duplicates were removed and the remaining studies were assessed for eligibility criteria by two researchers. Supplementary Table 1 and 2 present examples of the complete search strategy used in the electronic databases CAB Direct and PubMed. Reference lists of identified studies and related reviews were also hand-searched for relevant articles.
Data Extraction and Quality Evaluation
Based on the Cochrane template a data extraction form was developed (available from the authors upon request).25 The following information from each article was extracted: study details (authors, year, and county of publication), sample size and participant characteristics (age, gender, weight measurements at baseline), risk criteria for T2D (such as overweight/obese/prediabetic/diabetes risk score), duration of the intervention (from baseline to the end of intervention), active and maintenance phases, details of intervention procedures, outcome measurements (method, body mass index [BMI], body weight loss, blood glucose outcomes, and incidence of diabetes), author’s conclusions and study limitations.
The quality of selected research articles was evaluated according to Academy of Nutrition and Dietetics Evidence Analysis Library quality rating worksheet.26 This checklist includes criteria for evaluating the relevance and validity of included studies. Each study was assessed for quality independently by at least two reviewers and assigned an overall quality grade (categories: +, −, or 0). When present, differences in ratings between reviewers were discussed in order to reach a consensus. If agreement could not be established, a third reviewer was included to resolve differences.
Data Analysis
This review included the following outcome measurements: body weight, BMI, fasting blood glucose (FBG), 2-hour plasma glucose (2-h BG), and glycated hemoglobin (HbA1c). Reported imperial values were converted into metric units except for BG concentration, which was reported in mg/dl. Analyses were carried out using Stata Statistical Software: Release 13.27 Mean change was obtained by subtracting the baseline mean value from the mean at a subsequent measurement period (e.g., months 6 or 12). Separate analyses were conducted for active intervention phase changes, and for the overall intervention phase (i.e. active and maintenance phases) changes. Some of the missing mean standard deviations (SD) for the outcomes of interest were obtained by contacting authors. The rest of the missing values were calculated from correlation coefficients, confidence intervals or standard errors according to calculations outlined in the Cochrane handbook for systematic reviews of interventions (Section 16.1).28 Furthermore, both effect size (ES) and the non-standardized mean difference (NSMD) of primary outcomes (e.g., weight) were estimated and compared. An advantage of standardized effect sizes is that they can be used to compare magnitude of change across studies.29 Non-standardized mean difference allows the present results to be compared with related meta-analyses since they report NSMD.
To facilitate the model selection in this meta-analysis, statistical heterogeneity tests, I2 index were used to determine the magnitude of heterogeneity. 30 A random-effects model with DerSimonian and Laird’s technique was used when heterogeneity was confirmed.31,32 Meta-regression analyses were performed to explore heterogeneity and assess the relationship between relative weight loss, FBG reduction, change in 2-h BP and HbA1c (i.e., intervention group relative to control group) at 12 months and study characteristics, such as delivery agent (dietitian or non-dietitian), delivery channel (In-person or Technology-delivered), study design (RCT or QED), study location (US or non-US), percentage of female participants, mean age of the study sample, baseline average BMI, study setting (in primary setting or others), and group session (group-based or not). Furthermore, the existence of publication bias was assessed by a visual examination of funnel plots and test statistics from Begg’s and Egg’s tests.33
The average cost-effectiveness ratio (ACER), which refers to the average cost of an intervention per unit of output, was used to estimate average cost spent per effect (i.e., per kg weight lost). When sufficient information was available from the included studies, ACER was calculated to provide the average cost for 1 kg weight loss.
RESULTS
Characteristics of Studies
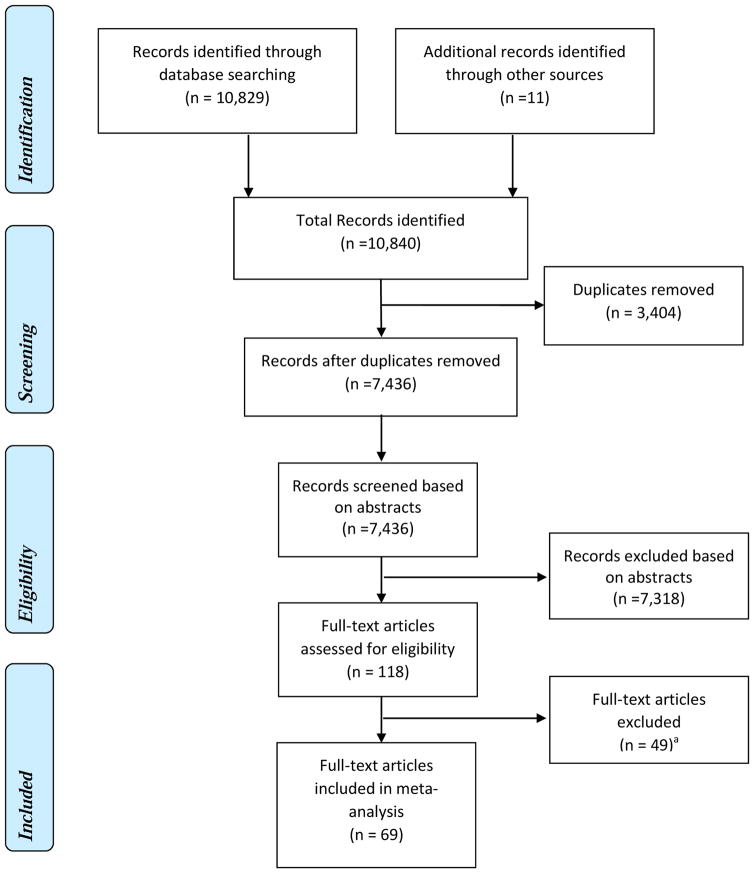
The database search resulted in a total of 10,840 abstracts. After removing duplicates (3,404 in total), a total of 7,436 abstracts went through the initial screening (See Eligibility Criteria). The initial screening resulted in 118 abstracts eligible for full-text article assessment. The full-text assessment resulted in the final sample of 69 studies included in our meta-analysis. Figure 1 depicts the complete search process.
Figure 1. Meta-analysis Flow Diagram: The effectiveness of lifestyle intervention including nutrition education for diabetes prevention.
aStudies did not meet the inclusion criteria: 19 studies included participants with diabetes; 1 study lasted less than 4 weeks; 5 studies did not include nutrition education; 13 studies did not aim to prevent diabetes; 11 studies did not report relevant outcomes for the meta-analysis, or had incomplete information.
The key characteristics of included studies are presented in Supplementary Table 3.15,24,25,34–99 Among included studies, there were 32 conducted in the US and 37 conducted in other countries. Study designs included RCT (n=41), pre-post (n=25), matched-cohort design (n=2), and non-randomized comparison (n=1). Most interventions included both dietary and physical activity lifestyle components, with four studies focusing solely on dietary changes.65,67,80,86 Intervention delivery agents included registered dietitians (33 studies), or non-dietitian agents such as other health professionals, lay leaders, and community health workers (36 studies). Active intervention phase ranged from two months (n=3) to 12 months (n=13), and maintenance phase ranged from 5 months (n=1) to 51 months (n=1). This analysis included a total of 22,009 participants with mean age of 52.7 years. Approximately 65.4% of participants were women. Participant’s race and ethnicity were mostly white and Hispanic or Latino.
The I2 index demonstrated that most of the overall effect sizes in weight, FBG, 2-h BG and HbA1c change at different durations were heterogeneous. When there was statistical evidence of heterogeneity at one or more time points, random effects models were used in the analysis. Otherwise, fixed effects models were used to calculate the average effect sizes. Results of change in BMI were similar to that of weight, so we chose to present the results of weight change [the results of BMI change are available from the authors upon request]. Negative effect sizes reflected a reduction in the variable of interest.
Main findings
The comparison of delivery agents and channels are the main focus of this review, however we will first briefly discuss the overall results (i.e., pooled comparison). The effect size and mean difference for weight at 12 months are presented as forest plots in Supplemental Figure 1 and 2, and the remaining overall results are available from authors upon request.
Weight
A total of 64 studies reported results related to change in weight. The overall effect sizes for weight change were all negative, reflecting a reduction in body weight, and the magnitudes ranged from 0.17 (95% CI: 0.04 to 0.30; p=0.012; I2= 86.83%, 95% CI: 80.42% to 91.14%) to 0.65 (95% CI: 0.49 to 0.82; p<0.001; I2= 98.75%, 95% CI: 98.52% to 98.94). The effect sizes over time showed curvilinear trends, with a peak value at six months for weight loss, although the trends were not statistically significant (p>0.05). Non-standardized mean differences in weight loss for intervention groups relative to control groups showed a decreasing trend over time. Changes in weight were negative, indicating a reduction in body weight, and the magnitude of change ranged from 1.17 kg (95% CI: 0.50 to 1.83; p= 0.001; I2= 73.02%, 95% CI: 56.29% to 83.34%) to 3.15 kg (95% CI: 2.08 to 4.22; p<0.001; I2= 97.61%, 95% CI: 97.11% to 98.02%). On average, participants receiving intervention including nutrition education experienced a reduction of 2.07 kg (95% CI: 1.52 to 2.62; p<0.001; I2=90.99%, 95% CI: 88.61% to 92.87%) in weight at 12 months.
Fasting blood glucose (FBG)
Forty-one studies reported FBG results. The average effect size of FBG change was negative, indicating a reduction, and the magnitude showed an increasing trend to 0.33 (95% CI: 0.14 to 0.52; p=0.00; I2= 94.38, 95% CI: 92.87% to 95.57%) at 12 months, followed by a decrease to 0.11 (95% CI: 0.04 to 0.18; p= 0.002; I2= 47.64%, 95% CI: 0.00% to 75.67%) at longer durations, but the trend was not statistically significant. Relative mean differences in FBG were all negative and the sizes of change range from 1.65 mg/dl (95% CI: 0.14 to 3.17; p=0.03; I2=56.83%, 95% CI: 9.21% to 79.46%) at 12 months and beyond to 3.84 mg/dl (95% CI: 1.35 to 6.33; p= 0.003; I2=99.18%, 95% CI: 98.99% to 99.34) at 6 months, with the greatest difference at 6 months.
2-h blood glucose (2-h BG) and Hemoglobin A1c (HbA1c)
Nineteen studies reported 2-h BG and 15 studies reported HbA1c. The overall effect size for 2-h BG was negative and the magnitude increased to 0.65 (95% CI: 0.03 to 1.27; p= 0.04; I2= 99.41%, 95% CI: 99.30% to 99.49%) at 12 months, but decreased to 0.06 (95% CI: 0.01 to 0.14; p= 0.078; I2= 38.40%, 95% CI: 0.00% to 74.08%) at longer durations. For HbA1c, the effect sizes were negative and the greatest magnitude was 0.34 (95% CI: 0.17 to 0.51; p<0.001; I2= 72.32%, 95% CI: 47.58% to 85.39%) at 12 months, but a smaller effect size of 0.29 (95% CI: 0.01 to 0.59; p=0.059; I2= 68.00%, 95% CI: 0.00% to 90.72%) was evident at longer durations. Relative mean differences were negative and showed a similar trend as that of effect size, with the largest difference of 7.21 mg/dl (95% CI: 1.95 to 16.36; p=0.123; I2= 97.7%, 95% CI: 97.04% to 98.20%) for 2-h PG at 12 months.
Intervention Cost
Among 69 studies, cost information was reported by only eight studies.35,36,44–46,93,98,100 Intervention costs primarily included material costs, intervention staff salaries, and others (i.e., cost for using pedometers and facilitator calls, cost relating to physical activity and cost for food shopping and preparation). The material cost was reported in only one study and was $1,075.09, including food, scales, and items distributed to 11 participants and paper handouts to 28 participants.35 Intervention labor cost varied by the delivery agent and by the intensity of counseling. In the two studies that reported these costs, diabetes educators were compensated by $275/year per participant while dietitians were compensated by $528/year per participant (at annual average exchange rate in 2014) for the intervention delivery.45,93 Dawes et al. reported that the cost for pedometers and facilitator calls per participant was C$35 and C$58 (Canadian dollars) respectively.98
Five of these studies provided intervention cost per participant.44–46,93,100 The average intervention cost per person was $385. Three of them were delivered by dietitians with a lower average cost per person of $314, compared to that of $491 delivered by health educators and community leaders. Vadheim et al. reported that the per-participant cost of a tele-health intervention was $90.17 less than an in-person intervention, because this approach could allow more individuals to participate.44
The absence of complete information made it challenging to compute the unit cost for direct comparison. However, four studies reported sufficient information to calculate the average cost effectiveness ratios (ACER). Table 1 presents cost information for the above studies with ACER. The lowest average cost for 1 kg weight loss over two months was $53.87 in a study that examined if intervention delivered by trained diabetes educators can reduce risk factors among individuals at risk for diabetes.46 The highest average cost for 1 kg weight loss was $1,005.36 over 12 months in a study that assessed an intervention including weight control and physical activity aimed at south Asian individuals in the UK.93
Table 1.
The effectiveness of lifestyle intervention including nutrition education for diabetes prevention: Average cost effectiveness ratios (ACER), weight loss in kg
| Reference | Duration (months) | Delivery Agent | Effectiveness Mean (kg) | Cost per person ($) | ACER ($) Mean |
|---|---|---|---|---|---|
| Davis-Smith, Boltri et al., 200735 | 12 | Volunteer healthcare professionals | −4.80 | - | - |
| Ackermann, Finch et al., 200836 | 12–14 | Wellness instructor | IGa: −5.67 CGb: −1.63 |
- | - |
| Vadheim, McPherson et al., 201044 | 4 | Dietitian | On-site: −6.50 Telehealth: −6.70 |
On-site: 560.17 Telehealth: 470.00 |
On-site: 86.18 Telehealth: 70.15 |
| Katula, Vitolins et al, 201145 | 12 | Dietitian | −5.70 | - | - |
| Kramer, McWilliams et al., 201146 | 12 | Health educators | −5.94 | 320.00 | 53.87 |
| Ockene, Tellez et al., 2012100 | 12 | Community individuals | −2.50 | 661.00 | 264.40 |
| Bhopal, Douglas et al., 201493 | 36 | Dietitian | IG: −1.01 CG: −0.31 |
IG: 328.35c CG: 311.66c |
IG: 325.10 CG: 1005.36 |
| Dawes, Ashe et al., 201598 | 6 | RD | −6.2 ±5.20 | 109.00d, e | 17.60 |
IG=intervention group
CG=control group
Conversion of English pound to US dollars, according to the current exchange rates (on 9 Sep, 2015).
Conversion of Canadian dollar to US dollar according to the current exchange rates (on 9 Sep, 2015).
Only intervention delivery cost was reported.
- Insufficient data for calculating ACER.
Subgroup Findings
Subgroup analyses for overall effect sizes for both active intervention phase and overall (active + maintenance) study phase by delivery agent and channel are presented in Table 2 and summarized below. For each outcome, the first row reports the effect sizes with the confidence intervals; the second row reports the I2 index and the confidence interval. Results of similar subgroup analyses by study design and location (US vs non-US), and for relative effect size (intervention group compared to control group) for delivery agent and channel, and study design and location are presented in Supplemental Tables 4, 5, and 6 respectively.
Table 2.
The effectiveness of lifestyle intervention including nutrition education for diabetes prevention: effect size according to delivery agent (dietitian vs non-dietitian) and delivery channel (in-person vs technology-delivered) over time, in the active intervention and overall (active + maintenance study phases)
| Outcome | 3 months | 6 months | 12 months | 13–60 months | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Active Intervention Phase | ||||||||
|
| ||||||||
| Delivery Agent | ||||||||
|
| ||||||||
| Dietitian | Non-dietitian | Dietitian | Non-dietitian | Dietitian | Non-dietitian | Dietitian | Non-dietitian | |
| Weight | −0.27*** (−0.36, −0.19)a | −0.20*** (−0.28, −0.12)a | −1.40 (−4.05, 1.24)a | −0.44*** (−0.56, −0.31)a | −0.16*** (−0.24, −0.07)a | −0.27*** (−0.45, −0.08)a | - | - |
|
|
||||||||
| 96.68b (95.38, 97.61)c | 90.61b (86.02, 93.70)c | 99.72b (99.64, 99.79)c | 90.38b (84.82, 93.90)c | 84.75b (71.74, 91.77)c | 95.62b (92.73, 97.36)c | - | - | |
|
| ||||||||
| FBGd | −0.18 (−0.44, 0.07)a | −0.30*** (−0.51, −0.10)a | −1.81 (−5.78, 2.16)a | −0.35** (−0.66, −0.04)a | −0.49 (−1.15, 0.16)a | 0.01 (−0.41, 0.43)a | - | - |
|
|
||||||||
| 94.18b (89.26, 96.85)c | 52.11b (0.00, 78.51)c | 99.87b (99.84, 99.90)c | 69.70b (28.92, 87.08)c | 97.39b (96.24, 98.19)c | 93.85b (85.43, 97.41)c | - | - | |
|
| ||||||||
| 2-h BGe | - | - | 0.06f,g (−0.08, 0.20)a | −0.22**f,g (−0.42, −0.01)a | −1.38 (−3.43, 0.68)a | −0.08f (−0.28, 0.12)a | - | - |
|
|
||||||||
| - | - | 0b (-) c | 0b (-)c | 99.77b (99.72, 99.81)c | 78.46b (30.76, 93.30)c | - | - | |
|
| ||||||||
| HbA1ch | - | −0.10f,g (−1.43, 1.23)a | - | −0.16f,g (−0.37, 0.04)a | −0.69g (−1.52, 0.14)a | - | - | |
|
|
||||||||
| - | 95.12b | - | 30.99b (0.00, 75.12)c | 99.77b (99.72, 99.81)c | - | - | ||
|
| ||||||||
| Delivery Channel | ||||||||
|
| ||||||||
| IPi | TECHj | IP | TECH | IP | TECH | IP | TECH | |
|
| ||||||||
| Weight | −0.22*** (−0.28, −0.15)a | −0.32*** (−0.50, −0.13)a | −0.88*** (−1.21, −0.55)a | −0.92** (−1.68, −0.15)a | −0.15*** (−0.22, −0.08)a | −0.63***g (−0.97, −0.29)a | - | - |
|
|
||||||||
| 96.26b (95.10, 97.15)c | 85.59b (73.55,92.15)c | 99.31b (99.16, 99.43)c | 89.64b (72.08, 96.16)c | 88.80b (82.36, 92.90)c | - | - | - | |
|
| ||||||||
| IP | TECH | IP | TECH | IP | TECH | IP | TECH | |
|
| ||||||||
| FBG | −0.25*** (−0.42, −0.07)a | −0.27g,g (−0.87, 0.34)a | −0.95 (−2.71, 0.80)a | - | −0.34 (−0.84, 0.15)a | - | - | - |
|
|
||||||||
| 88.01b (80.01, 92.81)c | 78.61b (31.37, 93.34)c | 99.58b (99.49, 99.65)c | - | 97.35b (96.34, 98.09)c | - | - | - | |
|
| ||||||||
| 2-h BG | - | - | −0.03f (−0.14, 0.09)a | - | −1.03 (−2.32, 0.26)a | - | - | - |
|
|
||||||||
| - | - | 40.72b (0.00, 79.95)c | - | 99.70b (99.64, 99.74)c | - | - | - | |
|
| ||||||||
| HbA1c | - | −0.10f (−1.43, 1.23)a | −0.11f,g (−0.33, 0.11)a | - | −0.69f (−1.52, 0.14)a | - | - | - |
|
|
||||||||
| - | 95.12b (− )c | 34.70b (0.00, 78.75)c | - | 88.13b (− )c | - | - | - | |
|
| ||||||||
| Overall Intervention Phase | ||||||||
|
| ||||||||
| Delivery Agent | ||||||||
|
| ||||||||
| Dietitian | Non-dietitian | Dietitian | Non-dietitian | Dietitian | Non-dietitian | Dietitian | Non-dietitian | |
|
| ||||||||
| Weight | −0.26*** (−0.34, −0.18)a | −0.20*** (−0.28, −0.12)a | −0.99 (−2.11, 0.12)a | −0.28** (−0.36, −0.19)a | −0.30*** (−0.40, −0.21)a | −0.26*** (−0.34, −0.18)a | −0.24** (−0.44, −0.04)a | −0.04**f (−0.08, −0.01)a |
|
|
||||||||
| 96.44b (95.09, 97.42)c | 90.61b (86.02, 93.70)c | 99.60b (99.50, 99.67)c | 88.51b (82.73, 92.35)c | 91.72b (88.29, 94.14)c | 94.71b (93.06, 95.96)c | 88.13b (81.51, 92.38)c | 56.71b (0.00 85.64)c | |
|
| ||||||||
| FBG | −0.18 (−0.44, 0.07)a | −0.34*** (−0.54, −0.14)a | −1.81f (−5.78, 2.16)a | −0.47**g (−0.78, −0.15)a | −0.42*** (−0.70, −0.14)a | −0.17 (−0.37, 0.04)a | −0.21***f,g (−0.29, −0.12)a | 0.04g (−0.07, 0.15)a |
|
|
||||||||
| 94.18b (89.26, 96.85)c | 53.46b (1.17, 78.09)c | 99.87b (99.84, 99.90)c | 69.28b (35.91, 85.28)c | 95.68b (94.14, 96.81)c | 87.61b (80.60, 92.09)c | 0.00b (0.00, 74.62)c | 0.00b (0.00, 89.60)c | |
|
| ||||||||
| 2-h BG | - | - | 0.06 (−0.08, 0.20)a | −0.22*** (−0.42, −0.01)a | −0.44*** (−0.51, −0.38)a | −0.09 (−0.22, 0.05)a | −0.13**g (−0.23, −0.04)a | 0.02f,g (−0.09, 0.12)a |
|
|
||||||||
| - | - | - | - | 99.65b (99.58, 99.70)c | 70.10b (30.02, 87.22)c | 0.00b (0.00, 79.20)c | - | |
|
| ||||||||
| Dietitian | Non-dietitian | Dietitian | Non-dietitian | Dietitian | Non-dietitian | Dietitian | Non-dietitian | |
|
| ||||||||
| HbA1c | −0.10f (−1.43, 1.23)a | - | - | −0.16g (−0.37, 0.04)a | −0.43*** (−0.70, −0.16)a | −0.26* (−0.55, 0.03)a | −0.38***f,g (−0.55, −0.20)a | - |
|
|
||||||||
| 95.12b (-)c | - | - | 30.99b (0.00, 75.12)c | 62.30b (0.00, 87.33)c | 74.29b (41.49, 88.71)c | 30.80b (-)c | - | |
|
| ||||||||
| Delivery Channel | ||||||||
|
| ||||||||
| IP | TECH | IP | TECH | IP | TECH | IP | TECH | |
|
| ||||||||
| Weight | −0.21*** (−0.27, −0.15)a | −0.32*** (−0.50, −0.13)a | −0.67*** (−0.86, −0.48)a | −0.62*** (−1.02, −0.22)a | −0.24*** (−0.30, −0.18)a | −0.41*** (−0.68, −0.14)a | −0.16*** (−0.31, −0.01)a | −0.21**g (−0.27, −0.15)a |
|
|
||||||||
| 96.09b (94.89, 97.01)c | 85.59b (73.55, 92.15)c | 99.02b (98.83, 99.18)c | 89.18b (77.50, 94.80)c | 92.81b (90.83, 94.36)c | 93.66b (89.35, 96.22)c | 89.01b (83.33, 92.76)c | 96.09b (94.89, 97.01)c | |
|
| ||||||||
| FBG | −0.27*** (−0.45, −0.10)a | −0.27f,g (−0.87, 0.34)a | −1.02 (−2.58, 0.53)a | − | −0.33*** (−0.55, −0.12)a | −0.33** (−0.58, −0.08)a | −0.13***g (−0.20, −0.06)a | |
|
|
||||||||
| 87.25b (79.10, 92.22)c | 78.61b (31.37, 93.34)c | 99.46b (99.34, 99.55)c | - | 94.96b (93.53, 96.07)c | 80.32b (48.11, 92.54)c | 40.58b (0.00, 73.76)c | - | |
|
| ||||||||
| 2-h BG | - | - | −0.03f,g (−0.14, 0.09)a | - | −0.71* (−1.42, 0.00)a | −0.27**f,g (−0.41, −0.13)a | −0.06g (−0.13, 0.02)a | - |
|
|
||||||||
| - | - | 40.72b (0.00, 79.95)c | - | 99.49b (99.40, 99.57)c | 0b (-)c | 46.63b (0.00, 78.87) | - | |
|
| ||||||||
| HbA1c | - | −0.10f (−1.43, 1.23)a | −0.11g (−0.33, 0.11)a | - | −0.40f,g (−0.62, −0.18)a | - | −0.29 (−0.59, 0.01)a | - |
|
|
||||||||
| - | 95.12b (-)c | 34.70b (0.00, 78.75)c | - | 68.90b (37.78, 84.46)c | - | 67.99b (0.00, 91.72)c | - | |
The 95% confidence intervals of effect size are in the parenthesis.
I square in heterogeneity test
The 95% confidence intervals of I square
FBG=fasting blood glucose
2-h BG=2-hour blood glucose
The number of studies is less than 4.
Indicates that fixed-effect model was used to calculate the summary mean difference in outcomes.
HbA1c=hemoglobin A1c
IP=in-person intervention
TECH=technology-delivered intervention with/without in-person contact
Statistical significance at 10% level
Statistical significance at 5% level.
Statistical significance at 1% level.
- Insufficient data for meta-analysis
Delivery agent
Weight
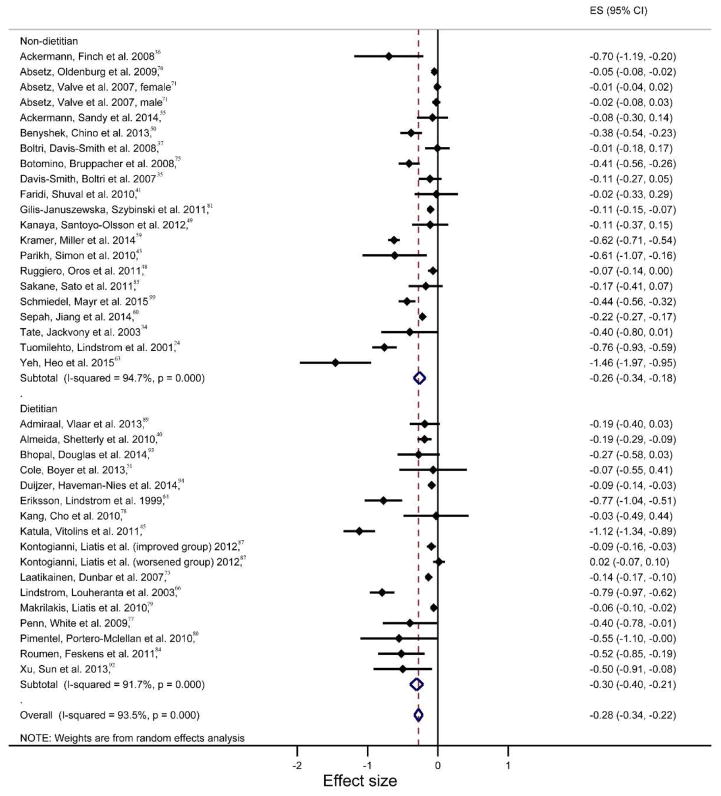
On average, dietitian-delivered interventions achieved the largest and statistically significant average effect size on weight reduction for the active phase of interventions at three months (Hedge’s g: −0.27, 95% CI: −0.36 to −0.19; p<0.001; I2= 96.68%, 95% CI: 95.38 % to 97.61%) and for the overall intervention phase at 12 months (Hedge’s g: −0.30, 95% CI: −0.40 to −0.21; p=0.025; I2= 91.72%, 95% CI: 88.29% to 94.14%) as compared to other durations (Table 2). Within the 12-month duration, the intervention phase and overall phase did not show statistically significant differences in effect sizes between dietitian-delivery and non-dietitian-delivery agents. As shown in the Figure 2 forest plot, there were relatively larger variations in effect sizes among non-dietitian delivered interventions (I2=94.71% 95% CI: 93.06% to 95.96%) as compared to dietitian-delivered ones (I2=91.72% 95% CI: 93.06% to 95.96%). For overall intervention phases lasting longer than 12 months, dietitian-delivered interventions demonstrated a greater effect size for weight reduction (Hedge’s g: −0.24, 95% CI: −0.44 to −0.04; p=0.019; I2= 88.13%, 95% CI: 81.51% to 92.38%)than non-dietitians (Hedge’s g: −0.04, 95% CI: −0.08 to −0.01; p=0.009; I2= 56.71%, 95% CI: 0.00 % to 85.64%) but the difference was not statistically significant due to the wider variations of effect sizes within dietitian-delivered intervention studies. Similar to overall effect sizes, all interventions across delivery agent type showed consistent significant effects on weight reduction across all program durations except for weight reduction at 6 months; no statistically significant differences across delivery agent types were noted (Supplementary Table 4). Mean reductions in weight of dietitian-delivery programs were larger than non-dietitian programs except for the 12 month duration (−1.90 kg, 95% CI: −2.73 to −1.61; p<0.001; I2= 91.14%, 95% CI: 87.38% to 93.78% for dietitian-delivery programs and −2.23 kg, 95% CI: −2.99 to −1.46; p<0.001; I2= 91.28%, 95% CI: 88.04% to 93.63% for non-dietitian-delivery programs) (Supplementary Table 4).
Figure 2.
Forest plot for the study effect sizes and the overall summary effect size for weight change at 12 months by delivery agent (non-dietitian vs dietitian). Each study is identified by author and year. Horizontal lines represent the effect size for each study. The whiskers extending to each side represent the study effect’s 95% CI. The diamond indicates the overall effect size.
FBG
Programs delivered by non-dietitians produced significant effect sizes for active intervention phases lasting three and six months (Hedge’s g: −0.30, 95% CI: −0.51 to −0.10; p=0.001; I2= 53.46%, 95% CI: 1.17% to 78.09% for those delivered by non-dietitians at six months), which was similar to the effect sizes at those time points for overall intervention phases (Table 2). For overall intervention phases lasting 12 months or longer, dietitian-delivered interventions produced significant average effect sizes (Hedge’s g: −0.21, 95% CI: −0.29 to −0.12; p<0.001; I2= 0.00%, 95% CI: 0.00% to 74.62% for programs delivered by dietitian and 0.04, 95% CI: −0.07 to 0.15; p=0.507; I2= 0.00%, 95% CI: 0.00% to 89.60% for those delivered by non-dietitians at 12 months and beyond) and the difference was statistically significant (p=0.0002).
2-h BG and HbA1c
A statistically significant effect size for 2-h BG for non-dietitian-delivered programs for active intervention phase was observed at 6 months (Hedge’s g: −0.22, 95% CI: −0.42 to −0.01; p=0.036; I2= 0.00%). Dietitian-delivered programs demonstrated significant effects for both outcomes at the longer durations No statistically significant effect for HbA1c for active intervention phase was present (Table 2). The subgroup comparison for HbA1c was only possible at 12 months for overall intervention phases, due to limited sample sizes (Table 2). Dietitian-delivered programs produced greater effect size for HbA1c than non-dietitian-delivered programs at 12 months (Hedge’s g: −0.43, 95% CI: −0.70 to −0.16; p=0.002; I2= 62.30%, 95% CI: 0.00% to 87.33% for programs delivered by dietitian and −0.26, 95% CI: −0.55 to 0.03; p=0.079; I2= 74.29%, 95% CI: 41.49% to 88.71% for those delivered by non-dietitians) (Table 2).
Delivery channel
Weight
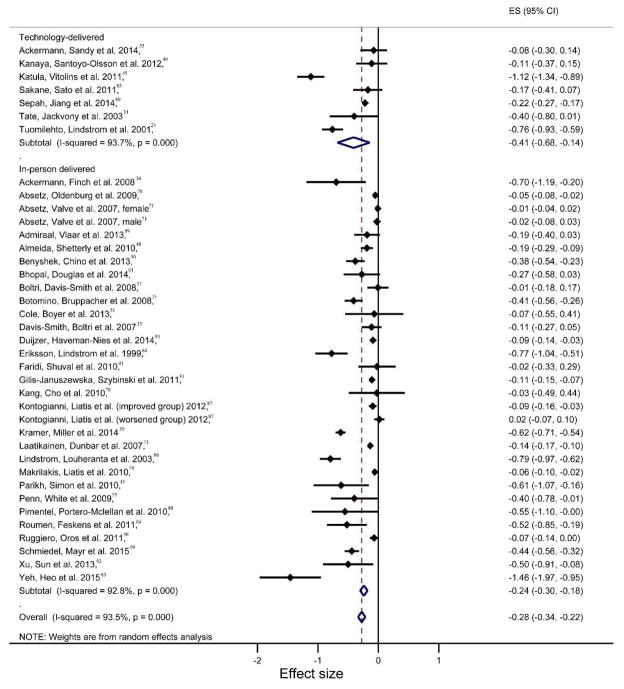
Effect sizes were generally larger for weight loss in technology-delivered interventions than in-person interventions in the active intervention phase, across all durations (Table 2). Similar comparison results were observed at 3, 12, and 13–60 months in the analysis of overall intervention phases. The largest effect sizes for both delivery channels were present at 6 months for overall intervention phases (Hedge’s g: −0.62, 95% CI: −1.02 to −0.22; p=0.002; I2= 89.18%, 95% CI: 77.50% to 94.80% for technology group and −0.67, 95% CI: −0.86 to −0.48; p<0.001; I2= 99.0%, 95% CI: 98.83% to 99.18% for in-person group). The only statistically significant larger weight loss the technology-delivered interventions produced as compared to in-person delivery interventions was at 12-month for the active intervention phase (Hedge’s g: −0.63, 95% CI: −0.97 to −0.29; p<0.001; I2= 61.1% for technology group and −0.15, 95% CI: −0.22 to −0.08; p<0.001; I2= 88.8%, 95% CI: 82.36% to 92.90% for in-person group). As depicted in the Figure 3 forest plot, there were many more studies using in-person delivered interventions, and they demonstrated a wider variation in effect size compared to technology-delivered interventions.
Figure 3.
Forest plot for the study effect sizes and the overall summary effect size for weight change at 12 months by delivery channel (technology-delivered vs in-person delivered). Each study is identified by author and year. Horizontal lines represent the effect size for each study. The whiskers extending to each side represent the study effect’s 95% CI. The diamond indicates the overall effect size.
FBG
For active intervention phases, only 3-month interventions showed statistically significant effect sizes for FBG changes for in-person delivery interventions (Hedge’s g: −0.25, 95% CI: −0.42 to −0.07; p=0.007; I2= 88.01%, 95% CI: 80.01% to 92.81%). The two delivery channels did not show statistical significance in effect sizes difference. For the overall intervention phase, the largest effect size for both delivery channels were noted as the same at 12 months (Hedge’s g: −0.33, 95% CI: −0.58 to −0.08; p=0.011; I2= 80.33%, 95% CI: 48.12% to 92.54% for technology group and −0.33, 95% CI: −0.55 to −0.12; p=0.003; I2= 94.46%, 95% CI: 93.53% to 96.07% for in-person group). Significant effect sizes were evident with reductions in FBG at durations longer than 12 months for in-person-delivered interventions (Hedge’s g: −0.13, 95% CI: −0.20 to −0.06; p<0.001; I2= 40.58%, 95% CI: 0.00% to 73.76%) (Table 2).
2-h BG and HbA1c
Comparisons of in-person and technology-delivered interventions were not possible for active intervention phases due to insufficient data (Table 2). A significant effect size was only detected for 2-h BG at 12 months for technology-delivered interventions for the overall intervention phase (Hedge’s g: −0.27, 95% CI: −0.41 to −0.13; p<0.001; I2= 0.00%).
Other factors
Weight
Interventions tested in RCT showed statistically significantly larger effect sizes for weight loss for programs with 6 month and 12 months as compared to interventions tested using QED for both active and overall phases (Supplementary Table 5). Both active and overall program effect sizes for weight loss were statistically significantly larger in US than in non-US studies except effect size at 12 months for active intervention (Supplementary Table 5).
FBG
Interventions using different study designs produced mixed effect sizes across different study durations, with no consistent trend (Supplementary Table 5). At 3 months and 12 months, interventions tested in RCT showed larger effect sizes for FBG than interventions using QED for both active and overall interventions. For example, the effect size at 12 month for overall programs was −0.53 (95% CI: −0.89 to −0.17; p=0.004; I2= 95.75%, 95% CI: 94.12% to 96.94%) for interventions tested in RCT and −0.11 (95% CI: −0.29 to 0.062; p=0.203; I2= 89.23%, 95% CI: 83.93% to 92.78%) for interventions using QED. US studies demonstrated statistically larger effect sizes for FBG change as compared to non-US studies for programs at 3 months for active interventions and at 3 and 12 months for overall interventions.
2-h BG and HbA1c
Only interventions tested in RCT showed a statistically significant effect size for 2-h BG at durations of 12 months and beyond (Hedge’s g: −0.13, 95% CI: −0.22 to −0.04; p=0.004; I2= 0.00%, 95% CI: 0.00% to 74.62%) (Supplementary Table 5) for overall intervention phases (active + maintenance). Non-US interventions demonstrated significant effect sizes for 2-h BG change at 12 months for non-US studies for overall intervention phases (Hedge’s g: −0.68, 95% CI: −1.32 to −0.04; p=0.039; I2= 99.45%, 95% CI: 99.35% to 99.53%). Non-US studies showed greater effect sizes for HbA1c compared to US studies at 12 months (Hedge’s g: −0.15, 95% CI: −0.20 to −0.10; p=0.027; I2= 56.43%, 95% CI: 0.00% to 83.86% for US studies and −0.44, 95% CI: −0.74 to −0.13; p=0.005; I2= 79.12%, 95% CI: 50.36% to 91.22% for non-US studies). No statistical difference was noted in the comparison between US and non-US studies on those outcomes.
Mean difference in outcomes showed similar results to effect sizes for overall intervention phases except the comparison result for weight. Interventions tested in RCT showed greater effect only at 6 months compared to interventions using QED (Supplementary Table 6).
Sources of heterogeneity: meta-regression
To provide insight as to which study characteristics were independent predictors of intervention effectiveness, meta-regressions on weight and FBG were conducted (Table 3 and Table 4). A meta-regression was performed in the full sample for 2-hr BG and HbA1c, which is presented in Table 5. There was not sufficient data available for a subsample analysis for 2-hr BG and HbA1C.
Table 3.
Meta-regression results: Effects of study features and participant characteristics on 12-month mean body weight change. Dependent variable: relative mean weight loss (intervention groups vs. control groups).
| Variable Name | Description | Data Summary Mean a (SD)b | All data Coefficient (SE) c | US Studies Coefficient (SE) c | Non-US Studies Coefficient (SE) c |
|---|---|---|---|---|---|
| Length | Duration of the nutrition education intervention (months) | 11.37 (9.56) | 0.02 (0.02) | 0.04 (0.04) | 0.04** (0.01) |
| Sample size | Sample size of the study | 299.64 (499.03) | −0.00 (0.00) | 0.00 (0.00) | −0.00 (0.00) |
| Percentage of women | Percentage of women | 62.90 (19.97) | 0.00 (0.01) | 0.05* (0.02) | −0.01 (0.01) |
| Setting | =1, if studies are conducted in primary care setting | 0.51 (0.50) | 1.08* (0.45) | 3.67*** (0.83) | −0.12 (0.38) |
| Delivery Agent | =1, if intervention is delivered by a dietitian | 0.52 (0.50) | −0.96* (0.39) | −2.44** (0.68) | −1.04* (0.40) |
| Study type | =1, if studies are RCT | 0.66 (0.48) | 0.92 (0.58) | 3.42** (1.03) | −0.47 (0.47) |
| Location | =1, if studies are conducted in United States | 0.53 (0.50) | −1.18* (0.49) | ||
| Delivery channel | =1, if intervention employs in-person delivery | 0.79 (0.41) | 0.17 (0.49) | −0.55 (0.60) | 1.60* (0.62) |
| Mean age | Average age of sample | 53.25 (6.99) | −0.04 (0.03) | −0.07 (0.05) | −0.06* (0.03) |
| Group-based | =1, if intervention is delivered in groups | 0.70 (0.46) | 1.01* (0.47) | 2.95*** (0.78) | 0.45 (0.37) |
| Baseline BMI | Average BMI at baseline | 31.21 (3.27) | −0.17* (0.08) | −0.08 (0.11) | −0.16* (0.08) |
| Constant | - | - | 3.82 (2.83) | −4.97 (4.87) | 6.28* (2.53) |
|
| |||||
| Adjusted R-squared: 53.94% | |||||
Mean=Mean value
SD=Standard deviation of included variables.
SE= Standard errors
Statistical significance at 10% level.
Statistical significance at 5% level.
Statistical significance at 1% level.
Table 4.
Meta-regression results: Effects of study features and participant characteristics on 12-month mean fasting glucose change. Dependent variable: relative mean fasting glucose reduction (intervention groups vs. control groups).
| Variable Name | Description | Data Summary Mean a (SD)b | All data Coefficient (SE) c | US Studies Coefficient (SE) c | Non-US Studies Coefficient (SE) c |
|---|---|---|---|---|---|
| Length | Duration of the nutrition education intervention (months) | 12.00 (9.98) | 0.04 (0.06) | 0.01 (0.15) | −0.02 (0.07) |
| Sample size | Sample size of the study | 197.95 (147.08) | 0.00 (0.00) | 0.00 (0.02) | 0.00 (0.01) |
| Percentage of women | Percentage of women | 65.29 (19.14) | −0.03 (0.03) | 0.08 (0.13) | −0.05 (0.03) |
| Setting | =1, if studies are conducted in primary care setting | 0.45 (0.50) | −0.21 (1.85) | −4.47 (5.69) | 0.61 (1.86) |
| Delivery Agent | =1, if intervention is delivered by a dietitian | 0.49 (0.50) | −1.60 (1.26) | −1.30 (5.63) | −2.77 (1.87) |
| Study type | =1, if studies are RCT | 0.58 (0.50) | −1.73 (1.51) | −0.10 (2.56) | −5.18* (1.89) |
| Location | =1, if studies are conducted in United States | 0.45 (0.50) | −1.45 (2.02) | ||
| Delivery channel | =1, if intervention employs in-person delivery | 0.85 (0.36) | 0.17 (1.58) | −1.27 (3.03) | 3.21 (2.60) |
| Mean age | Average age of sample | 53.94 (5.44) | 0.03 (0.11) | 0.14 (0.25) | −0.31 (0.16) |
| Group-based | =1, if intervention is delivered in groups | 0.82 (0.39) | 0.65 (1.55) | 3.02 (5.08) | −1.19 (1.80) |
| Baseline BMI | Average BMI at baseline | 31.10 (3.55) | 0.04 (0.22) | −0.32 (0.45) | 0.07 (0.29) |
| Constant | - | - | −2.83 (9.01) | −7.55 (18.68) | 17.50 (13.42) |
|
| |||||
| Adjusted R-squared = 35.55% | |||||
Mean=Mean value
SD=Standard deviation of included variables.
SE= Standard errors
Statistical significance at 10% level.
Statistical significance at 5% level.
Statistical significance at 1% level.
Table 5.
Meta-regression results: Effects of study features and participant characteristics on 12-month mean 2-hour blood glucose and HbA1c change. Dependent variable: relative mean 2-hour blood glucose (BG) and HbA1c reduction (intervention groups vs. control groups).
| Variable Name | Description | 2-BG as outcome | HbA1c as outcome | ||
|---|---|---|---|---|---|
| Data Summary Mean a (SD)b | All data Coefficient (SE) c | Data Summary Mean a (SD)b | All data Coefficient (SE) c | ||
| Length | Duration of the nutrition education intervention (months) | 17.42 (12.09) | 0.08 (0.11) | 12.28 (7.74) | −0.00 (0.00) |
| Sample size | Sample size of the study | 247.17 (136.12) | −0.00 (0.01) | 176.72 (169.66) | 0.00 (0.00) |
| Percentage women | Percentage of women | 60.92 (24.78) | 0.07 (0.05) | 52.39 (26.67) | −0.00 (0.00) |
| Setting | =1, if studies are conducted in primary care setting | 0.79 (0.41) | 5.29 (3.33) | 0.61 (0.50) | 0.02 (0.32) |
| Delivery Agent | =1, if intervention is delivered by a dietitian | 0.58 (0.50) | 2.75 (3.24) | 0.50 (0.51) | −0.14 (0.18) |
| Study type | =1, if studies are RCT | 0.67 (0.48) | −8.86 (4.52) | 0.94 (0.24) | 0.72 (0.39) |
| Location | =1, if studies are conducted in United States | 0.04 (0.20) | 2.66 (10.57) | 0.50 (0.51) | 0.08 (0.31) |
| Delivery channel | =1, if intervention employs in-person delivery | 0.88 (0.34) | −5.57 (4.23) | 0.89 (0.32) | −0.16 (0.33) |
| Mean age | Average age of sample | 54.45 (5.42) | −0.17 (0.35) | 52.92 (6.52) | −0.00 (0.01) |
| Group-based | =1, if intervention is delivered in groups | 0.67 (0.48) | 4.47 (4.15) | 0.78 (0.43) | 0.18 (0.13) |
| Baseline BMI | Average BMI at baseline | 29.77 (3.09) | 0.80 (0.65) | 29.92 (3.41) | 0.01 (0.02) |
| Constant | - | −24.29 (26.12) | −1.07 (0.79) | ||
| Adjusted R-squared | 93.49% | 100.00% | |||
Mean=Mean value
SD=Standard deviation of included variables.
SE= Standard errors
Statistical significance at 10% level.
Statistical significance at 5% level.
Statistical significance at 1% level.
a) Meta-regression on weight
Weight reduction (at 12 months) was chosen to be the outcome of interest, since it is the primary outcome of most diabetes prevention interventions in adults 7,24. Beyond the full sample meta-analysis, we also conducted a subsample meta-analysis by study location (US, Non-US) to further isolate location-specific contextual differences from the intervention effects. Studies included an average of 300 individuals, and 51% were conducted in a primary care setting. 52% of the studies also utilized dietitians for intervention delivery. Most studies (79%) were delivered in person, and most (70%) used a group-based intervention format. Mean baseline BMI was in the obese range (31.21 kg/m2).
Dietitian-delivered interventions demonstrated a larger relative weight loss (i.e., intervention group relative to control group) than programs delivered by non-dietitians in all groups (the full sample: −1.0 kg; the US subsample: −2.4 kg; the non-US subsample: −1 kg). Delivery channel was shown to be a statistically significant independent predictor of weight loss in the non-US subsample (Table 3).
Other independent predictors of weight change at 12 months included the study setting, as less relative weight reduction was noted in programs delivered in primary care settings in the full sample and in the US subsample. We observed lower relative weight loss in the full sample and in US studies using a group-based intervention format. Interventions conducted in the US demonstrated greater relative weight loss compared to non-US interventions (−1.2 kg). A longer study duration resulted in less weight loss, for non-US studies.
b) Meta-regression on FBG
FBG reduction (at 12 months) is a predictor of future diabetes and is commonly used as an enrollment criteria for diabetes prevention trials. 101 We also conducted meta-regression for the full sample and for subsamples of US and non-US studies. The average sample size for the full sample is smaller than that of the meta-regression on weight (198), and 45% of these studies were conducted in a primary care setting. About a half (49%) of the studies employed dietitians for intervention delivery. Most (85%) studies were delivered in person and most (82%) used a group-based intervention. Mean baseline BMI was in obese range (31.10 kg/m2). Only study type was shown to be a statistically significant independent predictor of FBG reduction in the non-US subsample (−5.2 mg/dl) (Table 4).
c) Meta-regression on 2-h BG and HbA1c
There was insufficient data for a meta-regression for subsamples of US and non-US studies, for the outcomes of 2-h BG and HbA1c. Table 5 presents the results of meta-regression for the full sample of those outcomes. The data summary was similar to those of weight and FBG. Only 4% of the studies with 2-h BG were conducted in the U.S. No statistically significant independent predictor was observed for 2-h BG or HbA1c.
Sensitivity Analysis and Publication Bias
A sensitivity analysis was performed to evaluate the robustness of the results. The sensitivity analysis, first restricted to studies with a positive rating and then to studies published within the past 10 years. The effect sizes of both sensitivity analyses did not substantially change from the primary results. For example, effect sizes of weight achieved the greatest magnitude at 6 months for both sensitivity analysis (High quality studies: −0.80, 95% CI: −1.12 to −0.48; p<0.001; I2= 98.94%, 95% CI: 98.74% to 99.11% and recent 10-year studies: −0.65, 95% CI: −0.82 to −0.49; p<0.001; I2= 98.85%, 95% CI: 98.52% to98.94 %), which is similar to the trend of overall interventions (−0.65, 95% CI: 0.17 to 0.28; p<0.001; I2= 98.75%, 95% CI: 98.52% to 98.94). Similar trends of effect sizes for all outcomes were observed in these two sensitivity analyses except for the greatest magnitude of effect size for 2-h BG occurred at 13 months for recent 10-year studies.
We included outcomes at 12 months for publication bias tests since it is only relevant if the number of studies is larger than 10.102 According to Egger’s test and funnel plots (Supplementary Figure 3), no statistically significant publication bias was observed for change in weight or FBG in the case of non-standardized mean differences.103–105 However, the Egger’s test showed evidence of publication bias for mean difference in 2-h BG (p=0.013) and HbA1c (p=0.004).
In terms of effect sizes, significant publication bias was evident for outcomes of weight (p=0.001) and HbA1c (p=0.043) (Supplementary Figure 4). One possible reason may be that only English language publications were included. Studies with positive results may be more likely to be published in international and English-language journals, whereas studies with negative findings tend to be published in local and regional journals in their native language.106
Study Quality
Supplementary Table 7 presents the results of study quality assessment. The two raters agreed that all the studies were eligible for plus (+) designation in the quality section. The second aspect was to examine the validity of the included studies; most were assigned a plus (+) in this section. Among all studies, 12 studies were rated as neutral quality and 57 studies were rated as high quality (Supplementary Table 7). The following aspects were sometimes not reported: blinding, representativeness of the population, number and reasons for withdrawals, and the validity of study measurements. Several studies did not clearly report their limitations or the funding sources. Discrepancies between the reviewers’ initial quality assessment focused on questions related to the comparableness of the study groups, and appropriateness of the statistical analysis.
Discussion
The overarching goal of this meta-analysis was to determine the effectiveness, costs, and cost-effectiveness of lifestyle interventions focused on diabetes prevention with a comparison of outcomes by delivery personnel (i.e., dietitian versus non-dietitian) and by delivery channel (i.e., technology versus in-person intervention delivery). Overall, we were able to replicate other systematic reviews that point towards the promise of these interventions to prevent diabetes.22 Specifically, small to medium effect sizes107 were documented across most outcomes (i.e., weight, 2-h BG and HbA1c) and varying intervention duration, indicating that lifestyle interventions were effective at reducing body weight, 2-h BG, and HbA1c. The observed effects on weight and blood glucose were clinically meaningful because sustained weight loss of 3–5% is likely to result in clinically meaningful reductions in blood triglycerides, blood glucose, and glycated hemoglobin and in the risk of developing T2D.108 Significant reductions in glycemic outcomes were also detected (e.g., reduction in FBG of 3.84 mg/dl at 6 months), which is a positive finding given that not all individuals participating in the reviewed studies met prediabetes diagnostic criteria (i.e., some were recruited based upon weight status or ADA risk screener scores). We also demonstrated in the meta-regression analysis that dietitian-delivered interventions may be more effective than those delivered by non-dietitian delivery agents, particularly for interventions carried out in the US. To further support this statement, we conducted a meta-regression analysis with the location dummy variable interacting with all variables and tested the interaction of the location dummy variable with the delivery agent dummy variable. The p-value for this interaction term is 0.061, indicating that interventions delivered by dietitians in the US studies have statistically significant greater reduction in weight change at the 10% level. However, this analysis did not indicate that delivery channel was a significant independent predictor of weight reduction. Unfortunately, we also document, as in other reviews of lifestyle interventions,109 that cost information is not regularly reported and that cost-reporting components varied across studies. For example, the total costs in one analysis consisted of three parts: cost for diabetes educators, program materials, and cost for a tool box; Bhopal et.al. also added indirect costs to the total cost. Hence, it is possible that the differences in ACER may be due to the lack of a standardized approach studies to complete cost analyses across studies rather than a true difference.93 Only eight of 69 studies reported intervention costs and only four provided the information necessary to determine ACERs—leaving these data tenuous to interpret with a high degree of confidence.
Our findings are consistent with those reported by Balk et al—combined diet and physical activity programs are effective at helping individuals at risk for T2D to reduce body weight and FBG.19 Schellenberg et al. conducted a meta-analysis of lifestyle interventions and reported that lifestyle interventions had an important impact on body weight and BMI for high-risk patients.110 Our analysis found that mean weight loss was 2.1 kg, which is similar to the 1.8 kg reported by Cardona-Morrell in 2010.18 According to the 2013 Guidelines for the Management of Overweight and Obesity in Adults,108 a sustained weight loss of 3–5% is likely to results in clinical meaningful reductions in blood glucose and in risk of developing T2D. This magnitude of weight loss reported overall for the studies reviewed was likely consistent with the 3% weight loss benchmark (e.g., 2.7 kg for an individual weighing 91 kg [200 lbs.]), therefore this magnitude of weight reduction appears to be clinically meaningful particularly for individuals who are at risk for T2D. Our study adds to the literature by summarizing the effect size across a number of relevant outcomes. We found that the effect size for different outcomes ranged from small (2-h BG and HbA1c within 6 months) to medium (weight and FBG at 12 months). 107,111 As such, our meta-analysis provides further evidence that when lifestyle interventions are implemented, practitioners and patients can expect modest improvements which translates into clinically meaningful change and reduced risk.
It is noteworthy that dietitian-delivered interventions were more effective at producing weight loss compared to other intervention delivery agents in the full sample, and particularly for interventions conducted in the US (Table 3). The US credentialing agency for Registered Dietitians (RD/RDN), the Commission on Dietetics Registration, has reciprocity agreements with only a limited number of countries who have similar dietetics education and credentialing as in the US: Dietitians of Canada; Dutch Association of Dieticians and Ministry of Welfare, Public Health and Culture; the Philippine Professional Regulation Commission; and the Irish Nutrition and Dietetic Institute 112. Thus, it is possible that dietitians practicing in other countries have received a different type of training than those in the US. This difference in training could explain the greater effectiveness (i.e., greater weight loss) of dietitian-led interventions in US studies. This issue warrants additional research. For example, it could be hypothesized that registered dietitians have training that allows them to more effectively communicate nutrition information, facilitate skill development, and develop strategies for implementation with their patients 113. It could also be that the training process associated with dietetics provides dietitians with tacit knowledge that aids in providing participants with the right services at the right time. From an organizational decision making perspective, this meta-analysis suggests that healthcare and community settings considering implementing a diabetes prevention program should utilize available dietitian resources.
We initially hypothesized that technology-delivered interventions would have equitable or smaller effects than in-person interventions. This hypothesis was based on the similar outcomes reported between technology-assisted interventions and in-person interventions at 12 months in a review conducted by Ali et al.16 We extended these findings to document no independent effects of delivery channel after controlling for other study factors. This, again, has implications for both practice and future research. Specifically, technology-support may be best delivered early in an intervention with in-person strategies used to support maintenance of changes. Alternatively, a fruitful area of research may examine the different technology-based strategies that are necessary for longer-term maintenance.
Additional findings of interest arose from our subgroup analysis. We found that study design was related to intervention outcomes, but in a complex way. We found in our univariate analyses that studies that used QED resulted in smaller effects than those that used RCT, however, when considered in our meta-regression, while accounting for a number of other factors, differences in study type was not associated with weight loss. This finding suggest that other factors beyond study design are likely the drivers of differential effect sizes. Furthermore, US studies on average documented larger weight loss as compared to those non-US studies which can be partially due to the heavier baseline samples in US (90.36 kg vs. 82.90 kg, respectively).
Related to design issues, our quality assessment of this body of literature was positive, but reporting could be improved by describing the method of handling withdrawals and missing values, whether blinding was used to prevent bias, or whether “intent to treat” was used in the analysis.
It is unfortunate that there was limited cost information provided across these studies. With some notable exceptions, 35,36,44–46,93,98,100 this body of literature does not include the information necessary to support clinical or community decision making relative to program uptake and implementation. 114 These preliminary data were, however, contrary to our hypothesis that dietitian-delivered interventions would be more expensive than those provided by non-dietitian delivery agents. The studies that did complete comparisons found that dietitian-delivered interventions are less expensive when considered by the amount of participant weight lost. This information underscores the need for community and clinical organizations to look beyond the simple cost of materials and delivery staff expertise and focus on the cost relative to intervention outcomes over time. Clearly, more evidence is needed to support that dietitian-delivered interventions and electronic-technology-interventions have a lower cost on average relative to changes in outcomes.
There are some limitations to this meta-analysis which should be acknowledged. Despite the large total number of papers included in this review, the number of studies available for some outcomes was limited, leading to insufficient studies available for some of the subgroup analyses. For example, there were insufficient data to analyze effect of 2-h BG and HbA1c beyond 12 months. In addition, a common limitation of meta-regression is that the analysis is often underpowered due to the limited number of available studies. We acknowledge this as a limitation of our investigation. The study selection was limited to English articles, which could be a source of the identified publication bias. Most of the studies using QED included in this review are before-and-after studies. Some of these studies did not report confounder-adjusted outcomes. It is possible that pre-post differences may result in potential bias. However, as mentioned previously, the overall quality of studies included was rated as positive. It is also possible that intervention design, as opposed to delivery agent (i.e., dietitian vs non-dietitian), could have explained differences in program effectiveness. Information about cost and cost-effectiveness information is quite limited in our body of literature and may lead to uncontrolled differences between available studies with cost information. Formal cost-reporting methods are needed in future studies to support a complete analysis of cost and cost-effectiveness of lifestyle interventions including nutrition education for prevention of diabetes.
Conclusions
This systematic review and meta-analysis indicated that diabetes prevention programs including nutrition education were associated with a reduced risk of diabetes, assessed by weight, FBG, 2-h BG and HbA1c. Dietitian-delivered intervention programs demonstrated greater effectiveness than those delivered by non-dietitian delivery agents. Technology-delivered interventions sometimes showed greater effectiveness compared to in-person delivered programs. These findings provide support for role of dietitians in diabetes prevention programs. However, our meta-analysis is limited in that it included only English language publications, and publication bias was present for some outcomes. Hence, the results should be interpreted with caution. We are unable to provide conclusive cost information due to the limited cost-reporting and inconsistent cost estimate approaches. Future research should include information about program costs, in order to better determine the cost-effectiveness of specific intervention features.
Supplementary Material
Supplementary Figure 1. Forest plot for the overall summary effect size for weight change at 12 months. Each study is identified by author and year. Horizontal lines represent the effect size for each study. The whiskers extending to each side represent the study effect’s 95% CI. The diamond indicates the overall mean difference.
Supplementary Figure 2. Forest plot for the overall summary mean difference relative to control group for weight change (in kg) at 12 months. Each study is identified by author and year. Horizontal lines represent the mean difference for each study. The whiskers extending to each side represent the study mean difference’s 95% CI. The diamond indicates the overall mean difference.
Supplementary Figure 3. Funnel plots for risk of publication bias: mean difference for weight, fasting and 2-hour blood glucose (BG), and HbA1c at 12 months against the SE. Scatter dots represent individual studies, dashed diagonal lines indicates pseudo 95% confidence interval.
Supplementary Figure 4. Funnel plots for risk of publication bias: effect size in weight, PBG, 2-h BG and HbA1c at 12 months against the SE. Scatter dots represent individual studies, dashed diagonal lines indicates pseudo 95% confidence interval.
Supplementary Table 1. Example of the database search strategy, CAB Direct
Supplementary Table 2. Example of the database search strategy, PubMed
Supplementary Table 3. The effectiveness and cost of lifestyle interventions including nutrition education for diabetes prevention: Characteristics of included studies (by publication date)
Supplementary Table 4. The effectiveness of lifestyle intervention including nutrition education for diabetes prevention: mean difference (intervention group compared to control group) in outcomes by subgroups (delivery agent and delivery channel)
Supplementary Table 5. The effectiveness of lifestyle intervention including nutrition education for diabetes prevention: effect sizes according to study design (randomized controlled trial vs quasi-experimental design) and study location (US vs Non-US) over time, in the active intervention and overall (active + maintenance study phases)
Supplementary Table 6. The effectiveness of lifestyle intervention including nutrition education for diabetes prevention: mean difference (intervention group compared to control group) in outcomes by subgroups (design and locations)
Supplementary Table 7. The effectiveness of lifestyle intervention including nutrition education for diabetes prevention: Study quality rating results
Acknowledgments
Funding Sources: This work was funded in part by NIH R18DK091811, the Virginia Tech Fralin Translational Obesity Research Center and Interdisciplinary Graduate Education Program, the Virginia Agricultural Experiment Stations and the USDA Hatch Program of the National Institute of Food and Agriculture.
Footnotes
COI: The authors have no financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.International Diabetes Federation (IDF) IDF Diabetes. 7. Brussels, Belgium: International Diabetes Federation; 2015. [Accessed June 1, 2016]. Available at: http://www.diabetesatlas.org Published 2015. [Google Scholar]
- 2.Gregg EW, Zhuo X, Cheng YJ, et al. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabs & Endo. 2014;2(11):867–874. doi: 10.1016/S2213-8587(14)70161-5. [DOI] [PubMed] [Google Scholar]
- 3.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 27 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 4.Alwan A, Armstrong T, Cowan M, Riley L World Health Organization. Noncommunicable diseases country profiles 2011. Geneva (CH): 2011. [Google Scholar]
- 5.Amos AF, McCarty DJ, Zimmet P. The Rising Global Burden of Diabetes and its Complications: Estimates and Projections to the Year 2010. Diab Med. 1997;14(S5):S7–S85. [PubMed] [Google Scholar]
- 6.American Diabetes Association. Economic costs of diabetes in the US in 2012. Diab Care. 2013;36(4):1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Prevention or Delay of Type 2 Diabetes. Diab Care. 2015;38(Supplement 1):S31–S32. doi: 10.2337/dc15-S008. [DOI] [PubMed] [Google Scholar]
- 9.Slawson DL, Fitzgerald N, Morgan KT. Position of the Academy of Nutrition and Dietetics: the role of nutrition in health promotion and chronic disease prevention. J Acad Nutr Diet. 2013;113(7):972–979. doi: 10.1016/j.jand.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Standards of Medical Care in Diabetes - 2016 Abridged for Primary Care Providers. Clin Diabetes. 2016;34(1):3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Foundations of Care: Education, Nutrition, Physical Activity, Smoking Cessation, Psychosocial Care, and Immunization. Diab Care. 2015;38(Supplement 1):S20–S30. doi: 10.2337/dc15-S007. [DOI] [PubMed] [Google Scholar]
- 12.Learn.org. [Accessed May 19, 2016];Dietitian: Career Profile, Employment Outlook, and Education Requirements. http://learn.org/articles/Dietitian_Career_Profile_Employment_Outlook_and_Education_Requirements.html.
- 13.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes Prevention in the Real World: Effectiveness of Pragmatic Lifestyle Interventions for the Prevention of Type 2 Diabetes and of the Impact of Adherence to Guideline Recommendations A Systematic Review and Meta-analysis. Diab Care. 2014;37(4):922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 14.Sherwood NE, Morton N, Jeffery RW, et al. Consumer preferences in format and type of community-based weight control programs. Am J Health Prom. 1998;13(1):12–18. doi: 10.4278/0890-1171-13.1.12. [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka Y, Gay CL, Joiner KL, et al. A Novel Diabetes Prevention Intervention Using a Mobile App: A Randomized Controlled Trial With Overweight Adults at Risk. Am J Prev Med. 2015;49(2):223–237. doi: 10.1016/j.amepre.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali MK, Echouffo-Tcheugui JB, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health affairs. 2012;31(1):67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 17.Yamaoka K, Tango T. Efficacy of Lifestyle Education to Prevent Type 2 Diabetes A meta-analysis of randomized controlled trials. Diab Care. 2005;28(11):2780–2786. doi: 10.2337/diacare.28.11.2780. [DOI] [PubMed] [Google Scholar]
- 18.Cardona-Morrell M, Rychetnik L, Morrell SL, et al. Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC public health. 2010;10(1):10, 653. doi: 10.1186/1471-2458-10-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balk EM, Earley A, Raman G, et al. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med. 2015:437–451. doi: 10.7326/M15-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Qu S, Zhang P, et al. Economic evaluation of combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med. 2015:452–460. doi: 10.7326/M15-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguiar EJ, Morgan PJ, Collins CE, et al. Efficacy of interventions that include diet, aerobic and resistance training components for type 2 diabetes prevention: a systematic review with meta-analysis. Int J Behav Nutr Phys Act. 2014;11(2) doi: 10.1186/1479-5868-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leeman J, Calancie L, Kegler MC, et al. Developing Theory to Guide Building Practitioners’ Capacity to Implement Evidence-Based Interventions. Health Educ & Beh. 2015 doi: 10.1177/1090198115610572. 1090198115610572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 24.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Yank V, Xiao L, et al. Translating the diabetes prevention program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–121. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Academy of Nutrition and Dietetics. [Accessed July 10, 2015];Evidence Analysis Library. 2012 https://www.andeal.org/evidence-analysis-manual.
- 27.StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 28.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Vol. 5. Wiley Online Library; 2008. [Google Scholar]
- 29.Cummings P. Arguments for and against standardized mean differences (effect sizes) Arch Ped Adol Med. 2011;165(7):592–596. doi: 10.1001/archpediatrics.2011.97. [DOI] [PubMed] [Google Scholar]
- 30.Borenstein M, Higgins JT. Meta-Analysis and Subgroups. Prev Sci. 2013;14(2):134–143. doi: 10.1007/s11121-013-0377-7. [DOI] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Jackson D, Bowden J, Baker R. How does the DerSimonian and Laird procedure for random effects meta-analysis compare with its more efficient but harder to compute counterparts? J Stat Plan Inf. 2010;140(4):961–970. [Google Scholar]
- 33.Sutton AJ, Duval S, Tweedie R, et al. Empirical assessment of effect of publication bias on meta-analyses. Bmj. 2000;320(7249):1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tate DF, Jackvony EH, Wing RR. Effects of internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: A randomized trial. JAMA. 2003;289(14):1833–1836. doi: 10.1001/jama.289.14.1833. [DOI] [PubMed] [Google Scholar]
- 35.Davis-Smith YM, Boltri JM, Seale JP, et al. Implementing a diabetes prevention program in a rural African-American church. J Natl Med Assoc. 2007;99(4):440–446. [PMC free article] [PubMed] [Google Scholar]
- 36.Ackermann RT, Finch EA, Brizendine E, et al. Translating the Diabetes Prevention Program into the community: the DEPLOY pilot study. Am J Prev Med. 2008;35(4):357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boltri JM, Davis-Smith YM, Seale JP, et al. Diabetes prevention in a faith-based setting: results of translational research. J Public Health Manag Pract. 2008;14(1):29–32. doi: 10.1097/01.PHH.0000303410.66485.91. [DOI] [PubMed] [Google Scholar]
- 38.Estabrooks PA, Smith-Ray RL. Piloting a behavioral intervention delivered through interactive voice response telephone messages to promote weight loss in a pre-diabetic population. Patient Educ Couns. 2008;72(1):34–41. doi: 10.1016/j.pec.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Amundson HA, Butcher MK, Gohdes D, et al. Translating the Diabetes Prevention Program Into Practice in the General Community Findings From the Montana Cardiovascular Disease and Diabetes Prevention Program. Diab Educator. 2009;35(2):209–223. doi: 10.1177/0145721709333269. [DOI] [PubMed] [Google Scholar]
- 40.Almeida FA, Shetterly S, Smith-Ray RL, et al. Reach and effectiveness of a weight loss intervention in patients with prediabetes in Colorado. Prev Chronic Dis. 2010;7(5):A103. [PMC free article] [PubMed] [Google Scholar]
- 41.Faridi Z, Shuval K, Njike VY, et al. Partners reducing effects of diabetes (PREDICT): a diabetes prevention physical activity and dietary intervention through African-American churches. Health Ed Res. 2010;25(2):306–315. doi: 10.1093/her/cyp005. [DOI] [PubMed] [Google Scholar]
- 42.Kramer MK, Kriska AM, Venditti EM, et al. A novel approach to diabetes prevention: evaluation of the Group Lifestyle Balance program delivered via DVD. Diabetes Res Clin Pract. 2010;90(3):e60–63. doi: 10.1016/j.diabres.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Parikh P, Simon EP, Fei K, et al. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. Am J Public Health. 2010;100(S1):S232–239. doi: 10.2105/AJPH.2009.170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vadheim LM, McPherson C, Kassner DR, et al. Adapted diabetes prevention program lifestyle intervention can be effectively delivered through telehealth. Diab Educ. 2010;36(4):651–656. doi: 10.1177/0145721710372811. [DOI] [PubMed] [Google Scholar]
- 45.Katula JA, Vitolins MZ, Rosenberger EL, et al. One-year results of a community-based translation of the diabetes prevention program. Healthy-living partnerships to prevent diabetes (HELP PD) project. Diab Care. 2011;34(7):1451–1457. doi: 10.2337/dc10-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kramer MK, McWilliams JR, Chen HY, Siminerio LM. A community-based diabetes prevention program: evaluation of the group lifestyle balance program delivered by diabetes educators. Diab Educ. 2011;37(5):659–668. doi: 10.1177/0145721711411930. [DOI] [PubMed] [Google Scholar]
- 47.Rosal MC, Lemon SC, Nguyen OH, et al. Translation of the diabetes prevention program lifestyle intervention for promoting postpartum weight loss among low-income women. Transl Behav Med. 2011;1(4):530–538. doi: 10.1007/s13142-011-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruggiero L, Oros S, Choi YK. Community-based translation of the diabetes prevention program’s lifestyle intervention in an underserved Latino population. Diab Educ. 2011;37(4):564–572. doi: 10.1177/0145721711411107. [DOI] [PubMed] [Google Scholar]
- 49.Kanaya AM, Santoyo-Olsson J, Gregorich S, et al. The Live Well, Be Well study: a community-based, translational lifestyle program to lower diabetes risk factors in ethnic minority and lower-socioeconomic status adults. Am J Pub Health. 2012;102(8):1551–1558. doi: 10.2105/AJPH.2011.300456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benyshek DC, Chino M, Dodge-Francis C, et al. Prevention of type 2 diabetes in urban American Indian/Alaskan Native communities: The Life in BALANCE pilot study. J Diabetes Mellitus. 2013;3(4):184–191. doi: 10.4236/jdm.2013.34028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cole RE, Boyer KM, Spanbauer SM, et al. Effectiveness of prediabetes nutrition shared medical appointments: prevention of diabetes. Diab Educ. 2013;39(3):344–353. doi: 10.1177/0145721713484812. [DOI] [PubMed] [Google Scholar]
- 52.Islam NS, Zanowiak JM, Wyatt LC, et al. A randomized-controlled, pilot intervention on diabetes prevention and healthy lifestyles in the New York City Korean community. J Comm Health. 2013;38(6):1030–1041. doi: 10.1007/s10900-013-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katula JA, Vitolins MZ, Morgan TM, et al. The healthy living partnerships to prevent diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med. 2013;44(4 Suppl 4):S324–S332. doi: 10.1016/j.amepre.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao L, Yank V, Wilson SR, et al. Two-year weight-loss maintenance in primary care-based Diabetes Prevention Program lifestyle interventions. Nutr Diabetes. 2013;3:e76. doi: 10.1038/nutd.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ackermann RT, Sandy LG, Beauregard T, et al. A randomized comparative effectiveness trial of using cable television to deliver diabetes prevention programming. Obesity. 2014;22(7):1601–1607. doi: 10.1002/oby.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cha E, Kim KH, Umpierrez G, et al. A Feasibility Study to Develop a Diabetes Prevention Program for Young Adults With Prediabetes by Using Digital Platforms and a Handheld Device. Diab Educator (2014) 2014 doi: 10.1177/0145721714539736. 0145721714539736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Islam NS, Zanowiak JM, Wyatt LC, et al. Diabetes prevention in the New York City Sikh Asian Indian community: a pilot study. Int J Env Res Pub Health. 2014;11(5):5462–5486. doi: 10.3390/ijerph110505462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaholokula JK, Wilson RE, Townsend CK, et al. Translating the Diabetes Prevention Program in Native Hawaiian and Pacific Islander communities: the PILI ‘Ohana Project. Transl Behav Med. 2014;4(2):149–159. doi: 10.1007/s13142-013-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kramer MK, Miller RG, Siminerio LM. Evaluation of a community diabetes prevention program delivered by diabetes educators in the United States: one-year follow up. Diab Res Clin Pract. 2014;106(3):e49–e52. doi: 10.1016/j.diabres.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Sepah SC, Jiang L, Peters AL. Translating the Diabetes Prevention Program into an Online Social Network: Validation against CDC Standards. Diabetes Educ. 2014;40(4):435–443. doi: 10.1177/0145721714531339. [DOI] [PubMed] [Google Scholar]
- 61.Tang TS, Nwankwo R, Whiten Y, Oney C. Outcomes of a church-based diabetes prevention program delivered by peers: a feasibility study. Diabetes Educ. 2014;40(2):223–230. doi: 10.1177/0145721713520569. [DOI] [PubMed] [Google Scholar]
- 62.Brokaw SM, Carpenedo D, Campbell P, et al. Effectiveness of an Adapted Diabetes Prevention Program Lifestyle Intervention in Older and Younger Adults. J Am Ger Soc. 2015;63(6):1067–1074. doi: 10.1111/jgs.13428. [DOI] [PubMed] [Google Scholar]
- 63.Yeh MC, Heo M, Suchday S, et al. Translation of the Diabetes Prevention Program for diabetes risk reduction in Chinese immigrants in New York City. Diab Med. 2015:547–551. doi: 10.1111/dme.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eriksson J, Lindström J, Valle T, et al. Prevention of Type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia. 1999;42(7):793–801. doi: 10.1007/s001250051229. [DOI] [PubMed] [Google Scholar]
- 65.Wein P, Beischer N, Harris C, Permezel M. A trial of simple versus intensified dietary modification for prevention of progression to diabetes mellitus in women with impaired glucose tolerance. Aust N Z J Obstet Gynaecol. 1999;39(2):162–166. doi: 10.1111/j.1479-828x.1999.tb03363.x. [DOI] [PubMed] [Google Scholar]
- 66.Lindstrom J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diab Care. 2003;26(12):3230–3236. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe M, Yamaoka K, Yokotsuka M, Tango T. Randomized controlled trial of a new dietary education program to prevent type 2 diabetes in a high-risk group of Japanese male workers. Diab Care. 2003;26(12):3209–3214. doi: 10.2337/diacare.26.12.3209. [DOI] [PubMed] [Google Scholar]
- 68.Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005;67(2):152–162. doi: 10.1016/j.diabres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet (British edition) 2006;368(9548):1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 70.Oldroyd JC, Unwin NC, White M, et al. Randomised controlled trial evaluating lifestyle interventions in people with impaired glucose tolerance. Diabetes Res Clin Pract. 2006;72(2):117–127. doi: 10.1016/j.diabres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 71.Absetz P, Valve R, Oldenburg B, et al. Type 2 Diabetes Prevention in the “Real World” One-year results of the GOAL Implementation Trial. Diab Care. 2007;30(10):2465–2470. doi: 10.2337/dc07-0171. [DOI] [PubMed] [Google Scholar]
- 72.Kilkkinen A, Heistaro S, Laatikainen T, et al. Prevention of type 2 diabetes in a primary health care setting: interim results from the Greater Green Triangle (GGT) Diabetes Prevention Project. Diabetes Res Clin Pract. 2007;76(3):460–462. doi: 10.1016/j.diabres.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 73.Laatikainen T, Dunbar JA, Chapman A, et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) Diabetes Prevention Project. BMC public health. 2007;7(1):249. doi: 10.1186/1471-2458-7-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barclay C, Procter KL, Glendenning R, et al. Can type 2 diabetes be prevented in UK general practice? A lifestyle-change feasibility study (ISAIAH) Br J Gen Pract. 2008;58(553):541–547. doi: 10.3399/bjgp08X319701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Botomino A, Bruppacher R, Krahenbuhl S, et al. Change of body weight and lifestyle of persons at risk for diabetes after screening and counselling in pharmacies. Pharm World Sci. 2008;30(3):222–226. doi: 10.1007/s11096-007-9174-3. [DOI] [PubMed] [Google Scholar]
- 76.Absetz P, Oldenburg B, Hankonen N, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL Lifestyle Implementation Trial. Diab Care. 2009;32(8):1418–1420. doi: 10.2337/dc09-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Penn L, White M, Oldroyd J, et al. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC public health 2009. 2009 Sep 16;9:342. doi: 10.1186/1471-2458-9-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang JY, Cho SW, Sung SH, et al. Effect of a continuous diabetes lifestyle intervention program on male workers in Korea. Diabetes Res Clin Pract. 2010;90(1):26–33. doi: 10.1016/j.diabres.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 79.Makrilakis K, Liatis S, Grammatikou S, et al. Implementation and effectiveness of the first community lifestyle intervention programme to prevent Type 2 diabetes in Greece. The DE-PLAN study. Diab Med. 2010;27(4):459–465. doi: 10.1111/j.1464-5491.2010.02918.x. [DOI] [PubMed] [Google Scholar]
- 80.Pimentel GD, Portero-Mclellan KC, Oliveira ÉP, et al. Long-term nutrition education reduces several risk factors for type 2 diabetes mellitus in Brazilians with impaired glucose tolerance. Nutr Res. 2010;30(3):186–190. doi: 10.1016/j.nutres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Gilis-Januszewska A, Szybinski Z, Kissimova-Skarbek K, et al. Prevention of type 2 diabetes by lifestyle intervention in primary health care setting in Poland: Diabetes in Europe Prevention using Lifestyle, physical Activity and Nutritional intervention (DEPLAN) project. Br J Diab Vasc Dis. 2011;11(4):198–203. [Google Scholar]
- 82.Moore SM, Hardie EA, Hackworth NJ, et al. Can the onset of type 2 diabetes be delayed by a group-based lifestyle intervention? A randomised control trial. Psych & Health. 2011;26(4):485–499. doi: 10.1080/08870440903548749. [DOI] [PubMed] [Google Scholar]
- 83.Nilsen V, Bakke PS, Gallefoss F. Effects of lifestyle intervention in persons at risk for type 2 diabetes mellitus - results from a randomised, controlled trial. BMC public health. 2011;11:893. doi: 10.1186/1471-2458-11-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roumen C, Feskens EJ, Corpeleijn E, et al. Predictors of lifestyle intervention outcome and dropout: the SLIM study. Eur J Clin Nutr. 2011;65(10):1141–1147. doi: 10.1038/ejcn.2011.74. [DOI] [PubMed] [Google Scholar]
- 85.Sakane N, Sato J, Tsushita K, et al. Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC public health 2011. 2011 Jan 17;11:40. doi: 10.1186/1471-2458-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salas-Salvadó J, Bulló M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diab Care. 2011;34(1):14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kontogianni MD, Liatis S, Grammatikou S, et al. Changes in dietary habits and their association with metabolic markers after a non-intensive, community-based lifestyle intervention to prevent type 2 diabetes, in Greece. The DEPLAN study. Diab Res Clin Pract. 2012;95(2):207–214. doi: 10.1016/j.diabres.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 88.Vermunt PW, Milder IE, Wielaard F, de Vries JH, Baan CA, van Oers JA, Westert GP. A lifestyle intervention to reduce Type 2 diabetes risk in Dutch primary care: 2.5-year results of a randomized controlled trial. Diabet Med. 2012;29(8):e223–231. doi: 10.1111/j.1464-5491.2012.03648.x. [DOI] [PubMed] [Google Scholar]
- 89.Admiraal WM, Vlaar EM, Nierkens V, et al. Intensive lifestyle intervention in general practice to prevent type 2 diabetes among 18 to 60-year-old South Asians: 1-year effects on the weight status and metabolic profile of participants in a randomized controlled trial. PLoS One. 2013;8(7):e68605. doi: 10.1371/journal.pone.0068605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sangeetha S, Fatimah A, Rohana AG, et al. Lowering dietary glycaemic index through nutrition education among Malaysian women with a history of gestational diabetes mellitus. Malays J Nutr. 2013;19(1):9–23. [PubMed] [Google Scholar]
- 91.Telle-Hjellset V, Raberg Kjollesdal MK, Bjorge B, et al. The InnvaDiab-DE-PLAN study: a randomised controlled trial with a culturally adapted education programme improved the risk profile for type 2 diabetes in Pakistani immigrant women. Br J Nutr. 2013;109(3):529–538. doi: 10.1017/S000711451200133X. [DOI] [PubMed] [Google Scholar]
- 92.Xu DF, Sun JQ, Chen M, et al. Effects of lifestyle intervention and meal replacement on glycaemic and body-weight control in Chinese subjects with impaired glucose regulation: a 1-year randomised controlled trial. Br J Nutr. 2013;109(3):487–492. doi: 10.1017/S0007114512001328. [DOI] [PubMed] [Google Scholar]
- 93.Bhopal RS, Douglas A, Wallia S, et al. Effect of a lifestyle intervention on weight change in south Asian individuals in the UK at high risk of type 2 diabetes: a family-cluster randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2(3):218–227. doi: 10.1016/S2213-8587(13)70204-3. [DOI] [PubMed] [Google Scholar]
- 94.Duijzer G, Haveman-Nies A, Jansen SC, et al. Feasibility and potential impact of the adapted SLIM diabetes prevention intervention in a Dutch real-life setting: The SLIMMER pilot study. Patient Educ Couns. 2014:101–107. doi: 10.1016/j.pec.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 95.Dunbar JA, Jayawardena A, Johnson G, et al. Scaling up diabetes prevention in Victoria, Australia: policy development, implementation, and evaluation. Diab Care. 2014;37(4):934–942. doi: 10.2337/dc12-2647. [DOI] [PubMed] [Google Scholar]
- 96.Parker AR, Byham-Gray L, Denmark R, et al. The Effect of Medical Nutrition Therapy by a Registered Dietitian Nutritionist in Patients with Prediabetes Participating in a Randomized Controlled Clinical Research Trial. J Acad Nutr Diet. 2014;114(11):1739–1748. doi: 10.1016/j.jand.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 97.Weir DL, Johnson ST, Mundt C, et al. A primary care based healthy-eating and active living education session for weight reduction in the pre-diabetic population. Prim Care Diabetes. 2014:301–307. doi: 10.1016/j.pcd.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 98.Dawes D, Ashe M, Campbell K, et al. Preventing Diabetes in Primary Care: A Feasibility Cluster Randomized Trial. Can J Diab. 2015;39(2):111–116. doi: 10.1016/j.jcjd.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Schmiedel K, Mayr A, Fießler C, et al. Effects of the Lifestyle Intervention Program GLICEMIA in People at Risk for Type 2 Diabetes: A Cluster-Randomized Controlled Trial. Diab Care. 2015;38(5):937–939. doi: 10.2337/dc14-2206. [DOI] [PubMed] [Google Scholar]
- 100.Ockene IS, Tellez TL, Rosal MC, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health. 2012;102(2):336–342. doi: 10.2105/AJPH.2011.300357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.American Diabetes Association and National Institute of Diabetes. Prevention or Delay of Type 2 Diabetes. Diab Care. 2004;27(suppl 1):s47–s47. doi: 10.2337/diacare.27.2007.s47. [DOI] [PubMed] [Google Scholar]
- 102.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 103.Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta-analysis: Prevention, assessment and adjustments. John Wiley & Sons; 2006. [Google Scholar]
- 104.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. Journal of clinical epidemiology. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 106.Higgins JPT, Green S, editors. The Cochrane Collaboration. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] [Google Scholar]
- 107.Cohen J. A power primer. Psychological bulletin. 1992;112(1):155. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 108.Jensen MD, Ryan DH. New obesity guidelines: promise and potential. JAMA. 2014;311(1):23–24. doi: 10.1001/jama.2013.282546. [DOI] [PubMed] [Google Scholar]
- 109.Harden S, Shoup J, Kinney K, et al. Fidelity to and comparative results across behavioral interventions evaluated through the RE-AIM framework: a systematic review. Syst Rev. 2015;4(1):1. doi: 10.1186/s13643-015-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schellenberg ES, Dryden DM, Vandermeer B, et al. Lifestyle Interventions for Patients With and at Risk for Type 2 Diabetes A Systematic Review and Meta-analysis. Ann Intern Med. 2013;159(8):543–551. doi: 10.7326/0003-4819-159-8-201310150-00007. [DOI] [PubMed] [Google Scholar]
- 111.Cohen J. Statistical power analysis for the behavioral sciences. Academic press; 2013. [Google Scholar]
- 112.Registration Eligibility Requirements For Dietitians. Commission on Dietetic Registration, Academy of Nutrition and Dietetics; 2016. [Acessed November 17, 2016]. Available at: https://www.cdrnet.org/certifications/registration-eligibility-requirements-for-dietitians Published 2016. [Google Scholar]
- 113.Nutrition Care Process. Academy of Nutrition and Dietetics; 2016. [Accessed November 17, 2016]. Available at http://www.eatrightpro.org/resources/practice/nutrition-care-process. Published 2016. [Google Scholar]
- 114.Glasgow RE, Klesges LM, Dzewaltowski DA, et al. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Ed Res. 2(5):688–694. doi: 10.1093/her/cyl081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Forest plot for the overall summary effect size for weight change at 12 months. Each study is identified by author and year. Horizontal lines represent the effect size for each study. The whiskers extending to each side represent the study effect’s 95% CI. The diamond indicates the overall mean difference.
Supplementary Figure 2. Forest plot for the overall summary mean difference relative to control group for weight change (in kg) at 12 months. Each study is identified by author and year. Horizontal lines represent the mean difference for each study. The whiskers extending to each side represent the study mean difference’s 95% CI. The diamond indicates the overall mean difference.
Supplementary Figure 3. Funnel plots for risk of publication bias: mean difference for weight, fasting and 2-hour blood glucose (BG), and HbA1c at 12 months against the SE. Scatter dots represent individual studies, dashed diagonal lines indicates pseudo 95% confidence interval.
Supplementary Figure 4. Funnel plots for risk of publication bias: effect size in weight, PBG, 2-h BG and HbA1c at 12 months against the SE. Scatter dots represent individual studies, dashed diagonal lines indicates pseudo 95% confidence interval.
Supplementary Table 1. Example of the database search strategy, CAB Direct
Supplementary Table 2. Example of the database search strategy, PubMed
Supplementary Table 3. The effectiveness and cost of lifestyle interventions including nutrition education for diabetes prevention: Characteristics of included studies (by publication date)
Supplementary Table 4. The effectiveness of lifestyle intervention including nutrition education for diabetes prevention: mean difference (intervention group compared to control group) in outcomes by subgroups (delivery agent and delivery channel)
Supplementary Table 5. The effectiveness of lifestyle intervention including nutrition education for diabetes prevention: effect sizes according to study design (randomized controlled trial vs quasi-experimental design) and study location (US vs Non-US) over time, in the active intervention and overall (active + maintenance study phases)
Supplementary Table 6. The effectiveness of lifestyle intervention including nutrition education for diabetes prevention: mean difference (intervention group compared to control group) in outcomes by subgroups (design and locations)
Supplementary Table 7. The effectiveness of lifestyle intervention including nutrition education for diabetes prevention: Study quality rating results