Percutaneous microwave ablation of lung tissue near the heart can be performed safely and effectively with adherence to specific antenna orientations and distances relative to the heart.
Abstract
Purpose
To determine how close to the heart pulmonary microwave ablation can be performed without causing cardiac tissue injury or significant arrhythmia.
Materials and Methods
The study was performed with approval from the institutional animal care and use committee. Computed tomographic fluoroscopically guided microwave ablation of the lung was performed in 12 swine. Antennas were randomized to either parallel (180° ± 20°) or perpendicular (90° ± 20°) orientation relative to the heart surface and to distances of 0–10 mm from the heart. Ablations were performed at 65 W for 5 minutes or until a significant arrhythmia (asystole, heart block, bradycardia, supraventricular or ventricular tachycardia) developed. Heart tissue was evaluated with vital staining and histologic examination. Data were analyzed with mixed effects logistic regression, receiver operating characteristic curves, and the Fisher exact test.
Results
Thirty-four pulmonary microwave ablations were performed with the antenna a median distance of 4 mm from the heart in both perpendicular (n = 17) and parallel (n = 17) orientation. Significant arrhythmias developed during six (18%) ablations. Cardiac tissue injury occurred with 17 ablations (50%). Risk of arrhythmia and tissue injury decreased with increasing antenna distance from the heart with both antenna orientations. No cardiac complication occurred with a distance of greater than or equal to 4.4 mm from the heart. The ablation zone extended to the pleural surface adjacent to the heart in 71% of parallel and 17% of perpendicular ablations performed 5–10 mm from the heart.
Conclusion
Microwave lung ablations performed more than or equal to 5 mm from the heart were associated with a low risk of cardiac complications.
© RSNA, 2016
Introduction
Percutaneous microwave ablation is an effective treatment option for patients with primary or oligometastatic lung cancer who are medically unfit to undergo surgical resection (1–3). The treatment of lung tumors in close proximity to the heart presents technical challenges, because precise antenna placement near vital mediastinal structures is essential to avoid complications related to nontarget tissue puncture or ablation. In addition, creating ablation zones near the heart may be unpredictable due to severe perfusion-mediated tissue cooling created by the heart and large pulmonary vessels, and because of the dynamic relationship between the ablation zone and the continuously beating heart. For instance, ablations performed too close to the heart may damage cardiac tissue or provoke dangerous arrhythmias (4), while placement of ablation applicators too far away from the heart may result in viable tumor left behind along the cardiac margin of the tumor (5). To date, in the treatment of lung tumors adjacent to the heart, this balance of treatment effectiveness versus safety has not been adequately quantified. Therefore, the purpose of our study was to determine how close to the heart a percutaneous pulmonary microwave ablation can be performed without causing cardiac tissue injury or a significant arrhythmia in a pig model.
Materials and Methods
Animal Subjects and Anesthesia
This study was performed with approval from our institutional animal care and use committee and was in compliance with National Research Council guidelines (6). Twelve female swine (mean weight, 70 kg; Arlington Farms, Arlington, Wis) were sedated with tiletamine and zolazepam (Telazol; Zoetis, Kalamazoo, Mich), atropine (Phoenix Pharmaceutical, St Joseph, Mo), and xylazine (AnaSed; Lloyd, Shenandoah, Iowa) administered by means of intramuscular injection. The animals were intubated, and anesthesia was maintained with inhaled isoflurane (Halocarbon Laboratories, River Edge, NJ) during mechanical ventilation. Intravenous fluids were administered through an auricle vein.
Ablation Protocol
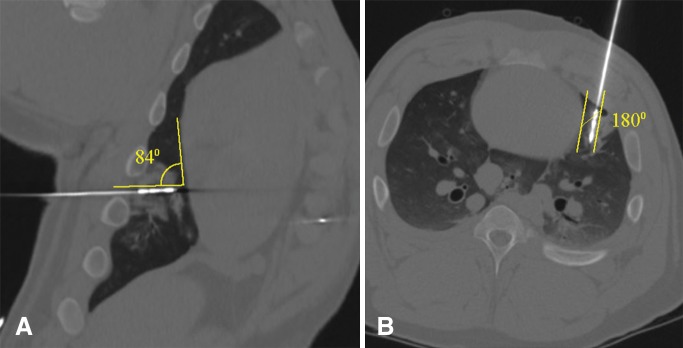
All ablations were performed by using a 17-gauge gas-cooled 2.45-GHz microwave ablation system (Certus 140 PR 15 antenna; NeuWave Medical, Madison, Wis). Two authors (F.L. and C.B.) are consultants for NeuWave Medical, which did not provide any support for the study. The authors who are not consultants for the company (remaining authors) had control of study data. Antennas were positioned by two authors (G.C. and E.N., each with 4 years of experience) with the use of computed tomographic (CT) fluoroscopic guidance. Each antenna insertion tract was positioned at either a perpendicular or parallel orientation relative to the surface of the heart. Perpendicular positioning was defined as a mean angle ± standard deviation of 90° ± 20°, and parallel orientation was defined as an angle of 180° ± 20° between the antenna shaft and the surface of the heart (Fig 1). The binary orientation of the antenna (perpendicular or parallel) was randomized for each ablation.
Figure 1:
Examples of perpendicular versus parallel antenna orientations relative to heart. A, Oblique coronal and, B, oblique axial multiplanar CT images from separate ablations reconstructed along axis of microwave antenna show perpendicular antenna orientation in, A, defined as a mean antenna-heart angle of 90° ± 20° and a parallel antenna orientation in B, defined as a mean antenna-heart angle of 180° ± 20°.
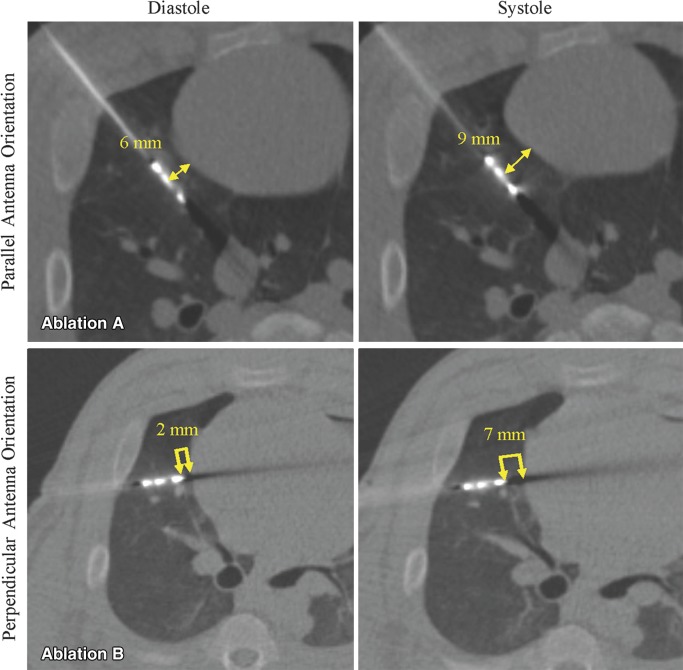
The distance between the heart and antenna (a) tip, if in perpendicular orientation, and (b) emission point (1 cm proximal to the tip), if in parallel orientation, was also randomized to 0.75 mm increments of 0–10 mm from the heart surface before each ablation (Fig 2). All percutaneous ablations were performed for 5 minutes at 65 W unless a significant arrhythmia developed, at which point the ablation was stopped. No more than three ablations were performed in each animal to prevent overlapping pulmonary ablation zones.
Figure 2:
CT fluoroscopy to record cardiac excursion and to measure antenna distance from heart during diastole. Axial CT fluoroscopic images from ablations performed with, Ablation A, parallel and, Ablation B, perpendicular orientation. Note change in antenna-heart distance from diastole to systole (double arrows). Antenna-heart distance was measured from antenna emission point for parallel ablations and from tip for perpendicular ablations.
CT Protocols
CT fluoroscopy (LightSpeed; GE, Milwaukee, Wis) with collimation of 2.5 mm, rotation time of 0.5 second, and 40 mA was used to position the microwave antennas according to the prescribed antenna-heart distance and orientation. A brief period of continuous CT fluoroscopy was performed for at least one full cardiac cycle after placement of each antenna to record the final position of the antenna relative to the surface of the beating heart (Fig 2). Once all of the ablations in an animal were complete, a chest CT (LightSpeed; GE; 1.25-mm section thickness, 0.625-mm interval, Smart mA, 120 kVp) examination was performed with the antennas in place to facilitate pulmonary ablation zone measurements along the axis of the antenna shaft and to evaluate for the presence of postablation complications.
Electrocardiographic Monitoring
Before the percutaneous ablations, four surface limb electrocardiographic (ECG) electrodes and a precordial electrode were placed on each study animal, and baseline cardiac rate and rhythm were recorded. An electronic ECG monitor (BM3; Bionet America, Tustin, Calif) was used to track the heart rate and rhythm continuously during the ablations. The baseline cardiac rhythm and the continuous ECG tracings were printed for blinded review.
During each ablation, one investigator (G.C.) continuously monitored the cardiac rhythm to identify the development of significant arrhythmia, which was defined as asystole, heart block, bradycardia, supraventricular tachycardia, or ventricular tachycardia or fibrillation. The ablation was terminated as soon as a significant arrhythmia was recognized on the cardiac monitor. If a significant arrhythmia developed, the ECG data were recorded until the rhythm returned to baseline. A board-certified cardiologist (P.M., with 16 years of experience) who was blinded to the position of the microwave antennas independently and retrospectively reviewed all ECG tracings. The specific cardiac rhythm and time until it developed and resolved were recorded.
Postablation Data Collection
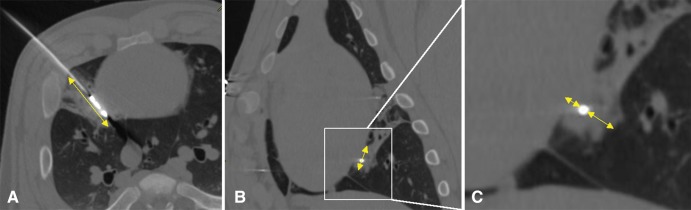
CT measurements and analysis.—The CT images were aligned along the long axis of the microwave antenna with the use of multiplanar reconstructions on a picture archiving and communications system workstation (McKesson Medical, San Francisco, Calif) and were reviewed by two authors in consensus (G.C. and E.N.). From these reconstructions, bone windows (Hounsfield unit window, 2000 HU; level, 250 HU) were used to digitally measure the angle that the antenna formed with the surface of the heart to confirm parallel or perpendicular antenna positioning (Fig 1). The zone of ground-glass opacity corresponding to each ablation zone was then measured by using digital calipers in three dimensions (length, width, and depth), per the accepted method of measuring pulmonary ablation zones at CT (7). The ablation zone volumes were calculated by using an ellipsoid assumption: V = Pi · L · [(W1 + W2) · (D1 + D2)/6]; where V is volume, L is the ablation zone length, W1 and W2 are the anterior and posterior radius of the ablation zone (if perpendicular antenna orientation) or medial and lateral radius of the ablation zone (if parallel antenna orientation), respectively; and D1 and D2 represent the cranial and caudal radius of the ablation zone (Fig 3). Whether the ground-glass opacity produced by each ablation extended to the pleural surface adjacent to the heart was also recorded. The CT images were also reviewed for the presence of pneumothorax, pericardial effusion, and pleural effusion.
Figure 3:
Multiplanar image reconstruction along axis of microwave antenna to measure ablation zone size. A, Oblique axial CT image shows elliptical ground-glass opacity from pulmonary ablation and provides total length of ablation zone (double arrow). B, Oblique coronal CT image provides cranial-caudal dimension of ablation zone (double arrow). C, Image is magnified view of ablation zone from, B, and provides dimensions of ablation zone medial and lateral to antenna. Note how ground glass-opacity of pulmonary ablation zone extends to heart surface in, C (double arrows).
The shortest distance between the antenna and the surface of the beating heart (representing the distance between the antenna and the heart in diastole) was measured retrospectively from the recorded continuous CT fluoroscopic images by using bone windows. The digital calipers were placed at the closest edge of the metallic microwave antenna and extended to the nearest surface of the heart (Fig 3c). These measurements of antenna-heart distance from the CT fluoroscopic images represented the study data used in the statistical analysis.
Gross dissection protocol and viability staining.—The animals remained anesthetized and ventilated for 3 hours after the last pulmonary ablation to allow histologic changes of cardiac tissue injury to occur (8). The animals were then sacrificed with an intravenous injection of Euthasol (390 mg/mL pentobarbital sodium and 540 mg/mL phenytoin sodium; Virbac, Fort Worth, Texas), and the heart and lungs were removed en bloc. The pericardium was removed and the heart was placed in a 400-mL solution of 1% tetrazolium chloride for 60 minutes at room temperature to stain viable epicardium (9–11). Normal tissue reduces tetrazolium chloride to a visible red pigment by means of the activity of cell membrane-bound diaphorases that use nicotinamide adenine dinucleotide, whereas necrotic tissue does not stain red. Digital photographs of the epicardium adjacent to a ruler were taken before and after staining with the 1% tetrazolium chloride solution.
The lung ablation zones were sliced along the orientation of the antennas, digitally photographed on a flatbed scanner, and three-dimensional measurements were made of the ablation zone length, width, and diameter by two authors (G.C. and E.N) in consensus. The outermost red-brown (hemorrhagic) rim of the ablation zones was included in the gross ablation zone measurements. The position of the central dark tan portion of the ablation zone, representing coagulation necrosis (12), was recorded with particular attention to whether this zone extended to the pleural surface adjacent to the heart. This observation was used as a surrogate for the ability to ablate the lung tissue effectively between the antenna and heart surface.
Histopathologic evaluation.—A transmural section of the cardiac chamber wall that corresponded to the location of the antenna in adjacent lung tissue was excised, placed in 10% formalin for fixation, then trimmed to maximize the amount of affected tissue, and was processed. Heart sections were embedded in paraffin, cut to 5-μm–thick sections, stained with hematoxylin and eosin, and examined by a blinded board-certified veterinary pathologist (D.S., with 11 years of experience). Cardiac tissue injury was defined as either a thermal burn on the epicardium before application of 1% tetrazolium chloride vital tissue stain, the presence of unstained epicardium at gross examination after application of the vital stain, or the presence of either epicardial or myocardial coagulation necrosis at histologic examination.
Statistical Methods
The primary outcomes of significant arrhythmia or cardiac tissue injury were compared between the two antenna orientations (parallel and perpendicular relative to the heart) by using a generalized estimating equation with the R package (13) “geepack” and function “geeglm.” The presence of significant arrhythmia as the outcome and distance to the heart as the predictor, while accounting for pig subject as a cluster value, was fit to assess the association between distance to the heart and presence of arrhythmia. A generalized estimating equation was fit separately for each of the two antenna orientations. Similar generalized estimating equations were fit for the presence of cardiac tissue injury. Receiver operating characteristic curves were then constructed and Youden method of optimizing sensitivity and specificity was used to calculate the best cutoff distance from the heart associated with primary outcome or lack thereof (14). The two-tailed Student t test was performed to compare gross and CT ablation zone sizes. The Fisher Exact test was used to compare the frequency of arrhythmias when an ablation was performed adjacent to the right versus the left ventricles.
Results
General Ablation Data
Thirty-five CT-guided percutaneous pulmonary microwave ablations were performed in 12 pigs. Fourteen (40%) ablations were performed in the left lung and 21 (60%) were performed in the right lung. One perpendicular ablation in the left lung was excluded because retrospective multiplanar reconstructed CT measurements revealed an antenna angle with the heart of less than 70°, which did not meet the experimental criteria for a perpendicular orientation. Thus, in 17 (50%) ablations, the probe was in a parallel orientation and in 17 (50%) ablations, the probe was in a perpendicular orientation for a study total of 34 ablations.
In 17 (50%) ablations, the pulmonary ablation zone was adjacent to the right ventricle; in 11 (32%) it was adjacent to the left ventricle. In four ablations (12%), the ablation zone was adjacent to the right atrium; in two (6%), it was adjacent to the left atrium. The median distance from the antenna tip to the heart in perpendicular orientation was 4 mm (range, 0–9.8 mm). The median distance from the antenna emission point when in parallel orientation was 3.9 mm (range, 0–9.9 mm).
Twelve of the 34 (35%) ablations were associated with a small grade 1 pneumothorax according to the Common Terminology Criteria for Adverse Events of the National Cancer Institute (15), which did not require chest tube placement. None of the pneumothoraces resulted in separation of the lung from the adjacent surface of the heart. No immediate postprocedure pericardial effusion, large intraparenchymal hemorrhage, or pleural effusion was observed.
Cardiac Arrhythmias
In six of 34 (18%) ablations, a significant arrhythmia developed and the ablation was terminated. Five of six (83%) of these arrhythmias were ventricular arrhythmias and one (17%) was a supraventricular tachycardia. No bradycardic rhythm, heart block, or asystole occurred. All significant arrhythmias were preceded by an ECG alteration before they developed (Table 1).
Table 1.
Subgroup of Ablations Resulting in Significant Cardiac Arrhythmia
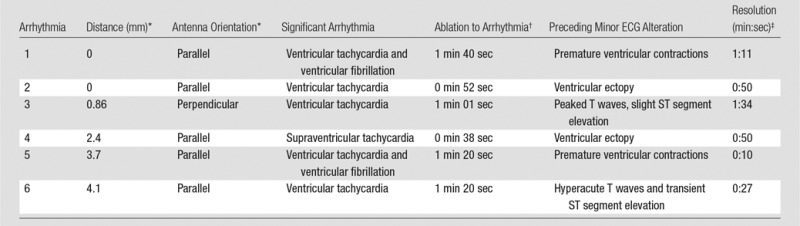
*In relation to the heart.
†Time from the start of ablation to arrhythmia.
‡Time from start to spontaneous resolution of arrhythmia.
The median time for a significant arrhythmia to develop into the ablation was 71 seconds (range, 38–100 seconds). After termination of the ablation, all significant arrhythmias self-terminated, converting to normal sinus rhythm a median time of 50 seconds later (range, 10–94 seconds). Four of the six (67%) significant arrhythmias developed after a pulmonary ablation adjacent to the left ventricle, and two (33%) developed after ablation adjacent to the right ventricle. Of all pulmonary ablations performed next to the left and right ventricles, four of 11 (36.3%) and two of 17 (11.8%) resulted in a significant arrhythmia, respectively (P = .174). All six (100%) of the ablations that resulted in a significant arrhythmia showed evidence of cardiac tissue injury at pathologic evaluation.
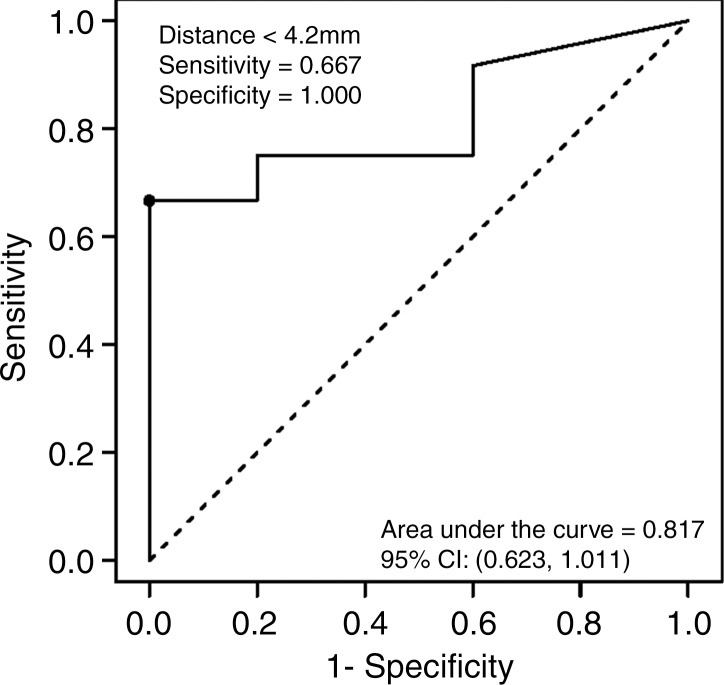
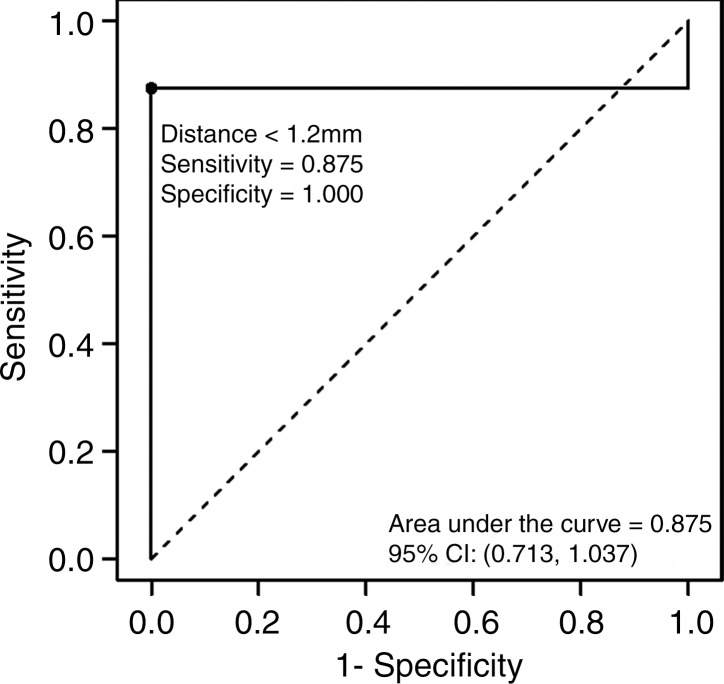
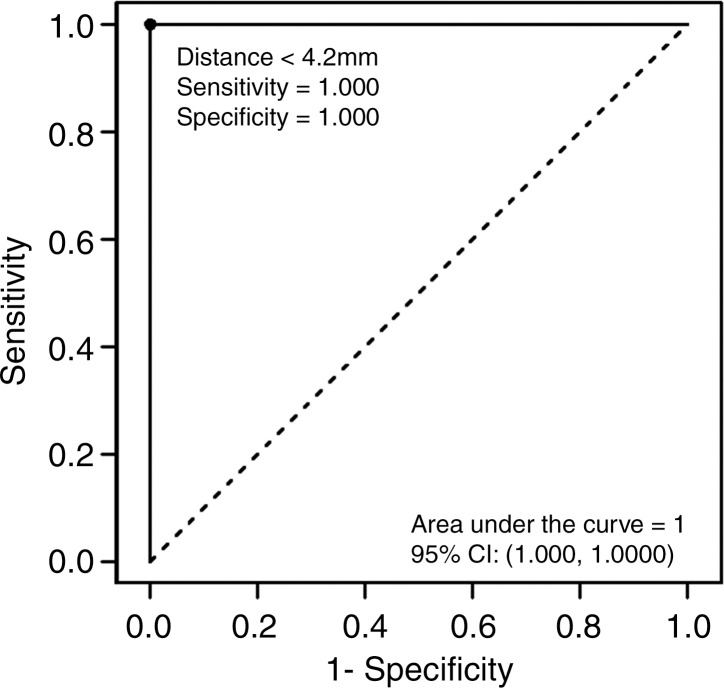
Logistic regression analysis of the data demonstrated a decreasing risk of significant cardiac arrhythmia with increasing antenna distance from the heart (Fig 4). The areas under the receiver operating characteristic curve generated from the parallel and perpendicular antenna orientations for distances less than 4.2 mm and 1.2 mm, respectively, were 0.817 mm2 and 0.875 mm2. No significant arrhythmia occurred at distances of more than 4.4 mm or 1.5 mm for parallel and perpendicular antenna orientations, respectively.
Figure 4a:
Graphs show receiver operating characteristic curves for (a) parallel and (b) perpendicular antenna distance. Logistic regression graphs show relationship between (c) parallel and (d) perpendicular antenna position and risk of significant cardiac arrhythmia. Several parallel ablations performed less than 5 mm from heart resulted in no significant arrhythmia (c). Single arrhythmia occurred among ablations performed with perpendicular antenna positioning (d). CI = confidence interval.
Figure 4b:
Graphs show receiver operating characteristic curves for (a) parallel and (b) perpendicular antenna distance. Logistic regression graphs show relationship between (c) parallel and (d) perpendicular antenna position and risk of significant cardiac arrhythmia. Several parallel ablations performed less than 5 mm from heart resulted in no significant arrhythmia (c). Single arrhythmia occurred among ablations performed with perpendicular antenna positioning (d). CI = confidence interval.
Figure 4c:
Graphs show receiver operating characteristic curves for (a) parallel and (b) perpendicular antenna distance. Logistic regression graphs show relationship between (c) parallel and (d) perpendicular antenna position and risk of significant cardiac arrhythmia. Several parallel ablations performed less than 5 mm from heart resulted in no significant arrhythmia (c). Single arrhythmia occurred among ablations performed with perpendicular antenna positioning (d). CI = confidence interval.
Figure 4d:
Graphs show receiver operating characteristic curves for (a) parallel and (b) perpendicular antenna distance. Logistic regression graphs show relationship between (c) parallel and (d) perpendicular antenna position and risk of significant cardiac arrhythmia. Several parallel ablations performed less than 5 mm from heart resulted in no significant arrhythmia (c). Single arrhythmia occurred among ablations performed with perpendicular antenna positioning (d). CI = confidence interval.
Cardiac Tissue Injury
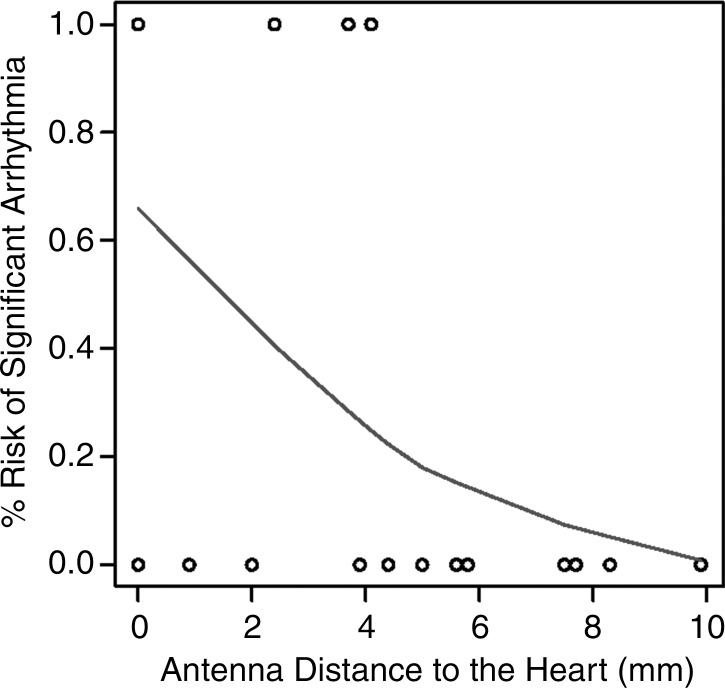
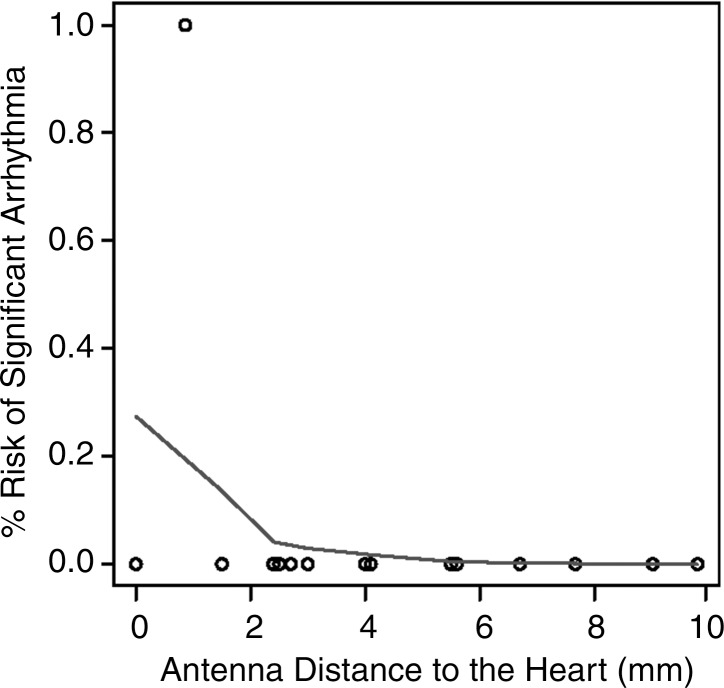
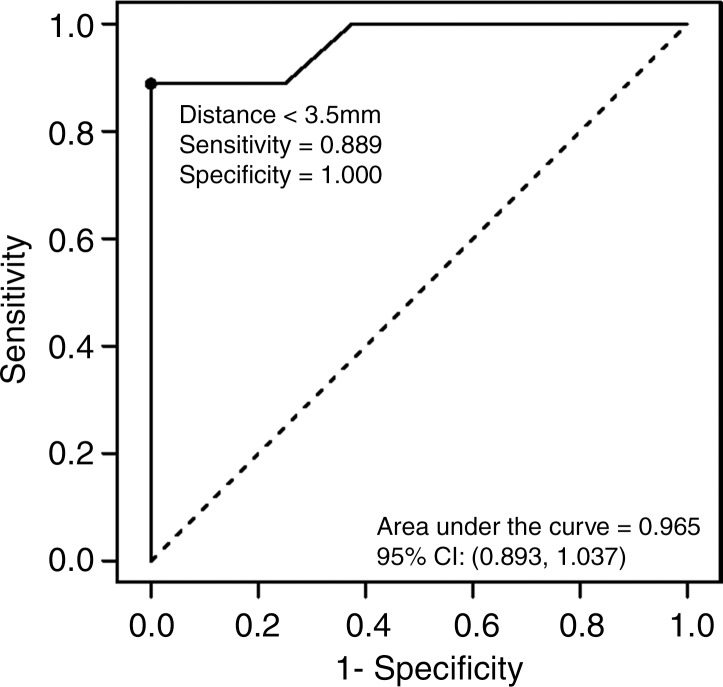
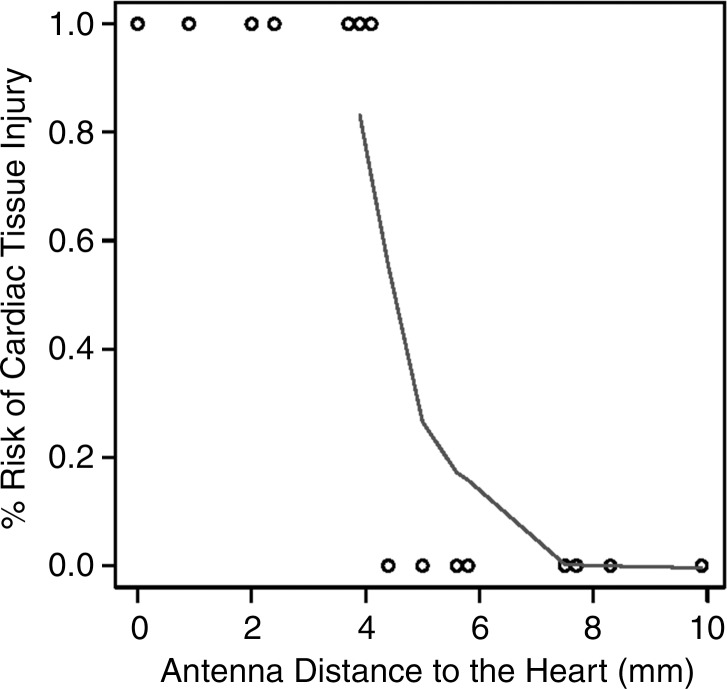
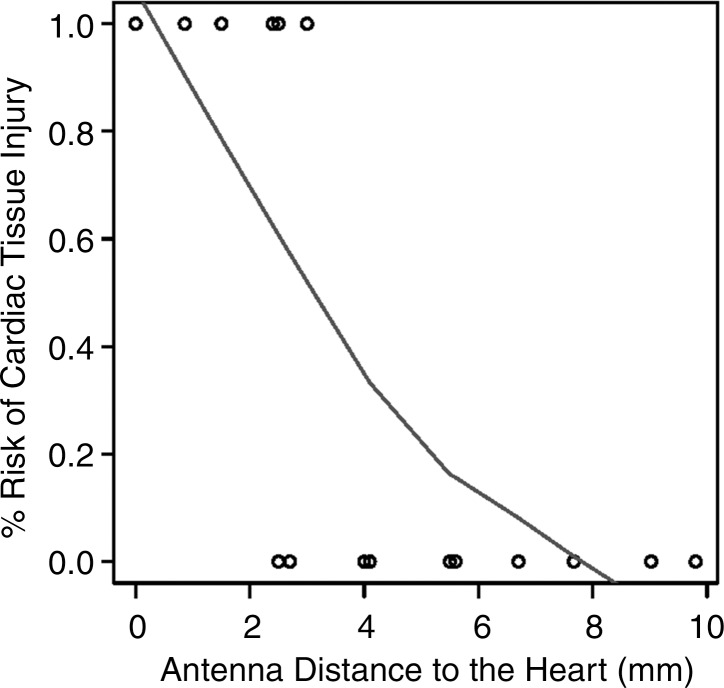
In 13 of the 34 (38%) ablations, a gross thermal burn was present on the epicardial surface of the heart after the vital stain was applied. The epicardial burns appeared either pale or dark purple with either elliptical or punctate morphology (Fig 5). In four additional cases, coagulation necrosis attributed to thermal injury that was initially occult, with vital staining seen at histologic evaluation. In total, 17 of 34 (50%) ablations, each with the antenna positioned less than 5 mm from the heart, resulted in gross or histologic evidence of tissue injury. No cardiac tissue injury occurred at distances of more than 4.4 mm and 4 mm, for parallel and perpendicular antenna orientations, respectively. The range of antenna-to-heart distances that did not result in cardiac tissue injury was 5–9.9 mm with a parallel antenna orientation and 2.5–9.8 mm with a perpendicular orientation (Fig 6).
Figure 5:
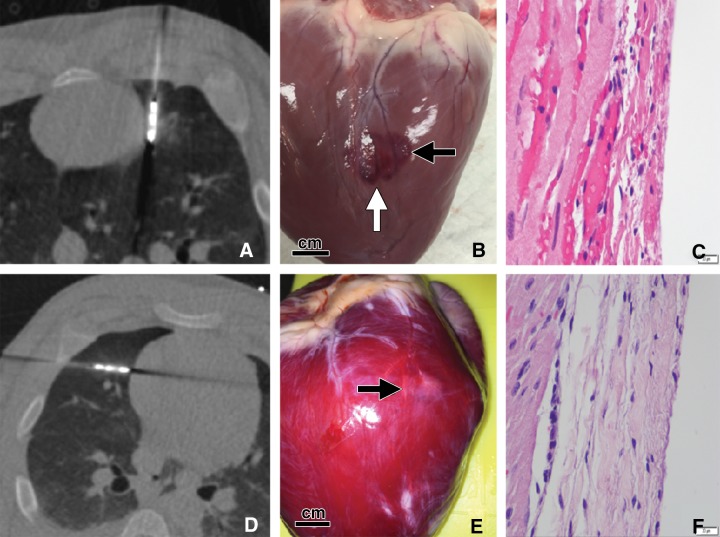
Two pulmonary ablations in swine performed less than 5 mm from heart. A–C, Microwave ablation performed with the antenna in a parallel orientation relative to the heart surface with the emission point 0 mm from the left ventricle. A, Axial CT image from CT fluoroscopy reveals emission point of antenna abutting heart surface. B, Gross photograph of heart surface after ablation and before application of vital stain reveals large, lobular dark purple burn on heart surface (black arrow; scale bar = 1 cm). Note cleft between two elliptical epicardial burns (white arrow), which is likely a result of continuous cardiac and respiratory motion during ablation. This ablation resulted in ventricular tachycardia that resolved after ablation was terminated. C, Photomicrograph of cardiac specimen shows severe acute myocardial and epicardial necrosis (hematoxylin-eosin stain, scale bar = 20 μm). D–F, Microwave ablation performed in perpendicular orientation relative to heart with antenna tip 0 mm from right ventricle. D, Axial CT image from CT fluoroscopy shows tip of antenna abutting heart surface. No arrhythmia developed during ablation. E, Gross photograph of heart surface after ablation and vital staining reveals pale, rounded lesion on left ventricular epicardium representing nonviable cardiac tissue (arrow) (scale bar = 1 cm). F, Photomicrograph of excised cardiac tissue specimen confirms focal coagulation necrosis of the epicardium (hematoxylin-eosin stain, scale bar = 20 μm).
Figure 6a:
(a, b) Receiver operating characteristic curves and (c, d) logistic regression graphs show relationship between antenna position and risk of cardiac tissue injury. There is high risk of causing cardiac tissue injury when microwave antenna is positioned less than approximately 4 mm from heart with either antenna orientation (a, b). This risk decreases with increasing antenna distance from heart at both antenna orientations (c, d).
Figure 6b:
(a, b) Receiver operating characteristic curves and (c, d) logistic regression graphs show relationship between antenna position and risk of cardiac tissue injury. There is high risk of causing cardiac tissue injury when microwave antenna is positioned less than approximately 4 mm from heart with either antenna orientation (a, b). This risk decreases with increasing antenna distance from heart at both antenna orientations (c, d).
Figure 6c:
(a, b) Receiver operating characteristic curves and (c, d) logistic regression graphs show relationship between antenna position and risk of cardiac tissue injury. There is high risk of causing cardiac tissue injury when microwave antenna is positioned less than approximately 4 mm from heart with either antenna orientation (a, b). This risk decreases with increasing antenna distance from heart at both antenna orientations (c, d).
Figure 6d:
(a, b) Receiver operating characteristic curves and (c, d) logistic regression graphs show relationship between antenna position and risk of cardiac tissue injury. There is high risk of causing cardiac tissue injury when microwave antenna is positioned less than approximately 4 mm from heart with either antenna orientation (a, b). This risk decreases with increasing antenna distance from heart at both antenna orientations (c, d).
Logistic regression demonstrated a decreasing risk of epicardial injury with increasing antenna distance from the heart. The areas under the receiver operating characteristic curve generated from each parallel and perpendicular orientation at the distances of less than 4.2 mm and 3.5 mm, respectively, were 1 and 0.964 mm2 (Fig 6).
Pulmonary Ablation Zones
In 24 of the 34 (71%) ablations, the heart or chest wall was at least partially included in the ablation zone margins, which limited the ability to measure the entire ablation zone and allowed underestimation of ablation zone volumes. Furthermore, ablation zone sizes were not corrected for degree of associated tissue contraction. The mean pulmonary ablation zone volumes measured grossly and at CT were 15.2 cm3 ± 5.9 (95% confidence interval: 13.3, 17.2) and 11.8 cm3 ± 7.5, (95% confidence interval: 9.3, 14.3), respectively (P = .048). Mean ablation zone diameters at gross evaluation and CT were 29.4 mm ± 5.8 (95% confidence interval: 27.5, 31.3) and 27.1 mm ± 4.6 (95% confidence interval: 25.6, 28.6), respectively (P = .809).
At postablation CT, the ground-glass opacity extended to the heart surface in all ablations (100%) performed with a parallel antenna orientation and positioned 5–10 mm from the heart (n = 7) (Fig 3c). At gross pathologic evaluation of the ablation zones, the zone of coagulation necrosis extended up to the pleural surface, contacting the heart in five (71%) of these ablations (Fig 7).In two (29%) cases, only the peripheral rim of hemorrhage indicative of potentially viable lung tissue (12) contacted the cardiac pleural surface (Table 2).Of the ablations performed with a perpendicular antenna orientation and positioned 5–10 mm from the heart (n = 6), one (17%) resulted in extension of the zone of coagulation necrosis to the pleural surface in contact with the heart.
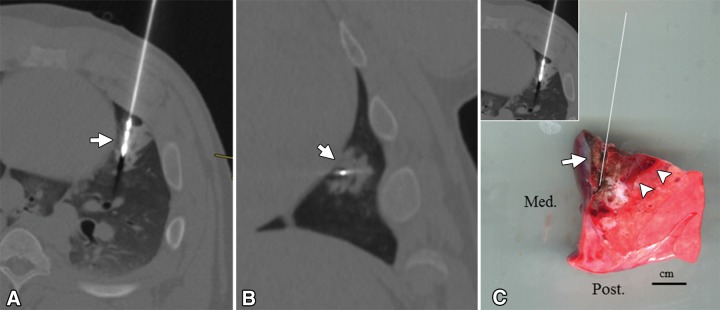
Figure 7:
Microwave pulmonary ablation extending to heart surface. A, Oblique transverse and, B, coronal postablation CT images show elliptical ground-glass ablation zone around distal microwave antenna extending to heart surface (arrow). C, Gross digital photograph of lung specimen oriented in transverse plane similar to that in, A (left upper corner image insert), with white line indicating path of antenna. Inner, necrotic portion of ablation zone extends to visceral pleural surface (arrow) along heart. For comparison, note outermost layer of same gross ablation zone that represents hemorrhage and potentially viable cells (arrowheads; scale bar = 1 cm). Med. = medial, Post. = posterior.
Table 2.
Percutaneous Microwave Ablations according to Distance from the Heart and Orientation
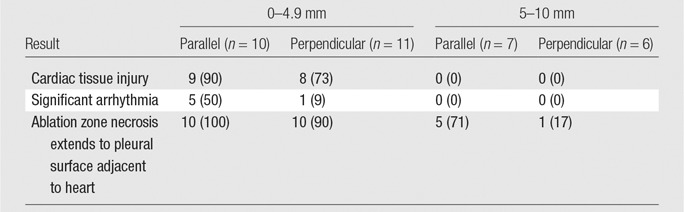
Note.—Data are number of subjects, with percentage in parentheses.
Discussion
The purpose of our study was to determine the closest distance to the heart at which a percutaneous pulmonary microwave ablation can be performed safely. We found that a distance of 5 mm or greater should be maintained between either the antenna emission point (if the antenna is positioned parallel to the heart surface) or the antenna tip (if positioned perpendicularly) to avoid cardiac tissue injury or significant arrhythmia. Shorter antenna-heart distances were highly predictive of cardiac complications. No inadvertent puncture of the heart or other vital mediastinal structure occurred during the study and no pericardial effusion was present at postablation CT examination.
Two varying antenna orientations were used for the ablations in our study. Different antenna approaches are often required during clinical cases of pulmonary ablation, depending on the location of the target lesion. The microwave antenna used in our study produces an elliptical ablation zone with a length of approximately 40 mm and a maximal diameter of 30 mm that extends approximately 3 mm off the tip of the antenna (7). Because of its shape and relationship to the antenna shaft, a larger surface area of the ablation zone contacts the heart with a parallel orientation than that with a perpendicular orientation. The increased contact area associated with parallel orientation is the likely reason for the higher rate of significant arrhythmias seen at a given distance. This suggests that physicians should place antennas farther from the heart when a parallel orientation is needed for a pulmonary ablation than when a perpendicular orientation is used.
All ablations resulting in a significant arrhythmia were performed less than 5 mm from the heart. When significant arrhythmias were encountered, the ablation was immediately stopped, and the heart rhythm spontaneously returned to normal sinus rhythm a median time of 50 seconds later. Despite the shortened ablation time period, epicardial necrosis was evident at pathologic evaluation after these pulmonary ablations. This finding suggests that although an arrhythmia may be reversible, associated permanent tissue injury is likely, and pulmonary ablations at less than 5 mm should be avoided. We believe that any degree of cardiac tissue injury should be avoided in patients with chronic cardiovascular disease, who comprise a large portion of patients referred for percutaneous tumor ablation. Minor ECG fluctuations preceded all significant arrhythmias, suggesting that these changes may herald a potentially dangerous arrhythmia, giving the operator a chance to stop the ablation immediately. This finding reinforces the need for the operator to monitor the ECG carefully and continuously in cases of ablation near the heart.
The safety of performing microwave ablations 5–10 mm from the heart is likely related to both the degree of perfusion-mediated tissue cooling (“heat sink”) from the highly vascular myocardium and to the variable exposure of the heart to the ablation zone. Although vascular heat sink has been shown to have an effect in radiofrequency pulmonary ablations near the heart (5,16), the variable exposure of the heart to the nearby pulmonary ablation zone as a result of continuous cardiac and diaphragmatic motion may also have a significant effect. Since all measurements for the study were made during cardiac diastole with the use of continuous CT fluoroscopy, the heart was observed to move away from the antenna in the axial plane during systole, increasing the antenna-heart distance with each contraction. In a similar way, because the lung bases move in conjunction with the diaphragm while the heart remains fixed in the mediastinum (thus moving the heart away from the antenna in the cranio-caudal axis), the microwave field may only intermittently contact the same location on the heart for the duration of the ablation. In fact, several of the epicardial thermal burns showed overlapping elliptical morphology with an intervening cleft at gross examination, which suggests that the microwave field contacted more than one area of the heart surface. This continuous motion between the pulmonary ablation zone and the heart appears to be a significant protective mechanism against cardiac thermal injury.
Although we found that an antenna distance of at least 5 mm protects the heart from a pulmonary ablation, we also intended to determine if the lung tissue between the antenna and heart would be susceptible to or resistant to thermal injury, given the significant effect of the adjacent cardiac heat sink. This has important implications for treatment of a mass immediately adjacent to the heart. We found that the lung tissue between the antenna and the heart was completely coagulated at pathologic evaluation in 71% of parallel ablations performed 5–10 mm from the heart. The ability to destroy lung tissue successfully at the pericardial margin of the pulmonary ablation zone, where authors of prior studies (5,16) have shown difficulty with radiofrequency ablation, may be related to the use of microwave energy, which, unlike radiofrequency energy, is not limited by the electrical conductivity of the lung and is less affected by vascular heat sink (17,18). The difference in susceptibility to thermal injury of the intervening lung and cardiac tissue also may be related to the fixed position of the lung as opposed to the dynamic position of the moving heart relative to the microwave field.
There were limitations to be acknowledged in our study. First, a single microwave antenna from one manufacturer was used for the pulmonary ablations, and we have not assessed how different or multiple antennas would perform adjacent to the heart. Furthermore, the safety of antenna orientations that are not either perpendicular or parallel relative to the heart was not assessed. Nevertheless, the ability to use microwave energy in lung tissue close to the heart without cardiac electrical interference is universal with microwave systems. Second, both human and swine hearts have variable levels of epicardial fat that acts as a thermal insulator. Combined with the degree of cardiac motion and wall thickness that differs with the chamber of the heart adjacent to the pulmonary ablation, a given antenna-heart distance may be a variable risk of cardiac complications. Third, this experimental study was performed in pigs with healthy lungs and no tumors. Swine are a commonly used animal model for procedures involving the human heart (9,10), given their similar anatomy and lower threshold for developing cardiac arrhythmias than that in humans (19), but differences between the species remain, and direct translation of data to a human population may yield different results. Finally, the pulmonary and cardiac lesions produced with our study were evaluated in the acute phase, a minimum of 3 hours after ablation. Although a survival study may reveal different changes in the heart tissue, the application of tetrazolium chloride vital stain to acute cardiac tissue has been shown to be sensitive and reliable by prior investigators (20) and to allow accurate prediction of the extent of necrosis, even at histologic evaluation in the chronic phase (10,21,22).
In summary, we found that percutaneous microwave ablation of lung tissue near the heart can be performed safely and effectively with adherence to specific antenna orientations and distances relative to the heart. This approach to treating lung tumors near the heart may prevent cardiac complications such as myocardial injury and significant arrhythmia. Microwave lung ablations performed greater than or equal to 5 mm from the heart are associated with a low risk of cardiac tissue injury and significant arrhythmias. Positioning the antenna 5–10 mm from the heart in a parallel orientation relative to the heart surface results in a high rate of coagulation necrosis of all intervening lung tissue.
Practical application: This experimental animal study provides in vivo safety and efficacy data of percutaneous microwave pulmonary ablation near the heart that may be applied to clinical ablations performed in a human population, although there are limitations inherent to a study performed in a healthy animal model.
Advances in Knowledge
■ Cardiac tissue injury and significant arrhythmias can be prevented by positioning the microwave antenna at least 5 mm from the heart with either a parallel or perpendicular orientation relative to the heart surface.
■ Microwave ablations performed less than 5 mm from the heart are associated with an approximately 50% risk of permanent cardiac tissue injury and an 18% risk of significant intraprocedural arrhythmia.
■ Complete coagulation necrosis of lung tissue immediately adjacent to the heart can be produced in 71% of cases by positioning the antenna 5–10 mm from the heart in a parallel orientation relative to the heart surface; this technique may reduce the incidence of viable tumor remaining on the cardiac margin of the tumor after an ablation.
Implication for Patient Care
■ Microwave ablation of lung tumors near the heart is safe with adherence to specific antenna positions and a standard power-time protocol.
Acknowledgments
Acknowledgment
The authors wish to thank Lisa Sampson, BS, for her assistance with research animal care and use throughout the study.
Received April 17, 2016; revision requested June 28; revision received July 16; accepted July 29; final version accepted August 11.
Supported by the Radiological Society of North America (RSNA Research Fellow Grant RF1503) and the National Institutes of Health Clinical Center (R01 CA149379).
Disclosures of Conflicts of Interest: G.A.C. disclosed no relevant relationships. E.N. disclosed no relevant relationships. P.J.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: stock/stock options from NueWave Medical. Other relationships: disclosed no relevant relationships. D.J.S. disclosed no relevant relationships. S.H. disclosed no relevant relationships. A.M.T. disclosed no relevant relationships. F.T.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: stock/stock options from Elucent; consultancy with Ethicon; board membership, grant/grants pending, and stock/stock options with Histosonics; patients and royalties from Medtronic/Covidien. Other relationships: disclosed no relevant relationships. C.L.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultancy and stock/stock options for NeuWave Medical and Symple Surgical and patents from Wisconsin Alumni Research Foundation. Other relationships: disclosed no relevant relationships.
Abbreviation:
- ECG
- electrocardiography
References
- 1.Belfiore G, Ronza F, Belfiore MP, et al. Patients’ survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol 2013;82(1):177–181. [DOI] [PubMed] [Google Scholar]
- 2.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. RadioGraphics 2005;25(Suppl 1):S69–S83. [DOI] [PubMed] [Google Scholar]
- 3.Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology 2011;260(3):633–655. [DOI] [PubMed] [Google Scholar]
- 4.Morrison PR, vanSonnenberg E, Shankar S, et al. Radiofrequency ablation of thoracic lesions: part 1, experiments in the normal porcine thorax. AJR Am J Roentgenol 2005;184(2):375–380. [DOI] [PubMed] [Google Scholar]
- 5.Iguchi T, Hiraki T, Gobara H, et al. Percutaneous radiofrequency ablation of lung tumors close to the heart or aorta: evaluation of safety and effectiveness. J Vasc Interv Radiol 2007;18(6):733–740. [DOI] [PubMed] [Google Scholar]
- 6.National Research Council . Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academies Press, 2011. [Google Scholar]
- 7.Brace CL, Hinshaw JL, Laeseke PF, Sampson LA, Lee FT, Jr. Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology 2009;251(3):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohl S, Medway DJ, Schulz-Menger J, Schneider JE, Neubauer S, Lygate CA. Refined approach for quantification of in vivo ischemia-reperfusion injury in the mouse heart. Am J Physiol Heart Circ Physiol 2009;297(6):H2054–H2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee AM, Aziz A, Sakamoto SI, Schuessler RB, Damiano RJ, Jr. Epicardial ablation on the beating heart: limited efficacy of a novel, cooled radiofrequency ablation device. Innovations (Phila) 2009;4(2):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaynor SL, Byrd GD, Diodato MD, et al. Microwave ablation for atrial fibrillation: dose-response curves in the cardioplegia-arrested and beating heart. Ann Thorac Surg 2006;81(1):72–76. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Jin GY, Goldberg SN, et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology 2004;230(1):125–134. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto A, Nakamura K, Matsuoka T, et al. Radiofrequency ablation in a porcine lung model: correlation between CT and histopathologic findings. AJR Am J Roentgenol 2005;185(5):1299–1306. [DOI] [PubMed] [Google Scholar]
- 13.Hojsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw 2006;15(2):1–11. [Google Scholar]
- 14.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3(1):32–35. [DOI] [PubMed] [Google Scholar]
- 15.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13(3):176–181. [DOI] [PubMed] [Google Scholar]
- 16.Bargellini I, Bozzi E, Cioni R, Parentini B, Bartolozzi C. Radiofrequency ablation of lung tumours. Insights Imaging 2011;2(5):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT, Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236(1):132–139. [DOI] [PubMed] [Google Scholar]
- 18.Dodd GD, 3rd, Dodd NA, Lanctot AC, Glueck DA. Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology 2013;267(1):129–136. [DOI] [PubMed] [Google Scholar]
- 19.Deodhar A, Dickfeld T, Single GW, et al. Irreversible electroporation near the heart: ventricular arrhythmias can be prevented with ECG synchronization. AJR Am J Roentgenol 2011;196(3):W330–W335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adegboyega PA, Adesokan A, Haque AK, Boor PJ. Sensitivity and specificity of triphenyl tetrazolium chloride in the gross diagnosis of acute myocardial infarcts. Arch Pathol Lab Med 1997;121(10):1063–1068. [PubMed] [Google Scholar]
- 21.Prasad SM, Maniar HS, Schuessler RB, Damiano RJ, Jr. Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J Thorac Cardiovasc Surg 2002;124(4):708–713. [DOI] [PubMed] [Google Scholar]
- 22.Prasad SM, Maniar HS, Moustakidis P, Schuessler RB, Damiano RJ, Jr. Epicardial ablation on the beating heart: progress towards an off-pump maze procedure. Heart Surg Forum 2002;5(2):100–104. [PubMed] [Google Scholar]