Rates of hepatocellular carcinoma (HCC) incidence are increasing worldwide, with 5-year survival remaining very low.1 Early diagnosis remains difficult, and aggressive growth and metastasis are major contributing factors to HCC’s high mortality rate. Thus, better understanding of the mechanisms of HCC aggressiveness and metastasis is crucial for developing more effective therapeutic strategies for the disease.
As with in other types of cancer, epithelial-mesenchymal transition (EMT), which has classically been considered a prerequisite for metastasis, has been implicated in cancer recurrence and metastasis and associated with shorter survival in HCC patients.2 EMT is promoted by loss of E-cadherin and up-regulation of many transcription factors, including Snail, Slug, and Twist. These changes allow degradation of the basement membrane, intravasation, and metastasis.3 However, some metastases do not possess a mesenchymal phenotype and instead more closely resemble their epithelial tumor progenitors. Additionally, EMT-independent migration has been reported for ovarian cancer cells in vitro.4 Although the former observations might be explained by a restoration of the epithelial phenotype by mesenchymal-epithelial transition, the latter cannot. This suggests that alternative modes of metastasis need to be considered.
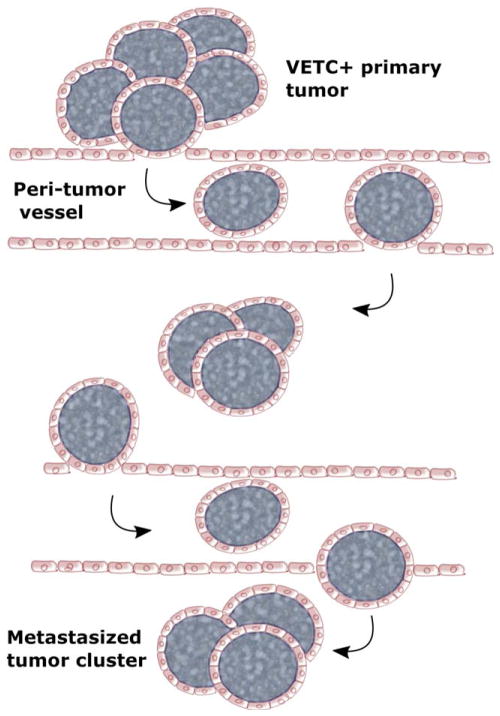
In this issue of Hepatology, Fang et al. present interesting data showing a previously unexplored process and mechanism of metastasis. Here, they describe a mode of HCC metastasis that is EMT independent, instead relying on a unique vascular pattern.5 This vascular structure, which they dubbed VETC (vessels that encapsulate tumor cluster), consists of a sinusoidal network of functional blood vessels that completely surrounds portions of the primary tumor (Fig. 1). Surprisingly, they found that the entire vessel-encapsulated tumor cluster is able to enter the bloodstream and metastasize within the liver and to the lung. This group had previously identified a link between this vascular pattern and increased micrometastases and shorter disease-free survival in HCC patients after resection.6 Here, they combined analysis of clinical samples, in vitro assays, and a patient-derived xenograft (PDX) mouse model to probe the mechanism of VETC-mediated metastasis.
Fig. 1.
A schematic model for VETC-mediated HCC spread. The whole tumor cluster, encapsulated by endothelial cells, can be released into the bloodstream and spread to other nearby or remote sites. This type of conserved tissue architecture with endothelium disguise apparently protects tumor cells against immune attack and anoikis.
In agreement with their previous observations, analysis of tumors from HCC patients revealed a significant correlation between presence of the VETC pattern and increased number of microemboli and portal vein tumor thromboses. This pattern may also have prognostic value, given that patients with VETC+ tumors tend to have increased rates of recurrence and shorter survival. From these observations, the investigators suggested that the VETC pattern may play a role in metastasis. Indeed, these endothelium-covered tumor clusters were found in the bloodstreams of VETC+ HCC patients, as well as in adjacent nontumor liver tissue. In the PDX model, VETC+ metastases were found in the bloodstream, liver, and lung.
Interestingly, tumor cells are able to induce this vascular pattern: Cells from VETC+ HCC tumors, when transplanted into mice, recruited mouse endothelial cells to form the VETC structure. To investigate the mechanism of this recruitment, the investigators compared expression of angiogenic factors between VETC− and VETC+ tumors. Both had similar levels of vascular endothelial growth factor (VEGF), but VETC+ tumor cells expressed much higher levels of angiopoietin 2 (Ang2), which is involved in tumor angiogenesis and may also be implicated in metastasis.7 To test whether this differential expression contributed to VETC formation, and whether this process also affected metastasis, the investigators used short hairpin RNA to knock down Ang2 in VETC-inducing HCC cells, which were then implanted into mice. Indeed, Ang2 knockdown reduced VETC formation postimplantation and decreased metastasis in the animal model. These results agree with another study by Mazzieri et al., which showed that antibody-mediated targeting of Ang2 inhibits angiogenesis and metastasis of mammary tumors in mice.8
To further examine the role of the VETC pattern in metastasis, the investigators analyzed expression of EMT-related factors in VETC− and VETC+ tumors; intriguingly, none of the VETC+ tumors showed loss of E-cadherin and up-regulation of Snail, Slug, and Twist that are the classical indicators of EMT. Knockdown of Snail or Slug reduced the metastasis rate of VETC−, but not VETC+, tumors in mice, suggesting that VETC+ tumor metastasis does not require an EMT. However, in vitro tests of invasiveness had a different result, with Slug/Snail knockdown markedly decreasing in vitro migration of VETC-inducing hepatoma cell lines. Additionally, the investigators report that Ang2 knockdown decreased rates of metastasis in vivo, but not of cell migration in vitro. These discrepancies suggest that this mechanism of metastasis may not require individual cells to be aggressively invasive.
From these observations, the investigators proposed a model in which certain tumor cells stimulate formation of the VETC pattern in an Ang2-dependent manner; this vascular pattern then facilitates entry of the entire tumor cluster into the bloodstream. In support of this, VETC+ tumor clusters were found in the bloodstreams and tumor-adjacent liver tissue of HCC patients. Additionally, mice with VETC-forming hepatoma cell transplants had these VETC+ emboli in the bloodstream, liver, and lungs. Furthermore, serial sections of tumors from VETC+ HCC patients revealed that endothelium-encased tumor clusters entered tumor stromal vessels. They also observed anastamosis of the VETC-patterned vessels with peritumor vessels; they suggest that this provides the tumor cluster with a route of entry into the bloodstream. These observations are consistent with the investigators’ model. Further investigation will be needed to delineate the processes of tumor cluster detachment, anastomosis, and entry into the bloodstream.
Together, these findings support a model of EMT-independent metastasis mediated by a vascular pattern that allows entire tumor clusters to enter the bloodstream (Fig. 1). The investigators suggest that several features of this mechanism may contribute to more efficient metastasis. These include a decreased requirement for stromal and vascular invasion, immune protection by the surrounding blood vessels, and increased survival resulting from maintenance of adhesion-dependent survival signals against anoikis. These possibilities might help explain the higher metastatic rates and shorter survival of VETC+ HCC patients. However, more mechanistic analyses are necessary to explain why and how this process follows an EMT-independent manner and also to determine whether this mode is mainly for intra-or extrahepatic metastasis in HCC patients.
The data presented in that report also argue that targeting Ang2 could ameliorate HCC progression by suppressing at least one mode of metastasis. Indeed, several Ang2 inhibitors are currently in clinical trials, with others in preclinical development.7 It will be interesting to determine whether stratifying patients’ VETC+ HCCs will achieve better therapeutic effect with Ang2 inhibitors. In mice, Ang2 targeting was effective even for cancers resistant to VEGF-targeting treatments.8 Sorafenib, the main treatment option for advanced HCC, which targets, among other pathways, VEGF receptor, has yielded only small increases in survival; the observations from this article suggest a much needed alternative therapeutic strategy for treating HCC. However, one needs to be cautioned that a potential role of Ang2 derived from the tumor microenvironment was not explored in this study. To identify the most critical factors for VETC structure assembly, it is important to launch a comprehensive, unbiased search using multiomics approaches.
The work by Fang et al. describes a novel mechanism of metastasis and may represent an exciting advance in understanding hepatocellular carcinogenesis. It will be particularly interesting to determine whether VETC-mediated metastasis exists in a particular subtype only or is common for HCCs and whether this process occurs in other solid tumor types.
Abbreviations
- Ang2
angiopoietin 2
- EMT
epithelial-mesenchymal transition
- HCC
hepatocellular carcinoma
- PDX
patient-derived xenograft
- VEGF
vascular endothelial growth factor
- VETC
vessels that encapsulate tumor cluster
Footnotes
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co-first authorship.
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Yamada S, Okumura N, Wei L, Fuchs BC, Fujii T, Sugimoto H, et al. Epithelial to mesenchymal transition is associated with shorter disease-free survival in hepatocellular carcinoma. Ann Surg Oncol. 2014;21:3882–3890. doi: 10.1245/s10434-014-3779-2. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao J, Zhu Y, Nilsson M, Sundfeldt K. TGF-β isoforms induce EMT independent migration of ovarian cancer cells. Cancer Cell Int. 2014;14:72. doi: 10.1186/s12935-014-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang JH, Zhou HC, Zhang C, Shang LR, Zhang L, Xu J, et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial–mesenchymal transition–independent manner. Hepatology. 2015;62:452–465. doi: 10.1002/hep.27760. [DOI] [PubMed] [Google Scholar]
- 6.Ding T, Xu J, Zhang Y, Guo RP, Wu WC, Zhang SD, et al. Endothelium-coated tumor clusters are associated with poor prognosis and micrometastasis of hepatocellular carcinoma after resection. Cancer. 2011;117:4878–4889. doi: 10.1002/cncr.26137. [DOI] [PubMed] [Google Scholar]
- 7.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17:347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2008;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]