Abstract
Introduction:
Diagnosing progressive disseminated histoplasmosis (PDH) in patients with systemic lupus erythematosus (SLE) is diagnostically challenging. Since PDH is lethal when untreated, awareness of this infection in patients with SLE is of utmost importance. To the best of our knowledge, this is the first description of a case of PDH in a patient with SLE in Europe.
Case presentation:
A 56-year-old woman of Surinamese descent with a history of SLE, presented with fever and polyarthritis. Although a flare of SLE was suspected initially, cultures of bone marrow and broncho-alveolar lavage fluid grew Histoplasma capsulatum.
Conclusion:
This case report highlights that physicians should be aware of progressive disseminated histoplasmosis in patients with SLE treated with immunosuppressive agents. The signs and symptoms can easily mimic a SLE flare, which would then be treated with more aggressive immunosuppression. Failure to recognize the infection will therefore invariably lead to death of the patient. Progressive disseminated histoplasmosis is usually not recognized by doctors in non-endemic areas such as Europe. However, globalisation and more frequent intercontinental traffic of immunocompromised patients currently increases the incidence of histoplasmosis in these areas. It is therefore of life-saving importance that doctors are aware of the features of the infection in areas where H. capsulatum is not endemic.
Keywords: histoplasmosis, disseminated, amphotericin B
Introduction
Histoplasma capsulatum is a soil-based fungus which is mostly associated with river valleys (e.g. Ohio and Mississippi river valleys), and places where bats live and birds roost, predominantly in (sub) tropical regions. After inhalation of spores, this microorganism can cause a broad range of clinical manifestations. The vast majority of infections (>90 %) are asymptomatic, but symptoms ranging from an influenza-like illness to death are also described (Goodwin et al., 1981). Contributing factors to the extent of the disease are higher inoculum size, increasing age and underlying disease. The use of immunosuppressive drugs is a risk factor for disseminated or fatal disease (Wheat et al., 1982). Adequate cell-mediated immunity is necessary to control a Histoplasma capsulatum infection after exposure (Porta & Maresca, 2000). Patients with systemic lupus erythematosus (SLE) have defects in their humoral and cellular immune systems (Tsokos, 1994). The combination of intrinsic immune system defects with chronic immunosuppressive therapy makes patients with SLE prone to develop progressive disseminated histoplasmosis (PDH) when infected. Although the reported incidence of invasive fungal infections in patients with SLE is low, 0.64–1.04 % (Weng et al., 2010), timely recognition of PDH is important because of the high mortality if untreated. Diagnosis is frequently delayed because symptoms are often attributed to SLE (Lim et al., 2013). Here we describe the first reported case of disseminated histoplasmosis in a patient with SLE in Europe.
Case report
A 56-year-old woman of Surinamese descent with a history of SLE and splenectomy was admitted to the Department of Rheumatology at the VU University Medical Center in Amsterdam because she had collapsed and had a fever. Her last visit to Suriname was in 2008. She had been treated with mycophenolate mofetil 1250 mg orally (PO) twice daily and prednisone 20 mg PO once daily. The dosage of both these drugs was recently increased because of exacerbation of her SLE-nephritis with a nephrotic syndrome. On admission, her temperature was 39.1 °C. She had polyarthritis with swelling and calor of her left knee and interphalangeal joints of her right hand. The white blood cell count was 7.9 × 109 l−1 (segmented neutrophils 7.50 × 109 l−1, lymphocytes 0.24 × 109 l−1), haemoglobin 7.5 mmol l−1, platelet count 419 × 109 l−1, and C-reactive protein 210 mg l−1. Nitrite in urine was positive. Antibiotic treatment was started with intravenous ceftriaxone, 2 g once a day, because a complicated urinary tract infection was suspected. Within one week of admission, the patient was admitted to the ICU because of respiratory insufficiency, and mechanical ventilation was started. Computed tomography (CT) of the lungs showed segmental pulmonary embolism, diffuse ground glass opacities and bilateral consolidations in the upper pulmonary lobes (Fig. 1). She also had progressive liver failure; bilirubin was 35 µmol l−1, alkaline phosphatase 950 U l−1, gamma-glutamyl transferase 6335 U l−1, aspartate aminotransferase 116 U l−1, and alanine transaminase 273 U l−1. A CT-scan of the abdomen revealed no explanation for the liver failure. The culture of the broncho-alveolar lavage (BAL) fluid was negativein the first days. PCRs for Legionella pneumophila, Mycobacterium tuberculosis, M. genus, Chlamydia pneumoniae, Chlamydia psittaci, Pneumocystis jiroveci, cytomegalovirus, Epstein-Barr virus and respiratory viruses were negative. With these negative microbiological test results, clinical deterioration of the patient was attributed to an exacerbation of SLE, and subsequently intravenous methylprednisolone pulse therapy was started in a daily dose of 1000 mg for three days. At first, her clinical condition improved and she was extubated. However within three days she was increasingly somnolent, in respiratory failure and she was intubated again. CT-scans of the lungs showed profound progression of the ground glass opacities. Histoplasmosis was considered in the differential diagnosis. However, at that moment fungal culture was negative, and with a low likelihood of H. capsulatum, no (toxic) pre-emptive therapy was initiated. A course of methylprednisolone pulse therapy was started again, but to no avail. Nine days after performing the BAL, culture of the BAL fluid grew a fungus, which was determined as H. capsulatum by the dimorphic character of the fungus, with a mycelial phase at 37 °C and a yeast phase at 30 °C. PCR performed on a bone marrow biopsy and culture of this biopsy were both positive for H. capsulatum. Antifungal therapy with liposomal amphotericin B (3.0 mg kg−1 daily) was started to treat this PDH, and immunosuppressive therapy was reduced to 20 mg prednisone per day. Two weeks after starting antifungal therapy the patient developed a lesion resembling pyogenic granuloma in her oral cavity from which she bled profoundly. A periodic acid-Schiff stain (PAS stain) of a needle biopsy of the gingiva showed encapsulated yeast cells and a haematoxylin-eosin stain of this biopsy showed 2–4 µm yeast buds and septated hyphae (Fig. 2). The patient could slowly be weaned from mechanical ventilation, and after one month on the ICU, she was extubated. After four weeks, liposomal amphotericin B was switched to itraconazole PO, 200 mg three times daily for three months, and then 200 mg once daily as lifelong suppressive therapy. Three months after she was diagnosed with PDH and treatment was started, the patient could be discharged from the hospital. Two months after her discharge her liver and kidney function tests had fully recovered.
Fig. 1.
Thoracic computed tomography (CT) of the lungs showing diffuse ground glass opacities and bilateral consolidations in the upper pulmonary lobe.
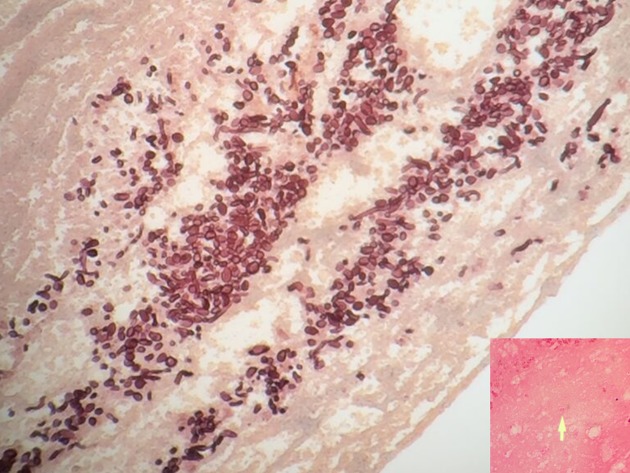
Fig. 2.
Histopathology of the gingival biopsy showing yeast buds and septate hyphae (haematoxylin-eosin stain). The inlet is a periodic acid-Schiff stain of the gingival biopsy showing encapsulated yeast cells.
Discussion
Symptoms of disseminated histoplasmosis mimic SLE-related symptoms, with focal lesions in the same organs affected by SLE. As a consequence, disseminated histoplasmosis is a diagnostic challenge for physicians. A complicating factor is that culturing H. capsulatum can take seven days to three weeks. H. capsulatum antigen testing on urine or serum can lead to a faster diagnosis with a reported sensitivity of 95–100 % (Connolly et al., 2007; Hage et al., 2010, 2011). As a consequence, diagnosing PDH solely by fungal culture can lead to a significant delay in initiating adequate antifungal therapy. In case the treating physician does not consider disseminated histoplasmosis, the dose of corticosteroids will likely be increased to treat a suspected flare of SLE. When symptoms temporarily improve by the increase in dosage of steroids, clinicians are put on the wrong track even more; the response to steroids affirms the initial diagnosis of SLE exacerbation. Even though the diagnosis of PDH was already considered in this patient three weeks after admission, a slight increase of SLE activity could not be excluded and symptoms were initially attributed to SLE. A case-based review on PDH in patients with SLE suggested that there is significant delay from onset of symptoms to time of diagnosis with a median time of 6.5 months (range 0.75–31 months) (Lim et al., 2013). Most of the patients in that review were using chronic immunosuppression, most commonly steroids with 56.3 % of the patients taking a dose of 20 mg prednisone or higher per day. In four out of the five fatal cases, the patients were using additional immunosuppressant drugs, like mycophenolate mofetil. Since the spleen plays an important role in our immune response it would be plausible that (functional) asplenia makes a patient prone to infection with H. capsulatum. However, splenectomy or asplenia does not seem to be a risk factor for an infection with H. capsulatum. Only sporadic cases have been reported of H. capsulatum infection in asplenic patients (Naina et al., 2010; Rose et al., 1982; Stone et al., 1990). Most case reports of disseminated histoplasmosis in patients with SLE are described in high-endemic areas (Table 1) (Lim et al., 2013). The patient in this case report is of Surinam descent, where H. capsulatum is also high-endemic (Colombo et al., 2011). In such high-endemic areas, physicians are more aware of PDH in patients with SLE than physicians in non-endemic areas. This case report describes the first reported case of disseminated histoplasmosis in a patient with SLE in Europe. The difficult diagnosis of PDH and the low awareness of European clinicians might well have resulted in under-reporting of PDH in European medical literature. Furthermore, the diagnosis was (partially) based on a positive fungal culture of BAL-fluid. The classical histopathological findings of the gingival biopsy showing yeast buds and septate hyphae and encapsulated yeast cells is a rarity. These findings make it a unique case.
Table 1. Cases of progressive disseminated histoplasmosis in systemic lupus erythematosus patients .
Clinical data of 17 cases described in the literature, plus the data of the current case report. PDH, Progressive disseminated histoplasmosis; SLE, systemic lupus erythematosus; M, male; F, female; u/a, unavailable; D, death; S, survived; bx, biopsy; hpe, histopathologic examination; cx, culture.
| Age (years)/ gender | Symptom | SLE flare | Antifungal treatment | Outcome | Initial presentation to diagnosis (time) | Initial method of diagnosis | Dose of steroid per day at onset of symptoms | Other immunosuppressants at onset of symptoms |
|---|---|---|---|---|---|---|---|---|
| 49/F | Dyspnea, productive cough, pleurisy, night sweats, weight loss | Yes | Amphotericin B | D | 4–5 months | Autopsy | Prednisone >20 mg | |
| 22/F | Fever, vision change | u/a | Amphotericin B | D | u/a | Autopsy | Steroids, dose not specified | Azathioprine |
| 23/F | Fever, night sweats, lethargy, pleurisy | No | Amphotericin B | S | 7 months | Histoplasmosis fixation antibody titer 1:128 | Prednisone 40–60 mg | |
| 65/M | Fever, fatigue, dyspnea | u/a | Amphotericin B | D | u/a | Bone marrow bx | Prednisone 20–60 mg | 6-Mercaptopurine |
| 23/F | Fever, malaise, weight loss | u/a | Amphotericin B | S | u/a | Liver bx | Prednisone 20–60 mg | |
| 35/M | Fever, malaise, dyspnea | u/a | No treatment | D | u/a | Autopsy | Prednisone 20–60 mg | 6-Mercaptopurine |
| 56/F | Fever, rash, headache, myalgia | Yes | Amphotericin B, fluorocytosin | S | 3 weeks | hpe (skin bx) | No steroids | |
| 53/F | Fever, confusion, fatigue, weakness, jaundice, papulonodular skin lesion | Yes | Amphotericin B, itraconazole | S | 6–7 months | cx (bone marrow bx) | Prednisone 30–80 mg | |
| 39/F | Headache, confusion | No | Amphotericin B, itraconazole | S | 6.5 months | hpe (cerebrospinal fluid) | Prednisone 20 mg | |
| 32/F | Fever, night sweats, lethargy, weight loss, irregular menses (ovarian dysfunction) | No | Ketoconazole | S | 2 years 7 months | hpe (ovary-surgical specimen) | Prednisone 10 mg | |
| 49/F | Nasal ulceration, cutaneous ulcer | u/a | Amphotericin B, itraconazole, voriconazole | S | 2 years | hpe (cutaneous ulcer, nasal ulceration bx) | Prednisone 5 mg | |
| 47/F | Nasal ulceration | No | Ketoconazole | S | u/a | cx (nasal septum bx) | Steroids, dose not specified | |
| 35/F | Hoarseness | u/a | Amphotericin B, itraconazole | S | 7 months | cx (nasal crusts) | Prednisone 10 mg | |
| 48/F | Fever, malaise, fatigue and arthralgias, swelling and stiffness of left hand, swelling of left ankle | Yes | Amphotericin B, miconazole | D | 6 months | hpe (palmar mass surgical specimen) | Methylprednisolone 16 mg | Azathioprine |
| 42/F | Swelling and stiffness of index finger of left hand | No | Itraconazole | S | 3 months | cx (synovium bx) | No steroids | |
| 32/F | Swelling, erythema in right forearm | No | Itraconazole | S | 2 months | cx (synovium bx) | Prednisone 30 mg | Mycophenolate mofetil |
| 56/F | Fever and polyarthritis | Yes | Liposomal Amphotericin B, itraconazole | S | 3 weeks | PCR (bone marrow bx), cx (BAL), hpe (gingival bx) | Prednisone 20 mg | Mycophenolate mofetil |
In conclusion, this case report highlights that physicians should be aware of progressive disseminated histoplasmosis in patients with SLE, treated with immunosuppressive agents. The signs and symptoms can easily mimic a SLE flare, which would then be treated with more aggressive immunosuppression. Failure to recognize the infection will therefore invariably lead to death of the patient. Progressive disseminated histoplasmosis is usually not recognized by doctors in non-endemic areas such as Europe. However, globalisation and more frequent intercontinental traffic of immunocompromised patients currently increases the incidence in these areas. It is therefore of life-saving importance that doctors in areas where H. capsulatum is not endemic are aware of the features of the infection, especially in patients from endemic areas. By creating awareness of this infection, faster diagnostic tests can be used to diagnose PDH. In this particular case, specific fungal staining, such as methenamine silver or periodic acid-Schiff stains (PAS), could have been performed on the BAL fluid or a bone marrow aspirate. Furthermore H. capsulatum antigen testing could have been performed on urine or serum for faster diagnosis (Connolly et al., 2007; Hage et al., 2010, 2011).
Acknowledgements
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review at the Editor-in-Chief of this journal.
Abbreviations:
- SLE
systemic lupus erythematosus
- CT
computed tomography
- BAL
broncho-alveolar lavage
- EBV
Epstein-Barr virus
- PDH
progressive disseminated histoplasmosis
- PCR
polymerase chain reaction
- PO
per os
References
- Colombo A. L., Tobón A., Restrepo A., Queiroz-Telles F., Nucci M. (2011). Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 49785–798. 10.3109/13693786.2011.577821 [DOI] [PubMed] [Google Scholar]
- Connolly P. A., Durkin M. M., Lemonte A. M., Hackett E. J., Wheat L. J. (2007). Detection of Histoplasma antigen by a quantitative enzyme immunoassay. Clin Vaccine Immunol 141587–1591. 10.1128/CVI.00071-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. A., Loyd J. E., Des Prez R. M. (1981). Histoplasmosis in normal hosts. Medicine 60231–266. 10.1097/00005792-198107000-00001 [DOI] [PubMed] [Google Scholar]
- Hage C. A., Bowyer S., Tarvin S. E., Helper D., Kleiman M. B., Wheat L. J. (2010). Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis 5085–92. 10.1086/648724 [DOI] [PubMed] [Google Scholar]
- Hage C. A., Ribes J. A., Wengenack N. L., Baddour L. M., Assi M., McKinsey D. S., Hammoud K., Alapat D., Babady N. E., other authors (2011). A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 53448–454. 10.1093/cid/cir435 [DOI] [PubMed] [Google Scholar]
- Lim S. Y., Kijsirichareanchai K., Winn R. (2013). Progressive disseminated histoplasmosis in systemic lupus erythematosus-an unusual presentation of acute tenosynovitis and a literature review. Clin Rheumatol 32135–139. 10.1007/s10067-012-2102-5 [DOI] [PubMed] [Google Scholar]
- Naina H. V., Thomas C. F., Jr., Harris S. (2010). Histoplasmosis and asplenia. Thorax 65372. 10.1136/thx.2008.100214 [DOI] [PubMed] [Google Scholar]
- Porta A., Maresca B. (2000). Host response and Histoplasma capsulatum/macrophage molecular interactions. Med Mycol 38399–406. 10.1080/mmy.38.6.399.406 [DOI] [PubMed] [Google Scholar]
- Rose F. B., Camp C. J., Chisdak M. (1982). Disseminated histoplasmosis and asplenia. Ann Intern Med 96790. 10.7326/0003-4819-96-6-790_1 [DOI] [PubMed] [Google Scholar]
- Stone M. M., Frenkel L. M., Howard D. H. (1990). Histoplasmosis after multiple trauma. Pediatr Infect Dis J 9747–749. [PubMed] [Google Scholar]
- Tsokos G. C. (1994). Lymphocytes, cytokines, inflammation, and immune trafficking. Curr Opin Rheumatol 6461–467. 10.1097/00002281-199409000-00002 [DOI] [PubMed] [Google Scholar]
- Weng C. T., Lee N. Y., Liu M. F., Weng M. Y., Wu A. B., Chang T. W., Lin T. S., Wang J. Y., Chang H. Y., Wang C. R. (2010). A retrospective study of catastrophic invasive fungal infections in patients with systemic lupus erythematosus from southern Taiwan. Lupus 191204–1209. 10.1177/0961203310368969 [DOI] [PubMed] [Google Scholar]
- Wheat L. J., Slama T. G., Norton J. A., Kohler R. B., Eitzen H. E., French M. L., Sathapatayavongs B. (1982). Risk factors for disseminated or fatal histoplasmosis. Analysis of a large urban outbreak. Ann Intern Med 96159–163. 10.7326/0003-4819-96-2-159 [DOI] [PubMed] [Google Scholar]