Abstract
Objective
We investigated the prognostic value of preoperative neutrophil-to-lymphocyte ratio (NLR) on germ cell testicular tumors (GCT).
Material and methods
The data of 53 patients who underwent inguinal orchiectomy were analyzed retrospectively. NLR was calculated from the preoperative complete blood cell counts. Receiver operating characteristic (ROC) analysis was performed to find the threshold values for NLR. Correlations between cancer-specific survival (CSS) and progression-free survival (PFS) and NLR were evaluated.
Results
The mean follow-up time was 23.55±18.06 months. The mean level of NLR was 3.08±1.81. Optimal threshold values of NLR was calculated as 3.55 for PFS (area under curve, AUC: 0.55) and 3.0 for CSS (AUC: 0.66). For patients with a NLR of <3.55 and NLR of ≥3.55, mean times-to-progression were 55.71 months (95% CI, 51.27–60.14) and 51.95 months (95% CI, 38.02–65.87, p=0.152), respectively. As well as, for patients with a NLR of <3.0 and NLR of ≥3.0, mean times-to-cancer specific death were 54.72 months (95% CI, 49.05–60.38) and 49.43 months (95% CI, 37.64–61.22, p=0.119), respectively.
Conclusion
Preoperative NLR is not a useful tool to predict the prognosis of patients with GCT.
Keywords: Inflammation, neutrophil-lymphocyte ratio, prognosis, survival, testicular cancer
Introduction
Testicular cancer (TC) is a relatively rare malignancy with more than 52,000 new cases and almost 10 000 deaths estimated worldwide for the year 2008. Testicular cancer makes up approximately 1% of all male cancer cases globally. However, TC is the most common cancer form in men aged 15–44 years in many countries, and has attained high or very high scores on the Human Development Index.[1] The histological type varies, although there is a clear predominance (90–95%) of germ cell testicular tumors (GCT) which can be subdivided into seminomatous and non-seminomatous GCT.[2]
Well-established risk factors for the development of TC include history of cryptorchidism, familial history of TC, presence of contralateral TC or testicular intraepitelial neoplasia, Klinefelter’s syndrome and infertility.[2,3] Some of the other risk factors with low incidence rates are scrotal trauma, inguinal hernia, atopy, history of infectious diseases, mumps orchitis, testicular torsion, increased scrotal temperature, varicocele, and human immunodeficiency virus infection.[3]
It has been demonstrated that inflammation plays a critical role in many aspects of cancer, including tumor development, progression, clinical presentation, and prognosis.[4] Several markers of systemic inflammatuar response, such as C-reactive protein, neutrophil or platelet counts, as well as the neutrophil-to-lypmhocyte ratio (NLR), have been shown to be independent prognostic factors in various human cancers.[5] As hematological tests are routinely conducted in cancer patients, the NLR represents a simple, robust and convenient parameter of the inflammatory response.[6] In a meta-analysis it has been reported that elevated NLR is a poor predictor for survival in patients with urinary system cancers.[7]
To the best of our knowledge no prior study has evaluated the association between systemic inflammation markers and prognosis of TC. In this study we aimed to investigate the potential prognostic impact of NLR in patients with GCT.
Material and methods
Data of 53 patients who underwent inguinal orchiectomy between 2008 and 2014 were analyzed retrospectively. Informed consent was obtained from all study participants. The study has been conducted in accordance with the ethical principles of Declaration of Helsinki.
Clinicopathological data including age, preoperative alpha-fetoprotein (AFP), human chorionic gonadotrophin (HCG), lactate dehydrogenase (LDH) levels, and complete blood cell counts (CBC), tumor side, size and histopathology, tumor stage according to 2009 TNM classification for TC of the International Union Against Cancer.[8] Prognostic factors for occult metastatic disease (tumor size and invasion of rete testis for seminomas; vascular/lymphatic invasion of the primary tumor, proliferation rate and percentage of embryonal carcinoma for nonseminomatous cancer), adjuvant therapy and follow-up status were recorded.
Patients with testicular stromal tumors, infectious or inflammatory signs and conditions, hematological diseases or other malignancies, cardiovascular diseases, end-stage renal disease, cerebrovascular disease, diabetes mellitus, smokers, corticosteroid or β-agonist users, and patients with missing data including preoperative CBC, tumor markers and pathology reports were excluded from the study.
Complete blood cell count was measured the day day prior to the surgery and the NLR was defined as absolute neutrophil count divided by the absolute lymphocyte count.
Different physicians in the same department performed similar follow-up schedule and adjuvant therapy protocols. After inguinal orchiectomy, all patients were included in a follow-up programme for physical examination and tumor marker analysis 4 times a year at an urooncology outpatient clinic. Also, chest X-ray and abdominopelvic tomography were performed twice a year. Cancer-specific survival (CSS) was defined as the time (in months) interval between the dates of surgery and cancer-related death. Progression-free survival (PFS) was defined as the time (in months) elapsed from the date of surgery to the recurrence of biochemically or radiologically confirmed distant metastases.
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Sciences version 21.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at a p value of <0.05. Receiver operating characteristic (ROC) curve analysis was performed to find the cut-off levels for NLR as a predictor of CSS and PFS, respectively. Youden’s index method was used to find the optimal cut-off value for NLR. Patients who were alive and progression-free were censored at the last follow-up date. Patients were grouped according to the cut-off levels of NLR. Chi-square, Fisher’s exact and two sample t-tests were used for intergroup comparisons. Kaplan-Meier method was used to evaluate the correlation between NLR and the time-to-event for CSS and PFS, respectively. Log-rank test was used to assess the statistically significant intergroup difference in NLR with respect to CSS and PFS.
Results
Demographic characteristics of patients were summarized in Table 1. Mean age of study population (n=53) was 38.85±15.70 years (range 16–81years). Of the Twenty-seven (50.9%) patients had seminomas and 14 (26.4%) mixed GCTs. Positive nodes were detected in 24 patients (45.3%) and lung metastasis was detected in 6 patients (11.3%). Mean follow-up time was 23.55±18.06 months (range 2–66 months). During the follow-up period, 5 patients (9.4%) showed disease progression and 7 (13.2%) died because of TC. No patient died because of other reasons except TC.
Table 1.
Demographic characteristics of the patients
| Mean±SD (range) | |
|---|---|
| Age, years | 38.85±15.70 (16–81) |
| Tumor size, cm | 4.48±2.22 (1–10) |
| Preoperative lymphocyte count, 103/uL | 2.02±0.78 (0.60–5.30) |
| Preoperative neutrophil count, 103/uL | 5.67±3.35 (2.3–25) |
| Neutrophil-to-lymphocyte ratio | 3.08±1.81 (3–48) |
| Follow-up time, months | 23.55±18.06 (2–66) |
| Total number of the patients, n (%) | 53 (100) |
| Seminoma | 27 (50.9) |
| Mixed germ cell tumor | 14 (26.4) |
| Embryonal carcinoma | 4 (7.5) |
| Teratoma | 3 (5.7) |
| Spermatocytic seminoma | 2 (3.8) |
| Yolk sac tumor | 2 (3.8) |
| Choriocarcinoma | 1 (1.9) |
| Tumor stage | n (%) |
| 1 | 28 (52.8) |
| 2A | 11 (20.8) |
| 2B | 5 (9.4) |
| 2C | 6 (11.3) |
| 3 | 0 |
| 4 | 3 (5.7) |
| Rete testis invasion | 2 (3.8) |
| Lymphovascular invasion | 3 (5.7) |
| Preoperative pathological lymph node metastasis | 24 (45.3) |
| Preoperative lung metastasis | 6 (11.3) |
| Preoperative non-pulmonary solid organ metastasis | - |
As shown in Figure 1, optimal cut-off value of NLR was 3.55 for PFS (AUC; 0.55) and 3.0 for CSS (AUC; 0.66). The patients were divided into two groups according to their NLR of <or≥3.55, a NLR of 3.55 was chosen as the threshold as it had the best sensitivity and specificity values for PFS in this study. There were no statistically significant differences between the groups in terms of any clinical or pathological variables except the lymph node metastasis at the time of diagnosis (Table 2).
Figure 1.
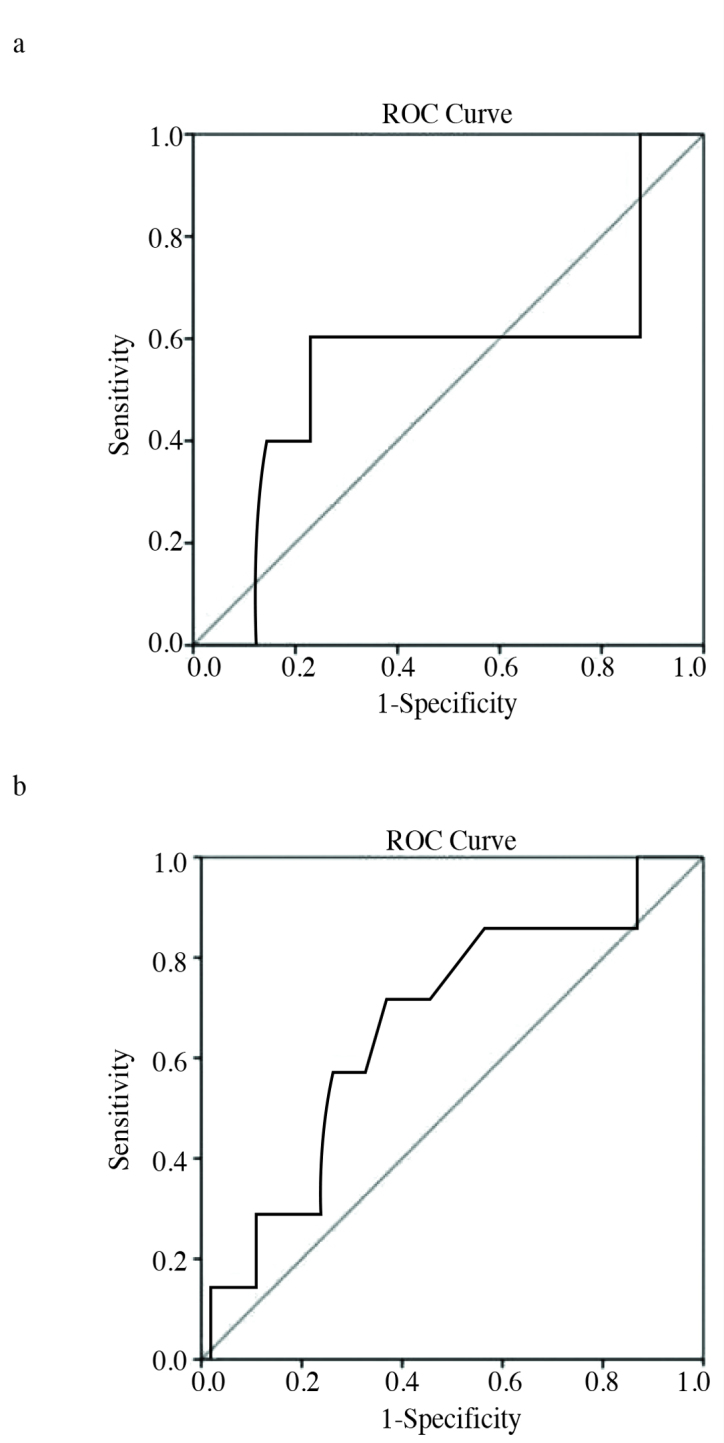
a, b. Receiver operating characteristic curves used to find the optimal cut-off value of neutrophil-to-lymphocyte ratio for progression-free survival (a) and cancer-specific survival (b)
Table 2.
Comparison of clinical parameters between the patients with a NLR of <3.55 and ≥3.55 (PFS cut-off)
| NLR <3.55 (n=39) | NLR ≥3.55 (n=14) | p | |
|---|---|---|---|
| Age, years, Mean±SD | 38.05±16.05 | 41.07±15.08 | 0.542 |
| Tumor size, cm, Mean±SD | 4.27±2.17 | 5.04±2.36 | 0.275 |
| Follow-up time, months, Mean±SD | 21.97±17.98 | 27.93±18.21 | 0.294 |
| Rete testis invasion, n (%) | 1 (2.6) | 1 (7.1) | 0.462 |
| Lymphovascular invasion, n (%) | 3 (7.7) | - | |
| Stage, n (%) | 0.10 | ||
| 1 | 20 (51.3) | 8 (44.4) | |
| 2A | 10 (25.6) | 1 (7.1) | |
| 2B | 5 (12.8) | 0 | |
| 2C | 3 (7.7) | 3 (21.4) | |
| 3 | 0 | 0 | |
| 4 | 1 (2.6) | 2 (14.3) | |
| Lymph node metastasis at the time of diagnosis, n (%) | 18 (46.2) | 6 (42.9) | 0.045 |
| Lung metastasis at the time of diagnosis, n (%) | 4 (10.3) | 2 (14.3) | 0.649 |
| Progression, n (%) | 3 (7.7) | 2 (21.4) | 0.599 |
| Time to progression, months, Mean±SD | 26.0±22.52 | 9.0±1.73 | 0.386 |
| Cancer-specific death, n (%) | 4 (10.3) | 3 (21.4) | 0.364 |
PFS: progression-free survival; NLR: neutrophil-to-lymphocyte ratio; SD: standard deviation
Neutrophil-to-lypmhocyte ratio was inversely related with lymph node involvement at the time of diagnosis (p=0.045). As shown in Figure 2A, patients with a NLR of <3.55 revealed a mean time-to-progression (TTP) of 55.71 months (95% CI, 51.27–60.14) and patients with a NLR of ≥ 3.55 revealed a mean TTP of 51.95 months (95% CI, 38.02–65.87) (p=0.152).
Figure 2.
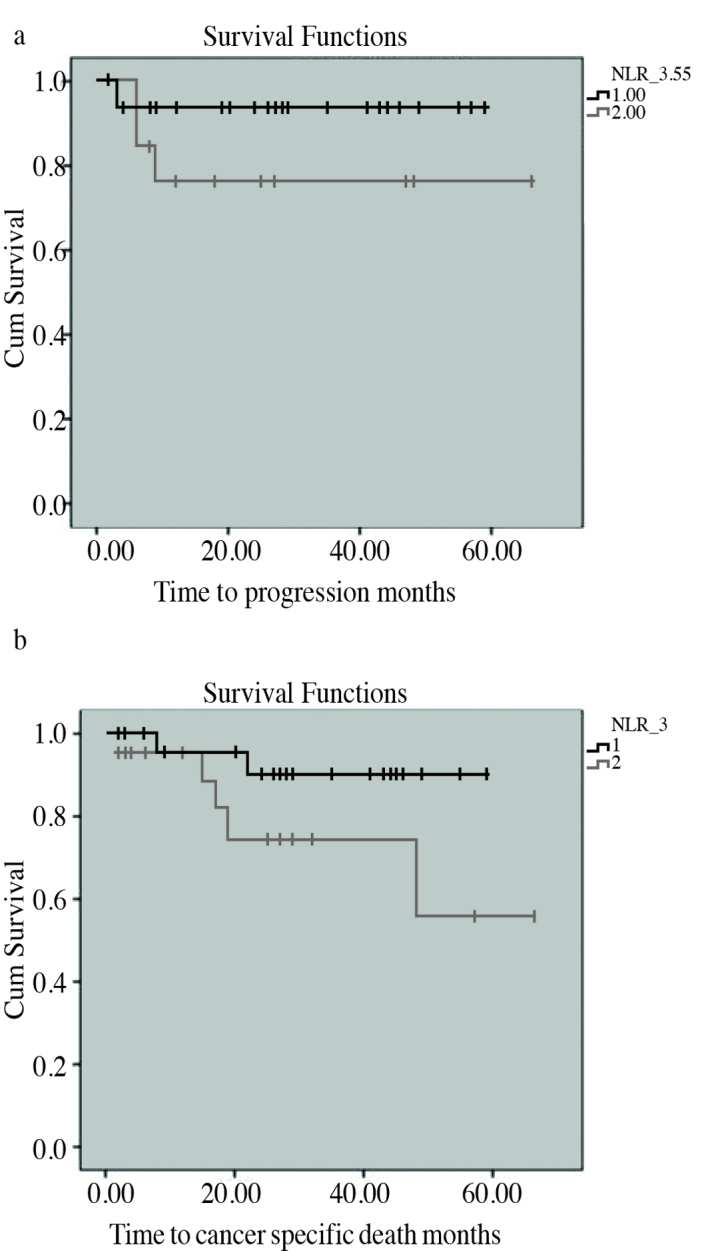
a, b. Kaplan-Meier curves used to evaluate the correlation between neutrophil-to-lymphocyte ratio and the time-to-event for progression-free survival (a) and cancer-specific survival (b)
Further the patients were divided into two groups according to a NLR of <or≥3.0, and a NLR of 3.0 was chosen as the threshold value as it had the best sensitivity and specificity values for CSS. There were no statistically significant differences between the groups in terms of any clinical or pathological variables (Table 3).
Table 3.
Comparison of clinical parameters between the patients with a NLR of <3.0 and ≥3.0 (CSS cut-off)
| NLR <3.0 (n=31) | NLR ≥3.0 (n=22) | p | |
|---|---|---|---|
| Age, years, Mean±SD | 37.97±15.5 | 40.1±16.2 | 0.632 |
| Tumor size, cm, Mean±SD | 4.38±2.37 | 4.60±2.03 | 0.734 |
| Follow-up time, months, Mean±SD | 23.42±17.76 | 23.73±18.89 | 0.952 |
| Rete testis invasion, n (%) | 1 (3.2) | 1 (4.5) | 0.062 |
| Lymphovascular invasion, n (%) | 3 (9.7) | - | |
| Stage, n (%) | 0.753 | ||
| 1 | 16 (51.6) | 12 (54.5) | |
| 2A | 8 (25.8) | 3 (13.6) | |
| 2B | 3 (9.7) | 2 (9.1) | |
| 2C | 3 (9.7) | 3 (13.6) | |
| 3 | 0 | 0 | |
| 4 | 1 (3.2) | 2 (9.1) | |
| Lymph node metastasis at the time of diagnosis, n (%) | 14 (45.2) | 10 (45.5) | 0.983 |
| Lung metastasis at the time of diagnosis, n (%) | 3 (9.7) | 3 (13.6) | 0.683 |
| Progression, n (%) | 2 (6.5) | 3 (13.6) | 0.638 |
| Time to progression, months, mean±SD | 15.0±6.97 | 22.0±12.51 | 0.737 |
| Cancer specific death, n (%) | 2 (6.5) | 5 (22.7) | 0.113 |
CSS: cancer specific survival; NLR: neutrophil-to-lymphocyte ratio; SD: standard deviation
As shown in Figure 2B, patients with a NLR of <3.0 revealed a mean time-to-cancer specific death (TTD) of 54.72 months (95% CI, 49.05–60.38) and patients with a NLR of ≥3.0 revealed a mean TTD of 49.43 months (95% CI, 37.64–61.22) (p=0.119).
Discussion
In the present study, we sought to investigate the potential association between the preoperative NLR and prognosis of GCT in patients treated with inguinal orchiectomy. Wei et al.[7] reported that elevated NLR indeed predicted a worse clinical outcome in a meta-analysis including 17 studies involving 3159 cases with urinary system cancers. In this study subgroup analyses revealed that poor OS with high NLR could be found in RCC and worse PFS/CSS in RCC, bladder cancer and urothelial carcinoma. To the best of our knowledge, our study represents the first trial determining the prognostic effect of preoperative NLR on GCT. In this study we found that the preoperative NLR is not significantly associated with PFS and CSS in the patients with GCT. By determining the optimal NLR cut-off levels of 3.55 and 3.0, patients with increased level of NLR did not show significantly lower rates of PFS and CSS, respectively.
Several studies reported the relationship between increased NLR and worse prognostic outcomes in various cancers including breast, colorectal and hepatocellular carcinomas, as well as, several urinary malignancies including prostate, bladder and renal cell carcinomas (RCC).[9–14] Proctor et al.[15] performed a Scottish Cancer Registry review across 11 different malignancies, including 8759 patients, and noted a significantly increased risk of cancer-specific (HR=1.76) and overall mortality (HR=1.77) in patients with a preoperative NLR>5. Our results could not demonstrate the presence of any significant relationship between the NLR and PFS or CSS.
Although chronic inflammation has a negative impact on cancer progression, various studies have shown that carcinogenesis itself activates chronic inflammation, which leads to the evaluation of inflammation markers as possible predictors of survival and cancer-related complications.[16] This inflammatory response reflects a non-specific response to tumor hypoxia, tissue injury and necrosis.[15–17] Suggested mechanisms to explain neutrophilia include release of granulocyte-colony stimulating factor (G-CSF) by tumor cells, and cancer inflammation through release of interleukin (IL)-1, IL-6 and tumor necrosis factor-alpha (TNF-α).[18,19] Neutrophils are recruited by (G-CSF), which is associated with tumor progression. In addition, IL-6 also mobilizes neutrophils into a circulating pool and can be measured to predict cancer stage and oncological outcome.[20,21] Relative neutrophilia increases the number of inflammatory markers including pro-angiogenic factors, growth factors, proteases and anti-apoptotic markers that support tumor growth and progression.[22,23] Furthermore, a relative lymphocytopenia may reflect a lower count of CD4+ T-helper lymphocytes, resulting in a suboptimal lymphocyte-mediated immune response to malignancy.[13] Both of these factors may contribute to aggressive tumor biology, cancer progression, and poor prognosis. On the other hand, we did not find any significant association between the NLR and prognosis of TC. This situation creates a doubt about the relationship between the chronic inflammation and development of TC. In our opinion, further studies are needed in order to reveal the underlying inflammatory response in testicular carcinogenesis.
Every patient with a suspect testicular mass must undergo inguinal exploration with exteriorization of the testis within its tunics. For seminoma stage I, tumor size (>4 cm) and invasion of the rete testis have been identified as predictors for relapse in a pooled analysis.[24,25] For non-seminoma stage I, vascular or lymphatic invasion of the primary tumour, higher proliferation rate (>70%) and higher percentage of embrional carcinoma (>40%) are the most important predictors of occult metastatic disease.[26,27] Our study we evaluated the possible relationship between NLR and risk factors for metastatic disease. According to our results, there was no significant association between the increased NLR and risk factors for occult metastatic disease such as higher tumor size, rete testis invasion and lymphovascular invasion.
Risk factors for metastatic disease are primary location of TC, elevation of tumor marker levels and presence of non-pulmonary visceral metastasis.[28] In our study, we found a negative correlation between the NLR (for PFS cut-off value of 3.55) and lymph node metastasis at the time of diagnosis.
There are some limitations of our study. Due to the retrospective design of the recent study, some of the patients with missing data were excluded. Second limitation is the relatively small number of patients recruited from a single center. Small sample size may also be responsible from lower prevalence of TC and the wide range of our exclusion criteria. Lastly, we had data only about medium-term follow-up outcomes of our patients.
In conclusion, in contrast to other urological cancers, preoperative NLR is not useful in order to predict survival rates of the patients with GCT. However, further large-scale, prospective randomized and multi-center studies with longer follow-up time are required to investigate the possible association between NLR and prognosis of GCTs.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - D.B., Ö.A.; Design - D.B., Ö.A.; Supervision - D.B.; Materials - İ.H.B., T.Y., V.Ş.; Data Collection and/or Processing - S.P., S.Y.; Analysis and/or Interpretation - Ö.A.; Literature Search - D.B.; Writing Manuscript - D.B.; Critical Review - Ö.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Znaor A, Lortet-Tieulent J, Jemal A, Bray F. International variations and trends in testicular cancer incidence and mortality. Eur Urol. 2013;65:1095–106. doi: 10.1016/j.eururo.2013.11.004. https://doi.org/10.1016/j.eururo.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, et al. EAU guidelines on testicular cancer: 2011 update. Eur Urol. 2011;60:304–19. doi: 10.1016/j.eururo.2011.05.038. https://doi.org/10.1016/j.eururo.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 3.Dieckmann KP, Pichlmeier U. Clinical epidemiology of testicular germ cell tumors. World J Urol. 2004;22:2–14. doi: 10.1007/s00345-004-0398-8. https://doi.org/10.1007/s00345-004-0398-8. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. https://doi.org/10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Roxburgh CS, Crozier JE, Maxwell F, Foulis AK, Brown J, McKee RF, et al. Comparison of tumour-based (Petersen Index) and inflammation-based (Glasgow Prognostic Score) scoring system in patients undergoing curative resection for colon cancer. Br J Cancer. 2009;100:701–6. doi: 10.1038/sj.bjc.6604926. https://doi.org/10.1038/sj.bjc.6604926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paramanathan A, Saxena A, Morris DL. Asystematic review and meta-analysis on the impact of pre-operative neutrophil/lymphocyte ratio on long term outcomes after curative intent resection of solid tumors. Surg Oncol. 2014;23:31–9. doi: 10.1016/j.suronc.2013.12.001. https://doi.org/10.1016/j.suronc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Wei Y, Jiang YZ, Qian WH. Prognostic role of NLR in urinary system cancers: a meta-analysis. PLoS One. 2014;9:e92079. doi: 10.1371/journal.pone.0092079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobin LH, Gospodariwicz M, Wittekind C. TNM classification of malignant tumors UICC International Union Against Cancer. 7th edn. Wiley-Blackwell; 2009. [Google Scholar]
- 9.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–24. doi: 10.1245/s10434-011-1814-0. https://doi.org/10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 10.Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiquchi Y, et al. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013;33:3291–4. [PubMed] [Google Scholar]
- 11.Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301–5. doi: 10.1097/SLA.0b013e318297ad6b. https://doi.org/10.1097/SLA.0b013e318297ad6b. [DOI] [PubMed] [Google Scholar]
- 12.Shafique K, Proctor MJ, McMillan DC, Qureshi K, Leung H, Morrison DS. Systemic inflammation and survival of patients with prostate cancer: evidence from the Glasgow Inflammation Outcome Study. Prostate Cancer Prostatic Dis. 2012;15:195–201. doi: 10.1038/pcan.2011.60. https://doi.org/10.1038/pcan.2011.60. [DOI] [PubMed] [Google Scholar]
- 13.Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, et al. Pretreatment neutrophil-to lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urethelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66:1157–64. doi: 10.1016/j.eururo.2014.02.042. https://doi.org/10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Ohno Y, Nakashima J, Ohori M, Hatano T, Tachibana M. Pretreatment neutrophil-to-lymphocyte ratio as as independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol. 2010;184:873–8. doi: 10.1016/j.juro.2010.05.028. https://doi.org/10.1016/j.juro.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–41. doi: 10.1016/j.ejca.2011.03.028. https://doi.org/10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–63. doi: 10.2217/fon.09.136. https://doi.org/10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 17.Chua TC, Chong CH, Liauw W, Zhao J, Morris DI. Inflammatory markers in blood and serum tumor markers predict survival in patients with epithelial appendiceal neoplasms undergoing surgical cytoreduction and intraperitoneal chemotherapy. Ann Surg. 2012;256:342–9. doi: 10.1097/SLA.0b013e3182602ad2. https://doi.org/10.1097/SLA.0b013e3182602ad2. [DOI] [PubMed] [Google Scholar]
- 18.Viers BR, Houston Thompson R, Boorjian SA, Lohse CM, Leibovich BC, Tollefson MK. Preoperative neutrophil-lymphocyte ratio predicts death among patients with localized clear cell renal carcinoma undergoing nephrectomy. Urol Oncol. 2014;32:1277–84. doi: 10.1016/j.urolonc.2014.05.014. https://doi.org/10.1016/j.urolonc.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. https://doi.org/10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tachibana M, Miyakawa A, Tazaki H, Nakamura K, Kubo A, Hata J, et al. Autocrine growth of transitional cell carcinoma of the bladder induced by granulucyte-colony stimulating factor. Cancer Res. 1995;55:3438–43. [PubMed] [Google Scholar]
- 21.Okamoto M, Hattori K, Oyasu R. Interleukin-6 functions as an autocrine growth factor in human bladder carcinoma cell lines in vitro. Int J Cancer. 1997;72:149–54. doi: 10.1002/(sici)1097-0215(19970703)72:1<149::aid-ijc21>3.0.co;2-d. https://doi.org/10.1002/(SICI)1097-0215(19970703)72:1<149::AID-IJC21>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Jablońska E, Kiluk M, Markiewicz W, Piotrowski L, Grabowska Z, Jabloński J. TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch Immunol Ther Exp (Warsz) 2001;41:63–9. [PubMed] [Google Scholar]
- 23.McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg. 1999;134:1325–31. doi: 10.1001/archsurg.134.12.1325. https://doi.org/10.1001/archsurg.134.12.1325. [DOI] [PubMed] [Google Scholar]
- 24.Shelley MD, Burgon K, Mason MD. Treatment of testicular germ-cell cancer: a cochrane evidence-based systematic review. Cancer Treat Rev. 2002;28:237–53. doi: 10.1016/s0305-7372(02)00059-2. https://doi.org/10.1016/S0305-7372(02)00059-2. [DOI] [PubMed] [Google Scholar]
- 25.Aparicio J, Germà JR, García del Muro X, Maroto P, Arranz JA, Sáenz A, et al. Second Spanish Germ Cell Cancer Cooperative Group. Risk-adapted management for patients with clinical stage I seminoma: the Second Spanish Germ Cell Cancer Cooperative Group study. J Clin Oncol. 2005;23:8717–23. doi: 10.1200/JCO.2005.01.9810. https://doi.org/10.1200/JCO.2005.01.9810. [DOI] [PubMed] [Google Scholar]
- 26.Bokemeyer C, Schmoll HJ. Treatment of clinical stage I testicular cancer and a possible role for new biological prognostic parameters. J Cancer Res Clin Oncol. 1996;122:575–84. doi: 10.1007/BF01221188. https://doi.org/10.1007/BF01221188. [DOI] [PubMed] [Google Scholar]
- 27.Albers P, Siener R, Kliesch S, Weissbach L, Krege S, Sparwasser C, et al. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumours: results of the German Testicular Cancer Study Group Trial. J Clin Oncol. 2003;21:1505–12. doi: 10.1200/JCO.2003.07.169. https://doi.org/10.1200/JCO.2003.07.169. [DOI] [PubMed] [Google Scholar]
- 28.International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]


