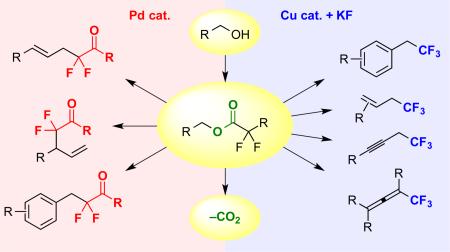
Abstract
Metal-catalyzed decarboxylative fluoroalkylation reactions enable the conversion of simple O-based substrates into biologically relevant fluorinated analogs. Herein, we present decarboxylative methods that facilitate the synthesis of trifluoromethyl- and difluoroketone-containing products. We highlight key mechanistic aspects that are critical for efficient catalysis, and that inspired our thinking while developing the reactions.
Keywords: Fluorine, Trifluoromethylation, Difluoroketone, Decarboxylation, Catalysis, Copper, Palladium
Graphical Abstract
1 Introduction
Fluorinated compounds possess desirable physical and chemical properties, and are important for biomedical, agrichemical, and materials research.1 While the field of synthetic organofluorine chemistry has grown in recent years,2 a need remains to develop more efficient strategies for accessing both common and underrepresented fluorinated motifs. One ongoing challenge involves the development of metal-catalyzed processes that convert common functional groups, such as alcohols, into fluorinated substructures using mild reaction conditions. To address these issues, we have employed decarboxylative strategies to enable synthesis of fluorinated molecules from simple and common alcohol groups.
Decarboxylative coupling of α,α-fluoroalkyl esters enables the formation of C–C(F)n bonds, and facilitates the synthesis of complex fluoroalkyl compounds.3 Beneficial aspects of this strategy include: 1) the generation of reactive fluoroalkyl metal species under mild reaction conditions; 2) the release of CO2 as a benign and easily separable by-product; and 3) the ability to use simple and inexpensive fluoroalkylcarboxylate derivatives as coupling partners.3–4 Given these advantages, our group pursued both Cu- and Pd-catalyzed decarboxylative strategies for installing fluoroalkyl groups.
2 Cu-catalyzed Decarboxylative Trifluoromethylation Reactions
2.1 Net Trifluoromethylation of Alcohols
Alcohols are a critical functional group for synthetic transformations, and the extensive number of alcohols found in bulk feedstock chemicals, natural products, screening libraries and therapeutic candidates provide a broad spectrum of potential substrates for transformations. These alcohols can serve as substrates for deoxyfluorination reactions to generate fluorinated target compounds;1 however, complementary deoxytrifluoromethylation reactions remain limited, and instead, most non-aryl–CF3 groups are installed by indirect conversion of OH-based substrates.
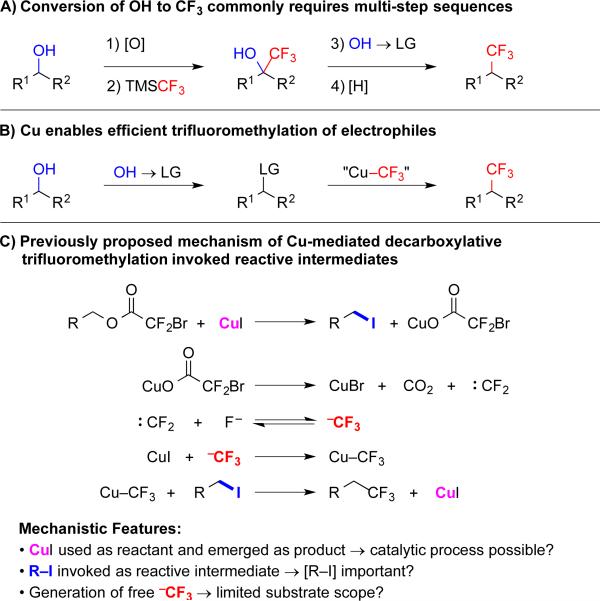
To convert alcohols to trifluoromethanes, the most common strategy involves: 1) oxidation to generate an aldehyde or ketone; 2) nucleophilic trifluoromethylation with TMSCF3; and 3) two-step deoxygenation (Scheme 1A). This sequence has been used in many discovery routes towards biological probes, therapeutic candidates, and agrichemicals, as well as in larger scale applications. Despite its use, several drawbacks limit this multi-step approach, including: 1) inefficient manipulation of oxidation states; 2) excess time and labor to conduct multiple steps; 3) generation of excess waste; 4) decreases in yields of desired products; and 5) incompatibility of functional groups that are sensitive to oxidation, reduction and/or strong nucleophiles. Considering these limitations, mild, streamlined and economical deoxytrifluoromethylation reactions would improve the scope of accessible molecules.3
Scheme 1.
Previous strategies for converting alcohols to trifluoromethanes do not tolerate functional groups that are sensitive to strong nucleophiles, oxidants and/or reductants
Considering this goal, a shortened and mild strategy might involve: 1) the conversion of an alcohol to an electrophile; and 2) nucleophilic substitution using Cu–CF3 (Scheme 1B).5–7 To develop such transformations, we have reinvestigated Chen's Cu-catalyzed decarboxylative trifluoromethylation reactions between sp3-electrophiles and halodifluoroacetates to provide access to target compounds in economical and green fashion.7,8 Halodifluoroacetates are appealing precursors to trifluoromethanes, because they are inexpensive and readily synthesized from industrial fluorinated building blocks, such as tetrafluoroethylene and vinylidene fluoride.9 In the presence of Cu and KF, halodifluoroacetates decarboxylate to generate [Cu–CF3], which reacts with electrophiles to create C–CF3 bonds. Previously, Chen demonstrated that alcohols can be converted to halodifluoroacetate esters, and upon treatment with stoichiometric Cu and KF, these electrophilic species undergo decarboxylative trifluoromethylation.7 However, reactions only tolerated the narrow subset of –Br, –Cl, and –NO2 functional groups, and required use of stoichiometric Cu. Considering these features, we sought to develop mild and functional group tolerant catalytic processes for trifluoromethylation of simple alcohol-derived substrates.
To develop these Cu-catalyzed methods, we studied Chen's proposed mechanism for Cu-mediated trifluoromethylation (Scheme 1C),7e and noticed three striking features: 1) CuI was used as a reactant, and emerged as a product. Therefore, catalytic use of Cu might be possible. 2) The pathway invoked alkyl iodides as reactive intermediates. Thus, increasing the concentration of the R–I electrophiles might prove critical. 3) The reactions formed solvent-separated :CF2 and −CF3 intermediates, which would react with a broad spectrum of functional groups, thus limiting the scope of accessible molecules. These three features inspired our efforts to develop general Cu-catalyzed decarboxylative trifluoromethylation reactions.
2.2 Mechanism of Decarboxylation Impacts Functional Group Tolerance
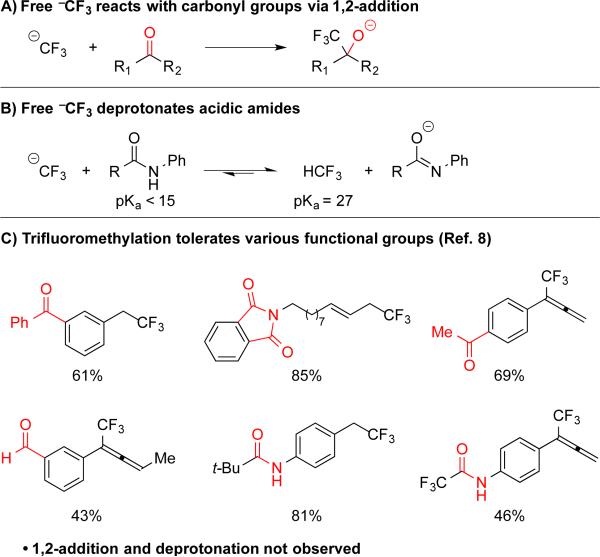
A critical aspect of our research involves developing efficient and mild methods that tolerate various functional groups found in drug-like molecules, agrichemicals and materials. Although Chen's Cu-mediated decarboxylative trifluoromethylation reactions provided a streamlined route, the proposed mechanism precluded broad functional group compatibility. Specifically, decarboxylation supposedly occurred via an outer-sphere process, which generated free −CF3 in solution (Scheme 1C). Since free −CF3 commonly reacts with aldehydes and ketones to generate 2,2,2,-trifluoroethanols (Scheme 2A),10 and deprotonates acidic amides (pKa < 15 in H2O)11 to generate fluoroform (pKa = 27 in H2O; Scheme 2B),12 reactions that would release free −CF3 in solution would not tolerate many important functional groups. However, we speculated that a Cu-catalyzed decarboxylation might instead occur within the solvent-sphere of the metal, and form Cu–CF3, which preferentially reacts with soft electrophiles.6c,13 According to this paradigm, the reaction would not generate free −CF3, which would improve the functional group compatibility.
Scheme 2.
Tolerance of carbonyl and acidic functional groups suggests that free −CF3 is not generated during Cu-catalyzed decarboxylative trifluoromethylation
As predicted, our optimized catalytic trifluoromethylation reactions do tolerate carbonyl groups and acidic protons.8 For compounds containing carbonyls (e.g. aldehydes, ketones, esters, imides), trifluoromethylation proceeded in modest to good yield, and products arising from 1,2-addition of −CF3 were not observed in purified product or the crude reaction mixtures (Scheme 2C). In addition, reactions of substrates bearing acidic amides furnished the desired trifluoromethanes, and no evidence of deprotonation or generation of HCF3 was observed (Scheme 2C). This data suggest that, unlike the previously proposed mechanism for Cu-mediated decarboxylation, our catalytic variant involved either a Cu-centered decarboxylation event, or at least a process that occurred within the solvent sphere of Cu. Additionally, the broad functional group compatibility extends the spectrum of accessible molecules relative to trifluoromethylation reactions using TMSCF3,5e–f,6f,10 which cannot tolerate carbonyls and acidic protons.6c Finally, the reactions also proceeded in the presence of many additional groups, such as aryl bromides, chlorides, fluorides, and triflates, protected amines, and heterocycles, which extend the utility of Chen's original protocol.
2.3 Allylic Trifluoromethylation Enabled by Activation Protocol
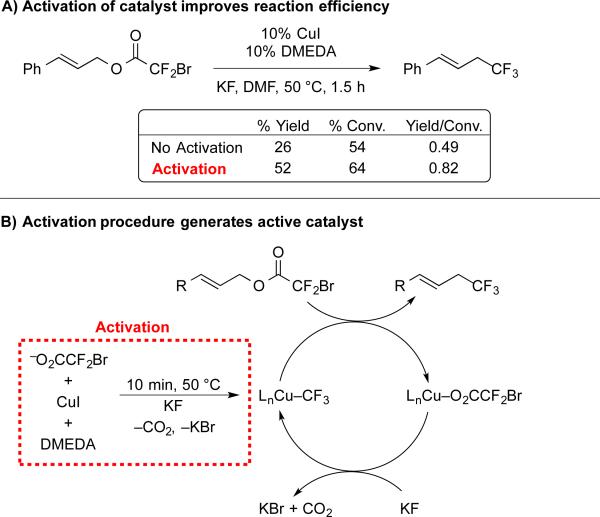
Initially, we developed a protocol to synthesize allylic trifluoromethanes, because allylic electrophiles possess high reactivity, and the final compounds serve as building blocks for assembling bioactive molecules.8a At the outset, we evaluated a broad series of N-, O-, and P-based ligands that would stabilize the [Cu–CF3] complex towards degradation and/or accelerate the reactions with electrophiles. We identified N,N’-dimethylethylenediamine (DMEDA) as a suitable bidentate ligand to accelerate the reaction, but also observed an induction period that decreased the yields of product. Specifically, during the first 30 minutes, 25–30% of the starting material decomposed, while generating <10% of product. After the first hour of the reaction, remaining substrate was more efficiently converted to product (~60% conv., ~50% yield). Thus, we speculated that in-situ generation of the active catalyst, presumably [(DMEDA)Cu–CF3], would improve the reaction. In order to access this active catalyst and circumvent the non-productive induction period, we explored a series of activation protocols. In practice, we generated the proposed complex in situ by heating NaO2CCF2Br, CuI, KF, and DMEDA for 10 min, prior to adding the substrate. This procedure improved the efficiency of the reaction (compared to a setup in which all reagents and substrates were combined and heated at once), particularly during the early phase of the reaction (Scheme 3). Finally since the previous stoichiometric process invoked allyl–I intermediates (Scheme 1C), we probed the necessity of I− for catalytic turnover. When the catalytic reaction was conducted with Cu[(MeCN)4PF6] instead of CuI, similar yields were obtained, indicating that allylic iodides are not essential intermediates for the process.
Scheme 3.
Activation enables efficient Cu-catalyzed decarboxylative trifluoromethylation of allylic bromodifluoroacetates
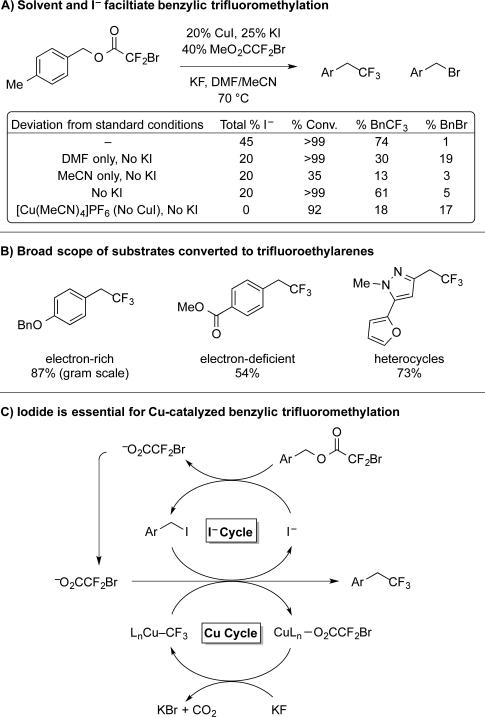
2.4 Generation of Iodide Intermediate Required for Benzylic Trifluoromethylation
Having developed a catalytic protocol for allylic trifluoromethylation, we explored similar conditions towards the transformation of benzylic bromodifluoroacetates into trifluoroethylarenes, which are found in various bioactive compounds.8b Unfortunately, benzylic electrophiles reacted inefficiently under conditions that enabled allylic trifluoromethylation, potentially because the activation of the benzylic electrophile may require additional energy to partially dearomatize at the transition state. To improve the reactions of benzylic electrophiles, a broad screen of conditions revealed two critical features, namely a specific solvent mixture and an I− additive (Scheme 4A). When assessing solvents, the use of DMF alone provided a highly reactive system that furnished modest yield of product and a benzyl bromide (Bn–Br) side-product. The use of MeCN alone provided a less reactive system that suppressed the Bn–Br side-product. Based on this data, we explored the use of a DMF/MeCN mixture to impart high reactivity to the catalyst system (DMF), while also minimizing side-products (MeCN). Indeed, the use of a 1:1 mixture of DMF:MeCN provided good yields of Bn–CF3, and minimized formation of Bn–Br.
Scheme 4.
Iodide and solvent are essential for benzylic trifluoromethylation
Similar to allylic trifluoromethylation, we observed an induction period in the benzylic transformation that destroyed the substrate. In this case, a time-course analysis of the reaction revealed non-productive decomposition of the substrate during the first hour of the reaction (30% conv., <2% yield). After 1 h, we observed buildup of Bn–I, along with the desired Bn–CF3. Further, a steady state concentration of Bn–I (max ~5%) persisted during the remainder of the reaction, and decreased during the final stages, suggesting the Bn–I could be the effective electrophile. We thus hypothesized that increasing [I−] would increase [Bn–I], and in turn circumvent the induction period. Thus, we added KI to the reaction, and observed an increased concentration of Bn–I (max ~10%). This additive accelerated the formation of product within the first 5 min of the reaction, and ultimately improved the final yield by 15%. Combined, these data implicate Bn–I as an intermediate in Cu-catalyzed decarboxylative trifluoromethylation of benzylic substrates (Scheme 1C, Scheme 4). The reaction tolerated a range of functional groups, including an acidic amide, ketone, ester, aryl bromide, and heterocycles such as furan, indole, and pyrazole. While previous Cu-catalyzed benzylic trifluoromethylation reactions were limited to electron-rich electrophiles,5f our method converted electron-rich, -neutral, and -deficient benzyl bromodifluoroacetates into trifluoroethylarenes.
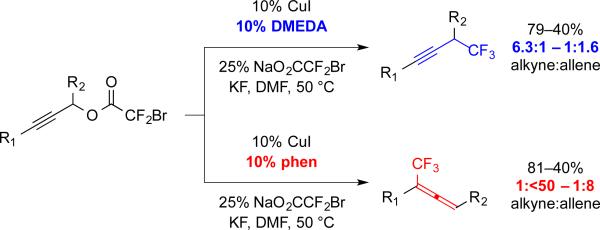
2.5 Ligand-controlled Regiodivergent Trifluoromethylation of Propargylic Electrophiles
Despite the rich history of Cu–CF3-based trifluoromethylation, no previous reactions have exploited ligands to control the regioselectivity of a transformation. In this area, we developed the first ligand-controlled regioselective process using Cu–CF3 and propargyl electrophiles. Historically, Cu-mediated trifluoromethylation reactions of propargylic electrophiles generated both propargyl trifluoromethane or trifluoromethylallene products, with regioselectivity dictated by the substructure of the substrate.6,8c,d
In contrast, our exploratory studies revealed that N-based ligands could dictate the regioselectivity of Cu-catalyzed propargylic trifluoromethylation towards selectively forming trifluoromethylallenes or propargyl trifluoromethanes. Initially as a control, ligand-free Cu-catalyzed reactions of propargyl bromodifluoroacetate generated a 3:1 mixture of propargyl : allene products.8c We speculated that ligands might influence the regiochemical outcome of these reactions, and thus, we screened a broad subset of N-, O-, S-, and P-based ligands. While most ligands did not significantly influence the regioselectivity of this reaction, bipyridyl-type ligands favored formation of trifluoromethylallenes.8c Specifically, 1,10-phenanthroline (phen) and terpyridine generated trifluoromethylallenes from 1°, 2°, and 3° electrophiles in good selectivity (>9:1, Scheme 5). Alternatively, a DMEDA-ligated system improved yields for propargylic trifluoromethylation, although the regioselectivity matched that of the unligated system (Scheme 5). These methods generated a range of useful products, including trifluoromethylallenes bearing substitution patterns that cannot be accessed using other Cu-mediated methods. Further, functionalization of the allenes generated a broader spectrum of products.
Scheme 5.
Ligands influence the regioselectivity of Cu-catalyzed trifluoromethylation of propargylic bromodifluoroacetates
2.6 Summary
We have developed a series of Cu-catalyzed reactions that convert sp3-hybridized O-based electrophiles into trifluoromethanes, and that tolerate many sensitive functional groups that are commonly found in bioactive molecules. Key improvements that enabled catalysis included: 1) the efficient generation of active [(diamine)Cu–CF3] complexes to avoid unproductive decomposition of substrate; 2) modulation of the concentration of the active electrophile; and 3) the use of N-based ligands to stabilize and alter the reactivity of [LnCu–CF3] species. Additionally, we believe that Cu-catalyzed decarboxylation occurs via an inner-sphere process that does not generate :CF2 and −CF3, since sensitive acidic and carbonyl functional groups are stable under the reaction conditions. Ultimately, these methods can be used for rapid and economical preparations of bioactive compounds on both discovery scale and larger applications. Further, these transformations inspire the development of additional catalytic decarboxylative trifluoromethylation reactions.
3 Pd-catalyzed Decarboxylative Alkylation of α,α-Difluoroketone Enolates
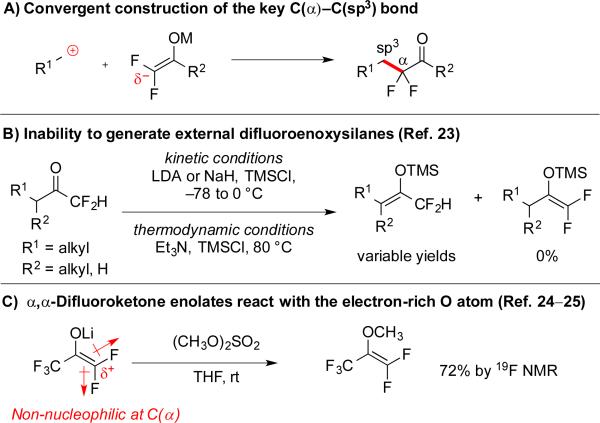
3.1 Convergent Routes towards α,α-Difluoroketones
Based on the success of our Cu-catalyzed trifluoromethylation reactions, we envisioned that the decarboxylative concept could be further extended to other fluoroalkyl nucleophiles, such as fluoroalkyl ketones. α,α-Difluoroketones exhibit unique physicochemical properties, and are found in many bioactive compounds,1c,d,14 including serine and aspartyl protease inhibitors. Thus, convergent and functional group tolerant strategies for forming these substructures can enable access to the next generation of biological probes and therapeutic candidates. Currently, the most common strategies for creating α,α-difluoroketones involve non-convergent functional group interconversions, such as electrophilic fluorination15 and deoxyfluorination,14a,16 that require harsh and/or oxidizing fluorinating reagents. Alternatively, Lewis acid-assisted alkylation of α,α-difluoroenoxysilanes can generate α-functionalized products, but only two groups have exploited this transformation that uses stoichiometric metals.17 Although Pd-catalyzed C(α)-C(sp2) bond-forming reactions of α,α-difluoroketone derivatives have been developed,18 catalytic coupling reactions of α,α-difluoroketones for constructing C(α)-C(sp3) bonds are underexplored. To address this challenge, we sought a direct convergent strategy to access this substructure that would generate the key C(α)–C(sp3) bond (Scheme 6A).
Scheme 6.
Generation of enolates and weak nucleophilicity of fluorinated enolates disfavor alkylation reactions of α,α-difluoroketone enolates with sp3-based electrophiles
Although SN2 reactions of non-fluorinated ketone enolates with sp3-hybridized electrophiles are a fundamental transformation for accessing α-substituted ketone derivatives,19 alkylation reactions of α,α-difluoroketone enolates with sp3-based electrophiles have been restricted by two problems. First, access to the desired terminal α,α-difluoroketone enolates is limited, because chemoselective deprotonation of α,α-difluoromethyl ketones preferentially generates internal non-fluorinated ketone enolates under both thermodynamic and kinetic conditions (Scheme 6B).20 Second, α,α-difluoroketone enolates display low nucleophilicity, and do not participate in traditional SN2-like reactions. Specifically, the strong inductive effect of the two fluorine atoms decreases the electron density at C(α),21 which renders this position non-nucleophilic (Scheme 6C). As a result, alkylation of α,α-difluoroenolates actually does not occur at C(α), but rather at the O atom to produce β,β-difluorovinyl ethers as the major product (Scheme 6C).22 Because of these two challenges, only two reports describe alkylation reactions of α,α-difluoroenoxysilanes with activated benzylic and allylic electrophiles, which both required stoichiometric metal additives to facilitate the reaction.17
To address these two challenges (the chemoselective formation of the α,α-difluoroenolate, and the intrinsically poor reactivity of the nucleophile), we applied a transition metal-catalyzed decarboxylative strategy that exploited: 1) a Pd-catalyzed decarboxylation to generate the desired Pd(II)-bound α,α-difluoroketone enolate; and 2) reductive elimination from [LnPdII(R)(enolate)] to form the key C(α)–C(sp3) bond, thus providing a convergent route for accessing α-alkyl-α,α-difluoroketones (Scheme 7). Using this strategy, we have developed both Pd-catalyzed benzylic and allylic substitution reactions of α,α-difluoroketone enolates. These reactions tolerate a broad spectrum of functional groups and heterocycles, such as amides, esters, carbamates, ketones, ethers, NO2, CF3, thiophenes, and pyrazoles.
Scheme 7.
A decarboxylative strategy chemoselectively generated the desired enolate and the C(α)–C(sp3)bond
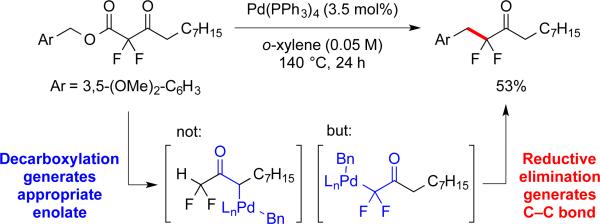
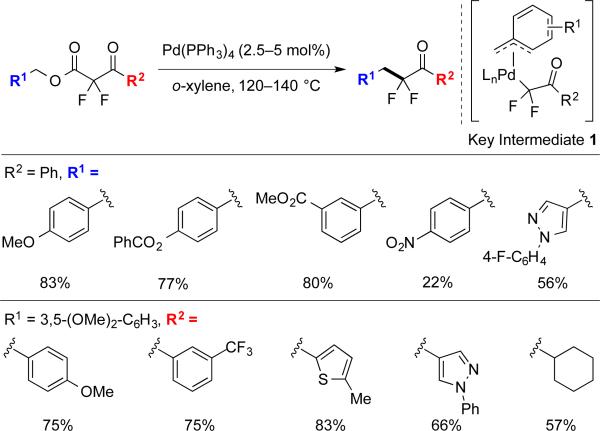
3.2 Pd-Catalyzed Decarboxylative Benzylation of α,α-Difluoroketone Enolates
A simple catalyst system of [Pd(PPh3)4] promoted the coupling reactions of benzylic substrates, with the electronic character of both α,α-difluoroketone enolate and benzylic fragment perturbing the reactivity of the system. Presumably, the electron-withdrawing effect from the two fluorine atoms improved the leaving-group ability of the α-carboxyl-α,α-difluoroketone, and accelerated the oxidative addition compared to less-activated mono- and non-fluorinated substrates, which do not react with this catalyst. Additionally, the electronic character of benzylic moieties also affected the system, with electron-rich benzylic esters affording higher yields than electron-poor substrates. This trend implies the involvement of [LnPd-(π-benzyl)(α,α-difluoroenolate)] intermediate 1,4b,23 with electron-donating groups stabilizing the π-system and favoring the reaction, and electron-withdrawing groups destabilizing the intermediate and disfavoring the reaction. Regardless, the fluorine effect also enabled reactions with electron-neutral, and -poor benzyl substrates (Scheme 8), whereas other transformations of nonfluorinated benzyl esters typically require an extended conjugated system, an electron-rich benzylic moiety, or a more powerful catalyst.24
Scheme 8.
Decarboxylative benzylation of substrates bearing distinct benzyl and ketone moieties
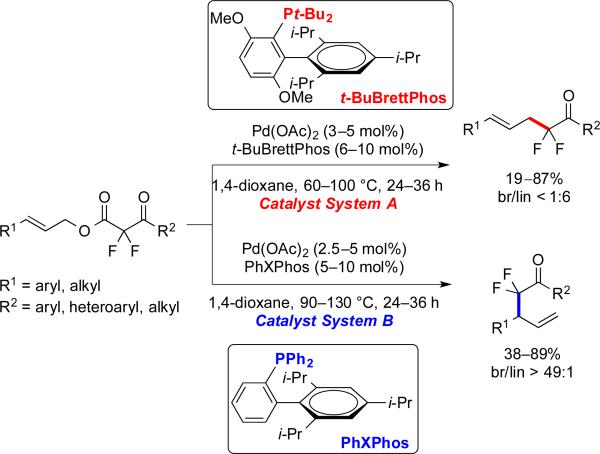
3.3 Pd-Catalyzed Decarboxylative Allylation of α,α-Difluoroketone Enolates
Additionally, we developed complementary Pd-catalyzed reactions of allylic substrates, which demonstrated an unique ligand-controlled regioselectivity. Specifically, Pd/monophosphine-based systems enabled access to both linear and branched α-allyl-α,α-difluoroketones (Scheme 9), with t-BuBrettPhos, an electron-rich and bulky ligand, producing linear products, and PhXPhos, an electron-deficient and smaller ligand, providing the uncommon and unexpected branched products. The formation of branched products was particularly intriguing,25 because Pd-catalyzed decarboxylative allylation of hard ketone enolates typically provide linear products.4b,26 Further, the ligand-controlled regioselectivity was observed for difluorinated substrates, but not for mono- and non-fluorinated substrates. Thus, the combination of the ligand and the electronic character of the α,α-difluoroketone enolate enabled access to the branched products.
Scheme 9.
Complementary catalytic systems generated the linear and branched α-allyl-α,α-difluoroketones
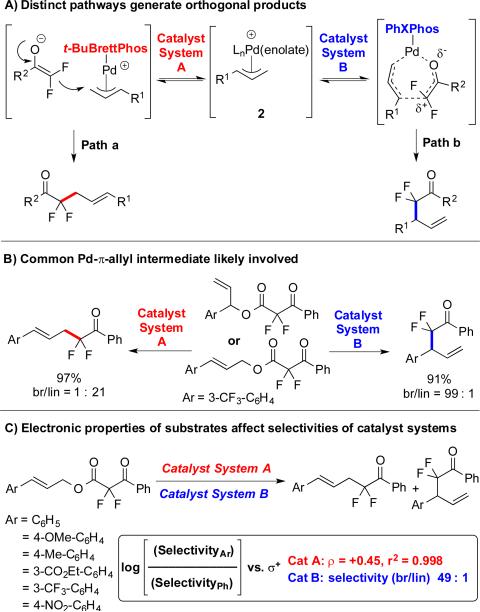
Our data implicate that both products likely result from a common Pd-π-allyl intermediate 2 (Scheme 10A). To confirm this intermediate, both linear and branched substrates were independently subjected to each catalyst system (Scheme 10B). Using catalyst system A, both linear and branched substrates formed linear products, and using catalyst system B, both linear and branched substrates provided branched products. This data discounted a memory effect, and implicated a common Pd-π-allyl intermediate, 2 (Scheme 10A). Evaluation of the relationship between electronic characters of cinnamyl moieties and regioselectivities of catalytic reactions suggests that the linear and branched products divert from this intermediate. For system A, the bulky electron-rich ligand might facilitate a dissociative pathway, in which the [LnPd(cinnamyl)]+ complex undergoes an outer-sphere attack by the α,α-difluoroketone enolate (Scheme 10A, Path a). For such a processes, the electronic nature of the cinnamyl-moiety can affect the distribution of linear and branched products, with electron-rich cinnamyl fragments displaying more SN1-like reactivity, which favors attack at the more hindered position.27 This scenario matches our observed linear free energy correlation (σ = +0.45, r2 = 0.998) between the electronic structure of cinnamyl moieties and the selectivity of br/lin products (Scheme 10C). In contrast, for system B, the electronic nature of cinnamyl-moiety did not affect selectivity with all substrates providing branched products (br/lin >49:1). This data disfavors an outer-sphere pathway, and instead, might support a mechanism proceeding through a sigmatropic–like rearrangement of a 7-membered [LnPd-(σ-allyl)(enolate)] intermediate (Scheme 10A, Path b).28 According to this paradigm, the small and electron deficient PhXPhos ligand might encourage association of the enolate ligand, and the fluorine atoms would sufficiently reduce the electron density at C(α) to allow attack by the allylic nucleophile. In support of the Pd-catalyzed rearrangement process, allyl α,α-difluoroenolethers undergo 3,3-sigmatropic Claisen rearrangement more easily than the non-fluorinated counterparts (80 °C/1 h/quantitative yield vs. 190 °C/6 h/75% yield).29 Ongoing collaborative computational work aims to probe these proposed pathways, or provide an alternate explanation for the observed reactivities.
Scheme 10.
Distinct mechanisms produce the linear and branched products
3.4 Summary
In conclusion, the Pd-catalyzed decarboxylative reactions provide convergent access to a variety of α-functionalized-α,α-difluoroketones bearing a broad spectrum of functional groups, and thus should enable access to biologically active compounds. Additionally, the strategy overcomes both challenges associated with alkylation reactions of α,α-difluoroketone enolates: chemoselective generation of α,α-difluoroketone enolate, and the formation of the key C(α)–C(sp3) bond. Finally, these reactions encourage further development of metal-catalyzed transformations of functionalized fluoroalkyl anions with sp3-based electrophiles.
Acknowledgments
We gratefully acknowledge the Herman Frasch Foundation for Chemical Research (701-HF12) and the NSF (CHE-1455163) for supporting these projects. We thank the NIGMS Training Grant on Dynamic Aspects of Chemical Biology (T32 GM08545) for a graduate traineeship (B.R.A.), and the University of Kansas, Office of the Provost, Department of Medicinal Chemistry, and General Research Fund (2506008) for additional financial assistance. We thank Mr. Douglas L. Orsi for creative contributions to the TOC figure.
Biography
 Ryan A. Altman received a B.S. in chemistry from Creighton University in 2003 and a Ph.D. in organic Chemistry from the Massachusetts Institute of Technology (MIT) in 2008. At MIT, he studied as a Pfizer and National Institutes of Health predocotoral fellow in the laboratory of Professor Stephen L. Buchwald. He completed his training at the University of California, Irvine as a National Institutes of Health postdoctoral fellow from 2008–2011 under the guidance of Professor Larry E. Overman, after which he accepted his current position as an assistant professor in the Department of Medicinal Chemistry at the University of Kansas. Dr. Altman's group primarily works at the interfaces of organometallic, organofluorine, and physical organic chemistries. However, the group is also initiating projects that explore physicochemical and biophysical perturbations imparted by fluorinated substructures, with the ultimate goal of enhancing distribution and metabolism properties of neurotherapeutic probes.
Ryan A. Altman received a B.S. in chemistry from Creighton University in 2003 and a Ph.D. in organic Chemistry from the Massachusetts Institute of Technology (MIT) in 2008. At MIT, he studied as a Pfizer and National Institutes of Health predocotoral fellow in the laboratory of Professor Stephen L. Buchwald. He completed his training at the University of California, Irvine as a National Institutes of Health postdoctoral fellow from 2008–2011 under the guidance of Professor Larry E. Overman, after which he accepted his current position as an assistant professor in the Department of Medicinal Chemistry at the University of Kansas. Dr. Altman's group primarily works at the interfaces of organometallic, organofluorine, and physical organic chemistries. However, the group is also initiating projects that explore physicochemical and biophysical perturbations imparted by fluorinated substructures, with the ultimate goal of enhancing distribution and metabolism properties of neurotherapeutic probes.
References
- 1.a Hiyama T. Organofluorine Compounds: Chemistry and Applications. Springer; New York: 2000. [Google Scholar]; b Kirsch P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity and Applications. Wiley-VCH; Weinhein: 2004. [Google Scholar]; c Bégué JP, Bonnet-Delpon D. Bioorganic and Medicinal Chemistry of Fluorine. Wiley-VCH; Weinheim: 2008. [Google Scholar]; d Ojima I. Fluorine in Medicinal Chemistry and Chemical Biology. Wiley-Blackwell; Chichester: 2009. [Google Scholar]; e Gouverneur V, Muller K. Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications. Imperial College Press; London: 2012. [Google Scholar]
- 2.a Tomashenko OA, Grushin VV. Chem. Rev. 2011;111:4475. doi: 10.1021/cr1004293. [DOI] [PubMed] [Google Scholar]; b Liu T, Shen Q. Eur. J. Org. Chem. 2012:6679. [Google Scholar]; c Liang T, Neumann CN, Ritter T. Angew. Chem., Int. Ed. 2012;52:8214. doi: 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]; d Liu X, Xu C, Wang M, Liu Q. Chem. Rev. 2015;115:683. doi: 10.1021/cr400473a. [DOI] [PubMed] [Google Scholar]; e Alonso C, Martínez de Marigorta E, Rubiales G, Palacios F. Chem. Rev. 2015;115:1847. doi: 10.1021/cr500368h. [DOI] [PubMed] [Google Scholar]
- 3.Qiao Y, Zhu L, Ambler BR, Altman RA. Curr. Top. Med. Chem. 2014;14:966. doi: 10.2174/1568026614666140202210850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a Baudoin O. Angew. Chem. Int. Ed. 2007;46:1373. doi: 10.1002/anie.200604494. [DOI] [PubMed] [Google Scholar]; b Weaver JD, Recio A, III, Grenning AJ, Tunge JA. Chem. Rev. 2011;111:1846. doi: 10.1021/cr1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Rodriguez N, Goossen LJ. Chem. Soc. Rev. 2011;40:5030. doi: 10.1039/c1cs15093f. [DOI] [PubMed] [Google Scholar]; d Behenna DC, Stoltz BM. Top. Organomet. Chem. 2013;44:281. [Google Scholar]; e Arseniyadis S, Fournier J, Thangevelu S, Lozano O, Prevost S, Archambeau A, Menozzi C, Cossy J. Synlett. 2013:2350. [Google Scholar]
- 5.a Kobayashi Y, Yamamoto K, Kumadaki I. Tetrahedron Lett. 1979;20:4071. [Google Scholar]; b Bouillon J-P, Maliverney C, Merenyi R, Viehe HG. J. Chem. Soc., Perkin Trans. 1991;1:2147. [Google Scholar]; c Tan L, Chen C.-y., Larsen RD, Verhoeven TR, Reider PJ. Tetrahedron Lett. 1998;39:3961. [Google Scholar]; d Larsson JM, Pathipati SR, Szabo KJ. J. Org. Chem. 2013;78:7330. doi: 10.1021/jo4010074. [DOI] [PubMed] [Google Scholar]; e Miyake Y, Ota S.-i., Nishibayashi Y. Chem. Eur. J. 2012;18:13255. doi: 10.1002/chem.201202853. [DOI] [PubMed] [Google Scholar]; f Miyake Y, Ota S.-i., Shibata M, Nakajima K, Nishibayashi Y. Org. Biomol. Chem. 2014;12:5594. doi: 10.1039/c4ob00957f. [DOI] [PubMed] [Google Scholar]
- 6.a Burton DJ, Hartgraves GA, Hsu J. Tetrahedron Lett. 1990;31:3699. [Google Scholar]; b Bouillon J-P, Maliverney C, Merényi R, Viehe HG. J. Chem. Soc. Perkin Trans. 1991;1:2147. [Google Scholar]; c Kawai H, Furukawa T, Nomura Y, Tokunaga E, Shibata N. Org. Lett. 2011;13:3596. doi: 10.1021/ol201205t. [DOI] [PubMed] [Google Scholar]; d Zhao TSN, Szabo KJ. Org. Lett. 2012;14:3966. doi: 10.1021/ol3017287. [DOI] [PubMed] [Google Scholar]; e Miyake Y, Ota S.- i., Shibata M, Nakajima K, Nishibayashi Y. Chem. Commun. 2013;49:7809. doi: 10.1039/c3cc44434a. [DOI] [PubMed] [Google Scholar]; f Jiang X, Qing F-L. Beilstein J. Org. Chem. 2013;9:2862. doi: 10.3762/bjoc.9.322. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Ji Y-L, Kong J-J, Lin J-H, Xiao J-C, Gu Y-C. Org. Biomol. Chem. 2014;12:2903. doi: 10.1039/c3ob42575d. [DOI] [PubMed] [Google Scholar]
- 7.a Chen Q-Y, Wu S-W. J. Chem. Soc., Chem. Commun. 1989:705. [Google Scholar]; b Su D-B, Duan J-X, Chen Q-Y. Tetrahedron Lett. 1991;32:7689. [Google Scholar]; c Duan J-X, Su D-B, Chen Q-J. J. Fluorine Chem. 1993;61:279. [Google Scholar]; d Chen Q-Y, Duan J-X. J. Chem. Soc., Chem. Commun. 1993:1389. [Google Scholar]; e Duan J-X, Chen Q-Y. J. Chem. Soc., Perkin Trans. 1994;1:725. [Google Scholar]; f Duan J-X, Su D-B, Wu J-P, Chen Q-Y. J. Fluorine Chem. 1994;66:167. [Google Scholar]; g Chen Q-Y. J. Fluorine Chem. 1995;72:241. [Google Scholar]
- 8.a Ambler BR, Altman RA. Org. Lett. 2013;15:5578. doi: 10.1021/ol402780k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ambler BR, Zhu L, Altman RA. J. Org. Chem. 2015;80:8449. doi: 10.1021/acs.joc.5b01343. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ambler BR, Peddi S, Altman RA. Synthesis. 2014;46:1938. doi: 10.1055/s-0033-1339128. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ambler BR, Peddi S, Altman RA. Org. Lett. 2015;17:2506. doi: 10.1021/acs.orglett.5b01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a England DC, Krespan CG. J. Org. Chem. 1968;33:816. [Google Scholar]; b Drivon G, Gillet J-P, Ruppin C. 2005 Atofina, U.S. 6906219 B2.
- 10.a Prakash GKS, Yudin AK. Chem. Rev. 1997;97:757. doi: 10.1021/cr9408991. [DOI] [PubMed] [Google Scholar]; b Zanardi A, Novikov MA, Martin E, Benet Buchholz J, Grushin VV. J. Am. Chem. Soc. 2011;133:20901. doi: 10.1021/ja2081026. [DOI] [PubMed] [Google Scholar]; d Lishchynskyi A, Novikov MA, Martin E, Escudero-Adán EC, Novák P, Grushin VV. J. Org. Chem. 2013;78:11126. doi: 10.1021/jo401423h. [DOI] [PubMed] [Google Scholar]; d Liu X, Xu C, Wang M, Liu Q. Chem. Rev. 2015;115:683. doi: 10.1021/cr400473a. [DOI] [PubMed] [Google Scholar]
- 11.Walter W, Voss J. In: The Chemistry of Amides. Patai S, editor. Interscience; London: 1970. Chapter 8. [Google Scholar]
- 12.Symons EA, Clermont MJ. J. Am. Chem. Soc. 1981;103:3127. [Google Scholar]
- 13.McClinton MA, McClinton DA. Tetrahedron. 1992;48:6555. [Google Scholar]
- 14.a Dreyer GB, Metcalf BW. Tetrahedron Lett. 1988;29:6885. [Google Scholar]; b Hamer RL, Freed B, Allen RC, Hoechst-Roussel Pharmaceuticals :1991. U.S. 5006563.; c Han C, Salyer AE, Kim EH, Jiang X, Jarrard RE, Powers MS, Kirchhoff AM, Salvador TK, Chester JA, Hockerman GH, Colby DA. J. Med. Chem. 2013;56:2456. doi: 10.1021/jm301805e. [DOI] [PubMed] [Google Scholar]; d Banville J, Remillard R, Balasubramanian N, Bouthillier G, Martel A, Squibb Bristol-Myers. 2002 U.S. 20020037875A1.
- 15.a Baudoux J, Cahard D. Organic Reactions. 2007;69:347. [Google Scholar]; b Differding E, Ruegg GM, Lang RW. Tetrahedron Lett. 1991;32:1779. [Google Scholar]; c Peng W, Shreeve JM. J. Org. Chem. 2005;70:5760. doi: 10.1021/jo0506837. [DOI] [PubMed] [Google Scholar]; d Pravst I, Zupan M, Stavber S. Synthesis. 2005:3140. [Google Scholar]
- 16.a Biju P. Synth. Commun. 2008;38:1940. [Google Scholar]; b Miwatashi S, Suzuki H, Okawa T, Miyamoto Y, Yamasaki K, Hitomi Y, Hirata Y, Shibuya A. Takeda Pharmaceutical Company Limited; Japan: 2013. WO 2013122028A1. [Google Scholar]
- 17.a Brigaud T, Doussot P, Portella C. J. Chem. Soc., Chem. Commun. 1994:2117. [Google Scholar]; b Lefebvre O, Brigaud T, Portella C. Tetrahedron. 1999;55:7233. [Google Scholar]; c Kobayashi S, Tanaka H, Amii H, Uneyama K. Tetrahedron. 2003;59:1547. [Google Scholar]
- 18.a Guo Y, Shreeve JM. Chem. Commun. 2007;34:3583. doi: 10.1039/b705137a. [DOI] [PubMed] [Google Scholar]; b Guo C, Wang R-W, Qing F-L. J. Fluor. Chem. 2012;143:135. [Google Scholar]; c Ge S, Chaladaj W, Hartwig J. J. Am. Chem. Soc. 2014;136:4149. doi: 10.1021/ja501117v. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Andersen TL, Frederiksen MW, Domino K, Skrydstrup T. Angew. Chem. Int. Ed. 2016;55:10396. doi: 10.1002/anie.201604152. [DOI] [PubMed] [Google Scholar]; e Zhao H-Y, Feng Z, Luo Z, Zhang X. Angew. Chem. Int. Ed. 2016;55:10401. doi: 10.1002/anie.201605380. [DOI] [PubMed] [Google Scholar]
- 19.a Stolz D, Kazmaier U. Metal Enolates as Synthons in Organic Chemistry. In: Zabicky J, editor. Chemistry of Metal Enolates. Chapter 7. John Wiley & Sons, Ltd.; London, UK: 2009. pp. 355–409. [Google Scholar]; b Stoltz BM, Bennett NB, Duquette DC, Goldberg AFG, Liu Y, Loewinger MB, Reeve CM. In: Comprehensive Organic Synthesis II (Second Edition) Knochel P, Molander GA, editors. Vol. 3. Elsevier; 2014. pp. 1–55. English. [Google Scholar]
- 20.a Yamana M, Ishihara T, Ando T. Tetrahedron Lett. 1983;24:507. [Google Scholar]; b Kuroboshi M, Ishihara T. Bull. Chem. Soc. Jpn. 1990;63:428. [Google Scholar]; c Liu Y-L, Zhou J. Chem. Commun. 2012;48:1919. doi: 10.1039/c2cc17140f. [DOI] [PubMed] [Google Scholar]
- 21.a Uneyama K. Fundamentals in Organic Fluorine Chemistry. Organofluorine Chemistry. Chapter. Vol. 1. Blackwell; Oxford, UK: 2006. p. p10. [Google Scholar]; b Qian C-P, Nakai T. J. Am. Chem. Soc. 1990;112:4602. [Google Scholar]
- 22.Qian C-P, Nakai T. Tetrahedron Lett. 1988;29:4119. [Google Scholar]
- 23.Trost BM, Czabaniuk LC. Angew. Chem., Int. Ed. 2014;53:2826. doi: 10.1002/anie.201305972. [DOI] [PubMed] [Google Scholar]
- 24.a Legros JY, Toffano M, Fiaud JC. Tetrahedron. 1995;51:3235. [Google Scholar]; b Torregrosa RP, Ariyarathna Y, Chattopadhyay K, Tunge JA. J. Am. Chem. Soc. 2010;132:9280. doi: 10.1021/ja1035557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.a Zheng W-H, Zheng B-H, Zhang Y, Hou X-L. J. Am. Chem. Soc. 2007;129:7718. doi: 10.1021/ja071098l. [DOI] [PubMed] [Google Scholar]; b Chen P-P, Peng Q, Lei B-L, Hou X-L, Wu Y-D. J. Am. Chem. Soc. 2011;133:14180. doi: 10.1021/ja2039503. [DOI] [PubMed] [Google Scholar]
- 26.a Oliver S, Evans PA. Synthesis. 2013;45:3179. [Google Scholar]; b Tsuji J, Yamada T, Minami I, Yuhara M, Nisar M, Shimizu I. J. Org. Chem. 1987;52:2988. [Google Scholar]
- 27.a Prétôt R, Pfaltz A. Angew. Chem. Int. Ed. 1998;37:323. doi: 10.1002/(SICI)1521-3773(19980216)37:3<323::AID-ANIE323>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]; b Hayashi T, Kawatsura M, Uozumi Y. Chem. Commun. 1997:561. [Google Scholar]
- 28.a Méndez M, Cuerva JM, Gómez-Bengoa E, Cárdenas DJ, Echavarren AM. Chem. Eur. J. 2002;8:3620. doi: 10.1002/1521-3765(20020816)8:16<3620::AID-CHEM3620>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; b Keith JA, Behenna DC, Mohr JT, Ma S, Marinescu SC, Oxgaard J, Stoltz BM, Goddard WA., III J. Am. Chem. Soc. 2007;129:11876. doi: 10.1021/ja070516j. [DOI] [PubMed] [Google Scholar]; c Keith JA, Behenna DC, Sherden N, Mohr JT, Ma S, Marinescu SC, Nielsen RJ, Oxgaard J, Stoltz BM. J. Am. Chem. Soc. 2012;134:19050. doi: 10.1021/ja306860n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.a Cresson P. Bull. Soc. Chim. Fr. 1964:2618. [Google Scholar]; b Metcalf BW, Jarvi ET, Burkhart JP. Tetrahedron Lett. 1985;26:2861. [Google Scholar]