Abstract
The identification of key driver mutations in melanoma has led to the development of targeted therapies aimed at BRAF and MEK, but responses are often limited in duration. There is growing evidence that MAPK pathway activation impairs antitumor immunity and that targeting this pathway may enhance responses to immunotherapies. There is also evidence that immune mechanisms of resistance to targeted therapy exist, providing the rationale for combining targeted therapy with immunotherapy. Preclinical studies have demonstrated synergy in combining these strategies, and combination clinical trials are ongoing. It is, however, becoming clear that additional translational studies are needed to better understand toxicity, proper timing, and sequence of therapy, as well as the utility of multidrug regimens and effects of other targeted agents on antitumor immunity. Insights gained through translational research in preclinical models and clinical studies will provide mechanistic insight into therapeutic response and resistance and help devise rational strategies to enhance therapeutic responses.
Keywords: Melanoma, BRAF, MEK, Targeted therapy, Checkpoint blockade, Combination therapy
Introduction
The discovery of activating mutations in the BRAF gene and development of therapeutics targeting this protein represents a major breakthrough in melanoma therapy and in cancer treatment as a whole [1]. Activating point mutations in the BRAF gene are present in over half of melanoma tumors, with the majority harboring a BRAFV600E mutation [2]. These mutations lead to constitutive activation of the MAPK signaling pathway and resultant reduced apoptosis, increased invasiveness, and increased metastatic behavior [3]. Therapeutic agents targeting oncogenic BRAF were developed within the past decade and clinical trials demonstrated unprecedented clinical responses over then standard-of-care dacarbazine [4], leading to the FDA approval of four agents and two combination regimens targeting the MAPK pathway [4–8]. Importantly, early efforts focused on BRAF inhibitor mono-therapy; however, resistance developed quickly in most patients, with a progression-free survival (PFS) of <6 months for these agents [4, 5]. Insights gained from translational research highlighted MAPK reactivation as a major resistance mechanism [9, 10] and led to therapeutic strategies cotargeting BRAF and MEK—leading to a near doubling in PFS [6, 7]. However, resistance still develops in the majority of patients, though a small fraction do achieve long-term disease control [11, 12].
The other major breakthrough in melanoma treatment is the success of immunotherapy, particularly with regard to immune checkpoint inhibitors, with three agents and one combinatorial regimen approved in the last 5 years, and many more in clinical trials [13, 14]. The first of these agents was ipilimumab [13], which is a checkpoint inhibitor antibody that targets the CTLA-4 molecule on the surface of T lymphocytes. While this regimen has a low overall response rate (~10 %), a significant proportion of patients derive long-term disease control with 20 % of patients surviving nearly 10 years [13, 15, 16]. More recently, several agents have been approved that target the immunomodulatory molecule programmed death receptor-1 (PD-1) on the surface of T lymphocytes (including pembrolizumab and nivolumab) [14, 17–19]. Treatment with this regimen is associated with higher response rates (~40 %) and with lower toxicity [19, 20], though the durability of these responses remains unknown. The combination of CTLA-4 and PD-1 blockade is also now FDA-approved for stage IV melanoma [21, 22], and this treatment is associated with high response rates (57.6 % in phase III trial), though it is also associated with high rates of toxicity [21].
Given the durable responses achieved with immunotherapy (particularly immune checkpoint inhibitors) [16] and the high response rates of targeted MAPK pathway inhibition (albeit with a shorter duration of response), investigators have started to assess the efficacy of combination therapy from these two classes of therapeutics. The rationale for combining these forms of therapy was initially clinically based; however, there is evolving scientific evidence substantiating this approach—mainly through a deeper mechanistic understanding of the interplay between genomic mutations and antitumor immunity and the impact of treatment with targeted therapy on the tumor immune microenvironment. To date, numerous preclinical and translational studies have demonstrated that targeting the BRAF/MAPK pathway has significant effects on antigen [23] and immunomodulatory molecule expression [24••], and that treatment with targeted therapy may synergize well with immunotherapy [25, 26••]. Details regarding these studies will be discussed herein. In addition, there are now a number of clinical trials underway to explore the crosstalk and synergy between these agents and these will be highlighted. Finally, we will discuss the complexities regarding proper timing, sequence, and toxicity related to combination therapy.
Key Concepts Underlying the Rationale for Combining Targeted Therapy and Immunotherapy
Targeting the BRAF/MAPK Pathway Can Abrogate an Immunosuppressive Tumor Microenvironment
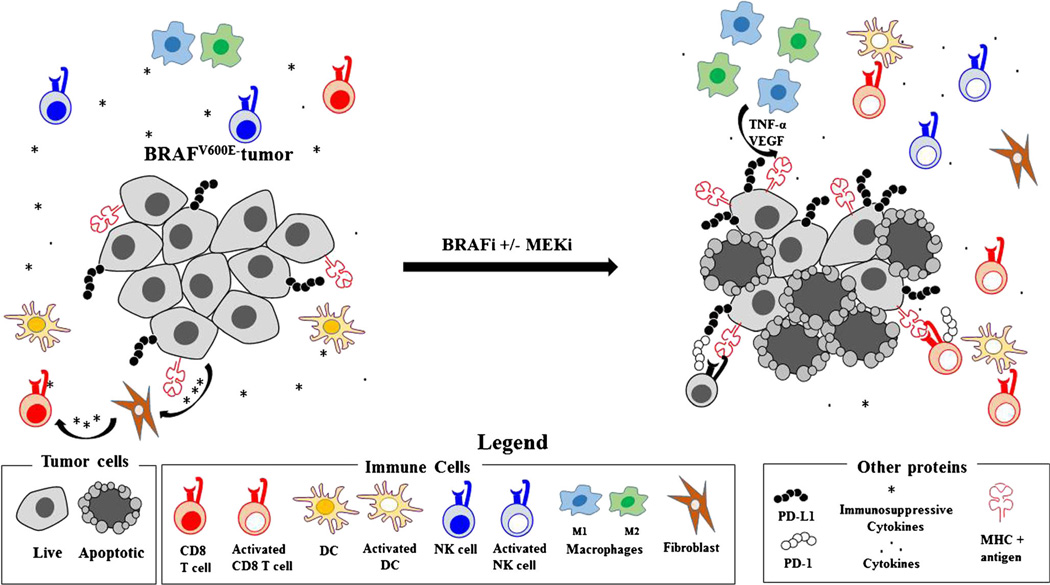
As illustrated in Fig. 1, MAPK inhibition contributes to a more favorable immune environment through a number of mechanisms, including suppression of immunosuppressive factors such as interleukin (IL)-10 [27], VEGF [28, 29••], PD-L1 [29••], myeloid derived suppressor cells (MDSCs) [30], regulatory T cells (Tregs) [31], and stromal fibroblast mediated inhibitory interactions with T cells [32]. The first report that MAPK pathway activation could contribute to immune escape was published in 2006 before the development of pharmacologic agents targeting the oncogenic BRAF mutation [33], and demonstrated that treating a BRAF mutant melanoma cell line with either a MEK inhibitor or RNAi against the BRAFV600E mutation was associated with decreased expression of immunosuppressive factors IL-6, IL-10, and VEGF [33]. Seminal in vitro work was then performed in the setting of BRAF-targeted therapy and showed that treatment with a BRAF inhibitor or MEK inhibitor in melanoma cell lines was associated with enhanced melanoma differentiation antigen (MDA) expression and enhanced reactivity to antigen-specific T lymphocytes [23]. The positive effect of BRAF inhibition on immunosuppressive proteins was subsequently corroborated in patients with metastatic melanoma that were treated with either BRAF inhibitor mono-therapy or combination with MEK inhibitor, in whom treatment caused a decrease in intratumoral IL-6 and IL-8 [24••]. An increase in exhaustion markers PD-1, PD-L1, and TIM-3 was also seen in this tissue-based study.
Fig. 1.
MAPK inhibition induces a favorable tumor microenvironment, with subsequent development of immune-mediated resistance to targeted therapy. Activated MAPK pathway in untreated BRAFV600E mutated melanoma promotes an immunosuppressive environment with high levels of immunosuppressive cytokines, low levels of MHC/melanoma antigen complexes, and low numbers of activated T cells, dendritic cells, and NK cells. Stromal components such as fibroblasts respond to tumor-secreted factors and suppress T cell activation. After MAPK pathway inhibition, immunosuppressive cytokine levels decrease and there is an increase in MHC/melanoma antigen complexes, mature dendritic cells, T, and NK cells. With time, immune mediators of resistance to targeted therapy develop, including PD-L1 upregulation on melanoma cells with resultant T cell exhaustion. An increase in both M1 and M2 macrophages is also seen with secretion of tumor promoting TNF-α and VEGF leading to increased melanoma tumor growth
Another mechanism through which BRAF/MAPK pathway blockade abrogates the immunosuppressive tumor microenvironment is through inhibition of tumor-associated fibroblasts (TAFs) via modulation of IL-1 [32]. This was first studied in vitro, where studies showed that transducing V600E mutant BRAF versus wild-type BRAF into melanoma cells induces transcription of IL-1α and IL-1β. Coculturing TAFs pretreated with IL-1α and IL-1β along with melanoma specific cytotoxic T cells resulted in suppressed T cell proliferation and function. The authors further demonstrated that this was mediated by upregulation of COX-2 and PD-1 ligands PD-L1 and PD-L2. BRAF inhibition was shown to abrogate TAF-induced T cell suppression in vitro. This mechanism was then validated in patients with metastatic melanoma on BRAF-targeted therapy in whom longitudinal biopsies were collected. These studies showed that BRAF inhibition prevented this induction of IL-1α and IL-1β transcription.
Another immunosuppressive mechanism at play in melanoma is through the chemokine CCL2, which is known to be involved in tumor progression and metastasis [34]. The role of CCL2 in the setting of BRAF-targeted therapy has been studied in preclinical models, demonstrating that BRAF inhibition decreases CCL2 gene expression and secretion, and that the magnitude of CCL2 suppression correlates with the magnitude of reduction in tumor size in a murine model of BRAF-mutant melanoma [35]. Mice treated with anti-CCL2 antibody showed reduced tumor growth and BRAF inhibition did not further contribute to tumor control, suggesting that lowering CCL2 is a mechanism by which BRAF inhibitors exert their antitumor effect. Similarly, BRAF inhibition was less effective in Ccr2−/− mice; Ccr2 is the receptor for CCL2 and was expressed primarily on Cd11b+ cells and CD4+ T regulatory cells, with near absence on other T or NK lymphocytes. BRAF inhibition therefore may impact recruitment of these CCR2+ tumor-infiltrating lymphocytes (TILs) via CCL2 suppression. Another group recently also found that CCL2 contributes to tumor proliferation and migration and that dual targeting of CCL2 and BRAF may be beneficial. However, in contrast to the prior study, they showed that BRAF inhibition led to an increase in CCL2 levels in both patient tumor and serum and contributed to BRAF treatment resistance [36]. Targeting CCL2 and downstream miRNAs with siRNA led to restoration of drug efficacy and cell apoptosis in resistant cell lines.
There is also now an emerging role of tumor-associated macrophages (TAMs) in the setting of BRAF inhibitor treatment [37], which may impact antitumor immunity. Macrophages are phagocytes that can be polarized as M1 or M2. The M1 type is classically activated and plays a role in the T helper (Th)1 cellular immune response and is thought of as “pro-inflammatory” while the M2 type is alternatively activated, plays a role in Th2 immune responses and is generally thought of as immunosuppressive and pro-tumoral [38]. In reality, these exist as a continuum rather than as two discrete entities [39]. One study exploring the interaction between macrophages and MAPK inhibitor treatment showed in a mouse model treated with MEK inhibitor as well as in tumor samples from patients treated with either BRAF inhibitor alone or BRAF/MEK inhibitor combination that treatment leads to an increase in number of intratumoral macrophages. Both M1 and M2 macrophages were increased and overall inhibited efficacy of targeted therapy as discussed further below [40••].
BRAF/MAPK Blockade Can Augment T Cell Infiltrate and Function
In addition to affecting the immunosuppressive tumor microenvironment, the MAPK pathway and BRAF inhibition affect both T cell intratumoral infiltration and function. As noted previously, MAPK inhibition has been shown to enhance T cell recognition due to increased antigen presentation [23]. Oncogenic signaling through the BRAF/MAPK pathway appears to impair MDA expression via transcriptional repression, and blockade of the pathway leads to enhanced expression of MDA—including gp100, MART-1, Trp-1, and Trp-2 [23]. Accordingly, antigen-specific T cell responses against these were enhanced with MAPK inhibitor treatment. In addition to enhancing mRNA expression of these antigens, it has been shown that the MAPK pathway affects antigen presentation via an MHC internalization pathway that is normally used by activated T cells for MHC-I trafficking [41].
Enhanced function of T cells has also been shown in the setting of BRAF inhibition. Initial reports focused on effect of MAPK inhibition on T cell proliferation and showed that while BRAF inhibition does not adversely affect T cell proliferative response, MEK inhibition leads to decreased proliferation [23]. Subsequent studies have shown that BRAF inhibition causes a paradoxical activation of T cells [42•], leading to enhanced T cell function. Similarly, BRAF inhibition causes T cell activation in a dose-dependent manner, measured by up-regulation of activation markers CD69 and Ki67; this increased activation corresponds to greater magnitude of ERK signaling. This is important for considering combination with immunotherapy since efficacy of checkpoint blockade is dependent not only on T cell presence but also activation.
In vivo mouse and human studies corroborate in vitro findings showing favorable effect of MAPK inhibition on intratumoral T cell infiltration and function. Wilmott et al. demonstrated an increase in CD8 and CD4 T cells in patient tumor samples collected 7 days after treatment with BRAF inhibition, with magnitude of CD8 T cell influx correlating to tumor size reduction and necrosis [43]. At time of tumor progression, a decrease in CD8 T cells was noted. With regard to the functionality of these T cells and their origin, Cooper et al. demonstrated that BRAF inhibition leads to a higher percentage of activated intratumoral CD8 T cells that secrete IFN-γ and TNF-α in a BRAF-mutant murine melanoma model [26••]. As no increase in Ki67+ T cells was seen with treatment, the influx of T cells into the microenvironment was attributed to cell migration from outside the tumor as opposed to proliferation of pre-treatment CD8 T cells. Notably, the efficacy of BRAF inhibition was dependent on this T cell response as administering anti-CD8 depleting antibody completely abrogated the response to BRAF inhibitors. In subsequent studies in patients, Cooper et al. also demonstrated that BRAF inhibition causes an increase in clonality of tumor infiltrating lymphocytes [44], suggesting that this is at least in part an antigen-specific response and an expansion of preexisting clones rather than a nonspecific infiltrative response to a dying tumor. Of note, no difference in CD8 TIL count was seen in patients on BRAF inhibitor monotherapy versus combined BRAF/MEK inhibition, suggesting that the addition of MEK inhibition to a BRAF inhibitor backbone does not adversely affect T cell infiltrate and expansion [24••].
Influence of BRAF/MAPK Pathway Blockade on Dendritic Cell Function
In addition to the direct effects on T cells, BRAF/MAPK pathway blockade may also augment T cell responses through its influence on dendritic cells (DCs). DCs are antigen-presenting cells that bridge the innate and adaptive immune systems and are critical for effective priming and activation of T cell responses. This has been studied in vitro and suggested that oncogenic BRAF may contribute to an immunosuppressive tumor microenvironment through suppression of DC maturation [33]. Immunosuppressive cytokines in the tumor microenvironment were shown to mediate decreased production of IL-12 and TNF-α and decreased expression of the costimulatory markers CD80, CD83, and CD86 [45•]. Importantly, most of these effects were reversible with pharmacologic inhibition of the BRAF/MAPK pathway, though DC cytokine production remained suppressed and this was reversed in BRAF mutant cells with either BRAF or MEK inhibition. Of note, coculturing DCs with BRAFWT cell lines also led to decrease in DC cytokine production but this was not reversed with BRAF or MEK inhibition. Culturing DCs directly with BRAF inhibitor did not show any toxic effects on DCs except at levels 50 times the IC50 of most BRAF mutant melanoma cell lines. In contrast, MEK inhibition showed decreased function of DCs including viability and priming capacity in a dose-dependent fashion, suggesting that BRAF inhibition may be preferable to MEK inhibition for optimizing DC cell function. Interestingly, other studies showed that while BRAF and MEK inhibition enhanced DC maturation, it also impaired their ability to cross-present antigens [46, 47].
Targeting the BRAF/MAPK Pathway May Enhance Natural Killer Cell Activity
In addition to the effects on T cells and DCs, BRAF/MAPK pathway inhibitors may also modulate natural killer (NK) cell activity. NK cells are cytotoxic lymphocytes that are traditionally part of the innate immune system. Their role in mediating response to BRAF inhibition has been explored, demonstrating that NK cells potentiate BRAF inhibitor response through a perforin-dependent pathway in a BRAF-mutant melanoma model [48•]. The authors showed that while lung metastases from mice depleted in CD4 or CD8 still showed response to BRAF inhibition, depletion of NK cells by neutralizing antibody treatment negated the efficacy of BRAF inhibition on tumor control. Combination treatment with BRAF inhibition and low-dose IL-2 led to improved BRAF efficacy with significantly lower burden of pulmonary metastases compared to monotherapy with either agent, providing support for combination trials of BRAF inhibition with NK stimulatory agents. Another study looking at the interaction between BRAF inhibition and NK cell function found that BRAF-resistant cell lines enhanced NK cell killing of melanoma cells [49]. MHC I was downregulated in BRAF-resistant cell lines, and this was associated with and at least partially contributed to the enhanced NK cell tumor killing. PD-L1 was also upregulated in BRAF-resistant cell lines but did not show that antibody-mediated inhibition of PD-1/PD-L1 interactions led to any detectable effect on NK cell-mediated cell lysis.
Immune Mechanisms of BRAF/MAPK Pathway Resistance
The immune microenvironment has been shown to be a powerful determinant of response to BRAF inhibition, both in determining initial response as well as development of resistance during treatment with BRAF inhibitors. As described above, part of the mechanism by which BRAF inhibition is effective is through altering the immune microenvironment [24••]. Therefore, any resistance mechanism that develops in the various components of the immune microenvironment in the context of BRAF/MAPK pathway inhibition can lead to treatment resistance. A few studies have explored the role of the immune microenvironment in MAPK inhibitor resistance [29••, 40••].
One of the first of these studies published showed that the immunosuppressive ligand for PD-1 (PD-L1) is upregulated in BRAF inhibitor-resistant melanoma cell lines compared to parental lines from which they were derived, and that this correlates with increased MAPK signaling. Importantly, PD-L1 expression remained elevated for 3 months even in the absence of BRAF inhibitor [29••]. Blocking MAPK pathway through ERK 1/2 siRNA, BRAF inhibitor, or MEK inhibitor suppresses this PD-L1 expression, and combining BRAF and MEK inhibition has additive effects.
Another potential immune-mediated mechanism of MAPK inhibitor resistance is through macrophage-derived secreted factors TNF-α and VEGF [37, 40••]. TNF-α is required for growth and survival of melanoma cells and has been shown to mediate resistance to BRAF/MAPK pathway inhibitors. Preclinical studies demonstrated that this is mediated through increased NF-κB signaling and downstream lineage transcription factor MITF expression. M1 and M2 macrophages were found to be the primary source of TNF-α and coculturing melanoma cells with macrophages caused resistance to MEK inhibition. This effect was lost when anti-TNF-α antibody was also added. As mentioned earlier, this group also demonstrated an increase in TAMs at time of resistance to BRAF/MAPK inhibitor treatment. Combining MEK inhibitor with IKK inhibitor (to block NF-κB signaling pathway) caused a decreased in this TNF-α expression in macrophages as well as decrease in macrophage counts. Another group showed that BRAF inhibitors paradoxically activate the MAPK pathway in macrophages resulting in VEGF production. VEGF subsequently reactivates the tumor MAPK pathway and stimulates growth of melanoma cells [37]. Targeting macrophages in mouse models enhanced efficacy of BRAF inhibition in this study.
In Vivo Evidence of Synergy with Combination BRAF Targeted and Immunotherapy
Preclinical Models
Synergy has been demonstrated when combining BRAF/MAPK-targeted therapy and immunotherapy in several preclinical models [26••, 35, 50]. Several regimens have been explored, including a regimen combining BRAF-targeted therapy with immune checkpoint inhibitors against PD-1 [26••]. In this study, treatment with BRAF inhibition and either anti-PD-1- or anti-PD-L1-based therapy significantly increased the number of CD8+ tumor infiltrating lymphocytes (TILs), and also improved their function—based on increased production of granzyme B, IFN-γ, and TNF-α (compared to BRAF inhibition alone) [26••]. Importantly, this was associated with a significant delay in tumor outgrowth and prolonged survival. Other studies have also demonstrated synergy, including in a BRAF-mutant murine model where BRAF inhibitors were combined with adoptive T cell therapy [50]. Results demonstrated superior antitumor response and survival in mice treated with the combination compared to those treated with single agent therapy. Unlike prior reports, BRAF inhibition did not affect antigen expression or cause an increase in T cells in the tumor, and no difference was observed in expansion or distribution of the adoptively transferred T cells. Despite these reports showing synergy, other reports have demonstrated none—including a report by Hoojikas et al. demonstrating no improvement in tumor control with combined BRAF inhibitor and CTLA-4-based therapy [51]. Of note, however, BRAF treatment in this study did not induce apoptosis but caused a decrease in CD8 cells following treatment. Knight et al. used the same mouse model and also found minimal single agent activity with α-PD-L1, α-CTLA-4, and α-TIM-3 but did see activity with anti-CD137 (4-1BB), which was enhanced when combined with BRAF inhibitor [35].
Combination therapy sequencing was also investigated in preclinical studies using CT26 mice. These studies confirmed that combining MEK inhibitors with checkpoint blockade immunotherapy resulted in improved tumor clearance due to increased CD8 infiltration. Furthermore, treatment with MEK inhibition followed by MEK inhibition +α-PD-1 was more successful than an α-PD-1 lead-in followed by α-PD-1 +MEK inhibition as measured by tumor burden and survival, suggesting that these combinations can have dramatically distinct effects depending on chosen sequence [52].
Clinical Trials
Based on the promising results from preclinical data and translational studies, several trials are underway assessing the effects of combining MAPK inhibition with immunotherapies (Table 1). These include combinations of BRAF±MEK inhibitors with immunotherapies, including checkpoint blockade (α-CTLA-4 and/or α-PD-1, α-PD-L1), adoptive T cell therapy, and cytokines such as IL-2 and/or interferon alpha-2b (IFN-α2b).
Table 1.
Clinical trials investigating combination MAPK inhibition and immunotherapy
| Agents | Phase | Status/preliminary results |
|---|---|---|
| Targeted + checkpoint inhibition | ||
| D + T → I; D + T → I + N; D → I + N; T → I + N, I alone, I + N (NCT01940809) |
I | Recruiting patients |
| D ± T + I (NCT01767454) | I | D + I was tolerable with no dose limiting toxicities or grades 3/4 hepa- totoxicity.Triple combination led to 2/7 patients developing colon perforation [53]. |
| V + I (NCT01400451) | I | 7/12 patients had hepatic dose limiting toxicities [54••]. |
| V ± cobimetinib + MPDL3280A (NCT01656642) | Ib | Recruiting patients |
| T ± D (D added to BRAF mutant, omitted from BRAF wild type) + MEDI4736 (NCT02027961) |
I/II | Treatment was relatively well tolerated with no unexpected toxicities [55••]. Preliminary results showed response rates with triple therapy, doublet concurrent therapy, and doublet sequential therapy of 69, 21, and 13 %, respectively, and disease control rates of 100, 79, and 80, respectively. |
| D + T + MK-3475 (NCT02130466) | I/II | Recruiting patients |
| V → I → V retreatment if no unacceptable toxicity and no progression (NCT01673854) |
II | 27 patients received sequential vemurafenib followed by ipilimumab therapy [56]. Thirty-three percent had grade 3/4 skin AEs, 22 %grade 3/4 GI AEs, and 4.3 % grade 3/4 hepatobiliary events. Median PFS 4.4 months, and overall survival 20.3 months |
| LGX818/MEK162 until PD → I + N; I + N until PD → LGX818/ MEK162; LGX818/MEK162 → I + N → LGX818/MEK162 (NCT02631447) |
II | Not open for recruitment |
| D + T until PD → I + N; I + N until PD → D + T (NCT02224781) | III | Suspended recruitment due to drug supply issues |
| Targeted + cytokine | ||
| V+ high dose interferon alpha-2b (NCT01943422) | I/II | Recruiting patients |
| V+ interferon alpha-2b + IL-2 (NCT01603212) | I/II | Ongoing, not recruiting |
| V+ pegylated-interferon (NCT01959633) | I/II | Recruiting patients |
| V+ aldesleukin (NCT01754376) | II | Grade 4 delirium seen in 1/6 evaluated patients. Grade 3 fatigue, hypo- tension, arthralgia, and capillary leak seen. Median PFS was 35.8 weeks [57]. |
| Targeted + adoptive T cell therapy | ||
| V + adoptive cell transfer (NCT01659151) | II | Recruiting patients |
D dabrafenib, T trametinib, V vemurafenib, I ipilimumab, N nivolumab, AEs adverse events, PFS progression-free survival
There are several important considerations for the optimal design of combination therapy trials. While data from the majority of the trials in Table 1 are immature, results from early studies highlight a potential for increased toxicity with combination therapy. A phase I study combining vemurafenib and ipilimumab in metastatic BRAFV600E mutated melanoma was stopped before completing accrual due to unexpected incidence of grade 2/3 hepatotoxicity, with 7/12 patients developing grade 2 or 3 transaminitis and 2 patients with grade 2 or 3 hyperbilirubinemia [54••]. Patients on this trial were treated with a 4-week run-in of vemurafenib followed by ipilimumab (3 mg every 3 weeks) with concurrent vemurafenib twice daily. Though none of the patients were symptomatic and toxicity was reversible with study drug discontinuation and/or administration of steroids, this study highlights the need for careful monitoring of toxicities in these targeted and immunotherapy combination trials. The same hepatotoxicity was not observed with sequential vemurafenib followed by ipilimumab but there was a higher incidence of grade 3–4 skin adverse events [56]. Using dabrafenib instead of vemurafenib in combination with checkpoint inhibitors targeting CTLA-4 also did not show significant hepatotoxicity; however, unexpected toxicity (i.e., colon perforations in 2/7 patients) was observed in the setting of treatment with combined dabrafenib + trametinib+ ipilimumab (NCT01767454) [53, 58], cautioning against the use of this specific combination.
Recently, the first study demonstrating successful combination of anti-PD-1/PD-L1 with targeted therapy was presented [55••]. The phase I study had three cohorts: cohort A treated with combination dabrafenib + trametinib + anti-PD-L1 agent MEDI4736, cohort B trametinib+MEDI4736, and cohort C with sequential trametinib +MEDI4736. Treatment was shown to be well tolerated with no significant increase in toxicities beyond what would be expected from targeted therapy or immunotherapy only. Preliminary analysis of patients dosed for at least 16 weeks showed response rates for cohorts A, B, and C were 69, 21, and 13 %, respectively, and disease control rates were 100, 79, and 80 %, respectively.
A second consideration in optimally combining targeted therapy and immunotherapy is the ideal timing and sequencing of therapies. Often, targeted therapy will be initiated first in patients with significant disease burden for rapid disease control, with immunotherapy considered as front-line therapy in patients with a lower disease burden, trading a slower onset of response for the potential benefit of long-term durable disease control. However, in the setting of more effective and rapidly acting immunotherapy regimens, practice patterns have changed. However, it is important to consider the translational evidence regarding the kinetics of the immune response to these agents, as it may ultimately help guide combination strategies. Data suggest that BRAF/MAPK targeted therapy favorably alters the immune environment within about 10–14 days; however, this effect is lost within several weeks of initiating therapy, suggesting that an optimal strategy may involve adding immunotherapy early in the course of treatment with BRAF/MAPK-targeted therapy [24••].
Several retrospective clinical studies have tried to help address the question of proper timing and sequence of therapy [59••]. One of these studies included a retrospective analysis of 274 patients treated with sequential BRAF inhibitor therapy and immunotherapy, with change of therapy at time of progression. Data from this study showed no statistically significant difference in outcomes between the 32 patients that received immune therapy first followed by targeted therapy and the 242 patients that received BRAF inhibition first followed by immune therapy [59••]. However, patients that had addition of ipilimumab after disease progression to BRAF inhibitors showed poor response and lack of benefit with therapy. Prospective clinical trials are now underway to address this, including the Intergroup/SWOG phase III study (NCT02224781) which is designed to investigate proper sequencing of combined targeted therapy (BRAF and MEK inhibition) and combined immunotherapy (CTLA-4 and PD-1 blockade). In this trial, patients are randomized to dabrafenib/trametinib followed by ipilimumab/nivolumab (with crossover at time of progression) or reverse order with a primary endpoint of overall survival. Another trial exploring this is the SECOMBIT trial (NCT02631447), which has a similar design but includes a third arm receiving an 8-week run-in of BRAF/MEK inhibition (encorafenib/binimetinib) followed by immune checkpoint blockade (ipilimumab/nivolumab) until progressive disease and subsequent targeted therapy (BRAF/MEK) until progressive disease. In addition to this, intermittent dosing of BRAF/MAPK pathway inhibitors is being explored [60], as there is evidence that intermittent dosing may be superior to continuous dosing—mainly by modulating clonal evolution of resistant cells [60].
Conclusions
There is growing evidence regarding immune effects of oncogenic mutations, and data clearly demonstrate that BRAF/MAPK pathway inhibition has a profound effect on antitumor immunity and the tumor microenvironment as a whole. These favorable effects are mediated through a number of different mechanisms, including effects on DC function, and NK cell activation. However, in addition to these favorable immune effects of BRAF/MAPK pathway blockade, immune mechanisms of therapeutic resistance to these agents also exist—including through the induced expression of immunomodulatory molecules within the tumor microenvironment, and also through stromal-mediated immunosuppression (via TAM and TAF, as well as MDSC). Taken together, these data provide a sound rationale for combination strategies with targeted therapy and immunotherapy, though complexities certainly exist regarding proper combination regimens and associated toxicity, as well as optimal timing and sequence of therapy. Clinical trials are ongoing and highlight these complexities; however, we cannot rely solely on clinical endpoints as we move forward in evaluating these regimens. Tremendous insights have been gained through longitudinal tissue- and blood-based analyses in patients during treatment with these agents as monotherapy, and these translational studies should be built into these trials to better inform mechanisms of response and resistance to these regimens and to gain insight into potential mechanisms of toxicity. In addition to this, we need to carefully study these regimens in preclinical models to gain mechanistic insight into potential synergy. Through these studies, we can move forward as a field in designing optimal combination strategies to enhance therapeutic responses and to combat resistance to therapy via a personalized approach.
Acknowledgments
Jennifer A. Wargo has received honoraria from Dava Oncology and has served on advisory boards for GlaxoSmithKline and Roche/Genentech.
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest Sangeetha M. Reddy declares that she has no conflict of interest.
Alexandre Reuben declares that he has no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as
• Of importance
•• Of major importance
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh M, Lin J, Hocker TL, Tsao H. Genetics of melanoma tumorigenesis. Br J Dermatol. 2008;158:15–21. doi: 10.1111/j.1365-2133.2007.08316.x. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 7.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 8.Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311:2397–2405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long GV, Fung C, Menzies AM, et al. Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat Commun. 2014;5:5694. doi: 10.1038/ncomms6694. [DOI] [PubMed] [Google Scholar]
- 10.Kwong LN, Boland GM, Frederick DT, et al. Co-clinical assessment identifies patterns of BRAF inhibitor resistance in melanoma. J Clin Invest. 2015;125:1459–1470. doi: 10.1172/JCI78954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 13.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 19.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or Monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 22.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 24. Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. BRAF/MAPK inhibition causes a more favorable immune microenvironment due to increased melanoma differentiation antigen expression as well as decrease in immunosuppressive cytokines. Also noted, however, in this translational human tissue based study is an increase in exhaustion markers PD-1, PD-L1, and TIM-3. The favorable changes are seen within 10–14 days and lost by several weeks after treatment.
- 25.Cooper ZA, Frederick DT, Ahmed Z, Wargo JA. Combining checkpoint inhibitors and BRAF-targeted agents against metastatic melanoma. Oncoimmunology. 2013;2:e24320. doi: 10.4161/onci.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cooper ZA, Juneja VR, Sage PT, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res. 2014;2:643–654. doi: 10.1158/2326-6066.CIR-13-0215. In vivo mouse model demonstrates that BRAF inhibition enhances number and function of intratumoral T cells and that following BRAF inhibition by checkpoint inhibition leads to improved tumor control and survival.
- 27.Dobreva ZG, Miteva LD, Stanilova SA. The inhibition of JNK and p38 MAPKs downregulates IL-10 and differentially affects c-Jun gene expression in human monocytes. Immunopharmacol Immunotoxicol. 2009;31:195–201. doi: 10.1080/08923970802626276. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Peng W, Xu C, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang X, Zhou J, Giobbie-Hurder A, et al. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598–609. doi: 10.1158/1078-0432.CCR-12-2731. BRAF inhibitor resistance is mediated by immune microenvironment with upregulation of PDL1 on tumor cells.
- 30.Schilling B, Paschen A. Immunological consequences of selective BRAF inhibitors in malignant melanoma: Neutralization of myeloid-derived suppressor cells. Oncoimmunology. 2013;2:e25218. doi: 10.4161/onci.25218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho PC, Meeth KM, Tsui YC, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNgamma. Cancer Res. 2014;74:3205–3217. doi: 10.1158/0008-5472.CAN-13-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalili JS, Liu S, Rodriguez-Cruz TG, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18:5329–5340. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAFMAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Knight DA, Synder LA, et al. A role for CCL2 in both tumor progression and immunosurveillance. Oncoimmunology. 2013;2:e25474. doi: 10.4161/onci.25474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight DA, Ngiow SF, Li M, et al. Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest. 2013;123:1371–1381. doi: 10.1172/JCI66236. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Vergani E, Di Guardo L, Dugo M, et al. Overcoming melanoma resistance to vemurafenib by targeting CCL2-induced miR-34a, miR-100 and miR-125b. Oncotarget. 2016;7:4428–4441. doi: 10.18632/oncotarget.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang T, Xiao M, Ge Y, et al. BRAF inhibition stimulates melanoma-associated macrophages to drive tumor growth. Clin Cancer Res. 2015;21:1652–1664. doi: 10.1158/1078-0432.CCR-14-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 39.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith MP, Sanchez-Laorden B, O’Brien K, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFalpha. Cancer Discov. 2014;4:1214–1229. doi: 10.1158/2159-8290.CD-13-1007. Macrophages increase in number in response to BRAF inhibition and present a mechanism of resistance, described in this paper to be mediated by TNF-α.
- 41.Bradley SD, Chen Z, Melendez B, et al. BRAFV600E Co-opts a conserved MHC class i internalization pathway to diminish antigen presentation and CD8+ T-cell recognition of melanoma. Cancer Immunol Res. 2015;3:602–609. doi: 10.1158/2326-6066.CIR-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Callahan MK, Masters G, Pratilas CA, et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunol Res. 2014;2:70–79. doi: 10.1158/2326-6066.CIR-13-0160. BRAF inhibition directly activates T cells through pardoxical MAPK activation.
- 43.Wilmott JS, Long GV, Howle JR, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 44.Cooper ZA, Frederick DT, Juneja VR, et al. BRAF inhibition is associated with increased clonality in tumor-infiltrating lymphocytes. Oncoimmunology. 2013;2:e26615. doi: 10.4161/onci.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ott PA, Henry T, Baranda SJ, et al. Inhibition of both BRAF and MEK in BRAF(V600E) mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer Immunol Immunother. 2013;62:811–822. doi: 10.1007/s00262-012-1389-z. BRAF mutated cells suppress DC function which is reversed with BRAF/MEK inhibition.
- 46.Vella LJ, Andrews MC, Pasam A, et al. The kinase inhibitors dabrafenib and trametinib affect isolated immune cell populations. Oncoimmunology. 2014;3:e946367. doi: 10.4161/21624011.2014.946367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vella LJ, Pasam A, Dimopoulos N, et al. MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells. Cancer Immunol Res. 2014;2:351–360. doi: 10.1158/2326-6066.CIR-13-0181. [DOI] [PubMed] [Google Scholar]
- 48. Ferrari de Andrade L, Ngiow SF, Stannard K, et al. Natural killer cells are essential for the ability of BRAF inhibitors to control BRAFV600E-mutant metastatic melanoma. Cancer Res. 2014;74:7298–7308. doi: 10.1158/0008-5472.CAN-14-1339. NK cells are a mediator of BRAF inhibitor response and present a possible target in designing future combination therapies.
- 49.Sottile R, Pangigadde PN, Tan T, et al. HLA class I downregulation is associated with enhanced NK-cell killing of melanoma cells with acquired drug resistance to BRAF inhibitors. Eur J Immunol. 2016;46:409–419. doi: 10.1002/eji.201445289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koya RC, Mok S, Otte N, et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer Res. 2012;72:3928–3937. doi: 10.1158/0008-5472.CAN-11-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hooijkaas A, Gadiot J, Morrow M, et al. Selective BRAF inhibition decreases tumor-resident lymphocyte frequencies in a mouse model of human melanoma. Oncoimmunology. 2012;1:609–617. doi: 10.4161/onci.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu L, Mayes PA, Eastman S, et al. The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PDL1, and CTLA-4. Clin Cancer Res. 2015;21:1639–1651. doi: 10.1158/1078-0432.CCR-14-2339. [DOI] [PubMed] [Google Scholar]
- 53.Puzanov I. Combining targeted and immunotherapy: BRAF inhibitor dabrafenib (D) +/− the MEK inhibitor trametinib (T) in combination with ipilimumab (Ipi) for V600E/K mutation-positive unresectable or metastatic melanoma (MM) J Transl Med. 2015;13(Suppl 1):K8. [Google Scholar]
- 54. Ribas A, Hodi FS, Callahan M, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–1366. doi: 10.1056/NEJMc1302338. The first phase 1 trial to report tolerability of combination targeted and checkpoint blockade showed unexpected grade 3 hepatotoxicity and highlighted the need for closer monitoring in combination clinical trials. Trial was closed early due to 7/12 patients developing grade 3 hepatotoxicity to combination vemurafenib and ipilimumab.
- 55. Ribas A, Butler M, Lutsky J, Lawrence DP, Robert C, et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol. 2015;33 abstr 3003. This phase 1 clinical trial is first study to demonstrate tolerability and efficacy with combination of MAPK targeted therapy and immunotherapy against PD-1/PD-L1 axis. High response rates and disease control rates were seen with combination BRAF and MEK inhibition along with anti-PD-L1 antibody with preliminary evidence of durable response.
- 56.Amin ALD, Salama AK, Koon HB, et al. A single-arm, open-label, phase II study to evaluate the safety of vemurafenib (VEM) followed by ipilimumab (IPI) in BRAF V600-mutated metastatic melanoma (MM) J Clin Oncol. 2015;33(suppl) abstr 9032. [Google Scholar]
- 57.Wargo JA, Lawrence DP, Cooper ZA, Frederick DT, et al. A phase II study of combined therapy with vemurafenib (vem) and high-dose interleukin-2 (aldesleukin; HD IL-2) in patients with metastatic melanoma. J Clin Oncol. 2015;33:e20074. [Google Scholar]
- 58.Puzanov I, Callahan MK, Linette GP. Phase 1 study of the BRAF inhibitor dabrafenib (D) with or without the MEK inhibitor trametinib (T) in combination with ipilimumab (Ipi) for V600E/K mutation-positive unresectable or metastatic melanoma (MM) J Clin Oncol. 2014;32(suppl):5s. abstr 2511. [Google Scholar]
- 59. Ackerman A, Klein O, McDermott DF, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120:1695–1701. doi: 10.1002/cncr.28620. This retrospective study of 274 metastatic melanoma patients treated with sequential BRAF targeted and immunotherapy suggests that treating with immunotherapy at time of BRAF inhibitor resistance is unlikely to be of clinical benefit.
- 60.Das Thakur M, Salangsang F, Landman AS, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494:251–255. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]