Abstract
Introduction:
Areas where leptospirosis and arboviruses are endemic largely overlap in the tropics. However, the number of arbovirus infections is usually much higher. The initial clinical presentation can be highly confusing; therefore, laboratory confirmation is key to an accurate diagnosis.
Case Presentation:
A 19–year–old man presented to a peripheral health centre with an acute febrile illness. Dengue was initially suspected, but the patient deteriorated to a shock syndrome. Leptospirosis as well as a co-infection with Zika virus were both confirmed in the laboratory, the latter being clinically masked in this dual infection.
Conclusion:
This case highlights the importance of not only considering the differential diagnosis of acute febrile syndromes, but also to consider the possibility of dual infections in the context of global spread of arboviruses. The specific context of travellers returning from endemic areas and pregnant women is also highlighted and discussed.
Keywords: leptospirosis, zika virus, shock syndrome, amoxycillin, differential diagnosis, dual infection
Introduction
In the last few years, an unprecedented wave of mosquito-borne viral infections has hit the Pacific Island Countries and Territories (Roth et al., 2014), and has also spread globally, putting new naïve populations at risk (Noel & Rizzo, 2014). As a result, a high level of awareness of arboviral diseases has reached the medical and public health communities leading to increased surveillance. In some tropical settings (e.g. in New Caledonia), all requests for laboratory diagnosis of any arboviral infection (dengue, chikungunya or Zika) in a hospitalized patient are systematically tested for all three viruses, as part of the public health surveillance scheme. A similar high awareness is not present for leptospirosis, a zoonotic bacterial disease, which is one of the possible differential diagnosis of acute febrile syndromes. We report a case of severe leptospirosis (with an initial suspicion of dengue) that was co-infected with Zika virus, emphasizingthe risk of Leptospira and arbovirus dual infections, already reported before (Sharp et al., 2012).
Case report
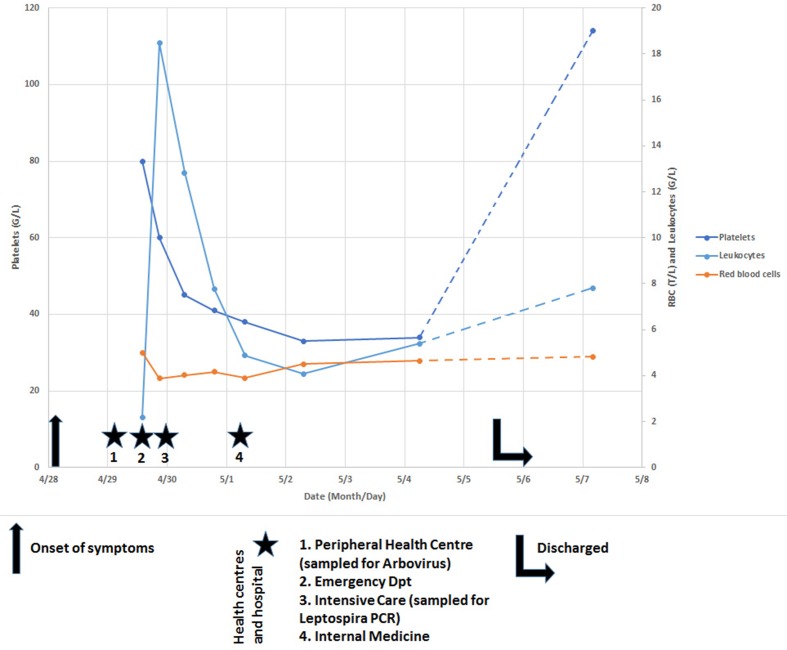
A 19-year–old male with previous good health and no significant past medical history, presented to a peripheral health centre 24 h after the onset of a febrile flu-like syndrome with fever, myalgia and painful cough. A suspicion of dengue led to a request for a laboratory diagnosis, and the patient was referred to the emergency department of New Caledonia central hospital, where he arrived 36 h after the onset of fever (Fig. 1). The pulmonary auscultation was normal, but the hemodynamic condition was unstable. Upon questioning, the patient reported a swim in a river on the North-East coast of New Caledonia two weeks before, a known exposure risk for leptospirosis in the Pacific (Berlioz-Arthaud et al., 2007). A blood culture and a Leptospira PCR were requested. The hemodynamic condition rapidly deteriorated, and the patient was transferred to the Intensive Care Unit, where he received noradrenalin, fluid infusion and was treated with Ceftriaxone and Gentamycin for a sceptic shock. Blood analysis revealed a thrombocytopenia 80×109 L−1, elevated C-Reactive Protein (CRP 241.5 mg L −1), Procalcitonin (PCT, 60.9 ng mL−1), Lactate (3.62 mmol L−1) and Lactate Dehydrogenase (LDH, 256 UI L−1).
Fig. 1.
Blood cell counts and timeline of medical history.
The next day, Leptospira PCR (Stoddard et al., 2009 returned a positive result with a quantified 192 bacteria/ml. The Leptospira lfb1 sequence used to identify the putative infecting serovar (Perez & Goarant, 2010) pointed to serogroup Icterohaemorrhagiae. The patient clinically improved, leading to discontinuation of noradrenaline support. Similarly, Ceftriaxone and Gentamycin were changed to Amoxycillin upon receipt of the positive Leptospira PCR result. The patient was transferred the next day to the Infectious Disease Department where Amoxycillin was continued. The laboratory blood results normalized, and patient was disharged after a week. However, there was persistent thrombocytopenia (34×109 L−1). At the time of discharge, the Zika RT-qPCR on the sample collected at first medical visit at the peripheral health centre returned a positive result (on both RNA targets(Lanciotti, 2008)), with an estimated 2×104 to 1.5×105viral RNA/ml. A full blood count showed a return to subnormal platelet count (114×109 L−1) two days later. The timeline of medical history is summarised in Fig. 1, the relevance of each symptom to both infectious agents is considered in Table 1.
Table 1. Clinical symptoms and laboratory results and their relevance to leptospirosis and Zika virus.
| Parameter | Leptospirosis | Zika Virus |
|---|---|---|
| Fever >38.5°C | +++ | + |
| Headache | ++ | + |
| Myalgia | +++ | +/- |
| Initial Leukocytopenia | ++ | + |
| Later Leukocytosis | +++ | - |
| Thrombocytopenia | +++ | + |
| Low RBC and hemoglobin | +++ | - |
| High CRP | +++ | - |
| High PCT | +++ | - |
| Increased Lactates & LDH | +++ | - |
-, +/-, +, ++ and +++ indicate the relavance for each parameter in increasing order (- not relevant; +++ fully relevant)
Discussion
Leptospirosis is endemic in New Caledonia, and the medical community is highly aware of this bacterial zoonosis. Epidemiological criteria are systematically considered for leptospirosis suspicion. However, because of unprecedented epidemics of arbovirus in the Pacific, and the larger size of outbreaks, arboviral diseases are often considered high up in the list of differential diagnoses of acute febrile syndromes. The patient presented early but probably failed to mention freshwater swimming, which is a well known risk factor in New Caledonia, and so leptospirosis was initially not considered in the differential diagnosis. His condition rapidly deteriorated to a septic shock with laboratory tests indicating a bacterial infection (CRP and PCT). Quantitative PCR and genotyping revealed a leptospiraemia caused by a strain from serogroup Icterohaemorrhagiae. The rapid presentation and antimicrobial treatment probably contributed to prompt recovery (Tubiana et al., 2013). Zika virus co-infection was discovered thanks to the systematic laboratory diagnosis of Zika, chikungunya and dengue, as part of the New Caledonian surveillance system but only when the patient was ready to be discharged. This virus probably contributed little to the patient’s clinical presentation although it possibly contributed to the thrombocytopenia (Table 1).
Although public and medical awareness are essential to arboviral diseases control and despite the tremendous media coverage of Zika and other arboviral infections in the last few years, careful differential diagnosis of acute fevers is essential during arbovirus outbreaks (Ellis & Imrie, 2008) to exclude leptospirosis and other antibiotic-treatable bacterial infections. This would help to minimize the rapid evolution to severe complications in leptospirosis cases.
The case reported here shows that Zika virus infection can easily be masked in dual infections by another, more symptomatic, viral or bacterial infection (Dupont-Rouzeyrol et al., 2015) because it is associated with few symptoms. Both leptosirosis and Zika virus might also be present in travellers returning from the tropics, and this should always be considered in such patients (Arcilla et al., 2012 , Maria, et al., 2016). This is important because it might not only lead to post-infectious neurological disorders (Cao-Lormeau et al., 2016), but also failure to arrange appropriate follow-up in pregnant women, if it is not diagnosed correctly. The case reported here highlights the importance of accurate diagnosis and management of dual infections with appropriate use of antibiotic therapy. This should especially be considered in returning travellers or when Zika infection is present in pregnant women.
Acknowledgements
Thanks are due to S. Geroult, O. O’Connor and A. Kem Seng for helping in biological investigations of this clinical case.
Abbreviations:
- CRP
C-Reactive Protein
- PCR
Polymerase chain reaction
- PCT
Procalcitonin
References
- Arcilla M., Wismans P. J., van Beek-Nieuwland Y., van Genderen P. J.(2012). Severe leptospirosis in a dutch traveller returning from the dominican republic, October 2011. Euro Surveill 17 pii: 20134. [PubMed] [Google Scholar]
- Berlioz-Arthaud A., Kiedrzynski T., Singh N., Yvon J. F., Roualen G., Coudert C., Uluiviti V.(2007). Multicentre survey of incidence and public health impact of leptospirosis in the western pacific. Trans R Soc Trop Med Hyg 101714–721. 10.1016/j.trstmh.2007.02.022 [DOI] [PubMed] [Google Scholar]
- Cao-Lormeau V. -M., Blake A., Mons S., Lastère S., Roche C., Vanhomwegen J., Dub T., Baudouin L., Teissier A.(2016). Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. The Lancet 3871531–1539. 10.1016/S0140-6736(16)00562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont-Rouzeyrol M., O'Connor O., Calvez E., Daurès M., John M., Grangeon J. P., Gourinat A. C.(2015). Co-infection with zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis 21381–382. 10.3201/eid2102.141553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T., Imrie A., Katz A. R., Effler P. V.(2008). Underrecognition of leptospirosis during a dengue fever outbreak in Hawaii, 2001-2002. Vector Borne Zoonotic Dis 8541–547. 10.1089/vbz.2007.0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R. S., Kosoy O. L., Laven J. J., Velez J. O., Lambert A. J., Johnson A. J., Stanfield S. M., Duffy M. R.(2008). Genetic and serologic properties of zika virus associated with an epidemic, yap state, micronesia, 2007. Emerg Infect Dis 141232–1239. 10.3201/eid1408.080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria A. T., Maquart M., Makinson A., Flusin O., Segondy M., Leparc-Goffart I., Le Moing V., Foulongne V.(2016). Zika virus infections in three travellers returning from South America and the caribbean respectively, to Montpellier, France, December 2015 to January 2016. Euro Surveill 21. 10.2807/1560-7917.ES.2016.21.6.30131 [DOI] [PubMed] [Google Scholar]
- Noël H., Rizzo C.(2014). Spread of chikungunya from the Caribbean to mainland Central and South America: A greater risk of spillover in Europe? Euro Surveill 1920855. 10.2807/1560-7917.ES2014.19.28.20855 [DOI] [PubMed] [Google Scholar]
- Perez J., Goarant C.(2010). Rapid leptospira identification by direct sequencing of the diagnostic PCR products in New Caledonia. BMC Microbiol 10325. 10.1186/1471-2180-10-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A., Mercier A., Lepers C., Hoy D., Duituturaga S., Benyon E., Guillaumot L., Souares Y.(2014). Concurrent outbreaks of dengue, chikungunya and zika virus infections - an unprecedented epidemic wave of mosquito-borne viruses in the pacific 2012-2014. Euro Surveill 19. 10.2807/1560-7917.ES2014.19.41.20929 [DOI] [PubMed] [Google Scholar]
- Sharp T. M., Bracero J., Rivera A., Shieh W. J., Bhatnagar J., Rivera-Diez I., Hunsperger E., Munoz-Jordan J., Zaki S. R.(2012). Fatal human co-infection with leptospira spp. and dengue virus, Puerto Rico, 2010. Emerg Infect Dis 18878–880. 10.3201/eid1805.111555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard R. A., Gee J. E., Wilkins P. P., McCaustland K., Hoffmaster A. R.(2009). Detection of pathogenic leptospira spp. through taqman polymerase chain reaction targeting the lipl32 gene. Diagn Microbiol Infect Dis 64247–255. 10.1016/j.diagmicrobio.2009.03.014 [DOI] [PubMed] [Google Scholar]
- Tubiana S., Mikulski M., Becam J., Lacassin F., Lefèvre P., Gourinat A. C., Goarant C., D'Ortenzio E.(2013). Risk factors and predictors of severe leptospirosis in New Caledonia. PLoS Negl Trop Dis 7e1991. 10.1371/journal.pntd.0001991 [DOI] [PMC free article] [PubMed] [Google Scholar]