Abstract
Objective
Ischaemic priapism is a rare condition characterised by little or no cavernosal blood flow, pain and rigidity of the penis. Immediate intervention is required to restore blood flow, prevent necrosis and erectile dysfunction. This review was conducted to determine the best course of treatment and identify areas in current guidelines to which improvements could be made.
Material and methods
PubMed, Ovid, MEDLINE (1946–December 2016) and the Cochrane Library were searched as sources for literature. Key studies in each of the areas of management were identified and analysed.
Results
A total of 45 articles were reviewed. The first step in treatment should be aspiration of corporeal blood. Further studies are needed to make firm recommendations as to whether irrigation should follow, as currently literature is inconclusive. If this fails to cause detumescence, sympathomimetics should be injected. The sympathomimetic of choice is phenylephrine as it is effective, specific and causes minimal cardiovascular side effects. It should be injected at a concentration of 100–500 μg/mL, with 1 mL being injected every 3–5 minutes for up to an hour (maximum 1mg in an hour). Surgical shunting is the next step, except in the cases of delayed priapism (48–72 hours duration) where immediate penile prosthesis insertion may be considered more appropriate. Distal shunts should be performed first, followed by proximal ones to minimise damage leading to erectile dysfunction. There exists little evidence recommending one shunting procedure over another. The final intervention is insertion of a penile prosthesis. Literature suggests that an inflatable prosthesis inserted immediately will yield the greatest patient satisfaction.
Conclusion
A review of the literature has highlighted areas in which further research needs to be done to make conclusive recommendations, including whether irrigation should accompany aspiration and efficacy of shunting procedures. Further studies are required to ensure that patients receive the treatment most likely to cause detumescence and maintain erectile function.
Keywords: Ischaemic, penis, priapism
Introduction
Priapism is a rare condition defined as an erection that is unrelated to, or persists after, sexual stimulation, continuing longer than 4 hours. [1,2] There exist 3 types of priapism: ischaemic (low-flow), non-ischaemic (high-flow) and stuttering (recurrent) priapism. The aetiology and classification of priapism determines how it should be treated.[3]
Ischaemic priapism is characterised by little or no cavernosal blood flow, pain and rigidity, with the features of compartment syndrome. It is a medical emergency and treatment should commence within 4 hours to preserve erectile function.[4] Stuttering priapism is a reoccurring form of ischaemic priapism. Non-ischaemic priapism is characterized by unregulated cavernosal blood flow, the absence of pain and, often, only a partially erect penis.[1] It is not an emergency, not requiring immediate treatment and may resolve spontaneously.
Ischaemic priapism, accounting for 95% of cases, will be the focus of this review.[2] Management of priapism has three main aims: to restore normal cavernosal blood flow, preserve erectile function and avoid fibrosis of cavernosal tissues.[5] Success of treatment is usually determined by how quickly detumescence is achieved to preserve future erectile function.
The American Urology Association (AUA) and the European Association of Urology (EAU) have published guidelines on the best course of treatment of ischaemic priapism.[1,6] Both recommend a stepwise escalation of treatment beginning with aspiration (with or without irrigation), then injection of sympathomimetics, followed by surgical shunting and finally insertion of penile prostheses. The recommendations differ and so the literature has been reviewed in conjunction with these guidelines to assess the best course of treatment.
Material and methods
The electronic databases PubMed, Ovid MEDLINE (1946–December 2016) and the Cochrane Library were searched as sources for literature. The search was limited to the English language. Suitable abstracts and full papers were reviewed to identify appropriate studies for inclusion in this narrative review. Keywords searched for included “priapism”, “ischaemic” and “treatment”.
Results and discussion
Incidence
Ischaemic priapism has an incidence between 0.34–1.5 per 100,00 person years.[7,8] However, incidence is markedly elevated in certain subgroups, particularly those with haematological abnormalities. Notably, those with sickle cell anaemia have an incidence rate of 3.6%, in those younger than 18 years old, and 42% in those older than 18 years old.[6,9]
Aetiology
There are various identifiable causes of priapism, however the majority of cases are idiopathic. Leading known causes are alcohol and drug abuse (21%), perineal trauma (12%) and Sickle Cell Disease (11%).[10] Common causes of priapism are summarised in Table 1.[1,10–15]
Table 1.
Common known causes of priapism
| Perineal Trauma[10] |
| Neurogenic Disorders (E.g. cauda equina, spinal cord injury)[11,12] |
| Haematological Disorders (E.g. Sickle Cell Disease, thalassaemia)[10] |
| Tumours[13] |
| Medications (E.g. vasoactive erectile agents, α-receptor antagonists, anticoagulants)[11,14] |
| Recreational Drugs (E.g. alcohol, marijuana, cocaine)[11] |
| Toxin Mediated Infections (E.g. Scorpion sting, malaria)[11] |
| Penile Tattooing[15] |
| Acute Infections[1] |
| Metabolic disorders (E.g. amyloidosis, diabetes)[11] |
| Shows some of the causes of priapism identified in the literature. |
Diagnosis
Diagnosis should be made as soon as possible after presentation; positive outcomes and future erectile function are closely related to the duration of tumescence. Often a diagnosis of priapism is self-evident and can be deduced from a brief history and examination. However, it is essential to discern between ischaemic and non-ischaemic priapism, as they require considerably different treatment strategies.[1,6]
A comprehensive history should include the duration of the erection, the circumstances under which it arose, whether there is any associated pain and if the patient is taking any medication. Any history of priapism should be identified.[16]
A physical examination of the penis should be performed to ascertain whether it is fully erect (as in ischaemic priapism) or partially erect (as in non-ischaemic priapism). Penile blood gases are vital for discriminating between the two types, with ischaemic priapism giving rise to acidotic, hypoxic, hypercarbic cavernous blood gases (pH≤ 7.25, pO2 < 30 mmHg, pCO2>60 mmHg).[1,11] These blood gases arise due to a build up of deoxygenated blood in the corpora cavernosa. This build up occurs as persistent relaxation of corporeal smooth muscle causes compression of the subtunical veins, which prevents outflow of deoxygenated blood via sinusoids. Consequently, intracorporeal pressure is increased to above the mean arterial pressure, compressing the cavernosal arteries and preventing arterial blood flow.[17] Perineal examination should also be performed to exclude any evidence of perineal trauma leading to non-ischaemic priapism.
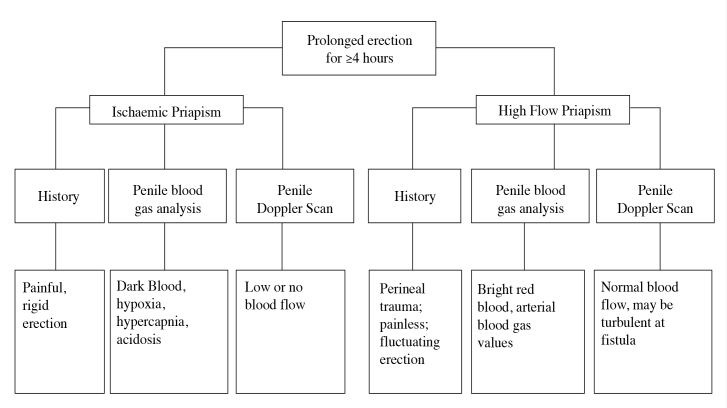
EAU guidelines suggest a penile doppler ultrasound to observe if penile blood flow is normal, and increased at the site of the fistula, as in non-ischaemic priapism, or decreased as in ischaemic priapism.[6]
Both the AUA (Table 2) and EAU (Figure 1) have outlined the diagnostic differences between ischaemic and non-ischaemic priapism.[1]
Table 2.
Diagnostic differences in ischaemic and non ischaemic priapism
| Ischaemic priapism | Non-ischaemic priapism | |
|---|---|---|
| Corpora cavernosa fully rigid | Usually present | Seldom present |
| Penile pain | Usually present | Seldom present |
| Abnormal cavernous blood gases | Usually present | Seldom present |
| Blood abnormalities and haematological malignancy | Sometimes present | Seldom present |
| Recent intracavernous vasoactive drug injections | Sometimes present | Seldom present |
| Chronic, well-tolerated tumescence without full rigidity | Seldom present | Usually present |
| Perineal trauma | Seldom present | Sometimes present |
Shows diagnostic differences in signs and symptoms between ischaemic and non-ischaemic priapism as outlined by the AUA[1]
Figure 1.
Differential diagnosis of priapism as detailed by the EAU. The characteristic diagnostic differences between ischaemic and high-flow priapism are detailed in three diagnostic categories: history, penile blood gas analysis and penile Doppler scan[6]
Conservative treatments
In the past, it has been recommended that conservative, non-medical treatments be the first line in management of priapism. However, due to a lack of evidence supporting their efficacy and the importance of resolving priapism promptly, these conservative treatments should be omitted. Conservative treatments that have been shown to be ineffective include applying heat packs or warm water, aerobic exercise and ejaculation.[6,9] It should also be noted that former treatments of applying cold water, ice packs and cold-water enemas can exacerbate priapism.[9] This may be counterintuitive to the lay population and should be made known to those at high risk, such sickle cell patients.
Aspiration
It is widely agreed that the first line of therapy should be aspiration, with or without irrigation.[1,6,11,18,19] The aim of aspiration is to relieve pressure in the corpora cavernosa in order to promote normal intracavernosal blood flow. Aspiration is contraindicated in patients with uncontrolled bleeding disorders or overlying cellulitis.
Local anaesthetic (e.g. 1% lidocaine) should be administered prior to aspiration. It is then suggested that a 23–30G needle or 16–18G wide-bore butterfly[6] is inserted perpendicularly into the base of the corpora cavernosa. Aspiration should then be performed using a 10 mL syringe attached to the 3-way stopcock until bright red arterial blood is obtained.[20] Following aspiration, the puncture site should be compressed to prevent haematoma formation.
Once aspirated, the EAU recommends that the corpora cavernosa be irrigated with 0.9% saline as the next step in the escalation of treatment.[6,11] However, the AUA makes no such recommendation, as the panel concluded that there is no real difference in resolution rates with or without irrigation.[1] There is an absence of studies comparing resolution rates with or without irrigation. Consequently, due to the lack evidence, it is difficult to conclude whether or not irrigation is beneficial.
Overall, aspiration has been found to yield a 36% resolution rate.[1] Hence, it is likely that treatment will need to be escalated to injection of sympathomimetics.
Sympathomimetics
Phenylephrine is the sympathomimetic of choice for treating priapism.[1,6,11,17] As an α1-adrenergic receptor agonist, phenylephrine causes α-mediated vasoconstriction within the corpora cavernosa. Due to its specificity, phenylephrine has minimal cardiac side effects, which may include tachycardia, palpations and hypertension. This makes it the preferential choice over less specific sympathomimetics of comparable efficacy, such as adrenaline, which activates both α and β-receptors. Other effective sympathomimetics include etilefrine, noradrenaline and metaraminol.[6,20,21]
Injection of phenylephrine in conjunction with aspiration (with or without irrigation) has been found to be effective in 81% of patients, whereas injection of phenylephrine alone has demonstrated 58% efficacy.[1] These results support use of sympathomimetic following aspiration as suggested by the EAU and AUA guidelines.
Sympathomimetics should be administered by inserting a 19G needle into the lateral aspect of one corpora cavernosa and injecting 1 ml phenylephrine, that has been diluted in normal saline at a ratio of 100–500 μg/mL.[6,17] Injections can be repeated every 3–5 minutes until detumescence occurs, with a maximum of 1mg administered within one hour.[6] For children and patients with high cardiovascular risk more conservative doses are recommended. Patients should be observed for side effects such as tachycardia and palpitations. Electrocardiogram and blood pressure monitoring are recommended in high cardiovascular risk patients.[1] Research has found that if sympathomimetics were to resolve priapism they would do so within an hour.[21] After this time sympathomimetics are deemed to be unsuccessful and treatment should be further escalated.[1,6] Failure of sympathomimetics may also be due to smooth muscle paralysis caused by compartment metabolic syndrome (such as hypoxia, glucopenia and acidosis).
An in vitro study has shown that increasing the dose of phenylephrine is unlikely to increase resolution rates. It demonstrated that a failure to respond to both recommended and high doses of phenylephrine usually suggests that apoptosis of smooth muscles cells has occurred. Hence, it is not advisable to increase the dose of phenylephrine as it will not increase resolution rates and will increase the likelihood of cardiovascular side effects.[22]
A recent study investigated whether a transient shunting technique would be appropriate to replace aspiration and injection of sympathomimetics.[18] Blood was shunted from the corpora cavernosa into the glans penis using 2 needles and external tubing. This procedure proved inferior to current practices achieving detumescence in 67% patients, compared to 81%[1] in patients with aspiration and sympathomimetics. However, it may provide an alternative treatment option for patients with high cardiovascular risk, for whom sympathomimetics are likely to cause complications.
A suggested sympathomimetic therapy for stuttering priapism is self-injection of metaraminol, an α1-receptor agonist, which also has low β-agonist effects.[23] As metaraminol is low acting and highly specific it is considered safe for self-injection. For patients with stuttering priapism, who are able to inject themselves, this course of treatment could prevent trips of hospital during episodes and reduce the duration of ischemia, thereby minimising cavernosal damage.
Surgical shunting
If sympathomimetics fail, treatment should be escalated to surgical shunting. There are 4 categories of shunt that can be performed: percutaneous distal (corpora-glandular), open distal (corpora-glandular), open proximal (corporospongiosal) and venous shunts.
There is a lack evidence recommending one shunting procedure over another. Both the AUA and the EUA recommend a stepwise escalation of shunting procedures, beginning with less invasive procedures with lower rates of complications and then moving onto more invasive techniques, beginning with distal and then escalating to proximal shunting if necessary.[1]
It is recommended to perform distal shunting first. Although proximal shunts have higher resolution rates than distal shunts, they also have higher rates of resulting erectile dysfunction, hence more conservative distal shunts are recommended first.[1] It must be noted, that the increased rates of erectile dysfunction associated with proximal shunting may be linked to the duration of priapism or the selection of patients, and not the procedure itself.
Types of distal shunts include Winter’s shunt[24], Ebbehoj’s procedure[25], T shunt[26], and Al-Ghorab’s shunt (and its modified form, the snake manouveur) (Table 3).[24,26–30]
Table 3.
Summary of distal shunting procedures and how they are performed
| Winter’s Shunt | A Travernol needle is used to form a fistula between the glans penis and corpora cavernosa[24] |
| Ebbehoj’s Procedure | A scalpel is laterally inserted into the glans penis piercing one or both of the corpora cavernosa[28] |
| T-shunt | A 10 blade is inserted into the glans penis lateral to the external urethral meatus and rotated 90 degrees. Deoxygenated blood is then “milked” out[26,29] |
| Al-Ghorab’s shunt | A 2 cm incision is made on the dorsum of the penis 1 cm distal to the coronal sulcus and a circular core of tunica albuginea approximately 5mm diameter is removed.[27,30] The Burnett snake maneuverer modification involves cannulating a 7/8 Heger through the removed tunica albuginea to the proximal limit of the corpora cavernosa. The dilator is then removed and the penis is compressed to remove stagnant blood. |
A brief overview of the procedural differences in the different types of distal shunts
In comparing studies it can be observed that Winter’s shunt has a notably lower resolution rate that other types of distal shunt, with a 66% resolution rate as compared to 74% in Al-Ghorab’s and 73% in Ebbehoj’s shunts.[1,31] Consequently, Winter’s shunt should not recommended over other distal shunt despite being reasonably simple and non-invasive. In distal shunting, surgeons should use their discretion and experience to choose from a T-shunt, Al-Ghorab’s and Ebbehoj’s shunts, due to a lack of conclusive studies.
It may also be noted that adaptations of both the T-shunt and Al-Ghorab’s procedure have been developed. “Tunnelling” in the T-shunt and “the snake manoeuver” in Al-Ghorab’s both use dilators to physically remove deoxygenated blood from the corpora cavernosa, showing resolution rates of 100% in both techniques.[5,27,29] As with all shunting procedures, the duration of priapism affects resolution rates; the sooner a shunting procedure is attempted the greater the efficacy. Hence, it is hard to compare resolution rates of these adapted shunts with other types due to variation in duration of priapism, as well as limited sample size.[5]
Should distal shunting fail to resolve priapism, the AUA recommends that treatment should be escalated to a proximal shunt, where blood is drained from the corpora cavernosa into the corpus spongiosum, such as a Quackel’s or Sacher shunt.[1] Again, there exists a shortage of evidence demonstrating the efficacy of these shunts. Recommendations from the AUA were made based on panel consensus and do not favour one shunt procedure over another.
If the proximal shunting fails it is recommended by the AUA that a venous shunt be performed, such as a Grayhack or Barry shunt.[1] In this type of shunting blood is drained for the corpora into the saphenous (Grayhack’s)[32] or superficial/deep dorsal veins (Barry’s).[33] These treatments are a last resort before the insertion of penile prostheses.
In the limited studies comparing the outcomes of shunting procedures there exists little conclusive evidence. Furthermore, whilst many studies demonstrate the efficacy of certain techniques,[26,34] few directly compare procedures and comparative studies that do exist yield inconclusive results. For example, a study comparing shunting procedures found Grayhack’s had the best outcomes, however due to patient heterogeneity it was not possible to reach firm conclusions.[32] Larger studies of comparable patients are required to make conclusive recommendations on the most effective shunting procedure for a given duration of priapism.
Penile prostheses
The EAU recommends the prompt insertion of penile prostheses in episodes of priapism >36 hours, as these usually result in permanent erectile dysfunction and penile deformity, and shunting surgery is of no benefit.[6,35] Some research has suggested that even though priapism may be resolved by more conservative treatments, after 24 hours of duration histological changes, like interstitial oedema, have begun to occur and there has been a loss of erectile function.[36] After 48 hours, smooth muscle necrosis begins and it has been suggested by Zacharakis et al.[37] that the best course of action after this time is to insert a penile prosthesis to maintain erectile function.
The success of penile prostheses is usually determined by patient satisfaction rates, which are generally high when complications are avoided. Major complications are found in 4% of cases and may include infection, mechanical failure and erosion.[38]
Penile prostheses can be either inflatable and malleable. Many makes and models of both types exist, with their own success rates.[39] Inflatable prostheses have two cylinders that are inserted into the body of the penis and a reservoir inserted into the scrotal sac. Erections are achieved by manual compression of the reservoir and fluid is transferred into the cylinders inflating them. Malleable prostheses are inserted in similar manner, with 2 semi rigid cylinders inserted into the body of the penis, but differ in that an individual is able to manipulate the penis into an erect state; there is no fluid reservoir. The penis is always erect, but can be orientated differently.[40]
A comparison of malleable and inflatable prostheses has shown that malleable prostheses are surgically easier, cheaper and less likely to fail mechanically.[41] Malleable prostheses tend to function more effectively as in some cases, due to fibrosis of tissues, inflatable prostheses are not able to overcome corporeal rigidity.[42] However, satisfaction rates have been found to be higher in inflatable prostheses than in malleable ones, most likely because inflatable prostheses give the patient the option of choosing when the penis is erect and are more aesthetically pleasing.[40] If feasible, inflatable prostheses should be recommended over malleable ones as new technology, and more recent prostheses, can aid in over coming corporeal fibrosis and patient satisfaction should be a main consideration when administering treatment.
Whilst in cases of ischaemic priapism, inflatable prostheses have been suggested to be the best choice, it has been shown that insertion of a malleable prosthesis could a cost effective measure to treat stuttering priapism.[43] Malleable prostheses have been found to resolve refractory priapism and, in long-term cases, be cost effective, resource effective and reduce hospital admissions. However, as the study conducted focused on cost-effectiveness, it is difficult to tell whether this strategy would be the best course of action for the patient in terms of satisfaction and penile function. Furthermore, it did not comment on whether inflatable prostheses would also be suitable and cost effect. As these have been shown to yield higher patient satisfaction rates than malleable ones, further studies investigating the cost effectiveness of inflatable prostheses as a treatment for stuttering priapism may be advisable.
Early prosthesis insertion when other strategies of management have failed, has been shown to yield higher rates of success and patient satisfaction than delayed insertion, with minimal complications.[44] It also resolves the issue of priapism itself. Early insertion has been found to be procedurally easier than delayed insertion as delayed insertion allows further fibrosis to take place.[2,35] Further advantages include reduced major scarring, lower risks of complications (e.g. infection or urethral injury) and less penile shortening. Prompt insertion has been found to yield a 96% satisfaction rate and capacity to have sexual intercourse. Conversely, delayed insertion leads to 60% satisfaction, primarily due to penile shortening and the inability of some patients to have penetrative sexual intercourse.[2]
A novel technique suggested in a 2015 study was the early insertion of a malleable prosthesis for a 3 month period and then exchanging the prosthesis for an inflatable one.[37] By inserting a malleable prosthesis immediately penile length was maintained and procedural difficulties that accompany insertion of an inflatable prosthesis were avoided. Changing the prosthesis at 3 months allowed upsizing of the cylinders and consequently an increase in penile length. The long-term maintenance, and in some cases, increase of penile length, as well as the other functional and cosmetic benefits of inflatable prosthesis, lead to increased patient satisfaction rates. This strategy could offer a new way of maintaining penile length with a technically simpler initial surgery, which make the subsequent insertion of an inflatable prosthesis easier. A potential limitation to this treatment strategy is that some patients may be adverse to a second surgery and would prefer to continue with a malleable prosthesis.
Conclusion
As a medical emergency, ischaemic priapism requires efficient diagnosis and immediate treatment. Future erectile function is dependent on quick resolution and once a diagnosis is made treatment should begin in a stepwise manner.
The first step should be aspiration, with or without irrigation. Further research needs to be done to establish the benefits of irrigation. If aspiration fails, injection of phenylephrine is recommended, followed by surgical shunting. It is recommended to begin with distal shunting and then escalate to proximal shunting, however evidence for the benefits of this strategy is limited. Comparative studies on resolution rates of each type of shunt are necessary to make conclusive recommendations.
Finally, a penile prosthesis should be inserted. Literature indicates that inflatable prostheses inserted immediate yield maximum patient satisfaction, despite increased risk of complications compared to malleable prostheses. However, complications can be minimised by new technologies and increased surgical skill.
Guidelines from the AUA and EAU mean current management of priapism is based partly on consensus of expert panels rather than clinical evidence. Further research into the areas highlighted would be beneficial to give support to these methods of management, and could improve patient outcomes.
Footnotes
Peer-review: This manuscript was prepared by the invitation of the Editorial Board and its scientific evaluation was carried out by the Editorial Board.
Author Contributions: Concept – K.A.; Design – K.A.; Supervision – K.A., P.D., N.R.; Data Collection and/or Processing – J.R.; Analysis and/or Interpretation – J.R., N.R.; Literature Search – J.R.; Writing Manuscript – J.R.; Critical Review – M.I.S., M.S.K., N.R., P.D., K.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Montague D, Jarow J, Broderick D, Dmochowski R, Heaton J, Lue T, et al. American Urology Association: Guidelines on Management of Priapism. J Urol. 2003;170:1318–24. doi: 10.1097/01.ju.0000087608.07371.ca. https://doi.org/10.1097/01.ju.0000087608.07371.ca. [DOI] [PubMed] [Google Scholar]
- 2.Zacharakis E, Garaffa G, Raheem AA, Christopher AN, Muneer A, Ralph DJ. Penile prosthesis insertion in patients with refractory ischaemic priapism: early vs delayed implantation. BJU Int. 2014;114:576–81. doi: 10.1111/bju.12686. https://doi.org/10.1111/bju.12686. [DOI] [PubMed] [Google Scholar]
- 3.Hisasue S, Kobayashi K, Kato R, Hashimoto K, Yamashita S, Takahashi S, et al. Clinical course linkage among different priapism subtypes: Dilemma in the management strategies. Int J Urol. 2008;15:1006–10. doi: 10.1111/j.1442-2042.2008.02153.x. https://doi.org/10.1111/j.1442-2042.2008.02153.x. [DOI] [PubMed] [Google Scholar]
- 4.Berger R, Billups K, Brock G, Broderick GA, Dhabuwala CB, Goldstein I, et al. Report of the American Foundation for Urologic Disease (AFUD) Thought Leader Panel for evaluation and treatment of priapism. Int J Impotence Res. 2001;13(Suppl 5):S39–43. doi: 10.1038/sj.ijir.3900777. [DOI] [PubMed] [Google Scholar]
- 5.Zacharakis E, Raheem AA, Freeman A, Skolarikos A, Garaffa G, Christopher AN, et al. The efficacy of the T-shunt procedure and intracavernous tunneling (snake maneuver) for refractory ischemic priapism. J Urol. 2014;191:164–8. doi: 10.1016/j.juro.2013.07.034. https://doi.org/10.1016/j.juro.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Salonia A, Eardley I, Giuliano F, Hatzichristou D, Moncada I, Vardi Y, et al. European Association of Urology Guidelines on Priapism. 2015. [Accessed 12/12, 2016]. Available at: http://uroweb.org/wp-content/uploads/15-_Priapism_LR.pdf. [DOI] [PubMed]
- 7.Kulmala RV, Lehtonen TA, Tammela TL. Priapism, its incidence and seasonal distribution in Finland. Scand J Urol Nephrol. 1995;29:93–6. doi: 10.3109/00365599509180545. https://doi.org/10.3109/00365599509180545. [DOI] [PubMed] [Google Scholar]
- 8.Eland IA, van der Lei J, Stricker BH, Sturkenboom MJ. Incidence of priapism in the general population. Urology. 2001;57:970–2. doi: 10.1016/s0090-4295(01)00941-4. https://doi.org/10.1016/S0090-4295(01)00941-4. [DOI] [PubMed] [Google Scholar]
- 9.Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R. Priapism: pathogenesis, epidemiology, and management. J Sex Med. 2010;7:476–500. doi: 10.1111/j.1743-6109.2009.01625.x. https://doi.org/10.1111/j.1743-6109.2009.01625.x. [DOI] [PubMed] [Google Scholar]
- 10.Pohl J, Pott B, Kleinhans G. Priapism: a three-phase concept of management according to aetiology and prognosis. Br J Urol. 1986;58:113–8. doi: 10.1111/j.1464-410x.1986.tb09008.x. https://doi.org/10.1111/j.1464-410X.1986.tb09008.x. [DOI] [PubMed] [Google Scholar]
- 11.Cherian J, Rao AR, Thwaini A, Kapasi F, Shergill IS, Samman R. Medical and surgical management of priapism. Postgrad Med J. 2006;82:89–94. doi: 10.1136/pgmj.2005.037291. https://doi.org/10.1136/pgmj.2005.037291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravindran M. Cauda equina compression presenting as spontaneous priapism. J Neurol Neurosurg Psychiatry. 1979;42:280–2. doi: 10.1136/jnnp.42.3.280. https://doi.org/10.1136/jnnp.42.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitley CA, Mosier AD, Keylock J, Nguyen D. Malignant priapism secondary to adenocarcinoma of the prostate. BMJ Case Rep. 2010 doi: 10.1136/bcr.07.2009.2135. pii: bcr0720092135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ylitalo P, Pasternack A. Priapism--side-effect of prazosin in patients with renal failure. Acta Med Scand. 1983;213:319–20. doi: 10.1111/j.0954-6820.1983.tb03743.x. https://doi.org/10.1111/j.0954-6820.1983.tb03743.x. [DOI] [PubMed] [Google Scholar]
- 15.Zargooshi J, Rahmanian E, Motaee H, Kohzadi M. Nonischemic priapism following penile tattooing. J Sex Med. 2012;9:844–8. doi: 10.1111/j.1743-6109.2011.02579.x. https://doi.org/10.1111/j.1743-6109.2011.02579.x. [DOI] [PubMed] [Google Scholar]
- 16.Summers A. Priapism: diagnosis and early referral in emergency departments. Emerg Nurse. 2007;14:26–9. doi: 10.7748/en2007.02.14.9.5.c4223. https://doi.org/10.7748/en2007.02.14.9.5.c4223. [DOI] [PubMed] [Google Scholar]
- 17.Bassett J, Rajfer J. Diagnostic and Therapeutic options for Ischaemic and Non-Ischeamic Pripaism. Rev Urol. 2010;12:56–63. [PMC free article] [PubMed] [Google Scholar]
- 18.Canguven O, Cetinel C, Horuz R, Tarhan F, Hamarat B, Goktas C. Transient distal penile corporoglanular shunt as an adjunct to aspiration and irrigation procedures in the treatment of early ischemic priapism. Korean J Urol. 2013;54:394–8. doi: 10.4111/kju.2013.54.6.394. https://doi.org/10.4111/kju.2013.54.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R. Priapism: pathogenesis, epidemiology, and management. J Sex Med. 2010;7:476–500. doi: 10.1111/j.1743-6109.2009.01625.x. https://doi.org/10.1111/j.1743-6109.2009.01625.x. [DOI] [PubMed] [Google Scholar]
- 20.Mantadakis E, Ewalt DH, Cavender JD, Rogers ZR, Buchanan GR. Outpatient penile aspiration and epinephrine irrigation for young patients with sickle cell anemia and prolonged priapism. Blood. 2000;95:78–82. [PubMed] [Google Scholar]
- 21.Roberts JR, Price C, Mazzeo T. Intracavernous epinephrine: a minimally invasive treatment for priapism in the emergency department. J Emerg Med. 2009;36:285–9. doi: 10.1016/j.jemermed.2007.10.051. https://doi.org/10.1016/j.jemermed.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 22.Muneer A, Minhas S, Freeman A, Kumar P, Ralph DJ. Investigating the effects of high-dose phenylephrine in the management of prolonged ischaemic priapism. J Sex Med. 2008;5:2152–9. doi: 10.1111/j.1743-6109.2008.00862.x. https://doi.org/10.1111/j.1743-6109.2008.00862.x. [DOI] [PubMed] [Google Scholar]
- 23.McDonald M, Santucci RA. Successful management of stuttering priapism using home self-injections of the alpha-agonist metaraminol. International Braz J Urol. 2004;30:121–2. doi: 10.1590/s1677-55382004000200007. https://doi.org/10.1590/S1677-55382004000200007. [DOI] [PubMed] [Google Scholar]
- 24.Winter CC. Cure of idiopathic priapism: new procedure for creating fistula between glans penis and corpora cavernosa. Urology. 1976;8:389–91. doi: 10.1016/0090-4295(76)90498-2. https://doi.org/10.1016/0090-4295(76)90498-2. [DOI] [PubMed] [Google Scholar]
- 25.Lund K, Ebbehoj J. Results of glando-cavernous anastomosis in 18 cases of priapism. Scand J Plast Reconstr Surg. 1980;14:269–72. doi: 10.3109/02844318009106720. https://doi.org/10.3109/02844318009106720. [DOI] [PubMed] [Google Scholar]
- 26.Brant WO, Garcia MM, Bella AJ, Chi T, Lue TF. T-shaped shunt and intracavernous tunneling for prolonged ischemic priapism. J Urol. 2009;181:1699–705. doi: 10.1016/j.juro.2008.12.021. https://doi.org/10.1016/j.juro.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Burnett AL, Pierorazio PM. Corporal “snake” maneuver: corporoglanular shunt surgical modification for ischemic priapism. J Sex Med. 2009;6:1171–6. doi: 10.1111/j.1743-6109.2008.01176.x. https://doi.org/10.1111/j.1743-6109.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- 28.Ebbehoj J. A new operation for priapism. Scand J Plast Reconstr Surg. 1974;8:241–2. doi: 10.3109/02844317409084400. https://doi.org/10.3109/02844317409084400. [DOI] [PubMed] [Google Scholar]
- 29.Garcia MM, Shindel AW, Lue TF. T-shunt with or without tunnelling for prolonged ischaemic priapism. BJU Int. 2008;102:1754–64. doi: 10.1111/j.1464-410X.2008.08174.x. https://doi.org/10.1111/j.1464-410X.2008.08174.x. [DOI] [PubMed] [Google Scholar]
- 30.Segal RL, Readal N, Pierorazio PM, Burnett AL, Bivalacqua TJ. Corporal Burnett “Snake” surgical maneuver for the treatment of ischemic priapism: long-term followup. J Urol. 2013;189:1025–9. doi: 10.1016/j.juro.2012.08.245. https://doi.org/10.1016/j.juro.2012.08.245. [DOI] [PubMed] [Google Scholar]
- 31.Nixon RG, O’Connor JL, Milam DF. Efficacy of shunt surgery for refractory low flow priapism: a report on the incidence of failed detumescence and erectile dysfunction. J Urol. 2003;170:883–6. doi: 10.1097/01.ju.0000081291.37860.a5. https://doi.org/10.1097/01.ju.0000081291.37860.a5. [DOI] [PubMed] [Google Scholar]
- 32.Tabibi A, Abdi H, Mahmoudnejad N. Erectile function and dysfunction following low flow priapism: a comparison of distal and proximal shunts. Urol J. 2010;7:174–7. [PubMed] [Google Scholar]
- 33.Barry JM. Priapism: treatment with corpus cavernosum to dorsal vein of penis shunts. J Urol. 1976;116:754–6. doi: 10.1016/s0022-5347(17)58998-3. [DOI] [PubMed] [Google Scholar]
- 34.Dangle PP, Patel MB, Pandya LK, Firlit CF. A modified surgical approach to the Al-Ghorab shunt - an anatomical basis. BJU Int. 2012;109:1872–4. doi: 10.1111/j.1464-410X.2012.11253.x. https://doi.org/10.1111/j.1464-410X.2012.11253.x. [DOI] [PubMed] [Google Scholar]
- 35.Burnett AL. Surgical management of ischemic priapism. J Sex Med. 2012;9:114–20. doi: 10.1111/j.1743-6109.2011.02446.x. https://doi.org/10.1111/j.1743-6109.2011.02446.x. [DOI] [PubMed] [Google Scholar]
- 36.Broderick GA, Gordon D, Hypolite J, Levin RM. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259–62. doi: 10.1016/s0022-5347(17)34928-5. [DOI] [PubMed] [Google Scholar]
- 37.Zacharakis E, De Luca F, Raheem AA, Garaffa G, Christopher N, Muneer A, et al. Early insertion of a malleable penile prosthesis in ischaemic priapism allows later upsizing of the cylinders. Scand J Urol. 2015:1–4. doi: 10.3109/21681805.2015.1059359. [DOI] [PubMed] [Google Scholar]
- 38.Anafarta K, Safak M, Beduk Y, Baltaci S, Aydos K. Clinical experience with inflatable and malleable penile implants in 104 patients. Urol Int. 1996;56:100–4. doi: 10.1159/000282820. https://doi.org/10.1159/000282820. [DOI] [PubMed] [Google Scholar]
- 39.Bettocchi C, Palumbo F, Spilotros M, Palazzo S, Saracino GA, Martino P, et al. Penile prostheses. Ther Adv Urol. 2010;2:35–40. doi: 10.1177/1756287209359174. https://doi.org/10.1177/1756287209359174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kilicarslan H, Kaynak Y, Gokcen K, Coskun B, Kaygisiz O. Comparison of patient satisfaction rates for the malleable and two piece-inflatable penile prostheses. Turk J Urol. 2014;40:207–10. doi: 10.5152/tud.2014.37108. https://doi.org/10.5152/tud.2014.37108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le B, Burnett AL. Evolution of penile prosthetic devices. Korean J Urol. 2015;56:179–86. doi: 10.4111/kju.2015.56.3.179. https://doi.org/10.4111/kju.2015.56.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertram RA, Carson CC, 3rd, Webster GD. Implantation of penile prostheses in patients impotent after priapism. Urology. 1985;26:325–7. doi: 10.1016/0090-4295(85)90176-1. https://doi.org/10.1016/0090-4295(85)90176-1. [DOI] [PubMed] [Google Scholar]
- 43.Tausch TJ, Zhao LC, Morey AF, Siegel JA, Belsante MJ, Seideman CA, et al. Malleable penile prosthesis is a cost-effective treatment for refractory ischemic priapism. J Sex Med. 2015;12:824–6. doi: 10.1111/jsm.12803. https://doi.org/10.1111/jsm.12803. [DOI] [PubMed] [Google Scholar]
- 44.Ralph DJ, Garaffa G, Muneer A, Freeman A, Rees R, Christopher AN, et al. The immediate insertion of a penile prosthesis for acute ischaemic priapism. Eur Urol. 2009;56:1033–8. doi: 10.1016/j.eururo.2008.09.044. https://doi.org/10.1016/j.eururo.2008.09.044. [DOI] [PubMed] [Google Scholar]