Summary
MicroRNAs (miRNAs) are involved in a plethora of important biological processes from embryonic development to homeostasis in adult tissues. Recently, miRNAs have emerged as a class of epigenetic regulators of metabolism and energy homoestasis. We have investigated the role of miRNAs in the regulation of adipogenic differentiation. In this report, we demonstrate that the miR-27 gene family is down-regulated during adipogenic differentiation. Over-expression of miR-27 specifically inhibits adipocyte formation, without affecting myogenic differentiation. We also find that expression of miR-27 results in blockade of expression of PPARγ and C/EBPα, the two master regulators of adipogenesis. Importantly, expression of miR-27 is increased in fat tissue of obese mice and is regulated by hypoxia, an important extracellular stress associated with obesity. Our data strongly suggest that miR-27 is a new class of adipogenic inhibitors and may play a role in the pathological development of obesity.
Keywords: adipocyte, differentiation, hypoxia, microRNA, obesity
Introduction
MicroRNAs (miRNAs) have emerged as an important class of post-transcriptional regulators of metabolism in several cell types including β-cells, muscle cells and adipocytes (1). They appear to impinge upon diverse aspects of cellular responses to metabolic demands or stresses from invertebrates to vertebrates. A forward genetic screening in Drosophila melanogaster provided the first example that miR-14 plays a critical role in the regulation of triacylglyceride metabolism in fruit flies (2). By a similar approach, miR-278 was recently identified as a potential regulator of energy metabolism in the fat body of fruit flies (3). In vertebrates, miR-375 and miR-376, both abundantly expressed in pancreatic β cells, are involved in the control of insulin secretion (4). Furthermore, the highly conserved miRNA, miR-1, has been found to exert significant influence on myogenic differentiation and muscle functions in invertebrates (5) as well as in mammals (6).
Adipose tissue functions are essential to energy metabolism because it is not only an energy depot (7), but also a source of endocrine factors (8, 9). Adipocytes are derived from mesenchymal stem or progenitor cells via a lineage-specific differentiation process called adipogenesis. Adipogenic differentiation is accomplished by a cascade of three major transcriptional events characterized by the transcriptional induction of (1) the early genes: C/EBPβ and C/EBPδ, (2) the determination genes: PPARγ and C/EBPα, also regarded as master regulators of adipogenesis, and (3) adipocyte-specific genes such as fatty acid synthase and fatty acid binding proteins (10-12). Epigenetic regulation of adipose functions mediated by miRNAs has been emerging as an important mechanism in the study of energy metabolism and obesity. By comparing miRNA profiles, Kajimoto et al. have found differential profiles of miRNA expression between preadipocytes and mature adipocytes (13), suggesting a role for miRNAs in the regulation of adipogenic differentiation. Consistent with this notion, microarray analysis has identified two classes of miRNAs, miR-143 and the miR-17/92 cluster, that are moderately (2-3 fold) increased during adipogenic differentiation (14, 15). Inhibition of miR-143 by an antisense oligonucleotide results in inhibition of adipogenesis in vitro (14), whereas over-expression of the miR-17/92 cluster moderately increases adipocyte formation in vitro (15). Although these studies have provided evidence for a role of miRNAs in adipogenesis, there is still no evidence regarding expression of miRNAs in adipose tissues, especially their regulation associated with obesity.
Adipose tissue undergoes a dramatic expansion in obesity, which eventually results in adipose tissue dysfunction. Our studies have shown that obese tissue becomes hypoxic or oxygen-deficient, and hypoxia facilitates inflammatory responses in adipocytes (16, 17). We have also shown that hypoxia strongly inhibits adipogenic differentiation (18, 19). However, it remains to be determined whether miRNAs are differentially regulated or play a role under obese conditions in vivo.
In the current study, we have investigated the role of miRNAs in adipogenic differentiation using the mouse embryonic fibroblast-derived 3T3-L1 preadipocytes (20) and mouse bone marrow-derived OP9 mesenchymal stem/progenitor cells (21). We found that expression of the miR-27 family genes (miR-27a and miR-27b) was down-regulated upon adipogenic differentiation. Over-expression of miR-27 resulted in robust and specific inhibition of adipogenic differentiation with the blockade of PPARγ and C/EBPα expression. Importantly, miR-27 expression was elevated in adipose tissue of genetically obese ob/ob mice. We also found that the environmental stress, hypoxia, was involved in the regulation of miR-27 expression. Our data suggest that the miR-27 gene family is potentially an important class of negative regulators of adipogenesis and may play a role in the regulation of adipose functions associated with obesity.
Results
miR-27 inhibits adipogenic differentiation
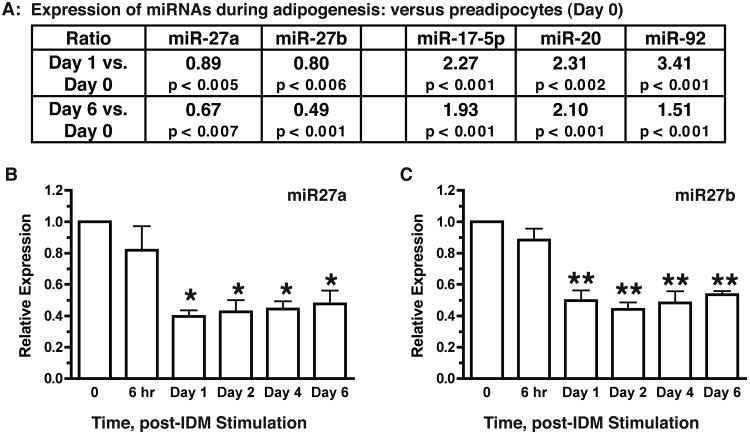
In order to investigate the role of miRNAs in the regulation of adipogenic differentiation, we performed a genome-wide microarray analysis of miRNA expression during adipogenic differentiation using the 3T3-L1 adipogenesis model. Our initial analysis revealed that the miR-27 gene family, consisting of miR-27a and miR-27b, was down-regulated during adipogenic differentiation (left panel, Fig. 1A). Consistent with the literature (15), genes of the miR-17/92 cluster, including miR-17-5p, miR-20, and miR-92, were increased during differentiation (right panel, Fig. 1A). We further investigated the kinetics of miR-27 expression during adipogenesis using qRT-PCR. As shown in Fig. 1C & D, expression of both miR-27a and miR-27b decreased by ≥50% within the first 24 hr of adipogenic stimulation compared to preadipocytes (time = 0) and remained at such reduced levels as differentiation progressed (6-day). These observations strongly suggest that miR-27 may negatively regulate adipogenic differentiation.
Fig 1.
Decreased expression of miR-27 during adipogenic differentiation. 3T3-L1 preadipocytes were grown to confluence. Adipogenic differentiation was initiated by treatment with the differentiation cocktail containing insulin, dexamethasone and isobutylmethylxanthine (IDM), as described in EXPERIMENTAL PROCEDURES. Total cellular RNA was prepared at indicated time points. A. MicroRNA profile analysis was performed by LC Sciences, Houston, TX. Ratios were calculated as mean ± SD from sextuplicate sampling. B & C. Expression of miR-27a and miR-27b was quantitatively assessed by SYBR Green-based qRT-PCR. Data shown are averages of four independent experiments (mean ± SD) and are analyzed using Student t-test (paired, 2-tailed). *p < 0.01; **p < 0.01, as compared to time = 0.
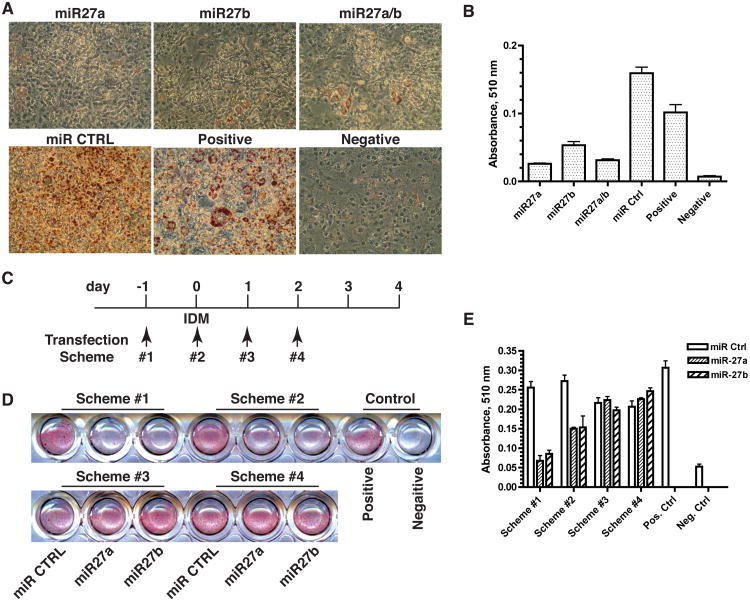
To investigate the role of miR-27 in adipogenesis, we transiently transfected 3T3-L1 preadipocytes with miRNA precursor molecules for miR-27a or miR-27b before adipogenic stimulation. The transfection efficiency approached 100% according to the uptake of a fluorescent small RNA duplex oligonucleotide control (siGLO Red, Dharmacon). Using qRT-PCR analysis, we found a >60-fold increase in mature miR-27a and miR-27b in the transfected preadipocytes. As shown in Fig. 2A, miR-27a, miR-27b or an equal molar mixture of miR-27a and miR-27b (miR27a/b) strongly inhibited adipogenic differentiation of 3T3-L1 preadipocytes, as demonstrated by a lack of intracellular fat accumulation. In contrast, the irrelevant miR control did not affect adipogenic differentiation. Quantitative analysis of intracellularly accumulated neutral lipids revealed statistically significant inhibition of adipocyte formation (Fig. 2B). Conversely, inhibition of the endogenous miR-27a or miR-27b using specific anti-miRs did not significantly affect adipogenesis (data not shown), suggesting that down-regulation of miR-27 is not sufficient to promote adipogenesis.
Fig 2.
Inhibition of adipogenic differentiation by miR-27. 3T3-L1 preadipocytes were grown to confluence and transfected with equal total amounts of each of the following miRNA molecules: miR-27a, miR-27b, miR-27a/miR-27b (1:1), miR control (Ctrl). Adipogenic differentiation was initiated at 24 hr post-transfection. Cells were fixed and stained with Oil Red O on Day 6 of differentiation (A). The amount of Oil Red O was quantified after extraction with isopropanol. Data shown in (B) are mean ± sem of a triplicate experiment. For the time course study, miRNA transfection was indicated in relationship to the start of the IDM-treatment at Day 0. (C). Cells were fixed and stained with Oil Red O on Day 4 of differentiation (D). Quantification of Oil Red O was shown in (E). Positive = differentiated L1 cells without miRNA transfection. Negative = undifferentiated 3T3-L1 cells. The results shown were confirmed by more than three independent experiments.
We performed a time course study to further gain insight into the role of miR-27 during different stages of adipogenesis (Fig. 2C-E). Transfection of miR-27a or miR-27b before the adipogenic stimulation by IDM (Scheme #1) resulted in near complete inhibition of adipogenic differentiation. Transfection of miR-27a or miR-27b at the same time with the IDM treatment (Scheme #2) resulted in partial but significant inhibition of adipogenesis. In contrast, transfection of miR-27a or miR-27b did not have significant effects on adipogenesis when performed after 24 hr or 48 hr of IDM treatment (Schemes #3 and #4). These results suggest that miR-27 exerts its inhibitory effects at or before the adipogenic commitment stage and that the IDM-induced genes appear to overcome the inhibitory effects of miR-27.
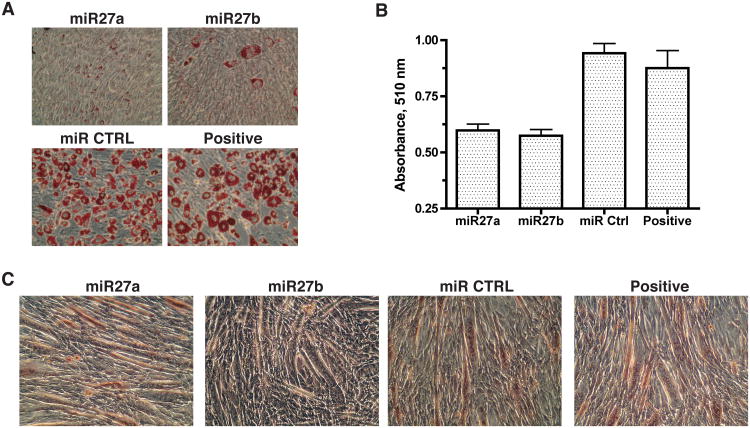
In order to ascertain that miR-27 inhibits adipogenesis in general and its activity is not limited to the embryonic fibroblast-derived 3T3-L1 preadipocytes, we used the OP9 multipotent mesenchymal stem cell line derived from mouse bone marrow as an independent model of adipogenesis. OP9 cells undergo adipogenic differentiation when treated with the same adipogenic stimulants. As shown in Fig. 3A & B, transfection with miR-27a or miR-27b resulted in significant inhibition of adipogenic differentiation of OP9 cells. This observation demonstrates that miR-27 has the potential to regulate the common essential genes or signal transduction pathways that regulate adipogenic differentiation of mesenchymal stem or progenitor cells from different tissue origins. To further determine whether miR-27 inhibits adipogenesis specifically, we investigated the effect of miR-27 on the myogenic differentiation of the C2C12 myoblast cells. As shown in Fig. 3C, formation of myofibers was not adversely affected by miR-27 over-expression, indicating that miR-27 does not play an important role in myogenic differentiation. These results together illustrate a critical and specific role of miR-27 in the regulation of adipogenic differentiation.
Fig 3.
Specificity of miR-27 toward inhibition of adipogenesis. A. The bone marrow-derived mesenchymal progenitor cells, OP9, were grown to confluence, transfected with indicated miRNAs or left untransfected (Positive). Adipogenic differentiation was initiated at 24 hr post-transfection. Cells were fixed and stained with Oil Red O on Day 6 of differentiation. B. Data shown are mean ± sem of a triplicate experiment. Positive = differentiated OP9 cells without miRNA transfection. One of three independent experiments is shown. C. C2C12 myoblast cells were transfected with indicated miRNAs or left untransfected (Positive). Myogenic differentiation was initiated at 48 hr post-transfection by maintaining the cells in culture medium containing 2% horse serum. Cells were fixed on day 4 of differentiation and stained with hematoxylin and eosin. One of two independent experiments is shown.
miR-27 prevents the induction of PPARγ and C/EBPα
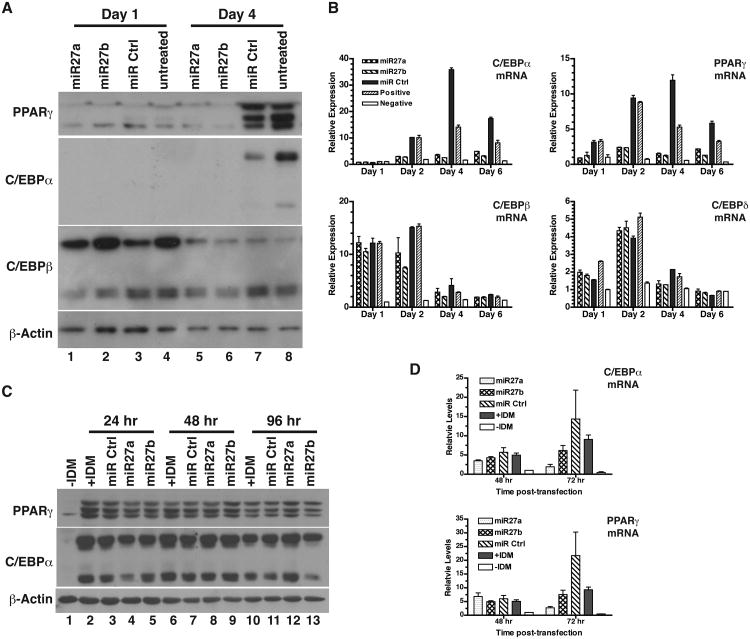
In order to delineate the mechanisms by which miR-27 inhibits adipogenic differentiation, we investigated the effect of miR-27 on the expression of the well-defined key transcription factors of adipogenic differentiation, including PPARγ, C/EBPα, and C/EBPβ. Their respective expression was determined in 3T3-L1 cells at the protein level using Western blot analysis on Day 1 and Day 4 of differentiation, a timeframe for observation of early genes and induction of C/EBPα and PPARγ. On Day 1, C/EBPβ was highly expressed, whereas C/EBPα and PPARγ were barely detectable, under control conditions (lanes 3 & 4, Fig. 4A). The expression of neither protein was affected by miR-27 within the first day of adipogenic stimulation. As adipogenesis progressed for 4 days, both C/EBPα and PPARγ were strongly increased; whereas C/EBPβ level was reduced, in the control cells (lanes 7 & 8, Fig. 4A). In the miR-27 transfected cells, C/EBPα and PPARγ expression was completely blocked after 4 days of adipogenic stimulation (lanes 5 & 6, Fig. 4A). In contrast, levels of C/EBPβ protein were not significantly affected by miR-27 either on Day 1 or Day 4 compared to the controls. By analysis of mRNA expression using qRT-PCR, we found that miR-27a and miR-27b were able to strongly inhibit the transcriptional induction of PPARγ within the first day of adipogenic stimulation (Day 1, Fig. 4B). Robust inhibition of both PPARγ and C/EBPα mRNA took place within 2 days of treatment. In contrast, expression of C/EBPβ and C/EBPδ, the two early genes during adipogenesis, was not affected by miR-27a or miR-27b compared to controls (miR Ctrl and Positive control, Fig. 4B). These data suggest that miR-27 inhibits adipogenic differentiation by blocking the transcription of the adipogenesis-determination genes PPARγ and C/EBPα mRNA.
Fig 4.
Inhibition of expression of PPARγ and C/EBPα in preadipocytes by miR-27. A. 3T3-L1 preadipocytes were transfected with miRNAs and then induced at 48 hr to undergo differentiation as described in Fig. 2. Whole-cell lysates were prepared at indicated time points for Western blot analysis. One of three independent experiments is shown. B. 3T3-L1 cells were treated as described in (A). Total RNA was prepared at indicated times and subjected to qRT-PCR analysis. Data shown were mean ± sem from three independent experiments. C. 3T3-L1 cells were subjected to the IDM-treatment for 2 days before transfection with indicated miRNA or left untransfected (Untreated). Whole-cell lysates were prepared at indicated time points for Western blot analysis. One of three independent experiments was shown. D. 3T3-L1 cells were treated as described in (C). Total RNA was prepared at indicated times and subjected to qRT-PCR analysis. Data shown were mean ± sem from three independent experiments.
It is predicted that the PPARγ gene contains a putative binding motif for miR-27a and miR-27b (www.microRNA.org). Because transcription of PPARγ is induced within 48 hr of IDM-stimulation (12), we investigated whether miR-27 could be down-regulated PPARγ expression in 3T3-L1 cells treated for 2 days with the adipogenic cocktail. The differentiating 3T3-L1 cells were transfected with miR-27a and miR-27b, respectively. PPARγ protein was detected at 24, 48 and 96 hr post-transfection. We found that approximately 100% transfection efficiency was achieved using siGLO Red as an indicator. Based on qRT-PCR analysis, a >30-fold increase in mature miR-27a and miR-27b was found in the IDM-stimulated preadipocytes at 48 hr after transfection. As shown in Fig. 4C, transfection of miR-27a or miR-27b failed to markedly decrease levels of PPARγ protein at each time-point of observation compared to the respective miR controls. The effects of miR-27 on the expression of C/EBPα protein also appeared to be unremarkable. Consistent with these observations, adipocyte formation, as observed by accumulation of fat droplets, was not blocked by miR-27 under these experimental conditions. On the other hand, miR-27a or miR-27b did not appreciably inhibit expression of PPARγ and C/EBPα mRNA, as observed at 48 hr after miR-27 transfection in the 2-day old differentiating 3T3-L1 cells (Fig. 4D). These data suggest that miR-27 may not directly repress PPARγ or C/EBPα mRNA. However, miR-27a appeared to repress the levels of PPARγ and C/EBPα mRNA at 72 hr after transfection (Fig. 4D), suggesting that miR-27a may target a yet unknown gene or pathway that negatively regulates the transcription of PPARγ and C/EBPα mRNA. Nonetheless, our data suggest that miR-27 does not repress the level of PPARγ protein in committed preadipocytes under physiologically relevant conditions.
Expression of miR-27 is elevated in obese mice
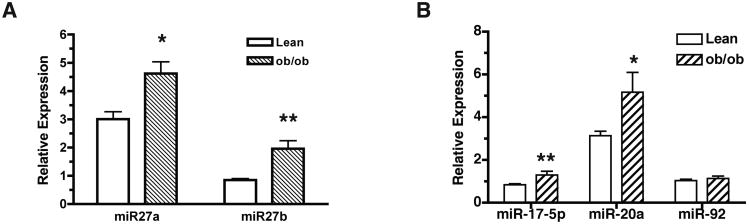
In order to gain insight into the potential biologically relevant role of miR-27 in the regulation of adipose tissue functions in vivo, we examined the expression of miR-27 in the genetically obese ob/ob mice. The expression of both miR-27a and miR-27b was significantly increased in the epididymal fat tissue from the ob/ob mice, compared to the genetically matched lean mice of the same gender and at the same age (Fig. 5A). It is worth mentioning that both miR-27a and miR-27b, although respectively located in chromosomes 8 and 13, are coordinately increased in obese tissue. In contrast, miR-17-5p, miR-20a and miR-92, miRNAs that are located in the same gene cluster appeared to be differentially regulated under obese conditions (Fig. 5B). These observations present the first evidence that obesity induces expression of a class of miR, such as miR-27, that has the potential to negatively regulate adipose tissue functions.
Fig 5.
Elevated expression of miR-27 in ob/ob mice. A & B. Total RNA was prepared from epididymal fat pads harvested from ob/ob mice and genetically matched lean mice. Levels of miRNA expression were analyzed by TaqMan quantitative PCR. Data are mean ± sem from four individual mouse of each group and are analyzed using Student t-test (unpaired 2-tailed). A. *p < 0.02, **p < 0.01 (ob/ob vs. lean); B. *p < 0.03, **p < 0.002 (ob/ob vs. lean).
Hypoxia regulates miR-27 expression
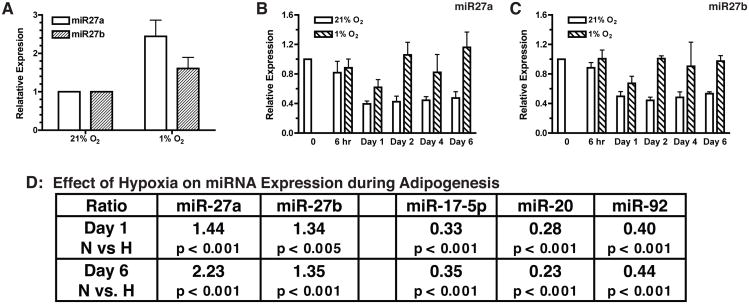
We and others have shown that hypoxia is a risk factor for adipose tissue malfunctions in obesity (17, 22). We have further shown that hypoxia inhibits adipogenesis (18, 19). The elevated miR-27 expression in the adipose tissue of ob/ob mice thus led us to hypothesize that hypoxia may play a role in the regulation of miR-27 expression. To test this hypothesis, we examined expression of miR-27 in differentiating preadipocytes under hypoxia. All hypoxia experiments were carried out at 1% O2, a hypoxic level of oxygenation similar to that found in obese mice (17). In preadipocytes, hypoxia increased miR-27a by approximately 2 fold and miR-27b by approximately 1.5 fold (Fig. 6A), consistent with observation that miR-27a expression was moderately increased by hypoxia in several cancer cell lines (23). During adipogenic differentiation under the control conditions (21% O2), expression of miR-27a and miR-27b was decreased after 24 hr of adipogenic stimulation (Fig. 6B & C). However, miR-27a and miR-27b remained at elevated levels under the hypoxic condition. This observation was further confirmed by miRNA microarray analysis (left panel, Fig. 6D). In comparison, the expression of the miR-17/92 cluster (miR-17-5p, miR-20, and miR-92), the expression of which is increased during normoxic adipogenesis (Fig. 1C and (15), was strongly inhibited by hypoxia (right panel, Fig. 6C). These results are consistent with the notion that hypoxia inhibits adipogenesis.
Fig 6.
Regulation of miR-27 expression by hypoxia. A. Confluent 3T3-L1 preadipocytes were incubated overnight at 21% or 1% O2. Levels of miR-27a and miR-27b were determined by quantitative RT-PCR. Data shown are mean ± sem from three independent experiments. B, C & D. Confluent 3T3-L1 preadipocytes were subjected to adipogenic differentiation under the same conditions as described in Fig. 1. For hypoxia treatment, 3T3-L1 cells were placed in a hypoxia incubator at 1% O2 immediately after addition of the IDM cocktail. The control was maintained in a standard incubator with 21% O2. The normoxia data are the same as shown in Fig. 1 and are included here for comparison. Expression of miR-27a and miR-27b at indicated time points was assessed by quantitative RT-PCR. Data shown in (B & C) are average of four independent experiments (mean ± sem). D. MicroRNA profile analysis was performed by LC Sciences, Houston, TX. Ratios were calculated as mean ± sem from sextuplicate sampling.
Discussion
In this report, we have identified miR-27a and miR-27b as a new class of adipogenic regulators that strongly inhibit adipogenesis. Although the gene loci of miR-27a and miR-27b are located in different chromosomes (for miR-27a: mouse chromosome 8 and human chromosome 19; for miR-27b: mouse chromosome 13 and human chromosome 9), our data reveal a concerted down-regulation of the miR-27 gene family during adipogenic differentiation of mesenchymal progenitor cells. Consistent with our observation, an independent study has found that miR-27a appears to be down-regulated upon adipogenic differentiation of 3T3-L1 preadipocytes (13). Our evidence indicates that the inhibitory effect of miR-27 on adipogenic differentiation is specific. Both miR-27a and miR-27b inhibit adipogenic conversion of mesenchymal progenitor cells from different tissue origins, such as the bone marrow-derived OP9 cells and the embryo-derived fibroblastic 3T3-L1 cells. On the other hand, neither miR-27a nor miR-27b significantly affects myogenic differentiation. Interestingly, a very recent study has shown that down-regulation of miR-27 increases intracellular lipid accumulation in hepatic stellate cells (24). Together, these findings suggest a role of miR-27 in multiple metabolic pathways. However, because miR-27 has the potential to target over three thousand genes, it is possible that miR-27 can regulate many other biological processes. It has been shown that miR-27a plays a role in cell cycle regulation in breast cancer cells (25) and facilitates growth of gastric cancer cells (26). On the other hand, miR-27b has been shown to regulate the expression of cytochrome P450, a drug-metabolizing enzyme, in cancer cells (27). It is possible that the biological function of miR-27 is manifested in a cell-type dependent manner and/or under certain patho-physiological conditions.
As compared to other reported miRNAs that have been investigated in adipogenesis, the miR-27 genes exhibit the strongest function as a class of negative regulators of adipogenesis. Wang et al. have shown that expression of the miR-17/92 cluster is moderately upregulated during adipogenesis (15). Over-expression of the miR-17/92 cluster moderately enhances adipogenic conversion, but does not initiate adipogenic differentiation of mouse 3T3-L1 preadipocytes in the absence of adipogenic hormones (15). A moderate increase in miR-143 has also been found during late stage (≥7 days) of adipogenic differentiation of human preadipocytes (14). Treatment with antisense oligonucleotides against miR-143 decreases lipid accumulation in adipocytes (14). However, Kajimoto et al. have shown that antisense inhibition of upregulated miRNAs does not affect adipogenic differentiation of 3T3-L1 cells (13). These observations, nonetheless, suggest the existence of extensive crosstalk or functional overlap among different miRNA genes.
The miR-27 genes appear to inhibit adipogenesis before preadipocytes become committed to terminal differentiation. The time course study (Fig. 2) has shown that miR-27a and miR-27b are capable of blocking adipogenic differentiation when introduced before or at the start of adipogenic stimulation by IDM. After 24 hr of IDM-stimulation, the miR-27 genes fail to suppress adipogenesis. Because robust transcriptional induction of PPARγ and C/EBPα generally occurs within 24-48 hr of adipogenic stimulation (11, 12, 28), our data suggest that the miR-27 genes are not capable of preventing the committed, PPARγ/C/EBPα-expressing preadipocytes from the terminal differentiation. Nonetheless, our observations indicate that miR-27 genes function by blocking the transcriptional induction of PPARγ and C/EBPα or by preventing preadipocytes from entering the stage of adipogenesis determination or commitment. The transcriptional repression of PPARγ and C/EBPα appears to be specific because C/EBPβ and C/EBPδ, which are expressed before the induction of PPARγ and C/EBPα, are unaffected by miR-27a or miR-27b.
It is predicted by bioinformatics that the PPARγ mRNA contains one putative binding site for miR-27a and miR-27b in its 3′ untranslated region. Our data, however, have shown that miR-27 does not repress PPARγ expression at the protein level, the gold-standard test for microRNA function, in maturing adipocytes. Because different miR-27-targeted genes have been identified in different cell types (24-27, 29), these observations suggest that the target recognition by microRNAs may be context dependent and/or cell-type specific. Alternatively, miR-27 could not overcome the strong transcriptional activation of PPARγ induced by IDM. Nonetheless, our data strongly suggest that the main mechanism by which miR-27 inhibits adipogenesis is to prevent the transcriptional induction of PPARγ in preadipcoytes before the adipogenic commitment stage.
The negative regulatory functions of miR-27a and miR-27b during adipogenesis prompted us to investigate whether the expression of miR-27a and miR-27b in adipose tissue is altered under pathological conditions. Using the epididymal fat tissue from the genetically obese ob/ob mice and the genetically matched lean mice, we have clearly demonstrated that the expression of both miR-27a and miR-27b is significantly increased in ob/ob mice (Fig. 5A). Although fat-derived primary stromal cells (also contain undifferentiated progenitor cells) have approximately 3-fold higher levels of miR-27a and miR-27b than primary mature adipocytes do, it is highly possible that both fat cells and stromal cells contribute to the overall increase of miR-27 in obese fat tissue, especially under stress conditions. Further investigation is warranted to clearly determine the contributions to miR-27 expression from different cell types and/or different types of cellular stresses in adipose tissue.
As compared to physiologically normal adipose tissues, obese fat tissues create dramatically different tissue microenvironments. We and others have found that obese fat tissues experience decreased tissue oxygenation or hypoxia (9, 17, 30). In this study, we have found that the expression of both miR-27a and miR-27b is maintained in preadipocytes under hypoxia (Fig. 6). This result is consistent with our previous findings that hypoxia inhibits adipogenesis (18, 31) and is also consistent with the finding that miR-27a expression is increased by hypoxia (23). However, it is worth noting that obese fat tissue does not only develop hypoxia, but also becomes inflammatory (8, 32). Inflammatory cytokines, such as TNFα, can also inhibit adipogenesis and adipocyte functions (33). It is highly likely that miR-27 expression in obese mice is subjected to regulation by multiple in vivo stresses. Nonetheless, our finding suggests a potential role of miR-27 in impairment of adipose functions associated with genetic obesity.
In summary, we have identified miR-27 as a new class of epigenetic regulators of adipogenesis. Our data have also presented a first example that obesity differentially regulates miRNA expression. The miR-27 genes may potentially play a role in the pathological progression of obesity-related diseases.
Experimental procedures
Tissue culture, differentiation, and transfection
Mouse 3T3-L1 preadipocytes, mouse bone marrow-derived OP9 cells, and mouse C2C12 myoblast cells were obtained from ATCC (American Type Culture Collections, Rockville, MD) and maintained in culture conditions recommended by ATCC. Briefly, 3T3-L1 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% calf serum. OP9 cells were grown in αMEM containing 20% fetal bovine serum (FBS). C2C12 cells were maintained in DMEM containing 10% FBS.
Adipogenic differentiation was carried out according to our previously published protocol (18, 19), confluent 3T3-L1 or OP9 cells were stimulated for 2 days in the differentiation medium: DMEM containing 10% FBS and IDM (10 μg/ml Insulin, 1 μM Dexamethasone and 0.5 mM Isobutylmethylxanthine). Cells were then maintained in DMEM containing 10% FBS and 1 μg/ml Insulin. The medium was replaced every other day. Mature adipocytes were visualized by staining with a 60% Oil Red O solution. For quantitative analysis, the intracellularly absorbed Oil Red O was extracted in 100% isopropanol and optical density was measured at 510 nm (18, 19).
Myogenic differentiation of C2C12 myoblasts was induced at approximately 70% confluence in DMEM containing 2% horse serum, and the differentiation medium was replaced every other day (31). Myofiber formation was examined microscopically with or without hematoxylin staining.
In hypoxia experiments, 3T3-L1 cells were maintained in a hypoxia chamber (Invivo 400, Ruskinn Inc., Cincinnati, OH) constantly maintained at 1% O2. Culture medium was replaced every other day inside the chamber.
For miRNA transfection, 3T3-L1, OP9 or C2C12 cells were plated one day before transfection at a concentration such that cells could reach confluence on the day of transfection. MicroRNA molecules (miR-27a, miR-27b or the non-targeting miR control, Applied Biosystems/Ambion, Austin, TX) were incubated in a solution containing DharmaFECT3 (Dharmacon) and then added to the confluent monolayer. Transfection efficiency was monitored using a fluorescent RNA duplex oligonucleotide (siGLO Red, Dharmacon) and was found to approach 100%.
Western blot analysis
Cell lysates were prepared on ice using 25 mM HEPES buffer, pH7.4, containing 1% NP-40, 150 mM NaCl, 2 mM EDTA, and 2 mM PMSF. Equal amounts of protein were subjected to SDS-PAGE under reducing conditions and analyzed with the following primary antibodies: polyclonal rabbit anti-PPARγ, anti-C/EBPα, anti-C/EBPβ (Santa Cruz Biotechnologies), anti-PPARα (Zymed Laboratories), and mouse monoclonal anti-β-actin (Sigma Aldrich).
Quantitative real-time PCR (qRT-PCR)
Total cellular RNA was isolated with Trizol reagent (Invitrogen). For analysis of miRNA expression in adipose tissue, total RNA was prepared using Trizol from minced epididymal fat pads harvested from the genetically obese ob/ob mice (male, 12-week old) with the genetically matched wild-type mice as control. Mice were provided with easy access to food and water. Animal protocols were approved by the Institutional Animal Use Committee.
Quantification of miRNA was performed using either the TaqMan method with the small RNA sno202 as an internal control (TaqMan MicroRNA Reverse Transcription Kit and TaqMan Universal PCR Master Mix, Applied Biosystems, Foster City, CA) or the SYBR Green method with 5S rRNA as the internal loading control (mirVana qRT-PCR miRNA Detection Kit, Applied Biosystems/ Ambion), according to the manufacture's recommended protocols.
Levels of mRNA were quantified in total cellular RNA using the SYBR Green method, with the two relatively stable endogenous genes UBC2 and 28S rRNA as controls for normalization. The following primers were used for PCR, and their specificities were validated by a single peak in their thermal dissociation curve. For C/EBPα (NM_007678), forward primer: 5′-CGCAA GAGCC GAGATA AAGC-3′; reverse primer: 5′-CGGTC ATTGT CACTG GTCAA CT-3′. For C/EBPβ (NM_009883), forward primer: 5′-AAGCT GAGCG ACGAG TACAA GA-3′; reverse primer: 5′-GTCAG CTCCA GCACC TTGTG-3′. For C/EBPδ (NM_007679), forward primer: 5′-TCCAC GACTC CTGCC ATGTA-3′, reverse primer: 5′- GCGGC CATGG AGTCA ATG-3′. For PPARγ (NM_011146), forward primer: 5′-GCCCA CCAAC TTCGG AATC-3′, reverse primer: 5′-TGCGA GTGGT CTTCC ATCAC-3′.
Acknowledgments
We thank Lisa Cabral for excellent editorial assistance. QL is supported by a fellowship from the Oak Ridge Institute for Science and Education. RMA is a visiting scientist from the Air Force Research Laboratory, Brooks City-Base, TX 78235-5107, USA.
References
- 1.Krutzfeldt J, Stoffel M. MicroRNAs: a new class of regulatory genes affecting metabolism. Cell Metab. 2006;4:9–12. doi: 10.1016/j.cmet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Xu P, Vernooy SY, Guo M, Hay BA. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13:790–795. doi: 10.1016/s0960-9822(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 3.Teleman AA, Maitra S, Cohen SM. Drosophila lacking microRNA miR-278 are defective in energy homeostasis. Genes Dev. 2006;20:417–422. doi: 10.1101/gad.374406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 5.Sokol NS, Ambros V. Mesodermally expressed Drosophila microRNA-1 is regulated by Twist and is required in muscles during larval growth. Genes Dev. 2005;19:2343–2354. doi: 10.1101/gad.1356105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nature genetics. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klaus S. Adipose tissue as a regulator of energy balance. Current drug targets. 2004;5:241–250. doi: 10.2174/1389450043490523. [DOI] [PubMed] [Google Scholar]
- 8.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 9.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? The British journal of nutrition. 2008;100:227–235. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- 10.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Rangwala SM, Lazar MA. Transcriptional control of adipogenesis. Annu Rev Nutr. 2000;20:535–559. doi: 10.1146/annurev.nutr.20.1.535. [DOI] [PubMed] [Google Scholar]
- 12.Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130:3122S–3126S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- 13.Kajimoto K, Naraba H, Iwai N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA (New York, N Y. 2006;12:1626–1632. doi: 10.1261/rna.7228806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, Dean NM, Freier SM, Bennett CF, Lollo B, Griffey R. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem. 2004;279:52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, Li X. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci U S A. 2008;105:2889–2894. doi: 10.1073/pnas.0800178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage Infiltration into Adipose Tissue May Promote Angiogenesis for Adipose Tissue Remodeling in Obesity. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 18.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 19.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 20.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 21.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, Bickel PE. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res. 2006;47:450–460. doi: 10.1194/jlr.D500037-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 23.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji J, Zhang J, Huang G, Qian J, Wang X, Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 2009;583:759–766. doi: 10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 26.Liu T, Tang H, Lang Y, Liu M, Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 2009;273:233–242. doi: 10.1016/j.canlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Tsuchiya Y, Nakajima M, Takagi S, Taniya T, Yokoi T. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res. 2006;66:9090–9098. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- 28.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 29.Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 2006;66:1277–1281. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- 30.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. International journal of obesity (2005) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 31.Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Mol Cell Biol. 2005;25:3040–3055. doi: 10.1128/MCB.25.8.3040-3055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 33.Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582:117–131. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]