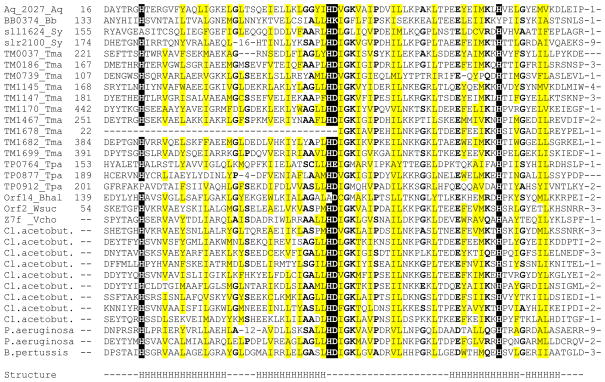
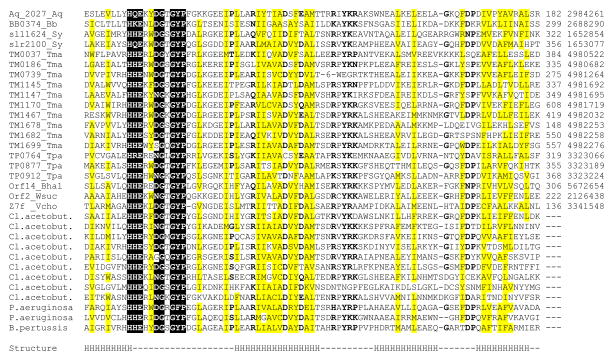
Recently, a superfamily of proteins containing a previously undetected domain with predicted metal-dependent phosphohydrolase activity has been described and designated the HD superfamily, after the principal conserved residues implicated in catalysis (Aravind and Koonin, 1998). In the course of our analysis of ancient conserved regions in microbial genomes (Koonin et al., 1998), we found a distinct version of this domain which is encoded in one to three copies in the genomes of Aquifex aeolicus, Borrelia burgdorferi, Synechocystis sp. and Treponema pallidum, but is dramatically expanded in the genomes of Thermotoga maritima and Clostridium acetobutylicum (Fig. 1). Compared with the consensus HD domain (Aravind and Koonin, 1998), this version contains a number of additional highly conserved residues; hereinafter we refer to it as the HD-GYP domain, after the characteristic sequence signatures (Fig. 1). This domain was also detected in previously uncharacterized proteins from Wolinella succinogenes (Kreis-Kleinschmidt et al., 1995), Bacillus halodurans (Takami et al., 1999), Pseudomonas aeruginosa and Bordetella pertussis (Fig. 1). The HD-GYP domain is missing in E. coli and B. subtilis. Remarkably, however, in other γ-proteobacteria, such as Shewanella putrefaciens and Vibrio cholerae, it is present in up to 8 copies (data not shown).
Figure 1. Multiple alignment of HD–GYP domains.
The proteins are listed under their names in complete genomes (left column) and their unique gene identification (gi) numbers in the GenBank protein database (right column); the numbers indicate positions of the first and the last residues in each protein, where available, and the distances between the aligned segments. Species name abbreviations are as follows: Aq, Aquifex aeolicus; Bb, Borrelia burgdorferi; Bhal, Bacillus halodurans; Cl. acetobut., Clostridium acetobutylicum; Sy, Synechocystis sp., Tma, Thermotoga maritima; Tpa, Treponema pallidum; Vcho, Vibrio cholerae; Wsuc, Wolinella succinogenes. Reverse shading indicates most conserved amino acid residues that are probably involved in metal and/or substrate binding. Grey shading indicates conserved uncharged amino acid residues, other conserved residues are in bold. The secondary structure of the HD domain is as predicted by PHDsec program (Rost and Sander, 1993); H indicates predicted α-helical segments, dash indicates a loop or the absence of confident prediction.
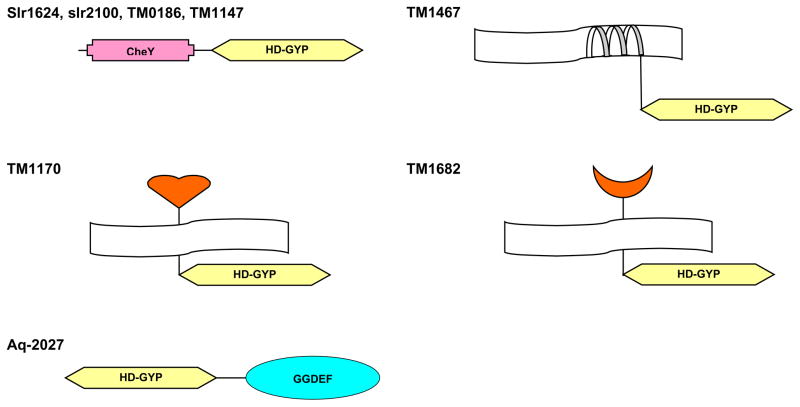
While none of the proteins that contain the HD-GYP domain has ever been characterized experimentally, the spectrum of the domains that are associated with HD-GYP in multidomain proteins (Fig. 2) suggests that it is probably involved in signal transduction. In Synechocystis sp., both copies of the HD-GYP domain are found in proteins that also contain CheY-like receiver domains of the two-component signal transduction system (Pao and Saier, 1995; Volz and Matsumura, 1991). A similar CheY – HD-GYP domain organization is found in two T. maritima proteins, TM0186 and TM1147 (Fig. 2). Two other proteins from T. maritima, TM1170 and TM1682, combine the HD-GYP domain with extracytoplasmic ligand-binding domains, which are closely related, respectively, to periplasmic solute-binding protein components of the ATP-dependent transport systems (Tam and Saier, 1993) and the extracytoplasmic part of methyl-accepting chemotaxis proteins of Bacillus subtilis, such as McpA and McpB (Hanlon and Ordal, 1994). Such a combination of an extracytoplasmic ligand-binding domain and a cytoplasmic HD-GYP domain, connected by a transmembrane segment, has the same topology as methyl-accepting proteins and many sensor kinases, which further supports the participation of the HD-GYP domain in signal transduction. Finally, in the Aq_2027 protein from A. aeolicus, the HD-GYP domain is found together with the GGDEF domain (Fig. 2). The latter domain has been recently identified in diguanylate cyclases and phosphodiesterases involved in the regulation of cellulose synthesis in Acetobacter xylinum (Tal et al., 1998) and in a variety of bacterial signalling proteins in combination with CheY, PAS, and HAMP domains (Hecht and Newton, 1995; Aravind and Ponting, 1999). The GGDEF domain is often associated with another uncharacterized domain, EAL, in particular, in diguanylate cyclases and phosphodiesterases (Tal et al., 1998; Aravind and Ponting, 1999). Remarkably, however, the combination of the HD-GYP and EAL domains is not seen in any of the currently available microbial genomes. Moreover, the number of copies of the HD-GYP domain in complete genomes generally correlates with prevalence of the GGDEF domain over the EAL domain (Table 1). Furthermore, the HD-GYP family of HD proteins so far is lacking in archaea and eukaryotes and so are the GGDEF and EAL domains. This suggests that HD-GYP domain might be also involved in cyclic diguanylate-mediated signaling. The HD superfamily is related to the cAMP/cGMP phosphodiesterases that are involved in eukaryotic signaling (Aravind and Koonin, 1998). Therefore is seems plausible that the HD-GYP family proteins are likely to possess a diguanylate phosphodiesterase activity and complement the function that, in the characterized diguanylate phosphodiesterases, is performed by the EAL domain. Such a function is compatible with the high sequence conservation of this domain as well as its unusual expansion in certain genomes. Indeed, cyclic diguanylate stimulates cellulose synthesis in Acetobacter xylinum in response to the lack of oxygen (Ross et al., 1991). Similarly, multiple HD-GYP domains in T. maritima, C. acetobutylicum, S. putrefaciens and V. cholerae might be involved in signaling the availability of various electron acceptors, including iron and sulfur (Nealson and Saffarini, 1994; Vargas et al., 1998). Remarkably, sugar metabolism in Thermotoga neapolitana has been found to be subject to catabolite repression (Galperin et al., 1997; Vargas and Noll, 1996), although this organism is devoid of the PTS system (Galperin et al., 1996; Nelson et al., 1999) and contains negligible amounts of cAMP (Vargas and Noll, 1996). Thus preferential utilization of certain sugars (e.g., glucose) in Thermotoga should be regulated by an elaborate regulatory system different from those found in, for example, E. coli or B. subtilis. Whatever its exact function, the HD-GYP domain is likely to play a crucial role in this novel regulatory mechanism.
Figure 2. Association of HD-GYP domain with other signaling domains.
CheY domain (Pao and Saier, 1995; Volz and Matsumura, 1991) and the periplasmic ligand-binding domain of TM1170 (Vyas et al., 1988) are well characterized; GGDEF domain (Hecht and Newton, 1995; Tal et al., 1998) and the MCP-like extracellular ligand-binding domain of TM1682 (Hanlon and Ordal, 1994) are less studied. The transmembrane portion of TM1467 does not show significant similarity to any characterized membrane protein.
Table 1.
Distribution of three domains implicated in signal transduction in complete microbial genomes
| Speciesa | Domains
|
||
|---|---|---|---|
| GGDEF | EAL | HD-GYP | |
| Escherichia coli | 19 | 18 | - |
| Rickettsia prowazekii | 1 | 1 | - |
| Bacillus subtilis | 4 | 2 | - |
| Mycobacterium tuberculosis | 1 | 2 | - |
| Synechocystis sp. | 23 | 13 | 2 |
| Borellia burgdorferi | 1 | 1 | 1 |
| Treponema pallidum | 1 | - | 3 |
| Aquifex aeolicus | 11 | 6 | 1 |
| Thermotoga maritima | 9 | - | 9 |
| Clostridium acetobutylicumb | 10 | 4 | 8 |
Genomes of bacteria Haemophilus influenzae, Helicobacter pylori, Chlamydia trachomatis, C. pneumoniae, Mycoplasma genitalium, M. pneumoniae, and archaea Methanococcus jannaschii, Methanobacterium thermoautotrophicum, Archaeoglobus fulgidus, Pyrococcus horikoshii, and Aeropyrum pernix do not contain any of these domains.
Preliminary data based on unfinished genome sequence (http://www.cric.com/sequence_center/bacterial_genomes)
Acknowledgments
Analysis of unfinished genome sequences was made possible by generous submission to the public databases of preliminary sequence data by Genome Therapeutics (C. acetobutylicum), the Sanger Centre (B. pertussis), The Institute for Genome Research (S. putrefaciens, V. cholerae) and the Pseudomonas sequencing project (P. aeruginosa).
References
- Aravind L, Koonin EV. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci. 1998;23:469–472. doi: 10.1016/s0968-0004(98)01293-6. [DOI] [PubMed] [Google Scholar]
- Aravind L, Ponting CP. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol Lett. 1999;176:111–116. doi: 10.1111/j.1574-6968.1999.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Galperin MY, Noll KM, Romano AH. The glucose transport system of the hyperthermophilic anaerobic bacterium Thermotoga neapolitana. Appl Environ Microbiol. 1996;62:2915–2918. doi: 10.1128/aem.62.8.2915-2918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin MY, Noll KM, Romano AH. Coregulation of beta-galactoside uptake and hydrolysis by the hyperthermophilic bacterium Thermotoga neapolitana. Appl Environ Microbiol. 1997;63:969–972. doi: 10.1128/aem.63.3.969-972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon DW, Ordal GW. Cloning and characterization of genes encoding methyl-accepting chemotaxis proteins in Bacillus subtilis. J Biol Chem. 1994;269:14038–14046. [PubMed] [Google Scholar]
- Hecht GB, Newton A. Identification of a novel response regulator required for the swarmer- to-stalked-cell transition in Caulobacter crescentus. J Bacteriol. 1995;177:6223–6229. doi: 10.1128/jb.177.21.6223-6229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV, Tatusov RL, Galperin MY. Beyond the complete genomes: from sequences to structure and function. Curr Opin Struct Biol. 1998;8:355–363. doi: 10.1016/s0959-440x(98)80070-5. [DOI] [PubMed] [Google Scholar]
- Kreis-Kleinschmidt V, Fahrenholz F, Kojro E, Kroger A. Periplasmic sulphide dehydrogenase (Sud) from Wolinella succinogenes: isolation, nucleotide sequence of the sud gene and its expression in Escherichia coli. Eur J Biochem. 1995;227:137–142. doi: 10.1111/j.1432-1033.1995.tb20369.x. [DOI] [PubMed] [Google Scholar]
- Nealson KH, Saffarini D. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu Rev Microbiol. 1994;48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- Nelson KE, Clayton RA, Gill SR, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Nelson WC, Ketchum KA, McDonald L, Utterback TR, Malek JA, Linher KD, Garrett MM, Stewart AM, Cotton MD, Pratt MS, Phillips CA, Richardson D, Heidelberg J, Sutton GG, Fleischmann RD, Eisen JA, Fraser CM. Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of Thermotoga maritima. Nature. 1999;399:323–329. doi: 10.1038/20601. [DOI] [PubMed] [Google Scholar]
- Pao GM, Saier MH., Jr Response regulators of bacterial signal transduction systems: selective domain shuffling during evolution. J Mol Evol. 1995;40:136–154. doi: 10.1007/BF00167109. [DOI] [PubMed] [Google Scholar]
- Ponting CP, Schultz J, Milpetz F, Bork P. SMART: identification and annotation of domains from signalling and extracellular protein sequences. Nucleic Acids Res. 1999;27:229–232. doi: 10.1093/nar/27.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P, Mayer R, Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol Rev. 1991;55:35–58. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost B, Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993;232:584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- Takami H, Masui N, Nakasone K, Horikoshi K. Replication origin region of the chromosome of alkaliphilic Bacillus halodurans C-125. Biosci Biotechnol Biochem. 1999;63:1134–1137. doi: 10.1271/bbb.63.1134. [DOI] [PubMed] [Google Scholar]
- Tal R, Wong HC, Calhoon R, Gelfand D, Fear AL, Volman G, Mayer R, Ross P, Amikam D, Weinhouse H, Cohen A, Sapir S, Ohana P, Benziman M. Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes. J Bacteriol. 1998;180:4416–4425. doi: 10.1128/jb.180.17.4416-4425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam R, Saier MH., Jr Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas M, Kashefi K, Blunt-Harris EL, Lovley DR. Microbiological evidence for Fe(III) reduction on early Earth. Nature. 1998;395:65–67. doi: 10.1038/25720. [DOI] [PubMed] [Google Scholar]
- Vargas M, Noll KM. Catabolite repression in the hyperthermophilic bacterium Thermotoga neapolitana is independent of cAMP. Microbiology. 1996;142:139–144. doi: 10.1099/13500872-142-1-139. [DOI] [PubMed] [Google Scholar]
- Volz K, Matsumura P. Crystal structure of Escherichia coli CheY refined at 1.7-A resolution. J Biol Chem. 1991;266:15511–15519. doi: 10.2210/pdb3chy/pdb. [DOI] [PubMed] [Google Scholar]
- Vyas NK, Vyas MN, Quiocho FA. Sugar and signal-transducer binding sites of the Escherichia coli galactose chemoreceptor protein. Science. 1988;242:1290–1295. doi: 10.1126/science.3057628. [DOI] [PubMed] [Google Scholar]