Abstract
Purpose of review
To provide a state-of-the-art update on some emerging measures of vitamin D status and discuss how assessment of these key vitamin D metabolites might improve prognostication of risk for cardiovascular disease (CVD) outcomes.
Recent findings
Vitamin D deficiency is a highly prevalent condition and relatively easy to treat with supplementation and/or modest sunlight exposure. A substantial body of experimental and epidemiological evidence suggest that vitamin D deficiency is a risk factor for CVD. Most epidemiologic studies to date have focused on total 25-hydroxyvitamin D [25(OH)D] concentrations, which is the established marker of vitamin D stores. However, there is emerging evidence that other novel markers of vitamin D metabolism may better characterize ‘true’ vitamin D status. Some key novel measures include bioavailable 25(OH)D, free 25(OH)D, 1–25 dihydroxyvitamin D, 24,25-dihydroxyvitamin D3 [24,25(OH)2D3], and ratio of 24,25(OH)2D3 to 25(OH)D [the vitamin D metabolic ratio]. Utilization of these biomarkers may enhance understanding of the association between vitamin D and CVD risk, and may provide explanation for the observation that 25(OH)D is a stronger CVD risk factor in whites than blacks
Summary
Novel measures of vitamin D status could potentially change clinical practice regarding how patients are currently screened for vitamin D status and defined as vitamin D deficient or not. However, whether measuring any of these alternate markers of vitamin D status can provide further insight regarding CVD risk beyond the traditionally measured 25(OH)D concentrations is uncertain at this time. This is an area where further research is strongly needed.
Keywords: Vitamin D, Biomarker, Cardiovascular Risk Factor
Introduction
Vitamin D is a fat-soluble vitamin that is critical for the maintenance of bone mineral density (BMD) [1, 2], but there is growing evidence that it may also reduce inflammation, play a role in regulating the renin-angiotensin-aldosterone system, improve insulin sensitivity, and modulate the immune system [3–8]. Specifically, there have been numerous epidemiologic studies that have demonstrated that vitamin D deficiency is associated with increased risk of cardiovascular diseases (CVD) [9], including coronary heart disease (CHD) [10, 11], congestive heart failure (HF) [12], peripheral arterial disease [13], and cerebral vascular disease [14, 15].
To date, virtually all epidemiologic studies linking low vitamin D with CVD risk have focused on total 25-hydroxyvitamin D levels [25(OH)D], which long withstanding has been viewed as the best marker of vitamin D stores. However, there is emerging evidence that other novel markers of vitamin D metabolism may better characterize vitamin D status. Were this the case, measurement of these novel markers might yield a stronger association between vitamin D and CVD risk, and may provide an explanation for the observation that the association between 25(OH)D and CVD is stronger in whites than blacks [10–12, 14]. Some of the novel measures at the forefront of these discussions include bioavailable 25(OH)D, free 25(OH)D, 1,25-dihydroxyvitamin D [1,25(OH)2D, i.e. calcitriol], 24,25-dihydroxyvitamin D3 [24,25(OH)2D3], and the ratio of 24,25(OH)2D3 to 25(OH)D, otherwise known as the vitamin D metabolic ratio (VMR). In fact, the U.S. Preventive Services Task Force in their 2015 recommendation statement not only concluded that there was insufficient evidence to balance the benefits and harms of screening for vitamin D deficiency among asymptomatic adults, but also that it is unclear whether total 25(OH)D is the best indicator of vitamin D status or whether bioavailable 25(OH)D should be used instead [16].
The major focus of this review article is to provide a state-of-the-art update on key emerging measures of vitamin D status and discuss how assessment of these novel vitamin D metabolites might improve prognostication of risk for CVD outcomes.
Vitamin D Metabolism: An Overview
There are two precursors to 25(OH)D; D3 (cholecalciferol), made in the skin and from animal food products, and D2 (ergocalciferol) which is derived from plant sources [6, 17]. A major source of vitamin D occurs when ultraviolet (UVB) exposure to skin results in the conversion of 7-dehydrocholestrol, a cholesterol precursor, to previtamin D3 which isomerizes to D3 [18]. Nutritional intake traditionally had provided a relatively small source of vitamin D, but there has been an increased use of over the counter supplements more recently [17, 19, 20].
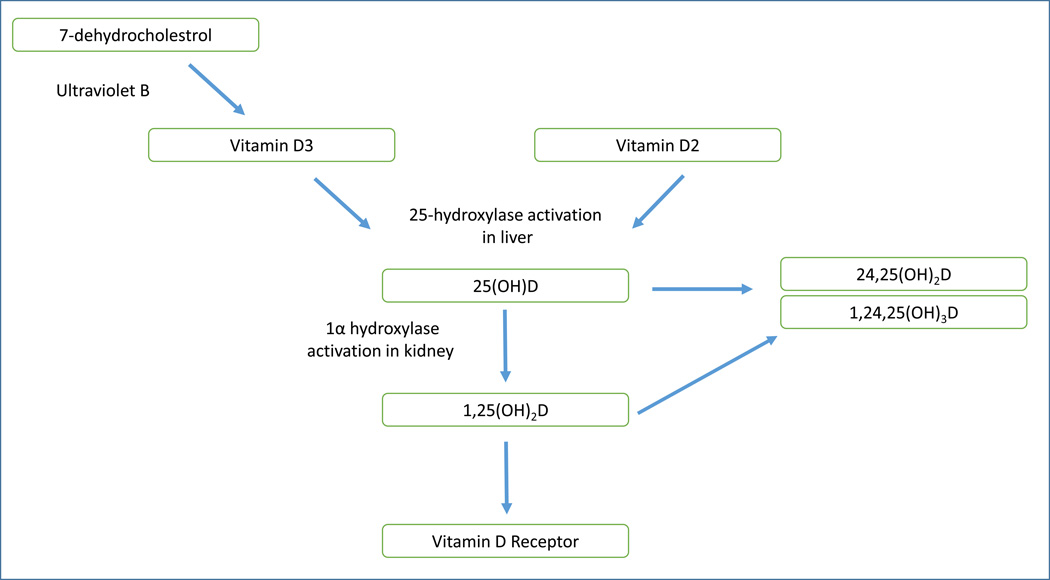
Cholecalciferol and ergocalciferol are hydroxylated to 25(OH)D predominantly in the liver. Although 25(OH)D is the major storage form of vitamin D, 1,25(OH)2D is the hormonally active metabolite of vitamin D. The conversion of 25(OH)D to 1,25(OH)2D takes place in the kidneys primarily by the 1α-hydroxylase enzyme, but this conversion can also be seen in vasculature as well. The 1α-hydroxylation is controlled by the effect of parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF-23) [21]. Figure 1 summarizes the vitamin D metabolism pathway in a simplified format.
Figure 1. Vitamin D Metabolism Pathway.
Vitamin D can be obtained in the form of D3 from conversion of 7-dehydrocholestrol in the skin after exposure to UVB or in dietary forms of D2 or D3. D2 and D3 are then hydroxylated to 25-hydroxyvitamin D in the liver. 25(OH)D circulates as the predominant storage form, with the majority bound to vitamin D binding protein (VDBP). Subsequently, the 25(OH)D is hydroxylated by the 1α hydroxylase in the kidney, converting it to the active 1,25(OH)2D. 1,25(OH)2D then circulates and binds to the vitamin D receptor (VDR) in various tissue cells including cardiovascular tissues which triggers cellular activity. Both 25(OH)D and 1,25(OH)2D can be catabolized with 24-hydroxylase activity and ultimately excreted by the kidneys.
In circulation, most of the 25(OH)D and 1,25(OH)2D is bound to vitamin D binding protein (VDBP). In fact, 85–90% of all circulating 25(OH)D is bound to VDBP, 10–15% is bound to albumin, and less than 1% of the total is free [22, 23]. Bioavailable vitamin D refers to the sum of the free and albumin-bound fractions of 25(OH)D. Because clinical assays do not distinguish between bound versus unbound 25(OH)D, it is possible that an individual with low total 25(OH)D may have adequate bioavailable 25(OH)D. Free vitamin can be estimated with a formula derived from the Vermeulen formula [22, 24, 25] as shown in Table 1 below. Some newer iterations of the formula for free vitamin D argues for using binding constants for rs7041 and rs4588 [26], which are single nucleotide polymorphism (SNP) variants related to VDBP, to account for differences in relative binding affinity, but there is currently no consensus opinion on using these iterations.
Table 1.
Key vitamin D equations for assessing alternate measures of vitamin D status
| Total Vitamin D (mol/L) = Free 25(OH)D + Albumin-bound 25(OH)D + VDBP-bound 25(OH)D Bioavailable Vitamin D (mol/L): Albumin-bound 25(OH)D + Free 25(OH)D Free 25(OH)D (mol/L) = total 25(OH)D / [1 + (Ka albumin* × albumin) + (Ka VDBP* × VDBP**)] = total 25(OH)D / [1 + (6 × 105 mol/L × albumin) + (7 × 108 mol/L × VDBP**)] VMR = 24,25(OH)2D3 / 25(OH)D |
K refers to the binding affinity constant for albumin and VDBP
Some iterations of equations for free 25(OH)D also take into account the different binding affinity conferred by different SNP variants of VDBP, not included here
The 25(OH)D form of vitamin D will eventually be activated to the 1,25(OH)2D form (calcitriol), which binds to the vitamin D receptor (VDR), found in nearly in every organ system including in cardiac tissue. After activation of the receptor, vitamin D is translocated to the nucleus for various protein syntheses which then in turns confer the primary health effects of vitamin D [6].
The metabolite 24,25(OH)2D3 is formed from 25(OH)D after 24-hydroxlation which is mediated via the cytochrome P450 (CYP24A1) enzyme, and has been proposed to be a metabolite largely destined for excretion. However, there is some debate about whether this 24,25(OH)2D3 compound is metabolically inactive or active. In essence, 24,25(OH)2D3 production is a key step in inactivating 25(OH)D and regulating 1,25(OH)2D (calcitriol) synthesis. Increased catabolism of 25(OH)D to 24,25(OH)2D3 occurs with higher 25(OH)D levels, such as seen with supplemental vitamin D treatment [27], and conversely, CYP24A1 is downregulated in vitamin D deficient states [28]. Thus 24,25(OH)2D3 levels may be a better marker of vitamin D sufficiency than 25(OH)D levels.
Because enzymatic synthesis of 24,25(OH)2D3 is proportional to its substrate (25(OH)D), levels of the two metabolites are highly correlated. Measuring the ratio of 24,25(OH)2D3 to 25(OH)D, otherwise known as the vitamin D metabolite ratio (VMR), may be a useful clinical marker of vitamin D metabolism and of response to vitamin D therapy [27, 29].
Why Study Vitamin D and CVD?
Vitamin D is a potentially important target of CVD risk for several reasons. First, vitamin D deficiency is relatively common in the adult population. Vitamin D concentrations are typically measured with serum 25(OH)D, which is the predominant circulating storage form. Vitamin D deficiency has generally been defined as 25(OH)D levels ≤20 ng/mL, with levels of 20–30 ng/mL considered insufficient, and levels ≥30 ng/ml considered optimal by the Endocrine Society [30], but these cut-points are not without controversy [31]. The Institute of Medicine (IOM) position is that levels ≥20 ng/ml should be adequate enough for health for the vast majority of Americans [32]. The National Health and Nutrition Examination Survey (NHANES) in the 2000’s analyzed the general American population and found a prevalence of vitamin D deficiency (levels <20 ng/ml) of about 40%, and that rates of deficiency were much higher in certain racial/ethnic groups, namely Blacks (80%) and Hispanics (60%) [33]. This data has been supported by other similar large population studies [34–36].
Second, there have been several large studies demonstrating a convincing association between vitamin D levels and cardiovascular outcomes [8, 9]. In a 2012 meta-analysis [9], which included 19 independent prospective studies with 6,123 CVD cases in 65,994 participants, Wang et al demonstrated a generally linear, inverse association between circulating 25(OH)D and risk of CVD. In a comparison of the lowest with the highest 25(OH)D categories, the pooled relative risk for total CVD was 1.52 (95% confidence interval, 1.30–1.77), for CVD mortality was 1.42 (1.19–1.71), for CHD was 1.38 (1.21–1.57), and for stroke was 1.64 (1.27–2.10). These associations were robust, remaining strong and significant when analyses were limited to studies that excluded participants with baseline CVD and were better controlled for season and confounding.
Findings from these epidemiologic studies align with in vitro studies using VDR and 1α-hydroxylase knock-out mice that have shown increased cardiovascular and metabolic risk [37, 38]. The activation of VDR is felt to be the key step that has a number of potential effects on the cardiovascular system including down-regulation of renin gene transcription [38–40], activation of vasodilatory and anti-thrombotic proteins [41–43], and inhibition of foam cell formation and increased cholesterol efflux in macrophages [44].
Third, there are numerous treatments for vitamin D deficiency ranging from increasing intake of nutritional sources such as milk, eggs, and fatty fish, to UVB radiation, and to supplements including vitamin D2 and D3 [1]. Finally, vitamin D levels in theory should be readily measureable, again making it a potentially ideal biomarker for screening. Presently, in patient populations where screening of vitamin D status is indicated, current guidelines recommend measurement of 25(OH)D, which has been associated with PTH, BMD, and fracture risk [45].
In sum, if low vitamin D status is confirmed to be causally associated with CVD, it makes it an attractive target for prevention given ease of screening and low-risk treatment options.
Limitations of Existing Vitamin D Research
Low vitamin D has not yet been definitely established as a true causal risk factor for CVD. It is unclear if vitamin D is a true risk factor that contributes directly to the development of CVD progression, or if it is simply a reflection of other health characteristics that are causally associated with CVD risk [46, 47]. Mendelian randomization studies have not supported a causal role of vitamin D in CVD pathogenesis [48, 49].
Additionally while animal models have suggested that vitamin D deficiency is associated with hypertension, endothelial dysfunction, and left ventricular dysfunction this association has not always been so conclusive in clinical trials. While a number of studies have demonstrated a positive association between vitamin D deficiency and CVD risk factors, there have been many other trials that have shown a null relationship.
For instance, hypertension has been one of the more well studied CVD outcomes in relation to vitamin D deficiency [50], but vitamin D supplementation in randomized controlled trials (RCTs) have not conclusively shown any clinically significant reduction in blood pressure despite more than 40 such trials investigating this question [51, 52]. Most recently, the VINDICATE trial, which recruited patients with chronic HF, showed that vitamin D supplementation did not improve the patients’ 6 minute walk test [53]. Jiang et al in their meta-analysis of vitamin D supplementation in the treatment of chronic HF also did not show any clinically significant change in LV function or exercise tolerance [53]. Additionally, meta-analyses of RCTs with vitamin D supplementation have not shown significant effects on endothelial dysfunction when flow-mediated dilatation, pulse wave velocity, and the augmentation index were used as indicators of arterial stiffness [54].
There remains very limited and inconclusive data on the role of vitamin D supplementation on CVD outcomes [55], and more RCTs need to be conducted. It is important to note that prior RCTs might be potentially limited by under-dosing of vitamin D supplementation [8]. Another criticism of prior vitamin D trials has been that most trials do not measure vitamin D levels at baseline and restrict recruitment to only those with low levels [46]. Vitamin D supplementation may only be beneficial in those with low levels at baseline. Fortunately, there are active large-scale trials underway to test whether 25(OH)D supplementation reduces CVD risk [55]. One of the largest studies in progress in the U.S. is the Vitamin D and Omega-3 Trial (VITAL) of nearly 26,000 participants that is looking at the role of vitamin D3 and omega-3 fatty acid (in combination or separately, versus placebo) for the prevention of CVD [56]. This RCT is anticipated to conclude in 2017 and hopefully will be informative in guiding clinical recommendations. Similar ongoing studies include D-Health in Australia, FIND in Finland, and VIDAL in the United Kingdom [55].
Finally, there have been recent concerns about whether 25(OH)D is the optimal marker for assessing vitamin D status. If we wish to screen for low vitamin D, to potentially intervene with the goal of preventing CVD, it is important to have a biomarker which accurately reflects of someone’s physiologically active vitamin D [57]. Initial studies that assessed the utility of 25(OH)D have been mostly validated for assessing bone health and may not be readily applicable in CVD settings [57, 58]. Also, new biomarkers have recently emerged, but these have not yet been evaluated prospectively in relation to CVD risk.
Racial Paradox of Vitamin D Levels and Potential Utility of Bioavailable/Free Vitamin D Levels
One of the concerns of 25(OH)D as a marker for serum vitamin D levels has been paradoxical findings by race. It has been well known that Blacks have lower levels of 25(OH)D because the melanin-rich skin reduces absorption of UVB light needed for vitamin D synthesis [18]. Yet despite the fact that Blacks living in the U.S. have roughly 30% lower 25(OH)D than their counterpart Whites, elderly African American women have significantly lower rates of fractures, roughly half of counterpart White American women [33, 59, 60]. Furthermore, using data from the MESA study, Robinson-Cohen et al raised concern that lower serum 25(OH)D was a risk factor for CHD in White and Chinese participants, but not in Blacks or Hispanics [10]. A similar finding of increased CHD risk associated with low 25(OH)D status in Whites but not Blacks was also noted in the ARIC study [11]. Furthermore racial differences in CVD risk conferred by 25(OH)D deficiency have also been noted for the outcomes of stroke [14], HF [12], and diabetes [61].
This has led to an intensified interest in the free hormone hypothesis, which is a general rule of thumb in endocrinology that free hormones in the serum are the physiologically active forms and their concentrations may be more reliable indicators of the hormonal effects, relative to total hormone levels. For other analogous endocrine hormones, clinicians frequently try to use measurements such as free thyroxine (T4) levels or free testosterone levels in a number of medical conditions that can affect the binding protein [24, 62].
To reconcile this paradox, Powe et al in 2013 published in the NEJM a study that concluded that the racial differences in 25(OH)D levels may be secondary to low VDBP levels in African Americans [26]. They used a monoclonal immunoassay to measure the concentration of VDBP to estimate bioavailable vitamin D and found that the bioavailable and free vitamin D levels in Blacks were similar to that of Whites after accounting for VDBP. This finding seemed promising as an explanation for the racial paradox.
However, the conclusions from Powe et al’s study have been very controversial and recently disputed especially regarding the method used to measure VDBP, which was used to estimate bioavailable 25(OH)D concentrations [63, 64]. Powe et al used a monoclonal sandwich assay to determine VDBP. Other studies have found that VDBP levels were significantly lower in Blacks when using certain monoclonal-based enzyme-linked immunosorbent assays (ELISA) but actually higher when using a polyclonal-based ELISA [59].
Genome-wide association studies (GWAS) of 25(OH)D levels have found that variants of genes involved in cholesterol synthesis, hydroxylation, and vitamin D transport influence vitamin D status [65]. There are two major polymorphisms for VDBP that generate three possible haplotypes (Gc1s, Gc1f, and Gc2) with genetic variation at GC, a gene that encodes the binding protein [63]. Of these, Blacks are more likely to have the Gc1f haplotype by a significant margin. Hoofnagle et al raised concern that there was no guarantee that monoclonal antibodies would recognize the same epitope in all the VDBP variants. In 187 participants with genotyped VDBP, Hoofnagle et al discovered that when they measured VDBP using a liquid chromatography-tandem mass spectrometric assay (LC-MS/MS), the concentrations of VBDP were similar to that of polyclonal assay, but the monoclonal assay resulted in very different (i.e. lower) results especially in the presence of the Gc1f haplotype [63, 64]. Their results found, in contrast to Powe’s conclusions, that the concentrations of VDBP were actually similar among Whites and Blacks and unfortunately could not explain the vitamin D racial paradox.
Furthermore, Nielson et al in two separate publications also showed that there is a strong dependence of calculated free 25(OH)D depending on the assay used for VDBP for Blacks, with the monoclonal assay conferring significant underestimation of the VDBP concentration compared to polyclonal assays [21, 66, 67].
In another recent study of 125 participants in the Chronic Renal Insufficiency Cohort (CRIC), Denburg et al compared measurement of serum VDBP using a monoclonal ELISA, a polyclonal ELISA, and LC-MS/MS [68]. They found that VDBP haplotype only explained ≤9% of the variability in VDBP concentrations when using LC-MS/MS or polyclonal ELISA but 85% of the variability of VDBP levels when using the monoclonal ELISA. Among the Gc1f homozygotes, VDBP levels were measured to be lower using monoclonal ELISA than when using the other assays. Again, VDBP levels did not differ by race when using LC-MS. Blacks had both lower levels of free 25(OH)D and bioavailable 25(OH)D which was in a reflection of their lower total 25(OH)D levels [68], since 25(OH)D is used to calculate free and bioavailable D.
The takeaway from the racial paradox controversy is that it is now widely accepted that VDBP levels are relatively similar across, arguing against the free hormone hypothesis to explain why 25(OH)D is a stronger CVD risk factor in Whites than Blacks. This highlights the need for further research.
As mentioned previously, these emerging studies have highlighted that the specific assay used for measurement of VDBP is absolutely critical for determination of free and bioavailable vitamin D levels. While free vitamin D can be calculated using concentrations of 25(OH)D and VDBP as described in the formula above, there are also newer commercially available assays that can directly measure free 25(OH)D [67]; however at this time, the utility of these assays remain uncertain. Regarding bioavailable vitamin D calculations, currently, the LC-MS method has been particularly promising for measurement of VDBP since it does not bind differentially by genotype, unlike the monoclonal immunoassay. Population-based studies using LC-MS measurement to calculate bioavailable vitamin D and free vitamin D levels have been applied in studying chronic kidney disease. For instance, in a study by Rebholz et al, lower levels of free and bioavailable vitamin D and higher levels of VDBP were associated with new onset end stage renal disease [69].
Measurement of 24,25(OH)2D3 and calculation of VMR may provide further insight into racial differences in the association of vitamin D status with health outcomes. Prior work by Berg et al in Clin Chem in 2015 found that Blacks compared to Whites have lower concentrations of both 25(OH)D3 and 24,25(OH)2D3, that these measures were strongly correlated with each other, and thus Blacks and Whites had similar median VMR values [28]. Their study provides additional support that total 25(OH)D may not be the best measure of vitamin D sufficiency in blacks and that alternate measures such as VMR should be considered.
Thus far, there has not been substantial research examining the relationship between free/bioavailable vitamin D with CVD outcomes [21]. At this time, whether measuring any of these alternate measures of vitamin D status can provide further prognostication regarding CVD risk above the traditionally measured 25(OH)D remains uncertain. This is an emerging area where further research is strongly needed
Novel Measurement of Vitamin D Status – Challenges and Limitations
While there has recently been a marked increased interest in using these novel measures of vitamin D status, they are not without their own limitations. Even measurement of 25(OH)D can be challenging since the assay needs to account for differences in the two major forms, 25(OH)D2 and 25(OH)D3. Both forms have different affinity to serum proteins and certain medical conditions such as pregnancy, high estrogen state, and renal failure can lead to incomplete dissociation of 25(OH)D from the serum proteins for assay measurement, which can lead to falsely low levels [25, 70, 71]. Although LC-MS is now considered the “gold standard” for 25(OH)D assessment, given the high cost with LC-MS instrumentation, immunoassays for 25(OH)D will likely remain the more commonly used assay in clinical practice for some time.
Ideally, calcitriol (i.e. 1,25(OH)2D) would be a useful marker of vitamin D activity since it is measuring the activated form of the hormone which is believed to confer the health benefits of vitamin D. But it generally remains a poor choice for assessment of vitamin D status because vitamin D deficient individuals can increase 1-alpha-hydroxylase expression through tight compensatory mechanisms involving PTH, phosphorous, and FGF-23, leading to normal levels of the active form even in the face of deficiency [72]. Also, 1,25(OH)2D has a very short half-life of about 4 hours and circulating levels are a 1000 times lower than 25(OH)D, making measurement technically challenging [73]. Furthermore, there is currently no accepted reference method for measurement [21, 73–75].
Concentrations of 24,25(OH)2D, the major catabolic product of 25(OH)D, are another promising marker of vitamin D activity. As mentioned above, 24,25(OH)2D has been demonstrated to be proportional to 25(OH)D breakdown; thus this or VMR may be a more accurate reflection of physiological vitamin D levels [28, 29, 76] as VMR is expected to decrease in patients with functional vitamin D deficiency. Unfortunately, there has been little to no clinical research examining the relationship of 24,25(OH)2D or VMR with BMD or CVD. In general, LC-MS is the only current method to measure these metabolite products and these concentrations are very low in the nanomolar range, making it even more challenging to measure [21].
Additionally, SNP variants in vitamin D metabolism may become more relevant in the future in assessing vitamin D status. In a GWAS meta-analysis by Ahn et al, the authors showed several promising polymorphism associated with 25(OH)D levels in these genes: GC (for VDBP), DHCR7 (7-dehydrocholestrol reductase involved in vitamin D synthesis pathway from sun exposure), and CYP2R1 (encoding cytochrome P450 family 2 that is involved in hydroxylation of vitamin D in liver) [77]. The strength of using genetically determined markers are that they remain stable across time, and are less likely to be affected by confounders. However, it has not been shown yet that measuring these SNPs improves cardiovascular risk prediction better than measuring serum 25(OH)D levels alone [15].
Conclusion
There is substantial experimental and epidemiological evidence to suggest that vitamin D is a potential casual risk factor for CVD. Vitamin D deficiency is highly prevalent and relatively easy to replete. However, the optimal approach for assessing vitamin D status is unclear. The racial paradox of vitamin D status in relationship to bone and CVD outcomes has particularly raised concern that the current method of 25(OH)D measurement may not be the most optimal measurement. New vitamin D metabolism markers – such as VDBP, bioavailable D, free vitamin D, 24,25(OH)2D, and VMR – may more accurately reflect vitamin D status than 25(OH)D, and may therefore be more relevant for understanding the physiology through which vitamin D influences CVD, and potentially for screening of vitamin D as a CVD risk factor. Of course, results from large and rigorously conducted RCTs are needed to determine whether vitamin D repletion reduces the risk of CVD outcomes.
Acknowledgments
FUNDING: Dr. Michos is supported by a grant R01NS072243 from NIH/NINDS and the Blumenthal Scholars Award in Preventive Cardiology. Dr. Lutsey is supported by R01HL103706 and R01HL103706-S1.
Footnotes
CONFLICTS OF INTEREST
Dr. Michos has no conflicts related to this topic. Unrelated to this work, she has been a consultant (modest) for Siemens Healthcare Diagnostics
No other authors declare a conflict of interest.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93(1):40–46. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33(3):456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SH, Baek MS, Yoon DS, et al. Vitamin D Inhibits Expression and Activity of Matrix Metalloproteinase in Human Lung Fibroblasts (HFL-1) Cells. Tuberc Respir Dis (Seoul) 2014;77(2):73–80. doi: 10.4046/trd.2014.77.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Kelly J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest. 2002;109(8):1091–1099. doi: 10.1172/JCI12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamka M, Wozniewicz M, Walkowiak J, Bogdanski P, Jeszka J, Stelmach-Mardas M. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: a systematic review with meta-analysis. Eur J Nutr. 2016;55(6):2163–2176. doi: 10.1007/s00394-015-1089-5. [DOI] [PubMed] [Google Scholar]
- 8.Lutsey PL, Michos ED. Vitamin D, calcium, and atherosclerotic risk: evidence from serum levels and supplementation studies. Curr Atheroscler Rep. 2013;15(1):293. doi: 10.1007/s11883-012-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179–188. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michos ED, Misialek JR, Selvin E, et al. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: The ARIC study. Atherosclerosis. 2015;241(1):12–17. doi: 10.1016/j.atherosclerosis.2015.04.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutsey PL, Michos ED, Misialek JR, et al. Race and Vitamin D Binding Protein Gene Polymorphisms Modify the Association of 25-Hydroxyvitamin D and Incident Heart Failure: The ARIC (Atherosclerosis Risk in Communities) Study. JACC Heart Fail. 2015;3(5):347–356. doi: 10.1016/j.jchf.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melamed ML, Muntner P, Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28(6):1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michos ED, Reis JP, Post WS, et al. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The NHANES-III linked mortality files. Nutrition. 2012;28(4):367–371. doi: 10.1016/j.nut.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider AL, Lutsey PL, Selvin E, et al. Vitamin D, vitamin D binding protein gene polymorphisms, race and risk of incident stroke: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Neurol. 2015;22(8):1220–1227. doi: 10.1111/ene.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeFevre ML. Force USPST: Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162(2):133–140. doi: 10.7326/M14-2450. [DOI] [PubMed] [Google Scholar]
- 17.Cashman KD. Vitamin D: dietary requirements and food fortification as a means of helping achieve adequate vitamin D status. J Steroid Biochem Mol Biol. 2015;148:19–26. doi: 10.1016/j.jsbmb.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57(4):588–593. doi: 10.1016/j.jaad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Gahche J, Bailey R, Burt V, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994) NCHS Data Brief. 2011;61:1–8. [PubMed] [Google Scholar]
- 20.Schleicher RL, Sternberg MR, Lacher DA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454–461. doi: 10.3945/ajcn.115.127985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrmann M, Farrell CL, Pusceddu I, Fabregat-Cabello N, Cavalier E. Assessment of vitamin D status - a changing landscape. Clin Chem Lab Med. 2016 doi: 10.1515/cclm-2016-0264. [DOI] [PubMed] [Google Scholar]
- 22.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 23.Yousefzadeh P, Shapses SA, Wang X. Vitamin D Binding Protein Impact on 25-Hydroxyvitamin D Levels under Different Physiologic and Pathologic Conditions. Int J Endocrinol. 2014;2014:981581. doi: 10.1155/2014/981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz JB, Lai J, Lizaola B, et al. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99(5):1631–1637. doi: 10.1210/jc.2013-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner D, Hanwell HE, Schnabl K, et al. The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol. 2011;126(3–5):72–77. doi: 10.1016/j.jsbmb.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Berg AH, Powe CE, Evans MK, et al. 24,25-Dihydroxyvitamin d3 and vitamin D status of community-dwelling black and white Americans. Clin Chem. 2015;61(6):877–884. doi: 10.1373/clinchem.2015.240051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cashman KD, Hayes A, Galvin K, et al. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin Chem. 2015;61(4):636–645. doi: 10.1373/clinchem.2014.234955. [DOI] [PubMed] [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 31.Rosen CJ, Abrams SA, Aloia JF, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilger J, Friedel A, Herr R, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111(1):23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 35.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(4 Suppl 5):S5, S97–101. [PubMed] [Google Scholar]
- 37.Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149(2):558–564. doi: 10.1210/en.2007-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103(3–5):521–524. doi: 10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiao G, Kong J, Uskokovic M, Li YC. Analogs of 1alpha,25-dihydroxyvitamin D(3) as novel inhibitors of renin biosynthesis. J Steroid Biochem Mol Biol. 2005;96(1):59–66. doi: 10.1016/j.jsbmb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74(2):170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 41.Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186(1):20–28. doi: 10.1016/j.atherosclerosis.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 42.Wakasugi M, Noguchi T, Inoue M, et al. Vitamin D3 stimulates the production of prostacyclin by vascular smooth muscle cells. Prostaglandins. 1991;42(2):127–136. doi: 10.1016/0090-6980(91)90072-n. [DOI] [PubMed] [Google Scholar]
- 43.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25(3):320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 44.Oh J, Weng S, Felton SK, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120(8):687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cauley JA, Greendale GA, Ruppert K, et al. Serum 25 hydroxyvitamin D, bone mineral density and fracture risk across the menopause. J Clin Endocrinol Metab. 2015;100(5):2046–2054. doi: 10.1210/jc.2014-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilz S, Verheyen N, Grubler MR, Tomaschitz A, Marz W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016;13(7):404–417. doi: 10.1038/nrcardio.2016.73. [DOI] [PubMed] [Google Scholar]
- 47.Lavie CJ, Lee JH, Milani RV. Vitamin D and cardiovascular disease will it live up to its hype? J Am Coll Cardiol. 2011;58(15):1547–1556. doi: 10.1016/j.jacc.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Manousaki D, Mokry LE, Ross S, Goltzman D, Richards JB. Mendelian Randomization Studies Do Not Support a Role for Vitamin D in Coronary Artery Disease. Circ Cardiovasc Genet. 2016;9(4):349–356. doi: 10.1161/CIRCGENETICS.116.001396. [DOI] [PubMed] [Google Scholar]
- 49.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330. doi: 10.1136/bmj.g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 51.Beveridge LA, Struthers AD, Khan F, et al. Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA Intern Med. 2015;175(5):745–754. doi: 10.1001/jamainternmed.2015.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arora P, Song Y, Dusek J, et al. Vitamin D therapy in individuals with prehypertension or hypertension: the DAYLIGHT trial. Circulation. 2015;131(3):254–262. doi: 10.1161/CIRCULATIONAHA.114.011732. [DOI] [PubMed] [Google Scholar]
- 53.Witte KK, Byrom R, Gierula J, et al. Effects of Vitamin D on Cardiac Function in Patients With Chronic HF:The VINDICATE Study. J Am Coll Cardiol. 2016;67(22):2593–2603. doi: 10.1016/j.jacc.2016.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez AJ, Scott D, Srikanth V, Ebeling P. Effect of vitamin D supplementation on measures of arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. Clin Endocrinol (Oxf) 2016;84(5):645–657. doi: 10.1111/cen.13031. [DOI] [PubMed] [Google Scholar]
- 55.Manson JE, Bassuk SS. Vitamin D research and clinical practice: at a crossroads. JAMA. 2015;313(13):1311–1312. doi: 10.1001/jama.2015.1353. [DOI] [PubMed] [Google Scholar]
- 56.Pradhan AD, Manson JE. Update on the Vitamin D and OmegA-3 trial (VITAL) J Steroid Biochem Mol Biol. 2016;155(Pt B):252–256. doi: 10.1016/j.jsbmb.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volmer DA, Mendes LR, Stokes CS. Analysis of vitamin D metabolic markers by mass spectrometry: current techniques, limitations of the "gold standard" method, and anticipated future directions. Mass Spectrom Rev. 2015;34(1):2–23. doi: 10.1002/mas.21408. [DOI] [PubMed] [Google Scholar]
- 58.Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. 2015;55(9):1193–1205. doi: 10.1080/10408398.2012.688897. [DOI] [PubMed] [Google Scholar]
- 59.Aloia J, Mikhail M, Dhaliwal R, et al. Free 25(OH)D and the Vitamin D Paradox in African Americans. J Clin Endocrinol Metab. 2015;100(9):3356–3363. doi: 10.1210/JC.2015-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20(2):185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 61.Reis JP, Michos ED, Selvin E, Pankow JS, Lutsey PL. Race, vitamin D-binding protein gene polymorphisms, 25-hydroxyvitamin D, and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2015;101(6):1232–1240. doi: 10.3945/ajcn.115.107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(Pt A):132–137. doi: 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoofnagle AN, Eckfeldt JH, Lutsey PL. Vitamin D-Binding Protein Concentrations Quantified by Mass Spectrometry. N Engl J Med. 2015;373(15):1480–1482. doi: 10.1056/NEJMc1502602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Henderson CM, Lutsey PL, Misialek JR, et al. Measurement by a Novel LC-MS/MS Methodology Reveals Similar Serum Concentrations of Vitamin D-Binding Protein in Blacks and Whites. Clin Chem. 2016;62(1):179–187. doi: 10.1373/clinchem.2015.244541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nielson CM, Jones KS, Chun RF, et al. Free 25-Hydroxyvitamin D: Impact of Vitamin D Binding Protein Assays on Racial-Genotypic Associations. J Clin Endocrinol Metab. 2016;101(5):2226–2234. doi: 10.1210/jc.2016-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nielson CM, Jones KS, Bouillon R, et al. Role of Assay Type in Determining Free 25-Hydroxyvitamin D Levels in Diverse Populations. N Engl J Med. 2016;374(17):1695–1696. doi: 10.1056/NEJMc1513502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denburg MR, Hoofnagle AN, Sayed S, et al. Comparison of Two ELISA Methods and Mass Spectrometry for Measurement of Vitamin D-Binding Protein: Implications for the Assessment of Bioavailable Vitamin D Concentrations Across Genotypes. J Bone Miner Res. 2016;31(6):1128–1136. doi: 10.1002/jbmr.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rebholz CM, Grams ME, Lutsey PL, et al. Biomarkers of Vitamin D Status and Risk of ESRD. Am J Kidney Dis. 2016;67(2):235–242. doi: 10.1053/j.ajkd.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hollis BW. Editorial: The determination of circulating 25-hydroxyvitamin D: no easy task. J Clin Endocrinol Metab. 2004;89(7):3149–3151. doi: 10.1210/jc.2004-0682. [DOI] [PubMed] [Google Scholar]
- 71.Lankes U, Elder PA, Lewis JG, George P. Differential extraction of endogenous and exogenous 25-OH-vitamin D from serum makes the accurate quantification in liquid chromatography-tandem mass spectrometry assays challenging. Ann Clin Biochem. 2015;52(Pt 1):151–160. doi: 10.1177/0004563214533316. [DOI] [PubMed] [Google Scholar]
- 72.Somjen D, Weisman Y, Kohen F, et al. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111(13):1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 73.Strathmann FG, Laha TJ, Hoofnagle AN. Quantification of 1alpha,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57(9):1279–1285. doi: 10.1373/clinchem.2010.161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seiden-Long I, Vieth R. Evaluation of a 1,25-dihydroxyvitamin D enzyme immunoassay. Clin Chem. 2007;53(6):1104–1108. doi: 10.1373/clinchem.2006.077560. [DOI] [PubMed] [Google Scholar]
- 75.Lauridsen AL, Vestergaard P, Hermann AP, et al. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. 2005;77(1):15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 76.Tai SS, Nelson MA. Candidate Reference Measurement Procedure for the Determination of (24R),25-Dihydroxyvitamin D3 in Human Serum Using Isotope-Dilution Liquid Chromatography-Tandem Mass Spectrometry. Anal Chem. 2015;87(15):7964–7970. doi: 10.1021/acs.analchem.5b01861. [DOI] [PubMed] [Google Scholar]
- 77.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]