Abstract
Background
The combination of low-dose radiation therapy with PARP inhibition enhances anti-tumor efficacy through potentiating DNA damage. We combined low-dose fractionated whole abdominal radiation (LDFWAR) with ABT-888 in patients with peritoneal carcinomatosis with a dose escalation in ovarian and fallopian cancer patients (OV).
Methods
Patients were treated with veliparib, 40–400mg orally BID on days 1–21 of 3 28-day cycles on 6 dose levels. Dose levels 5 and 6 included only OV patients. LDFWAR consisted of 21.6Gy in 36 fractions, 0.6 Gy twice daily on days 1 and 5 for weeks 1–3 of each cycle. Circulating tumor material and quality of life were serially assessed.
Results
32 pts were treated. Median follow-up was 45 months (10–50). The most common treatment-related grade 3 and 4 toxicities were lymphopenia (59%), anemia (9%), thrombocytopenia (12%), neutropenia (6%), leukopenia (6%), nausea (6%), diarrhea (6%), anorexia (6%), vomiting (6%) and fatigue (6%). The maximum tolerated dose was determined to be 250mg PO BID. Median PFS was 3.6 months and median OS was 9.1 months. In OV patients, OS was longer for platinum-sensitive patients (10.9mo) compared to platinum-resistant patients (5.8mo). QoL decreased for all groups during treatment. Germline BRCA status was known for 14/18 patients with OV cancers, 5 of whom were BRCA mutation carriers. One objective response (3%) was observed.
Conclusion
ABT-888 plus LDFWAR is tolerable with gastrointestinal symptoms, fatigue and myelosuppression as the most common toxicities. The single observed objective response was in a germline BRCA mutated, platinum-sensitive patient.
Introduction
We previously reported the results of a phase I study combining low-dose whole fractionated abdominal radiation (LDFWAR) with veliparib (ABT-888) in patients with advanced solid tumors and peritoneal carcinomatosis[1]. The rationale for this approach was good preclinical evidence that, PARP inhibitors may act as sensitizing agents for DNA-damaging modalities such as chemotherapy and radiotherapy beyond their well-established anti-tumor effect in cancers with BRCA mutations [2–10]. In addition, it had been demonstrated in prior early phase clinical trials that the combination of LDFWAR with chemotherapy in patients with small bowel cancers, pancreatic cancer and ovarian cancer was well tolerated[11, 12]. Therefore, using the same dosing of LDFWAR as previously published, we anticipated our proposed combination to have manageable toxicity. Consistent with this previously established data, a maximum tolerated dose (MTD) was not reached in our original study, which consisted of 22 patients with peritoneal carcinomatosis of varying origins including colorectal cancer, peritoneal mesothelioma, pancreatic cancer, gastric cancer, appendiceal cancer, small bowel cancers and cholangiocarcinoma.
No objective responses were observed during the original trial. However, there was durable stabilization of disease (≥24 weeks) in 7 of 22 patients, 4 of whom had ovarian or fallopian tube cancers (OV). At the time of the initial analysis, it was noted that the patients in the OV subset had a median OS of 17.5 months. BRCA mutation carrier status was known for only half the patients in this cohort. Somatic BRCA mutation status was unknown. We hypothesized that the OV subset of patients were plausibly the most likely to be afflicted with a homologous recombination deficit (HRD), and therefore may have been the most sensitive to the combination of PARP inhibition and DNA-damaging radiotherapy.
The original protocol was amended and the study was reopened with two additional dose levels (DL5 and DL6) for patients with advanced ovarian or fallopian tube cancers. In this follow-up manuscript, we report the final, complete results of our phase I study.
Patients and Methods
Study Design
The primary objective was to assess the safety profile of veliparib and LDFWAR in patients with peritoneal carcinomatosis. Secondary objectives included disease response and quality of life (QoL) assessment. Analysis of ɣ-H2AX levels in serial circulating tumor cell (CTC) and circulating endothelial cells (CEC) were exploratory objectives. Germline BRCA status for OV patients in DL 1–4 was collected as available by chart review. Patients in DL5 and DL6 were specifically queried about germline BRCA mutational status. Assessment for somatic BRCA mutations was not performed.
Eligibility Criteria
Eligible patients in DL1-DL4 had an unresectable or metastatic solid tumor malignancy with the presence of peritoneal carcinomatosis documented either via imaging, operative notes, clinical notes or symptoms. Measureable disease was not required as an eligibility criterion. Any number or prior treatments was permitted. Extra-abdominal disease was permitted so long as peritoneal disease was dominant. Patients in DL5-DL6 had to have advanced peritoneal, ovarian or fallopian tube cancers.
Patients had adequate organ function, an Eastern Cooperative Oncology Group (ECOG) performance status of ≤1 and a life expectancy of greater than 3 months. Exclusion criteria included prior treatment with PARP inhibition, prior abdominal radiation therapy (prior pelvic radiation was acceptable as long as there was no overlap between radiation fields), previous malignant bowel obstruction (except if at diagnosis) or uncontrolled ascites. The protocol was approved by the institutional review boards of the participating institutions, and written informed consent was obtained from all patients prior to performing study-related procedures in accordance with federal and institutional guidelines.
Drug/Radiotherapy Administration
Veliparib was provided by the Cancer Therapy Evaluation Program (CTEP) through a Clinical Trials Agreement between Abbott Laboratories and the NCI Division of Cancer Treatment and Diagnosis. Patients were treated with veliparib by mouth in 6 escalating doses [dose levels (DL) 1–4: 40mg PO BID (DL1), 80mg PO BID (DL2), 120mg PO BID (DL3), 160mg PO BID (DL4), 250mg PO BID (DL5) and 400mg PO BID (DL6)]. Patients received veliparib on days 5–21 of the first 28-day cycle and on days 1–21 of the subsequent 2 cycles. LDFWAR was delivered using anterior and posterior open fields, in two daily fractions of 60 cGy on days 1 and 5 (minimum 4 hours between fractions) for weeks 1–3 of each cycle, with posterior kidney shielding used to keep kidney doses < 20 Gy. The field borders were as follows: superiorly 1 cm above the dome of the diaphragm at the patient’s maximum comfortable expiration and inferiorly either at the inferior border of the obturator foramina or 2 cm below the lowest extension of disease. Lateral borders extended at least 2 cm beyond skin. In some cases and extended source to skin distance (SSD) was needed to cover the entire area. Radiation treatment was standardized between the participating centers.
The trial was amended during the initial accrual period to allow ovarian/fallopian tube cancer patients who had obtained substantial benefit from the treatment to continue on single-agent veliparib at a dose of 400 mg PO BID until progression of disease at the discretion of the principal investigator. These patients were required to either have a germline BRCA mutation or a strong family history of BRCA-associated malignancies.
We enrolled successive cohorts of 3 patients each using a standard 3+3 design [13]. Dose escalations occurred no sooner than 4 weeks after the last patient on the dose level had begun therapy. DLTs were defined as any grade 4 toxicity; any grade 3 toxicity with the exception of nausea, vomiting or diarrhea that improved to grade ≤ 2 within 3 days of receiving maximal medical support and any grade 3 electrolyte abnormality that did not correct to grade ≤ 2 within 48 hours. Asymptomatic lymphopenia or leukopenia of any grade was not considered to be a DLT.
On-study evaluation and safety assessment
Patients underwent a complete clinical assessment and imaging at baseline. Patients had weekly physical exams, adverse event (AEs) evaluation, and laboratory studies. Response was assessed every 8 weeks by CT with intravenous contrast and using RECIST 1.1 criteria[14].
QoL was measured by the European Organization for the Research and Treatment of Cancer core quality of life questionnaire, QLQ-C30 at baseline and every 2 cycles.
Circulating Tumor Cells
Our exploratory translational hypothesis was that ɣ-H2AX expression would increase from baseline with exposure to DNA-damaging radiation and that this expression level would increase when ABT-888 was added to radiation exposure. Blood draws for CTCs were obtained prior to treatment and during cycle 1 on days 1 (after radiation), 3, 5 (prior to radiation) and 12. Details regarding sample analysis have been previously decribed.1 Samples in DL 5–6 were additionally evaluated for circulating endothelial cells (CECs) using a more sensitive EpCAM independent platform. CTCs are EpCAM+, CK+, DAP1+ or CD45− while CECs are CD146+, CD105+, DAP1+ or CD45−.
Statistical analysis
Proportions are reported with exact 95% binomial confidence intervals. Event time distributions were estimated with the method of Kaplan and Meier[15] and compared using the log-rank statistic[16] or the proportional hazards regression model[17]. Item scores were linearly transformed to a 0 to 100 scale with the five functional scales and global QoL. There was no imputation of missing data. Changes in QoL and subdomains of the QLQC-30 standardized questionnaire pre-treatment and during cycle 2 of treatment were analyzed with paired t-tests. Two sample QoL comparisons were made with two sample t-tests.
Results
Patients and treatment (Table 1)
Table 1.
Baseline Characteristics of All Treated Patients
| Characteristic | DL1 | DL2 | DL3 | DL4 | DL5 | DL6 | All Dose Levels |
|---|---|---|---|---|---|---|---|
| N = 3 | N = 6 | N = 6 | N = 7 | N = 6 | N = 4 | N = 32 | |
| Age – yr | |||||||
| Median | 65 | 57 | 59 | 56 | 57 | 58 | 58 |
| Range | 62–86 | 50–75 | 40–78 | 42–74 | 47–78 | 56–65 | 40–86 |
| Sex – no (%) | |||||||
| Male | 2 (66) | 0 | 3 (50) | 3 (43) | 0 | 0 | 8(25) |
| Female | 1 (33) | 6 (100) | 3 (50) | 4 (57) | 6(100) | 4(100) | 24(75) |
| ECOG – no (%) | |||||||
| 0 | 0 | 0 | 3 (50) | 2 (29) | 4(66) | 2(50) | 11(34) |
| 1 | 3 (100) | 6 (100) | 3 (50) | 5 (71) | 2(33) | 2(50) | 21(66) |
| Primary Site of Disease –no (%) | |||||||
| Non-Gyn | 3(100) | 2(33) | 3(50) | 6(86) | 0 | 0 | 14(44) |
| Ovary or Fallopian | 0 | 4 (66) | 3(50) | 1 (14) | 6(100) | 4(100) | 18(56) |
| Stage of Disease | |||||||
| II | 0 | 0 | 1(17) | 0 | 1(17) | 0 | 2(6) |
| III | 0 | 0 | 0 | 1(14) | 2(33) | 3(75) | 6(19) |
| IV | 3(100) | 6(100) | 5(83) | 4(57) | 2(33) | 1(25) | 21(66) |
| Unknown | 2(28) | 1(17) | 0 | 3(9) | |||
| # Prior Tx | |||||||
| 1 | 2(66) | 3(50) | 1(17) | 2 (29) | 1(17) | 0 | 9(28) |
| 2-3 | 0 | 0 | 3(50)) | 5 (71) | 1(17) | 0 | 9(28) |
| 4+ | 1(33) | 3(50) | 2(33) | 0 | 4(66) | 4(100) | 14(44) |
Thirty-two patients were enrolled between September 8th, 2011 and February 23rd, 2015. Median age was 58 (range, 55–65), 59% had been treated with ≥3 prior lines of therapy (range, 1–12). Eighteen patients had OV cancers (56%). BRCA status was known for 14/18 patients, with 5 being BRCA mutation carriers. Nine OV patients were platinum-sensitive. Supportive care, including antiemetic therapy, was prescribed as per institutional guidelines.
Thirteen of the patients (41%) received all 3 planned cycles, with first imaging reassessment conducted after the second cycle per protocol. Upon completion of 3 cycles, 4 patients continued with veliparib monotherapy for 2–10 cycles, one still ongoing. These were in the following dose levels by BRCA carrier status: DL2 (BRCA neg), DL3 (BRCA pos), DL5 (BRCA neg) and DL6 (BRCA pos).
Reasons for premature discontinuation of therapy included progression of disease (10 patients; 31%), adverse events (7 patients; 22%) and withdrawal of consent (1 patient; 3%).
Dose escalation
In the initial report, DL1-4 were enrolled and MTD was not reached; two patients in DL2 had protracted thrombocytopenia. In the amended study assessing new dose levels, 1 of 3 patients at DL5 experienced grade 3 protracted nausea and vomiting requiring admission and intravenous rehydration. Three more patients were enrolled at DL5 without incident. Four patients were enrolled in DL6; 1 experienced grade 3 diarrhea.
Safety
Thirty-two patients were evaluable for toxicity. Across all dose levels and grades, the most common treatment-related AEs included hematological toxicities, gastrointestinal toxicities, and fatigue.
One patient had protracted grade 2 thrombocytopenia at dose DL2 that did not meet criteria for a DLT by the original protocol, but was deemed a DLT at the discretion of the investigators . There was a DLT at DL5 in the form of grade 3 nausea. There was a DLT at DL6 in the form of grade 3 diarrhea. There were no other DLTs at DL6. However, 75% (3/4) of patients at DL6 required a dose reduction during cycle 1. Therefore, DL5 was considered to be the MTD by the investigators. The details of dose holds and reductions are outlined in Table 2.
Table 2.
ABT-888 dose reductions and holds
| Dose Level (mg ABT-888)/Patient | Dose Reductions | #Dose Holds/Patient | Reason |
|---|---|---|---|
| DL1 (40mg PO BID) | |||
| Patient 1 | None | 1 | Neutropenia |
| Patient 2 | None | None | |
| Patient 3 | None | None | |
| DL2 (80mg PO BID) | |||
| Patient 4 | None | None | |
| Patient 5 | None | None | |
| Patient 6 | None | 1 | Neutropenia |
| Patient 7 | None | None | |
| Patient 8 | None | 2 | Nausea/vomiting |
| Patient 9 | None | None | |
| DL3 (120mg PO BID) | |||
| Patient 10 | None | 1 | Nausea, anorexia, vomiting, fatigue |
| Patient 11 | None | 1 | Diarrhea |
| Patient 12 | None | 1 | Dizziness |
| Patient 13 | None | None | |
| Patient 14 | None | 1 | Thrombocytopenia |
| Patient 15 | None | None | |
| DL4 (160mg PO BID) | |||
| Patient 16 | None | None | |
| Patient 17 | None | None | |
| Patient 18 | None | None | |
| Patient 19 | None | None | |
| Patient 20 | None | 2 | Thrombocytopenia |
| Patient 21 | None | None | |
| Patient 22 | None | None | |
| DL5 (250mg PO BID) | |||
| Patient 23 | None | 2 | Nausea |
| Patient 24 | Reduced to 250mg PO QAM, 150mg PO QPM | 2 | Nausea/vomiting |
| Patient 26 | None | None | |
| Patient 28 | None | 2 | Thrombocytopenia |
| Patient 29 | None | None | |
| Patient 30 | None | None | |
| DL6 (400mg PO BID) | |||
| Patient 31 | Reduced to 250mg PO BID at C1D22 | 1 | Nausea/vomiting |
| Patient 32 | Reduced to 250mg PO BID at C4D1 | 1 | Thrombocytopenia |
| Patient 33 | Reduced to 250mg PO BID at C1D15 | 1 | Nausea, vomiting, fatigue |
| Patient 34 | Reduced to 250mg PO BID at C1D15 | 1 | Diarrhea, fatigue |
Clinical activity
Thirty-two patients were evaluable for response. One objective partial response was observed in a platinum-sensitive BRCA-mutated OV patient. This patient had not yet progressed as of the cut-off date of January 6th, 2016 and remains on treatment.
Median PFS was 3.63 months and median OS was 9.18 months. Patients with OV cancers had a PFS and OS of 4.6 and 9.35 months compared to 2.5 and 8.92 months in non-OV patients, respectively. These differences were non-significant between the groups.
In the OV cancer patients, 5/14 patients with known BRCA mutation status were mutation carriers. BRCA mutation carriers had a PFS 4.47 months compared to PFS 3.58 months in the non-BRCA carriers and an OS of 10.15 months compared to 7.89 months in non-BRCA carriers. OV Patients who were platinum-sensitive had a PFS of 7.92 months compared to 3.58 months in platinum-resistant patients. Platinum-sensitive patients had an OS of 10.94 months compared to 5.78 months in platinum-resistant patients (Figure 1).
Figure 1. Progression-free survival (PFS) and overall survival (OS): all ovarian cancer patients by platinum-sensitivity status.
Kaplan-Meier survival curves of PFS (A) and OS (B) in all patients by platinum sensitivity status.
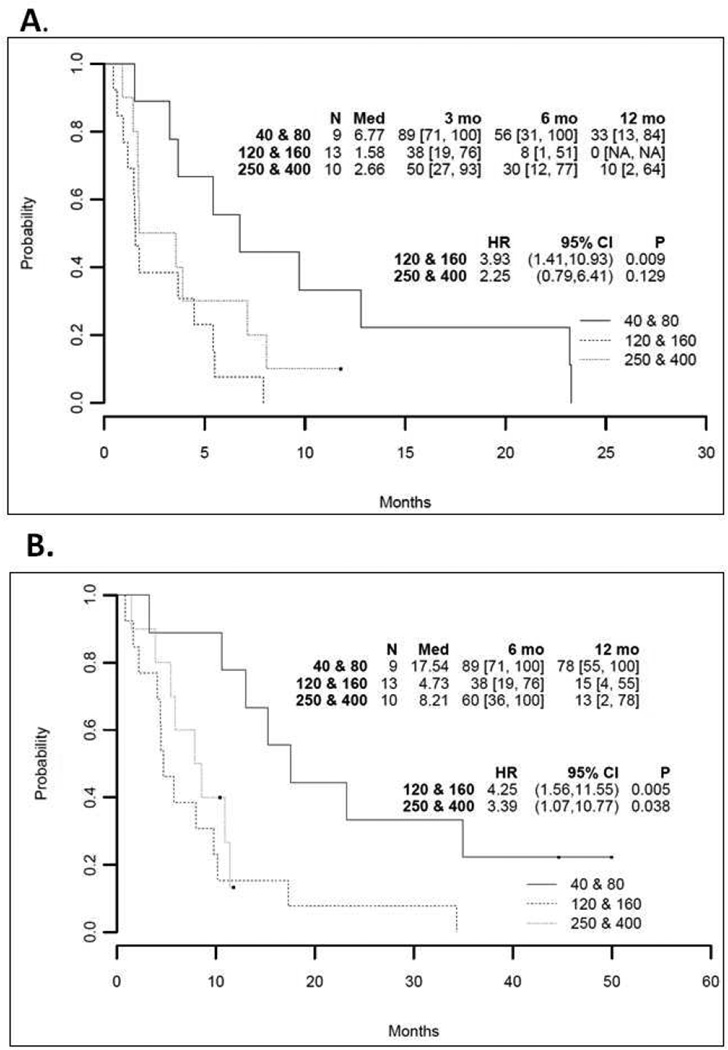
Patients treated on DL1 and DL2 had a median PFS 6.77 months and median OS 17.54 months. Patients treated on DL3 and DL4 had a median PFS 1.58 months and median OS 4.73 months. Patients treated on DL5 and DL6 (our OV-only dose escalation) had a median PFS 2.66 months and median OS 8.21 months (Figure 2).
Figure 2. Progression-free survival (PFS) and overall survival (OS): all patients by dose level.
Kaplan-Meier survival curves of PFS (A) and OS (B) in all patients by treatment dose level.
QoL Assessment
Global QoL, physical and role function were significantly reduced during treatment while symptoms of appetite loss and fatigue significantly increased. Conclusions are limited due to a large number of missing data points.
CTC data
Forty-two percent (13/31) of patients for whom CTCs were collected had fewer than 2 CTCs at the baseline evaluation. There was no evidence of a significant correlation between treatment and DNA damage response based on eighteen patients who had ≥2 CTCs in the baseline assessment.
Conclusion
This is the final report of our phase I study of veliparib plus LDFWAR in patients with peritoneal carcinomatosis. Our original study was suggestive of possible efficacy in the OV subset. We hypothesized that this effect might be due to an HRD within those patients, rendering them more sensitive to a combination of PARP inhibitors and DNA damaging agents such as radiation[18, 19]. Therefore, we added two additional dose levels, aiming to reach single agent veliparib dosing, for OV patients only. The highest dose level (veliparib 800mg/day) did not meet protocol criteria for intolerability by DLT, but due to frequent nausea and diarrhea seen at this dose level and a very high rate of dose reduction during cycle 1, we established DL5 as the recommended phase 2 dose. We note a higher rate of cytopenias than perhaps expected, which was plausibly due to the amount of bone marrow and splenic tissue irradiated using our technique.
In this study, patients in our lowest two dose levels demonstrated longer median PFS and OS than expected and longer than those at the higher dose levels. It is possible that patients in the lower dose levels had lower toxicity which had beneficial ramifications in terms of being able to tolerate subsequent therapy and overall health. Previous studies have also shown a potential for benefit to be seen in lower versus higher dose levels with targeted therapy, where biological inhibition of a target is the goal rather than cytotoxicity[20].
The trend of improved OS in OV patients compared to non-OV patients seen in our first report was not confirmed with additional data and follow-up. Our final results suggest there may be an advantage to platinum-sensitivity. Indeed, the single objective response observed in this trial was in a heavily pretreated, platinum-sensitive OV patient with a germline BRCA mutation. Sensitivity to platinum agents is well known to confer a sensitivity to PARP inhibitors[21, 22], which is consistent with the outcome observed in this patient. Additionally, platinum sensitive patients may have received subsequent effective therapy with platinum-based regimens following their participation on the trial, adding to their OS. Independent effects of platinum-sensitivity and germline BRCA mutation on OS or PFS were not possible to evaluate in this study.
Any observed benefit may be due to PARP inhibition alone, although it is notable that our efficacy in the unselected OV population appears lower than those observed with other PARP inhibitors[23, 24]It seems unlikely that LDFWAR provided additional advantage, though confirmation of this finding would require further exploration in a randomized trial of BRCA+, platinum-sensitive patients. At this time we do not plan to move this combination into phase II testing.
In conclusion, veliparib plus LDFWAR is a tolerable regimen with primary toxicities of myelosuppression, fatigue and gastrointestinal complaints. Notably, fewer than half the patients were able to complete the therapy, either due to progression of disease or adverse events. QoL data was therefore limited, but was suggestive that increased fatigue and decreased appetite were problematic during therapy. There may be benefit in patients with platinum-sensitive and/or BRCA-related ovarian cancers with dominant peritoneal disease. Our maximum-tolerated dose and recommended phase 2 dose for any future explorations is veliparib 250mg PO BID. Regarding translational science, any future studies should include somatic genomic testing and homologous recombination deficiency scoring as additional biomarkers for possible response.
Highlights.
Combining PARP inhibition with radiation potentiates DNA damage in tumor cells
This effect may be enhanced in those with an underlying DNA damage deficiency
Combining PARP inhibition with radiation can be tolerable
A single response was observed in a platinum-sensitive, BRCA-mutation+ patient
Acknowledgments
This study was supported by the following grants U01-CA-132123 (Princess Margaret Phase I Consortium) and by the P30 UMGCC Cancer Center Support Grant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reiss KA, et al. A Phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. Clinical Cancer Research. 2015;21(1) doi: 10.1158/1078-0432.CCR-14-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalmers AJ. Poly(ADP-ribose) polymerase-1 and ionizing radiation: sensor, signaller and therapeutic target. Clin Oncol (R Coll Radiol) 2004;16(1):29–39. doi: 10.1016/s0936-6555(03)00223-1. [DOI] [PubMed] [Google Scholar]
- 3.Tentori L, et al. Inhibition of poly(ADP-ribose) polymerase prevents irinotecan-induced intestinal damage and enhances irinotecan/temozolomide efficacy against colon carcinoma. FASEB J. 2006;20(10):1709–1711. doi: 10.1096/fj.06-5916fje. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese CR, et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 2004;96(1):56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 5.Miknyoczki SJ, et al. Chemopotentiation of temozolomide, irinotecan, and cisplatin activity by CEP-6800, a poly(ADP-ribose) polymerase inhibitor. Mol Cancer Ther. 2003;2(4):371–382. [PubMed] [Google Scholar]
- 6.Nowsheen S, Bonner JA, Yang ES. The poly(ADP-Ribose) polymerase inhibitor ABT-888 reduces radiation-induced nuclear EGFR and augments head and neck tumor response to radiotherapy. Radiother Oncol. 2011;99(3):331–338. doi: 10.1016/j.radonc.2011.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert JM, et al. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res. 2007;13(10):3033–3042. doi: 10.1158/1078-0432.CCR-06-2872. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer NG, James E, Wahl RL. Poly(ADP-ribose) polymerase inhibitors combined with external beam and radioimmunotherapy to treat aggressive lymphoma. Nucl Med Commun. 2011;32(11):1046–1051. doi: 10.1097/MNM.0b013e32834a369b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dungey FA, Loser DA, Chalmers AJ. Replication-dependent radiosensitization of human glioma cells by inhibition of poly(ADP-Ribose) polymerase: mechanisms and therapeutic potential. Int J Radiat Oncol Biol Phys. 2008;72(4):1188–1197. doi: 10.1016/j.ijrobp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Tuli R, et al. Effect of inhibition of poly-(ADP ribose) polymerase on gemcitabine and radiationinduced cytotoxicity of pancreatic cancer cells, in 2011 Gastrointestinal Cancers Symposium. 2011. [Google Scholar]
- 11.Regine WF, et al. Low-dose radiotherapy as a chemopotentiator of gemcitabine in tumors of the pancreas or small bowel: a phase I study exploring a new treatment paradigm. Int J Radiat Oncol Biol Phys. 2007;68(1):172–177. doi: 10.1016/j.ijrobp.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 12.Kunos CA, et al. Low-dose abdominal radiation as a docetaxel chemosensitizer for recurrent epithelial ovarian cancer: a phase I study of the Gynecologic Oncology Group. Gynecol Oncol. 2011;120(2):224–228. doi: 10.1016/j.ygyno.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101(10):708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 16.Mantel N, Haenszel W. Statistical Aspects of the Analysis of Data from Retrospective Studies of Disease. Journal of the National Cancer Institute. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 17.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34(2) 187-+ [Google Scholar]
- 18.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 19.Gelmon KA, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, openlabel, non-randomised study. Lancet Oncol. 2011;12(9):852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 20.Ranson M, Jayson G. Targeted antitumour therapy--future perspectives. Br J Cancer. 2005;92(1):S28–S31. doi: 10.1038/sj.bjc.6602606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong PC, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 22.Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33(12):1397–1406. doi: 10.1200/JCO.2014.58.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledermann J, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 24.Mirza MR, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375(22):2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 25.Study of niraparib in combination with pembrolizumab (MK-3475) in patietns with triple negative breast cancer or ovarian cancer (KEYNOTE-162) 2016. Jan 13, 2016. [Google Scholar]
- 26.Gemcitabine hydrochloride and cisplatin with or without veliparib or veliparib alone in treating patients with locally advanced or metastatic pancreatic cancer. 2016. Jan 27, 2016. [Google Scholar]