Abstract
Social anxiety disorder (SAD) is characterized at a neurobiological level by disrupted activity in emotion regulation neural circuitry. Previous work has demonstrated amygdala hyperreactivity and disrupted prefrontal responses to social cues in individuals with SAD (Kim et al., 2011). While exposure-based psychological treatments effectively reduce SAD symptoms, not all individuals respond to treatment. Better understanding of the neural mechanisms involved offers the potential to improve treatment efficacy. In this study, we investigated functional connectivity in emotion regulation neural circuitry in a randomized controlled treatment trial for SAD. Participants with SAD underwent fMRI scanning while performing an implicit emotion regulation task prior to treatment (n=62). Following 12 weeks of cognitive behavioral therapy, acceptance and commitment therapy, or wait-list, participants completed a second scan (n=42). Psychophysiological interaction analyses using amygdala seed regions demonstrated differences between SAD and healthy control participants (HC; n=16) in right amygdala-vmPFC connectivity. SAD participants demonstrated more negative amygdala-to-vmPFC connectivity, compared to HC participants, an effect that was correlated with SAD symptom severity. Post-treatment symptom reduction was correlated with altered amygdala-to-vm/vlPFC connectivity, independent of treatment type. Greater symptom reduction was associated with more negative amygdala-to-vm/vlPFC connectivity. These findings suggest that effective psychological treatment for SAD enhances amygdala-prefrontal functional connectivity.
Keywords: psychophysiological interactions, amygdala, prefrontal cortex, fMRI, randomized controlled trial, CBT, ACT
1. Introduction
Social anxiety disorder (SAD) is characterized by a fear of being judged or scrutinized by others in social situations (Kessler et al., 2009). While psychological treatments, including cognitive behavioral therapy (CBT) and acceptance and commitment therapy (ACT), have been shown to be efficacious for SAD in randomized controlled trials (Craske et al., 2014; Rodebaugh et al., 2004), many individuals do not respond, or retain residual symptoms and impairment after treatment. Better understanding of the mechanisms of efficacious treatment change, such as associated changes in neural activity, may ultimately aid the development of more targeted interventions.
1.1 SAD and emotion regulation
The prevailing neurobiological model of anxiety disorders posits that amygdala hyperreactivity to fearful or threatening stimuli is associated with heightened emotional reactivity, while disrupted processing in prefrontal regions is linked to impairments in emotional regulation (Berkman and Lieberman, 2009; Freitas-Ferrari et al., 2010; Kim et al., 2011). Neuroscientific investigation of SAD has repeatedly shown heightened amygdala reactivity to social or emotional cues (Birbaumer et al., 1998; Cooney et al., 2006; Evans et al., 2008; Phan et al., 2006; Stein et al., 2002), the extent of which has been shown to correlate with symptom severity (Cooney et al., 2006; Goldin et al., 2009b; Phan et al., 2006; Shah and Angstadt, 2009).
Compared to the body of work investigating emotional reactivity in SAD, few studies have assessed disruptions in emotion regulation. Across these studies, there is a general trend for disrupted (increased or decreased) levels of activity in prefrontal regions (dorsolateral and ventrolateral prefrontal cortex, dl/vlPFC, dorsal anterior cingulate cortex, dACC) among individuals with SAD, relative to healthy control participants when explicitly instructed to engage in a regulatory strategy (for recent meta-analyses, see Brühl et al., 2014; Zilverstand et al., 2016). However, findings are not entirely consistent, with two recent studies demonstrated no significant differences in prefrontal activity during regulation between groups of SAD and healthy control participants (Burklund et al., 2015; Gaebler et al., 2014).
Data from one of these studies (Burklund et al., 2015; upon which the analyses in the current paper are also based) was acquired using an implicit, rather than an explicit, emotion regulation strategy (affect labeling). Affect labeling, the act of putting feelings into words, is considered an ‘incidental’ or ‘implicit’ form of emotion regulation and has been shown to be an effective regulatory strategy, diminishing the intensity of emotional reactions to labeled stimuli (Kircanski et al., 2012; Lieberman et al., 2011; Niles et al., 2015; Tabibnia et al., 2008). It is commonly used in the laboratory to investigate emotional regulation as it provides a way to measure activation in emotion regulation circuitry independent of the effort or intentionality that is typically required to engage in voluntary regulation (Creswell et al., 2007; Lieberman et al., 2007; Payer et al., 2012). Both explicit and incidental forms of emotion regulation have been shown to increase PFC and decrease amygdala activity in healthy participants (Burklund et al., 2014; Delgado et al., 2008; Hariri et al., 2000; Lieberman et al., 2007; Ochsner et al., 2002). It is notable, therefore, that when task demands are minimal, amygdala reactivity was found to be heightened in individuals with SAD, relative to healthy individuals, but there was no significant difference in right vlPFC activity during implicit emotion regulation (Burklund et al., 2015). One explanation for this effect is that dysregulated amygdala activity in SAD during implicit emotion regulation may be attributable to disrupted communication, or functional connectivity, between amygdala and prefrontal cortex, rather than a failure to activate prefrontal regions per se.
Previous functional connectivity studies have shown that while viewing face stimuli, greater SAD symptom severity was associated with greater amygdala to fusiform gyrus and amygdala to superior temporal sulcus connectivity in one study (Frick et al., 2013), or amygdala to dACC/dorsal medial PFC connectivity in another (Demenescu et al., 2013). Functional connectivity studies of emotion regulation found that while reappraising negative self-beliefs, participants with SAD demonstrated altered amygdala-prefrontal connectivity relative to HC participants. Greater prefrontal activity (in both dlPFC and right vlPFC) was associated with reduced amygdala activity, indicative of an inverse connection, to a greater extent in healthy control than SAD participants (Goldin et al., 2009a). A similar effect was demonstrated in resting state functional connectivity analyses, showing reduced correlation in amygdala and vmPFC activity in patients with SAD, compared to healthy adults (Hahn et al., 2011). Finally, one study of effective connectivity within this circuitry (using dynamic causal modelling) demonstrated impairments in bidirectional connectivity from vmPFC to amygdala in patients with SAD while perceiving emotional cues (Sladky et al., 2015a).
1.2 Treatment studies
Psychological treatments for SAD aim to alter emotion regulation capacities, albeit through different approaches. CBT teaches ‘reappraisal’, the intentional re-framing of negative or unpleasant thoughts or experiences (Craske, 2010). ACT promotes ‘acceptance’, the acknowledgement that emotional experiences are fleeting and can be viewed with a sense of perspective (Hayes et al., 1999). Existing studies assessing the neural correlates of CBT for SAD have investigated differences in emotional reactivity and explicit reappraisal. In a study of internet-delivered CBT (iCBT) for SAD, treatment-related reductions in amygdala reactivity to affective faces were associated with i) increases in mOFC activity (i.e., inverse connectivity) and ii) decreases in ventral and dorsal lateral PFC activity (i.e., positive connectivity) (Månsson et al., 2013). Two studies comparing CBT to wait-list groups of SAD patients demonstrated treatment-related increases in i) inverse connectivity between the dmPFC and left amygdala while reappraising negative self-beliefs (Goldin et al., 2013), and ii) positive connectivity among prefrontal regions including medial PFC, dmPFC, left dACC, left dlPFC and left vlPFC when reappraising social criticism (Goldin et al., 2014). These studies have all focused on explicit emotion regulation, requiring intentional engagement with a regulatory strategy. It is unknown whether treatment for SAD impacts functioning within amygdala-prefrontal neural circuitry during incidental emotion regulation, when task demands are reduced, and how such connectivity might be affected by different treatment strategies.
1.3 Aims and hypotheses
In the current study, we aimed to investigate the effects of psychological therapy for SAD on neural functional connectivity during incidental emotion regulation. We also assessed differences in functional connectivity across two treatments conditions (CBT and ACT) compared to a wait-list (WL) control group. Data in this study was obtained as part of a larger RCT for SAD (Craske et al., 2014). It was hypothesized that individuals who experienced reduction of SAD symptoms following psychological treatment (CBT or ACT) would demonstrate improved prefrontal ‘down-regulation’ of amygdala reactivity as evidenced by greater inverse functional connectivity.
2. Methods
Data were collected as part of a RCT of CBT and ACT for social anxiety disorder. Full details of methods and outcomes for the RCT are reported elsewhere (Craske et al., 2014). Below is a brief description of methodology relevant to the current study.
2.1 Participant recruitment and screening
Participants were recruited through the University of California, Los Angeles (UCLA) Anxiety and Depression Research Center, flyers posted throughout the UCLA community, newspaper and internet advertisements. Participants provided informed consent prior to assessment and the research protocol was approved by the UCLA Office for the Protection of Human Research Subjects. Participants were aged 18–45 years old, English speaking and right-handed (see Table 1 for demographic details by group). Exclusion criteria were: standard MRI exclusions (pregnancy, claustrophobia, non-removable metal, serious medical conditions or brain damage); history of bipolar disorder, substance-use disorders, suicidality, psychosis or psychiatric hospitalizations; modifications to psychotropic medication (past month for benzodiazepines, past 3 months for SSRIs/SNRIs and heterocyclics); current cognitive or behavioral psychotherapy for anxiety disorder or modifications to other psychotherapies in the past 6 months. Of the participants included in this analysis, 17.7% were currently were stabilized on psychotropic medication at the beginning of the study (3 in the CBT group, 4 in the ACT group and 4 in the wait-list control group).
Table 1.
Participant demographic information
| Pre-treatment assessment | Post-treatment assessment | ||||||
|---|---|---|---|---|---|---|---|
| HC | SAD | SAD | |||||
| - | CBT | ACT | WL | CBT | ACT | WL | |
| N | 16 | 20 | 24 | 18 | 13 | 16 | 13 |
| Age mean years (SD) |
27.47 (6.59) |
27.80 (7.30) |
27.46 (5.93) |
26.54 (6.52) |
26.77 (6.85) |
26.93 (5.10) |
27.11 (6.26) |
| Gender (M/F) | 7/9 | 12/8 | 11/13 | 10/8 | 7/6 | 9/7 | 8/5 |
| Mean LSAS Score (SD) |
17.76 (6.21) |
79.86 (15.55) |
87.69 (19.17) |
74.77 (20.13) |
55.99 (22.80) |
58.91 (22.07) |
71.65 (16.91) |
2.2 Diagnostic and self-report measures
Diagnosis of SAD was based on Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for current, principal or co-principal diagnosis of SAD, with a clinical severity rating (CSR) of 4 or higher, indicating clinically significant severity. Diagnostic evaluations were conducted using the Anxiety Disorders Interview Schedule-IV (ADIS IV; Brown et al., 1994) by trained interviewers. Participants in the healthy control (HC) group had no current or past psychiatric diagnoses. SAD severity was assessed using the Liebowitz Social Anxiety Scale–Self-Report Version (LSAS-SR, a measure with high reliability and validity; Baker et al., 2002; Fresco et al., 2001). The LSAS-SR is a 24-item measure that assesses fear and avoidance of social interactional and performance situations and was completed as part of a laboratory session conducted 1–2 weeks before fMRI sessions.
2.3 Treatment procedure
A subset of participants described in Craske et al., 2014 [3] participated in an fMRI component of this RCT. One hundred participants with SAD were stratified by age and gender and randomized to CBT (n = 40), ACT (n = 34), or wait list (WL; n = 26). Seventy-one of these participants completed pre-treatment fMRI scanning along with 17 HC participants. Fifty-three SAD participants completed a second fMRI scanning session 12 weeks later after completing CBT or ACT treatment, or on wait-list (the WL group was offered their choice of CBT or ACT treatment free of charge after completing the second fMRI session). Table 2 provides details of final sample sizes and reasons for excluded datasets. Participants in the CBT and ACT groups received 12 weekly 1-hr individual therapy sessions. Therapy for both treatment conditions was based on detailed treatment manuals for anxiety (CBT (Hope et al., 2000), ACT (Eifert and Forsyth, 2005); see (Craske et al., 2014) for full details). In brief, both treatments involved exposure to feared social cues but differed in the framing of the intent of the exposure. CBT exposure was focused on explicit cognitive restructuring of negative thoughts and evaluations. ACT exposure was focused on mindful acceptance, the practice of experiencing anxiety-related thoughts as part of the broader, ongoing stream of present experience. Only participants with full treatment compliance (i.e. completed all 12 sessions of treatment) were included in the reported analyses.
Table 2.
Details of participants included at each time point. NL = no LSAS-SR data, BS = bad scan (defined as: >10% of images with global signal intensity >2.5SD of mean, or affected by motion of more than 2.5mm in any direction), BB = bad baseline data, i.e. baseline data excluded due to NL or BS.
| HC | SAD | ||||
|---|---|---|---|---|---|
| CBT | ACT | WL | TOTAL | ||
| Completed pre-treatment fMRI session |
17 | 26 | 25 | 20 | 88 |
| Excluded | 1 (BS) |
6 (3NL, 3BS) | 1 (BS) | 2 (BS) | 10 |
| Included in baseline analysis | 16 | 20 | 24 | 18 | 78 |
| Lost to follow-up | - | 3 | 8 | 2 | 14 |
| Complete post fMRI session | - | 17 | 19 | 17 | 53 |
| Excluded | - | 4 (2BS, 1NL, 1BB) |
3 (2NL, 1BB) |
4 (2BS, 1NL, 1BB) | 11 |
| Included in treatment analysis | - | 13 | 16 | 13 | 42 |
2.4 fMRI task, acquisition and data analysis
2.4.1 Affect labeling task
Full details of the affect labeling task are described elsewhere (along with findings from a GLM analysis of pre-treatment data; Burklund et al., 2015). In brief, participants observed photographs of emotional facial expressions and geometric shapes and were instructed to complete simple labeling and matching tasks (affect labeling, gender labeling, affect matching and shape matching). In the labeling conditions, participants were asked to respond via button press to select which of two words best match the affect or gender of the face displayed (match conditions require selection of matching images rather than matching words). The current study focused on assessment of implicit emotion regulation capacity, as indexed by the contrast of affect label versus gender label. This contrast isolates activity specific to emotion-based linguistic processing while controlling for processes involved with emotion perception, response selection and verbal processing (as described in Lieberman et al., 2007). Stimuli were presented in a blocked design, with four blocks of each condition type and six trials per block. Each trial lasted 5 seconds, with stimuli presented for the entire trial length, with a 10 second inter-trial-interval during which a fixation crosshair was presented. Blocks began with a 3 second instruction cue. Condition order was counterbalanced across participants.
2.4.2 fMRI acquisition parameters
Magnetic resonance images were acquired using a Trio 3.0 Tesla MRI scanner at the UCLA Ahmanson-Lovelace Brain Mapping Center. For each participant, a high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, TR=5000ms, TE=34ms, matrix size=128×128, resolution=1.6mmx1.6mmx3mm, FOV=200mm, 36 slices, 3mm thick, flip angle=90°, bandwidth=1302Hz/Px) was acquired coplanar with the functional scans. Four functional runs were acquired, with a total of 344 volumes (gradient-echo, TR=3000ms, TE=25ms, flip angle=90°, matrix size=64×64, resolution=3.1mmx3.1mmx3.0mm, FOV=200mm, 36 axial slices, 3mm thick, bandwidth=2604 Hz/Px).
2.4.3 fMRI data analysis
Imaging data were analyzed using SPM8 (Wellcome Trust Center for Neuroimaging, University College London, UK, http://www.fil.ion.ucl.ac.uk). Functional images for each participant were realigned to correct for head motion, co-registered to the high-resolution structural images, normalized into a standard stereotactic space as defined by the Montreal Neurological Institute and smoothed with an 8mm Gaussian kernel FWHM. Experimental blocks were modeled using a boxcar function convolved with the canonical hemodynamic response. Motion parameters were included in the model as regressors of no interest. Linear contrasts of affect label vs gender label and affect match vs shape match were computed at the first-level for each participant using a fixed-effects model. Treatment-related differences in neural activity during affect-labeling compared to gender labeling were investigated using a paired-samples t-test for pre- and post-treatment scans among SAD participants. Given strong a priori hypotheses regarding the functioning of the amygdala, a small volume correction was used to assess changes in amygdala reactivity. Multiple comparison correction was performed using 3dClustStim (AFNI: http://afni.nimh.nih.gov/afni/), which conducts a Monte Carlo simulation. Using 10,000 iterations and an alpha level of 0.05, a voxelwise threshold of p<.005 (1-tailed) combined with a minimum cluster size of 4 voxels was determined for the amygdala.
Psychophysiological interaction (PPI) analyses were conducted using right and left amygdala seed regions to assess functional connectivity of these regions in a task-dependent manner, implemented using generalized PPI (gPPI) within SPM8 (McLaren et al., 2012). Other approaches for connectivity analysis typically focus on correlations between specific regions of interest during task active periods. PPI performs a more rigorous interaction analysis to investigate how activity in a seed region of interest is correlated with activity across the whole brain as a function of task. That is, only voxels in which there is a significant change in the extent of correlation of activity with the seed region during task-active periods, compared to baseline, will be detected. Task-specific changes in functional connectivity are subsequently interpreted as regions working in concert to achieve a task-related function. Analyses were repeated for both Pre- and Post-treatment scans for each participant, producing whole-brain images reflecting right or left amygdala functional connectivity for the contrast ‘affect label - gender label’ for each participant. A whole brain two-sample t-test was used to investigate group differences in functional connectivity between SAD and HC participants at baseline.
Analyses of treatment-related change in functional connectivity were performed on change scores in symptom data [‘LSAS-SR (Post)’ – ‘LSAS-SR (Pre)’] and neural data [‘Affect label > Gender Label (Post)’ – ‘Affect label > Gender Label (Pre)’; computed using imcalc, SPM8]. A one-way ANOVA with a covariate of interest (LSAS-SR) was used to investigate the relationship between change in symptom levels and change in functional connectivity across groups. Post-hoc analyses were conducted to investigate specific between-groups differences for CBT vs. ACT, CBT vs. WL and ACT vs. WL. In all PPI analyses, whole-brain correction for multiple comparisons using an alpha level of 0.05 determined a voxelwise threshold of p<.005 combined with a minimum cluster size of 40 voxels (two-tailed tests; 3dClustSim, AFNI).
3. Results
Pre-treatment task-based activity in this study is the subject of another paper (see Burklund et al., 2015). In brief, increased rvlPFC activity and decreased amygdala activity was observed in the HC group during affect labeling compared to gender labeling. The SAD group demonstrated increased activity in both the rvlPFC and amygdala, and in a direct comparison, only amygdala activity was significantly greater in the SAD than HC group). In both groups, increased activity was also observed in occipital lobe regions and the cerebellum, while in the HC group, there was increased insula activity and in the SAD group there was increased posterior medial frontal gyrus and middle temporal gyrus activity. Both groups showed decreased activity in ventromedial and cingulate cortex as well as temporal and occipital areas, among other regions (see Supplementary Materials; Table S1 and http://scan.oxfordjournals.org/content/10/2/199/suppl/DC1 for full results).
3.1 SAD symptom severity pre- and post-treatment
A one-way ANOVA confirmed no significant differences in LSAS-SR score based on allocation of treatment group (CBT, ACT or WL; F(2, 61) = 2.52, p = .09) prior to beginning of treatment, consistent with results for the full sample of the parent study [3]. Post-hoc Bonferroni comparisons also confirmed no significant differences between pairs of groups (all p > .10). A one-way ANOVA of symptom change (Pre - Post treatment LSAS-SR score) demonstrated a significant main effect of group (CBT, ACT or WL; F(2, 41) = 12.78, p < .001) with post-hoc Bonferroni pairwise comparisons demonstrating significantly greater symptom reduction in CBT than WL (p = .003), in ACT than WL (p < .001) and no significant difference between CBT and ACT (p = .81), also consistent with [3].
3.2 Amygdala activity during implicit emotion regulation pre- and post-treatment
Pre-/post-treatment changes in amygdala reactivity were investigated as part of the current study. A paired-samples t-test demonstrated a significant decrease in amygdala activity among SAD participants for the affect label vs. gender label contrast after treatment, compared to before treatment (p < .005, 1-tailed, 15 voxels, peak voxel t = 3.37, MNI coordinates, 36, 2 −26).
3.3 Pre-treatment PPI analysis of amygdala connectivity
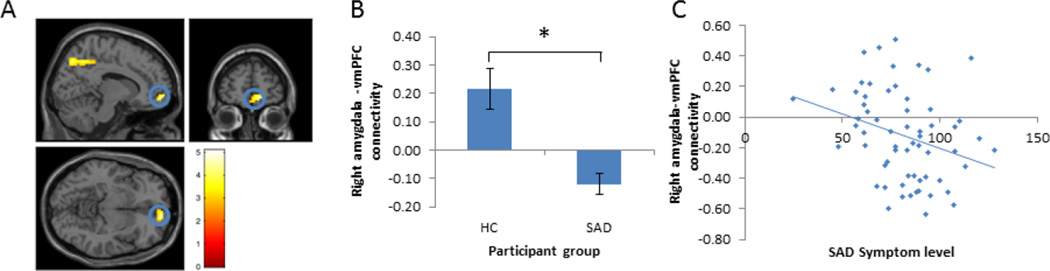
Using the right amygdala as a seed region, there were significant differences between HC and SAD groups in functional connectivity with vmPFC, insula, superior parietal cortex, inferior frontal gyrus/premotor cortex and posterior cingulate cortex (see Figure 1, Table 3). Across these regions, there was greater positive functional connectivity with the amygdala in HC than SAD participants. Within the SAD group, level of right amygdala-vmPFC connectivity was significantly negatively correlated with LSAS-SR score (r = −.29, n = 64, p = .02); the higher the LSAS-SR score, the more negative the amygdala-to-vmPFC connectivity during affect labeling (Figure 1). Using the left amygdala as a seed region resulted in no significant clusters.
Figure 1.
Differences between SAD patients and healthy controls (HC) in functional connectivity during implicit emotion regulation (affect labeling > gender labeling). A) Prior to treatment, there were differences in right amygdala functional connectivity between HC and SAD participants during affect labeling, including in the vmPFC. B) Right amygdala-to-vmPFC functional connectivity was positive in HC participants and negative in SAD participants during affect labeling, based on mean connectivity estimates across all voxels within the suprathreshold vmPFC cluster identified (* denotes significance at the whole brain level, as established during whole brain analysis, α = .05, p < .005, k > 40; error bars represent mean +/− standard error). C) Within the SAD group, symptom severity was correlated with amygdala-vmPFC functional connectivity, such that higher symptom levels were associated with more negative connectivity (r = −.29, n = 64, p = .02).
Table 3.
Results of whole brain PPI analysis using right and left amygdala seed regions, ‘affect label – gender label’ contrast showing differences in functional connectivity during emotion regulation between HC vs. SAD and correlated with LSAS-SR score prior to treatment.
| Amygdala seed (L/R) |
Anatomical region | Brodmann’s Area |
MNI coordinates: x,y,z | t | k | ||
|---|---|---|---|---|---|---|---|
| Healthy Control > SAD patients (affect label - gender label) | |||||||
| R | Superior parietal cortex | 7 | 15 | −49 | 46 | 5.09 | 19 6 |
| R | Superior parietal cortex | 7 | −9 | −76 | 40 | 3.91 | 40 |
| R | Inferior frontal gyrus/premotor cortex |
44/6 | −57 | 8 | 22 | 4.52 | 64 |
| R | vmPFC/OFC | 11 | 12 | 56 | −8 | 4.63 | 52 |
| R | Insula | 48 | −42 | −13 | 16 | 3.86 | 51 |
| R | Posterior cingulate cortex | 23 | −6 | −25 | 28 | 3.33 | 45 |
| R | Superior parietal cortex | 7 | 15 | −49 | 46 | 5.09 | 19 6 |
| R | Superior parietal cortex | 7 | −9 | −76 | 40 | 3.91 | 40 |
| L | No suprathreshold clusters | ||||||
| All Participants (HC and SAD) Baseline correlation with LSAS-SR | |||||||
| R | vmPFC/OFC | 10/11 | 9 | 59 | −5 | − 4.55 |
16 1 |
| R | Inferior frontal gyrus/premotor cortex | 44/6 | −60 | 8 | 22 | − 4.46 |
49 |
| R | Inferior parietal cortex | 40 | 51 | −67 | 40 | − 3.34 |
43 |
| R | Superior parietal cortex | 7 | 15 | −52 | 46 | − 4.46 |
47 |
| L | Inferior frontal gyrus | 44/6 | −54 | 14 | 22 | − 4.02 |
14 7 |
| L | Inferior parietal cortex | 40/2 | 54 | −28 | 28 | − 4.47 |
71 |
| L | Posterior cingulate | 6/31 | 9 | −34 | 52 | − 4.86 |
45 |
A whole-brain correlation of LSAS-SR score with right and left amygdala connectivity additionally demonstrated a negative association between SAD symptoms and right amygdala connectivity to vmPFC, IFG and parietal cortex, and left amygdala connectivity to IFG, inferior parietal cortex and posterior cingulate cortex. In each of these associations, higher levels of SAD symptomatology were associated with reduced amygdala connectivity.
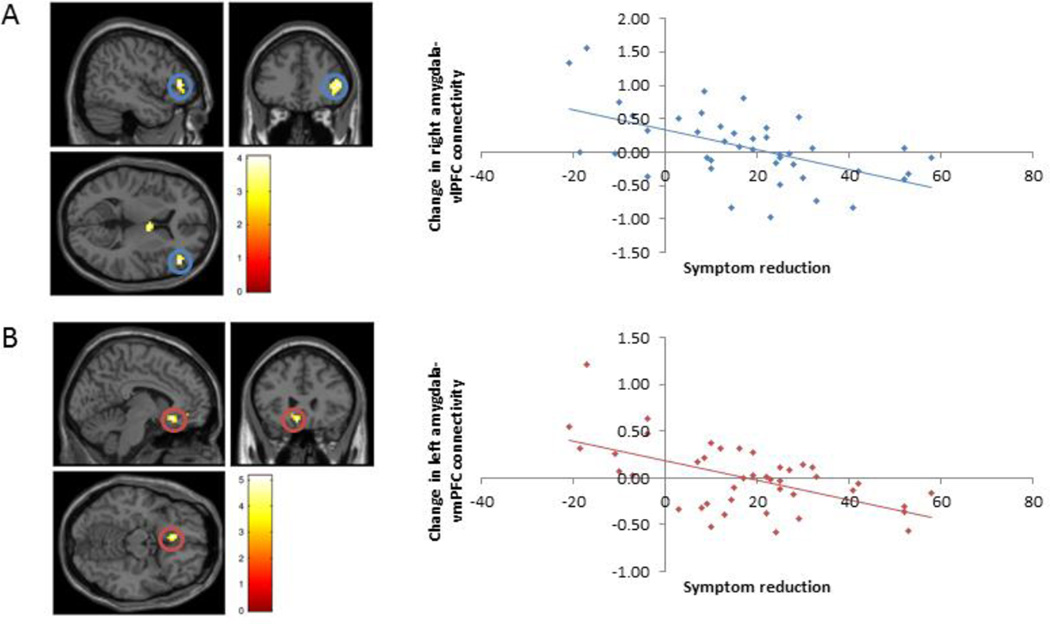
3.4 Treatment-related changes in amygdala connectivity
Across all SAD participants, there was an increase in functional connectivity between right amygdala and visual cortex, parietal regions and primary motor cortex, but no significant changes in left amygdala functional connectivity after treatment, compared to before (see Table 4). Using the right amygdala as a seed region, greater LSAS-SR score reduction was associated with more negative change (i.e. reduced positive/greater inverse connectivity) in amygdala-vlPFC functional connectivity from pre- to post-treatment (Figure 2, Table 4). The same analysis with the left amygdala demonstrated a similar pattern with amygdala-vmPFC functional connectivity (see Figure 2, Table 4). Comparisons between treatment groups demonstrated no significant clusters related to the main effect of treatment group (CBT vs. ACT vs. WL). Post-hoc pairwise comparisons using the right amygdala seed region demonstrated one significant cluster for WL > CBT, located in the inferior temporal gyrus (and no clusters for CBT > WL). Using the left amygdala as seed region, there was one significant cluster for WL > CBT, located in the left dlPFC (and again no significant clusters for CBT > WL). There were no significant differences in functional connectivity using either the left or right amygdala as seed regions between groups of ACT vs. WL or CBT vs. ACT.
Table 4.
Pre- to post-treatment changes in functional connectivity from whole brain PPI analyses using right and left amygdala seed regions (contrast: ‘Post (Affect Label – Gender Label) - Pre (Affect Label – Gender Label)’). Results are presented for: i) pre- to post-treatment changes, ii) pre- to post-treatment changes correlated with symptom reduction across all groups, iii) group differences in functional connectivity pre- to post-treatment. There were no significant suprathreshold clusters for left or right amygdala seed regions for the comparisons: CBT > WL, ACT > WL, WL > ACT , CBT > ACT or ACT > CBT.
| Amygdala seed (L/R) |
Anatomical region | Brodmann’s Area |
MNI coordinates: x,y,z | t | k | ||
|---|---|---|---|---|---|---|---|
| Pre-Post changes in functional connectivity | |||||||
| R | Visual cortex | 17/18 | 21 | −82 | 10 | 4.24 | 94 |
| R | Parietal lobe/angular gyrus | 40/39 | −57 | −55 | 25 | 3.68 | 45 |
| R | Primary motor cortex | 6 | 33 | 14 | 22 | 4.86 | 58 |
| R | Parietal cortex | 7 | −21 | −79 | 43 | 3.99 | 24 4 |
| All groups (Post-Pre) correlation with change in LSAS-SR (Post-Pre) | |||||||
| R | vlPFC | 45 | 48 | 38 | 7 | 4.05 | 48 |
| L | vmPFC | 10/11 | −6 | 26 | −14 | 5.15 | 54 |
| L | Supplementary motor area | 32 | 6 | 5 | 49 | − 4.11 |
68 |
| WL (Post-Pre) > CBT(Post-Pre) | |||||||
| R | Inferior temporal gyrus | 19 | 42 | −67 | 10 | 4.08 | 46 |
| L | dlPFC | 8/9 | −36 | 26 | 23 | 5.00 | 59 |
Figure 2.
Treatment-related changes were observed in amygdala-prefrontal functional connectivity during affect labeling. Using the right amygdala as a seed region, greater symptom reduction was associated with more negative functional connectivity with right vlPFC from pre- to post-treatment (A). Similarly, using the left amygdala as a seed region, greater symptom reduction was associated with more negative functional connectivity with vmPFC from pre-to-post treatment (B). Together, results indicate that larger reductions in anxiety were associated with stronger negative amygdala-prefrontal connectivity at post- relative to pre-treatment. [Blue indicates changes in right amygdala functional connectivity; Red indicates changes in left amygdala functional connectivity; correlations are significant based on whole brain analyses, p < .005, clusters thresholded at k > 40]
4. Discussion
In this paper, we report three major results. First, we observed differential right amygdala-to-vmPFC functional connectivity between HC and SAD patients during an affect labeling task. In HC participants, we observed positive functional connectivity, while in SAD patients, we observed negative functional connectivity. Second, the strength of this right amygdala-vmPFC connectivity was correlated with symptom severity among SAD participants such that greater symptom severity was associated with more negative functional connectivity. Third, in post-treatment analyses, SAD symptom reduction was specifically associated with altered right amygdala-right vlPFC and left amygdala-vmPFC functional connectivity such that anxiety reductions over time were associated with stronger inverse functional connectivity between amygdala and prefrontal regions. These results suggest that one consequence of CBT and ACT may be to strengthen neural systems supporting emotion regulatory abilities.
4.1 Pre-treatment differences in functional connectivity
We demonstrated positive right amygdala-vmPFC functional connectivity during affect labeling in healthy participants, but inverse functional connectivity among individuals with SAD. We further demonstrate that the level of SAD symptomatology was significantly associated with connectivity strength, with higher symptom levels of SAD associated with more negative right amygdala-vmPFC functional connectivity during affect labeling. Meta-analyses of explicit emotion regulation have demonstrated a different pattern of effects. Compared to healthy adults, individuals with SAD had reduced activity in lateral prefrontal regions, as well as reduced inverse connectivity between these regions and the amygdala (see Brühl et al., 2014 for review).
Previous work has suggested that differing task demands may influence the recruitment of different prefrontal sub-regions for regulatory purposes, i.e. during explicit regulation, lateral PFC regions may be implicated, while for more implicit regulation, medial PFC regions are involved (see Sladky et al., 2015a).
In support of this interpretation, a resting-state study demonstrated reduced amygdala-vmPFC connectivity (Hahn et al., 2011) and an effective connectivity study of emotional reactivity found a decreased forward connection from amygdala to OFC in SAD individuals compared to healthy adults (Sladky et al., 2015a). These findings have been interpreted as representing impaired automatic recruitment of vmPFC/OFC regions for regulatory functions in SAD. Affect labeling, however, does not perfectly align with this pattern. Although considered an implicit or ‘incidental’ regulation approach, recruitment of lateral prefrontal regions is typically observed (Lieberman et al., 2007). Here, we observed differential connectivity between amygdala and both medial and lateral regions of PFC, highlighting the need for further investigation to understand differential contributions of prefrontal sub-regions during different types of regulation.
4.2 Post-treatment changes in functional connectivity
Investigation of changes in functional connectivity associated with symptom reduction, independent of treatment group (CBT, ACT or WL), demonstrated altered connectivity between right amygdala and right vlPFC as well as between left amygdala and vmPFC. Greater symptom reduction was associated with more negative right amygdala-vlPFC and left amygdala-vmPFC functional connectivity from pre- to post-treatment during affect labeling. Notably, this pattern of effects was observed only with the inclusion of the symptom change covariate, suggesting that changes in amygdala-prefrontal connectivity are dependent upon an individual’s response to treatment. These findings suggest efficacious treatment (as indexed by symptom reduction) is associated with more negative amygdala-PFC connectivity during affect labeling. This increase in inverse prefrontal-amygdala connectivity is consistent with findings from studies of the impact of CBT on explicit emotion regulation (Goldin et al., 2014). It is notable that prior to treatment, individuals with SAD demonstrated more inverse connectivity between right amygdala and vmPFC, while treatment changes were linked to stronger inverse connectivity between left amygdala and vmPFC. These effects require further investigation, but may point to heterogeneity in the functioning of different subregions of the vmPFC, or differences in the role of amygdala-prefrontal connectivity across hemispheres.
4.3 Limitations
A central tenet of current models of disrupted emotion regulation in anxiety disorders considers prefrontal regions to effectively ‘down-regulate’ amygdala hyper-reactivity. PPI functional connectivity analyses, however, are correlational in nature. A change in correlation of activity between amygdala and PFC can therefore plausibly reflect both the feedforward effect of amygdala activity on prefrontal regions and/or the feedback effect of prefrontal regions on amygdala activity. Previous work using effective connectivity methods (which do allow inference on directionality) has demonstrated bidirectional disruption in amygdala-to-vmPFC connectivity during emotional reactivity in SAD (Sladky et al., 2015a), while affect labeling was found to specifically increase inverse connectivity from right vlPFC to amygdala in healthy adults (Torrisi et al., 2013). It might therefore be hypothesized that SAD is associated with disrupted reciprocal amygdala-vmPFC connectivity (during emotional reactivity and regulation) and that effective treatment specifically promotes prefrontal downregulation of amygdala. Future effective connectivity analyses of treatment effects would allow specific investigation of this.
It should be noted that substantial between-subject heterogeneity was observed in changes in functional connectivity. While participants with greatest symptom reduction demonstrated more negative amygdala-prefrontal functional connectivity, participants with less or no symptom reduction demonstrated effects in the opposite direction (more positive amygdala-prefrontal connectivity). This variance may be in part related to potential hetereogeneity in disrupted amygdala reactivity among individuals with social anxiety disorder. Prior research has described different aspects of dysfunctional amygdala reactivity including: a temporal delay in amygdala reactivity in SAD (Campbell et al., 2007); more sustained amygdala reactivity in learned fear responses (Andreatta et al., 2015); and over-generalization of amygdala reactivity to non-threatening cues (Cooney et al., 2006). While the relationships between these types of disruption are not well understood at this stage, it is possible that different types of disrupted amygdala reactivity constitute different ‘neural profiles’ of SAD and in turn, might be characterized by different patterns of functional connectivity with prefrontal cortical regions. A better understanding of these individual differences hold potential for improving our mechanistic understanding of anxiety disorders, and their effective treatment, at a neurobiological level.
Due to the small proportion of individuals in this study taking psychotropic medications, it was not possible to investigate medication status as a potential moderator of treatment effects. This would be particularly relevant for future work as recent studies have demonstrated that administration of psychotropic medications in healthy volunteers can impact amygdala functional connectivity, with different substances affecting connectivity with different regions. Administration of (S)-citalopram was found to be associated with enhanced downregulation of amygdala by orbitofrontal cortex, as demonstrated using dynamic causal modeling (Sladky et al., 2015b), ketamine administration was found to modulate connectivity between amygdala and pregenual anterior cingulate cortex (Scheidegger et al., 2016) and psilocybin administration reduced top-down amygdala to primary visual connectivity (Kraehenmann et al., 2016). It might be hypothesized that each of these treatments could impact different stages of emotional processing and investigations of how these medications impact functional connectivity in individuals experiencing disrupted emotion regulation be an important next step. Comparison of the effects of pharmacological and psychological interventions would be of particular interest when considering how therapeutic approaches might be combined to optimally target particular systems thought to be dysregulated in affected individuals.
An additional limitation of work presented here is that the sample size was too small to investigate the impact of comorbidities on treatment-related changes in emotion regulation neural circuitry. Recent work has demonstrated marked differences in neural activity associated with affect labeling in individuals with comorbid depression (Burklund et al., 2014). It is plausible that comorbidities similarly impact changes in functional connectivity. The sample size was in part affected by the number of participants lost to follow-up, missing data and imaging data removed due to motion. Participant attrition is a major challenge in multi-visit studies such as that described here and high levels of anxiety in subjects may have additionally contributed to greater amounts of motion during scans. Future studies might aim to address these concerns with additional strategies to ensure completion of all sessions.
4.4 Implications for understanding of emotion regulation neural circuitry
Here we showed disrupted functional connectivity between the amygdala and medial areas of the PFC in SAD and altered connectivity between amygdala and both medial and lateral areas of PFC following treatment. Medial regions of PFC are broadly considered to be recruited for autonomous emotion regulation while lateral regions are thought to be necessary for cognitive reappraisal and voluntary downregulation (Ochsner and Gross, 2005; Phillips et al., 2008). Were this functional separation to hold true, findings presented here would suggest an impairment in neural circuitry supporting incidental emotion regulation during affect labeling pre-treatment. Treatment-related symptom reduction might be thought to act through compensatory mechanisms, altering engagement of both medial ‘incidental’ and lateral ‘voluntary’ emotion regulation regions. These systems are, however, widely regarded to be reciprocally linked, acting in concert to support voluntary and automatic processing and reactions to emotional stimuli (Phillips et al., 2008). Further investigation of these possibilities would be required to understand this effect more thoroughly.
4.5 Conclusion
In sum, we present differences in functional connectivity between right amygdala and vmPFC between healthy control and SAD patients, and treatment-related changes in amygdala-to-vl/vmPFC functional connectivity during incidental emotion regulation. These findings further implicate frontoamygdalar circuitry in disrupted emotion regulation functioning in social anxiety disorder. We also demonstrate for the first time that greatest symptom reduction, whether achieved from CBT or ACT, was associated with more negative amygdala-vl/vmPFC functional connectivity during emotion regulation. Future work should aim to replicate this effect and compare different measures of emotion regulation capacity before and after treatment to improve our mechanistic understanding of the functioning of this neural circuitry and how it responds to treatment. In addition, similar analyses might be used prospectively to investigate predictors of treatment response.
Supplementary Material
Highlights.
Amygdala-prefrontal functional connectivity in social anxiety disorder was assessed
SAD symptomatology was associated with more negative amygdala-vmPFC connectivity
Following psychological therapy amygdala-prefrontal connectivity changed
Symptom reduction was linked to more inverse amygdala-vm/vlPFC connectivity
Acknowledgments
The authors wish to acknowledge the contributions of Lily Brown, Andrea Niles, Carolyn Davies and Benjamin Tabak in the preparation of data and discussion of analyses.
Funding and Disclosure
This project was funded by the National Institutes of Mental Health 1 R21 MH081299 (PIs: Craske, Lieberman and Taylor).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing financial interests in relation to the work described and declare no conflict of interest.
References
- Andreatta M, Glotzbach-Schoon E, Mühlberger A, Schulz SM, Wiemer J, Pauli P. Initial and sustained brain responses to contextual conditioned anxiety in humans. cortex. 2015;63:352–363. doi: 10.1016/j.cortex.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Baker SL, Heinrichs N, Kim H-J, Hofmann SG. The Liebowitz social anxiety scale as a self-report instrument: a preliminary psychometric analysis. Behaviour research and therapy. 2002;40:701–715. doi: 10.1016/s0005-7967(01)00060-2. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Using Neuroscience to Broaden Emotion Regulation: Theoretical and Methodological Considerations. Soc Personal Psychol Compass. 2009;3:475–493. doi: 10.1111/j.1751-9004.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9:1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Brown TA, Barlow DH, Di Nardo PA. Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV): Client Interview Schedule. Graywind Publications Incorporated. 1994 [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neuroscience & Biobehavioral Reviews. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Burklund LJ, Craske MG, Taylor SE, Lieberman MD. Altered emotion regulation capacity in social phobia as a function of comorbidity. Soc Cogn Affect Neurosci. 2015;10:199–208. doi: 10.1093/scan/nsu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund LJ, Creswell JD, Irwin MR, Lieberman MD. The common and distinct neural bases of affect labeling and reappraisal in healthy adults. Front Psychol. 2014;5:221. doi: 10.3389/fpsyg.2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DW, Sareen J, Paulus MP, Goldin PR, Stein MB, Reiss JP. Time-varying amygdala response to emotional faces in generalized social phobia. Biological psychiatry. 2007;62:455–463. doi: 10.1016/j.biopsych.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugène F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Research: Neuroimaging. 2006;148:55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Craske MG. Cognitive behavior therapy. Ametican Psychological Association. Washington, DC: 2010. [Google Scholar]
- Craske MG, Niles AN, Burklund LJ, Wolitzky-Taylor KB, Vilardaga JC, Arch JJ, Saxbe DE, Lieberman MD. Randomized controlled trial of cognitive behavioral therapy and acceptance and commitment therapy for social phobia: outcomes and moderators. J Consult Clin Psychol. 2014;82:1034–1048. doi: 10.1037/a0037212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demenescu L, Kortekaas R, Cremers H, Renken R, van Tol M, van der Wee N, Veltman D, den Boer J, Roelofs K, Aleman A. Amygdala activation and its functional connectivity during perception of emotional faces in social phobia and panic disorder. Journal of psychiatric research. 2013;47:1024–1031. doi: 10.1016/j.jpsychires.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Eifert GH, Forsyth JP. Acceptance and commitment therapy for anxiety disorders: A practitioner’s treatment guide to using mindfulness, acceptance, and values-based behavior change. New Harbinger Publications. 2005 [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression and anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JE, Trzesniak C, Santos Filho A, Machado-de-Sousa JP, Chagas MHN, Nardi AE, Crippa JAS. Neuroimaging in social anxiety disorder: a systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Fresco D, Coles M, Heimberg RG, Liebowitz M, Hami S, Stein M, Goetz D. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological medicine. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Frick A, Howner K, Fischer H, Kristiansson M, Furmark T. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Translational psychiatry. 2013;3:e312. doi: 10.1038/tp.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler M, Daniels JK, Lamke J-P, Fydrich T, Walter H. Behavioural and neural correlates of self-focused emotion regulation in social anxiety disorder. Journal of psychiatry & neuroscience: JPN. 2014;39:249. doi: 10.1503/jpn.130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological psychiatry. 2009a;66:1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of general psychiatry. 2009b;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA psychiatry. 2013;70:1048–1056. doi: 10.1001/jamapsychiatry.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Weeks J, Heimberg RG, Gross JJ. Impact of cognitive-behavioral therapy for social anxiety disorder on the neural bases of emotional reactivity to and regulation of social evaluation. Behaviour research and therapy. 2014;62:97–106. doi: 10.1016/j.brat.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. New York: Guilford Press; 1999. Acceptance and commitment therapy. [Google Scholar]
- Hope DA, Heimberg RG, Juster HA. Managing social anxiety: A cognitive-behavioral therapy approach client workbook. Graywind Publications. 2000 [Google Scholar]
- Kessler RC, Ruscio AM, Shear K, Wittchen H-U. Epidemiology of anxiety disorders, Behavioral neurobiology of anxiety and its treatment. Springer; 2009. pp. 21–35. [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural brain research. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, Lieberman MD, Craske MG. Feelings into words: contributions of language to exposure therapy. Psychol Sci. 2012;23:1086–1091. doi: 10.1177/0956797612443830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenmann R, Schmidt A, Friston K, Preller KH, Seifritz E, Vollenweider FX. The mixed serotonin receptor agonist psilocybin reduces threat-induced modulation of amygdala connectivity. NeuroImage: Clinical. 2016;11:53–60. doi: 10.1016/j.nicl.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Inagaki TK, Tabibnia G, Crockett MJ. Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion. 2011;11:468–480. doi: 10.1037/a0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson KN, Carlbring P, Frick A, Engman J, Olsson C-J, Bodlund O, Furmark T, Andersson G. Altered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder. Psychiatry Research: Neuroimaging. 2013;214:229–237. doi: 10.1016/j.pscychresns.2013.08.012. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles AN, Craske MG, Lieberman MD, Hur C. Affect labeling enhances exposure effectiveness for public speaking anxiety. Behav Res Ther. 2015;68:27–36. doi: 10.1016/j.brat.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of cognitive neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in cognitive sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Payer DE, Baicy K, Lieberman MD, London ED. Overlapping neural substrates between intentional and incidental down-regulation of negative emotions. Emotion. 2012;12:229–235. doi: 10.1037/a0027421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodebaugh TL, Holaway RM, Heimberg RG. The treatment of social anxiety disorder. Clinical Psychology Review. 2004;24:883–908. doi: 10.1016/j.cpr.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Scheidegger M, Henning A, Walter M, Lehmann M, Kraehenmann R, Boeker H, Seifritz E, Grimm S. Ketamine administration reduces amygdalo - hippocampal reactivity to emotional stimulation. Human brain mapping. 2016 doi: 10.1002/hbm.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SG, Angstadt M. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. Journal of psychiatry & neuroscience: JPN. 2009;34:296. [PMC free article] [PubMed] [Google Scholar]
- Sladky R, Hoflich A, Kublbock M, Kraus C, Baldinger P, Moser E, Lanzenberger R, Windischberger C. Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for FMRI. Cereb Cortex. 2015a;25:895–903. doi: 10.1093/cercor/bht279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R, Spies M, Hoffmann A, Kranz G, Hummer A, Gryglewski G, Lanzenberger R, Windischberger C, Kasper S. (S)-citalopram influences amygdala modulation in healthy subjects: a randomized placebo-controlled double-blind fMRI study using dynamic causal modeling. NeuroImage. 2015b;108:243–250. doi: 10.1016/j.neuroimage.2014.12.044. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of general psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Lieberman MD, Craske MG. The lasting effect of words on feelings: words may facilitate exposure effects to threatening images. Emotion. 2008;8:307–317. doi: 10.1037/1528-3542.8.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi SJ, Lieberman MD, Bookheimer SY, Altshuler LL. Advancing understanding of affect labeling with dynamic causal modeling. Neuroimage. 2013;82:481–488. doi: 10.1016/j.neuroimage.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, Goldstein RZ. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.