This case report presents 3 cases of C septicum, a rare complication of HUS, focusing on key diagnostic signs and the importance of early antibiotic initiation.
Abstract
Clostridium septicum is an anaerobic bacterium that causes rapidly progressive myonecrosis, bacteremia, and central nervous system infection. It has been reported as a complication of Escherichia coli hemolytic uremic syndrome (HUS) in 8 children worldwide; 5 children died, and the 3 reported survivors had surgically treated disease. We present 3 cases of C septicum complicating HUS in children, including the first 2 reported cases of survival without surgical intervention. All patients presented with classic cases of HUS with initial clinical improvement followed by deterioration. Patient 1 had rising fever, tachycardia, and severe abdominal pain 24 hours after admission. She developed large multifocal intraparenchymal cerebral hemorrhages and died 12 hours later. Autopsy revealed C septicum intestinal necrosis, myonecrosis, and encephalitis. Patient 2 had new fever, increasing leukocytosis, and severe abdominal pain on hospital day 4. She was diagnosed with C septicum bacteremia and treated with metronidazole, meropenem, and clindamycin. Patient 3 had new fever and increasing leukocytosis on hospital day 3; blood cultures grew C septicum, and she was treated with penicillin. Patients 2 and 3 improved rapidly and did not require surgery. C septicum is a potential co-infection with E coli. It thrives in the anaerobic environment of E coli–damaged intestinal mucosa and translocates to cause systemic infection. Fever, tachycardia, a rising white blood cell count, and abdominal pain out of proportion to examination are key findings for which physicians should be vigilant. Timely evaluation by anaerobic blood culture and early initiation of antibiotics are necessary to prevent fatalities.
Clostridium septicum is an anaerobic gram-positive bacillus that causes bacteremia, myonecrosis, bowel necrosis, intracranial abscesses, and encephalitis.1 As with Shiga toxin–producing Escherichia coli (STEC), C septicum lives in the intestines of herbivores and may be transmitted to humans by a fecal–oral route.2 It may also colonize the ileocecal region of healthy humans.3 The chief virulence factor of C septicum, α-toxin, causes tissue necrosis and intravascular hemolysis.4 C septicum disease is rapidly progressive, with symptoms developing 6 to 48 hours after inoculation5 and death possible within 24 to 48 hours of symptom onset.6 Survival for myonecrosis is 57% to 59%, typically with debridement or amputation,5 but is only 0% to 36% with nonsurgical bacteremia or central nervous system disease.6
STEC-associated hemolytic uremic syndrome (HUS) is defined as hematocrit <30% with evidence of hemolysis (eg, schistocytes on a peripheral blood smear), platelet count <150 K/mm3, and elevated serum creatinine level in a patient with STEC infection.7 C septicum has been reported as a complication of STEC-HUS in 8 children,1,2,8–13 but the only 3 reported survivors had disease amenable to surgical debridement.5 We report 3 additional cases of C septicum complicating HUS, including 2 children with bacteremia who survived with antibiotic therapy alone.
Case Reports
Patient 1
Patient 1 was a 3-year-old, previously healthy female who lived on a cattle farm and was admitted with 4 days of bloody diarrhea. She was febrile to 38.8°C, listless, and her abdomen was exquisitely tender. A stool culture specimen obtained 2 days before admission was positive for STEC O157:H7. Her white blood cell (WBC) count was 45.5 K/mm3, hematocrit was 25.4% with schistocytes on a peripheral blood smear, platelet count was 18 K/mm3, and creatinine level was 1.3 mg/dL. Abdominal radiograph and ultrasound demonstrated diffuse colitis.
The patient’s fever initially improved, but 16 hours after admission, her temperature rose to 39.1°C. She continued to report abdominal pain, although her examination remained benign. Twenty-four hours after admission, her heart rate increased to 140 to 150 beats/min; red blood cells and platelets were transfused for worsening anemia and thrombocytopenia.
Five hours later, the patient suddenly became nonresponsive with a fixed and dilated left pupil. She was intubated and treated for intracranial hypertension. A computed tomography scan of the brain revealed large multifocal intraparenchymal hemorrhages with uncal herniation (Fig 1). She subsequently developed profound shock with severe lactic acidosis and multiorgan failure. A repeat abdominal radiograph showed no free air or pneumatosis. The patient died 36 hours after admission.
FIGURE 1.
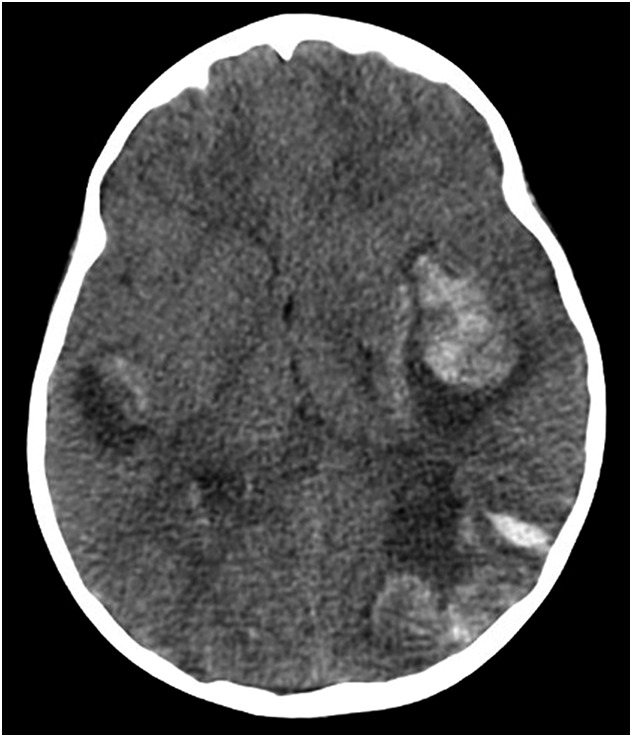
In contrast to the basal ganglia changes seen with neurologic involvement in HUS, the head computed tomography scan of patient 1 revealed multiple large foci of intraparenchymal hemorrhage, with a 4-mm midline shift and uncal herniation.
The patient’s autopsy revealed glomerular thrombi, fragmented erythrocytes, and visceral organ petechial hemorrhages consistent with thrombotic microangiopathy. STEC was found in postmortem colonic stool samples, confirming STEC-HUS; a perimortem ADAMTS13 activity level of 39% and normal results of coagulation studies ruled out thrombotic thrombocytopenic purpura and disseminated intravascular coagulation as alternative etiologies.
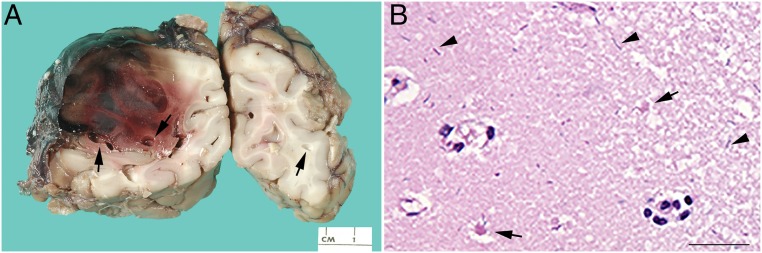
Her autopsy also revealed abdominal wall myonecrosis, hemorrhagic necrosis of the colon, and encephalitis. There were extensive cerebral intraparenchymal hemorrhages, necrosis, and marked pneumocephalus (Fig 2A). Large gram-positive bacilli were present within the colonic wall, abdominal wall, and cerebral cortex (Fig 2B); microbial polymerase chain reaction confirmed C septicum.
FIGURE 2.
Autopsy findings in patient 1 highlight changes consistent with intracranial C septicum. A, Coronal sections of the cerebrum with pneumocephalus (arrows) and intraparenchymal hemorrhage/necrosis. B, Microscopic image of the cerebral cortex with scattered rod-shaped bacteria (arrowheads) with minimal surrounding inflammation and evidence of cerebral infarct, including ischemic neurons (arrows); hematoxylin and eosin stain, 50 µm.
Patient 2
Patient 2 was a 4-year-old, previously healthy female admitted with 3 days of abdominal pain, 1 day of bloody diarrhea, and fever to 38.6°C. She had recently visited a petting zoo. On hospital day 2, she developed STEC-HUS with hematocrit 28.5%, schistocytes on a peripheral blood smear, a platelet count of 28 K/mm3, a creatinine level of 1.9 mg/dL, and stool culture specimen from admission positive for STEC O157:H7. Hemodialysis was initiated on hospital day 4 for anuria.
After initial defervescence, the patient developed a new fever to 39°C and worsening abdominal pain on hospital day 4. Her heart rate rose to 130 to 140 beats/min, even when afebrile. Her WBC count rose from 13.4 K/mm3 on admission to 38.8 K/mm3. Abdominal radiograph showed inflamed small bowel but no free air. Anaerobic blood culture specimen grew C septicum at 12 hours. She was immediately started on metronidazole 4 mg/kg intravenously (IV) every 6 hours and meropenem 20 mg/kg IV every 24 hours (meningitic dose) with doses adjusted for renal failure.
The patient remained hemodynamically stable and defervesced after 1 day of antibiotics. She continued to have hemolysis; metronidazole was therefore changed to clindamycin 10 mg/kg IV every 8 hours to target Clostridium α-toxin production. Hemolysis improved after the change in antibiotic, and the WBC count normalized. Antibiotics were discontinued after a 14-day course, dialysis was discontinued on day 16 of admission, and she was discharged from the hospital on day 19 with no apparent sequelae of the bacteremia.
Patient 3
Patient 3 was a 6-year-old, previously healthy female admitted with 7 days of bloody diarrhea and 2 days of fever, abdominal pain, and irritability. Results of laboratory tests revealed a platelet count of 39 K/mm3, schistocytes, a creatinine level of 2.2 mg/dL, and stool culture positive for STEC O157:H7; hematocrit fell to 27.5% 18 hours after admission. She had had no known animal exposures. She started hemodialysis on hospital day 2 for anuria.
The patient’s irritability and fevers initially resolved, but on hospital day 3, she developed new fever to 39.3°C and worsening abdominal pain. Her WBC count rose from 11.3 K/mm3 on admission to 27.4 K/mm3. C septicum grew from an anaerobic blood culture specimen at 9 hours. She initially received vancomycin and gentamicin; therapy was changed to penicillin G 20 000 U/kg IV every 6 hours once C septicum was identified. Two days after starting antibiotics she defervesced, and her WBC count normalized. The patient remained hemodynamically stable and completed 7 days of penicillin G monotherapy. Hemodialysis was discontinued on day 14 of admission, and she was discharged from the hospital on day 21.
Discussion
We report the first 2 cases of survival without surgery among children with C septicum complicating HUS. C septicum is rare in children, with <50 cases reported. Most cases involve immunosuppressed children who develop C septicum of the gastrointestinal tract or extremities. Outcomes seem to be strongly associated with treatment course; fatality among surgically treated patients is 23.8%, but without surgery, this rate rises to 87%.5
Eight cases of C septicum complicating HUS have been reported previously (Table 1). Gastrointestinal disease was common, but 6 of these 8 children also had brain involvement, an otherwise rare finding.14 Five of the children died. All 3 of the survivors underwent surgery; 1 patient had amputation of the arm due to myonecrosis, and 2 patients had drainage of brain abscesses.1,2,8–13 There is also 1 reported case of Clostridium sordellii bacteremia in a child with HUS who died of septic shock despite antibiotic therapy.15
TABLE 1.
Reported Cases of C septicum Infection in HUS
| Reference | Patient | Onset After HUS, d | Symptoms | Location | Method of Diagnosis | Antibiotics | Outcome | |
|---|---|---|---|---|---|---|---|---|
| With surgical intervention | ||||||||
| Previously published cases | ||||||||
| 1 | 19 mo, M | 2 | New fever, arm swelling and ecchymosis | Arm myonecrosis | Wound culture | Penicillin, clindamycin | Survival | |
| 2 | 2.5 y, M | 6 | Irritability, drowsiness, hypertonicity | Brain abscess | Brain biopsy | Meropenem, metronidazole | Survival | |
| 11 | 2 y, M | 5 | Abdominal pain, irritability, seizure | Brain abscess | Abscess culture | Penicillin, metronidazole | Survival | |
| Without surgical intervention | ||||||||
| Previously published cases | ||||||||
| 8 | 2 y, M | 0.5 | Acute neurologic deterioration | Brain parenchyma, lung, bowel, kidney, liver | Blood culture, postmortem tissue | None | Death | |
| 9 | 2 y, M | 1.5 | Irritability, confusion, leukocytosis, abdominal wall fasciitis, shock | Bacteremia, abdominal wall fascia | Blood culture | Cefuroxime, metronidazole | Death | |
| 10 | 2 y, F | 0 | Fever, seizures, acute neurologic deterioration | Brain parenchyma, meninges, bowel | CSF, blood culture, postmortem tissue | Penicillin, metronidazole | Death | |
| 12 | 16 mo, M | 2 | Fever, abdominal rash, acute neurologic deterioration | Brain parenchyma, bowel, visceral organs | Skin culture, postmortem blood | Ampicillin, clindamycin, gentamycin | Death | |
| 13 | 4 y, M | 2 | Seizure, acute neurologic deterioration | Brain parenchyma, meninges, bowel | Blood culture, postmortem tissue and CSF | None | Death | |
| Present cases | ||||||||
| Patient 1 | 3 y, F | 1 | Rising fever, tachycardia, abdominal pain, acute neurologic deterioration | Brain parenchyma, bowel | Postmortem tissue | None | Death | |
| Patient 2 | 4 y, F | 4 | New fever, tachycardia, abdominal pain, leukocytosis | Bacteremia | Blood culture | Clindamycin, meropenem | Survival | |
| Patient 3 | 6 y, F | 3 | New fever, abdominal pain, leukocytosis | Bacteremia | Blood culture | Penicillin | Survival | |
CSF, cerebrospinal fluid; F, female; M, male.
Because E coli and C septicum are both present in herbivore feces, they may be acquired simultaneously from a common source. Alternatively, C septicum has been reported in the feces of 2.8% of healthy humans.5 E coli gastroenteritis is thought to predispose to C septicum co-infection by causing areas of damaged, poorly perfused intestine, creating conditions for anaerobic C septicum to thrive and translocate across intestinal mucosa.6,9
C septicum produces α-toxin, which causes tissue necrosis and intravascular hemolysis.4 Symptoms are nonspecific and include pain out of proportion to examination, fever, and significant tachycardia.16 Diagnosis of C septicum is made by culture of blood or draining fluid. Treatment consists of surgical debridement and antibiotic therapy, typically with high-dose penicillin G (250 000–400 000 U/kg/d IV). Meropenem, metronidazole, and chloramphenicol are alternative therapies. The addition of clindamycin may be beneficial by inhibiting toxin synthesis.2,3,17
Likely critical to the survival of our 2 patients was early collection of anaerobic blood culture specimens and prompt initiation of antibiotics. All 3 patients improved after admission, then developed new or worsening fever, tachycardia, WBC counts >25 K/mm3, and severe abdominal pain despite reassuring examination. These symptoms may be attributed to an established HUS diagnosis but should prompt further evaluation for bacteremia. Antibiotics are typically avoided in patients with STEC-related diarrhea due to the association of antibiotics with the development of HUS18; however, we are unaware of any literature suggesting that antibiotic therapy worsens outcomes of existing HUS. Therefore, there is no reason to delay the initiation of antibiotics if sepsis is suspected.
In patients 2 and 3, C septicum was identified in the anaerobic culture specimen only. In pediatrics, routine use of anaerobic blood culture is controversial and may not be standard in all settings.19,20 Given the risk of C septicum, we believe that all patients with HUS and concern for bacteremia should have anaerobic culture specimens drawn. Adequate blood volume collection is also important for diagnosis. Insufficient blood volume is a common problem with pediatric blood cultures, whereas adequate blood volume is associated with a higher proportion of positive culture results.19
Rapid treatment of patient 2 was facilitated by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry, a newer technique that allows identification of bacteria directly from a positive anaerobic blood culture specimen in ∼1 hour.21 MALDI-TOF has been associated with earlier identification of organisms22 and decreased mortality in adults.21 The use of modern blood culture techniques and MALDI-TOF for this patient showed that C septicum may no longer be as difficult to grow and identify as has been previously reported.5
Alternatively, our 2 surviving patients may suggest that C septicum co-infection in HUS is not as rare as previously thought. Blood cultures are generally not part of the evaluation of HUS, and we are unaware of any literature reporting the rates of bacteremia in this setting. The 2 surviving patients may represent a larger cohort with undiagnosed C septicum who develop few symptoms and clear the infection without antibiotics.
Conclusions
We report the first 2 children with HUS and C septicum infection to survive without surgery and add to the literature describing C septicum as a rare but potentially fatal complication of HUS. Increased awareness of this potential complication can help clinicians monitor for subtle clinical symptoms, initiate prompt diagnostic testing, and consider early initiation of antibiotics that may decrease the mortality of this disease.
Glossary
- HUS
hemolytic uremic syndrome
- IV
intravenously
- MALDI-TOF
matrix-assisted laser desorption ionization–time of flight
- STEC
Shiga toxin–producing Escherichia coli
- WBC
white blood cell
Footnotes
Drs Engen and Killien identified the relevant cases and jointly drafted the manuscript; Dr Davis drafted the portions of the manuscript related to pathology, provided the pathology images, and assisted in revising the entire manuscript; Drs Symons and Hartmann oversaw the manuscript creation and critically reviewed the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This research was supported in part by NIH training grant 5 T32 DK007662. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hunley TE, Spring MD, Peters TR, Weikert DR, Jabs K. Clostridium septicum myonecrosis complicating diarrhea-associated hemolytic uremic syndrome. Pediatr Nephrol. 2008;23(7):1171–1175 [DOI] [PubMed] [Google Scholar]
- 2.Williams EJ, Mitchell P, Mitra D, Clark JE. A microbiological hazard of rural living: Clostridium septicum brain abscess in a child with E coli 0157 associated haemolytic uraemic syndrome. BMJ Case Rep. 2012;2012:bcr2012006424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinzon-Guzman C, Bashir D, McSherry G, Beck MJ, Rocourt DV. Clostridium septicum gas gangrene in a previously healthy 8-year-old female with survival. J Pediatr Surg. 2013;48(4):e5–e8 [DOI] [PubMed] [Google Scholar]
- 4.Kennedy CL, Krejany EO, Young LF, et al. The α-toxin of Clostridium septicum is essential for virulence. Mol Microbiol. 2005;57(5):1357–1366 [DOI] [PubMed] [Google Scholar]
- 5.Smith-Slatas CL, Bourque M, Salazar JC. Clostridium septicum infections in children: a case report and review of the literature. Pediatrics. 2006;117(4). Available at: www.pediatrics.org/cgi/content/full/117/4/e796 [DOI] [PubMed] [Google Scholar]
- 6.Hermsen JL, Schurr MJ, Kudsk KA, Faucher LD. Phenotyping Clostridium septicum infection: a surgeon’s infectious disease. J Surg Res. 2008;148(1):67–76 [DOI] [PubMed] [Google Scholar]
- 7.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073–1086 [DOI] [PubMed] [Google Scholar]
- 8.Martin SE, Allen SD, Faught P, Hawley DA, Bonnin JM, Hattab EM. A 2-year-old boy with hemolytic uremic syndrome and pneumocephalus. Brain Pathol. 2012;22(1):121–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnham M, Weightman N. Clostridium septicum infection and hemolytic uremic syndrome. Emerg Infect Dis. 1998;4(2):321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broughton RA, Lee EY. Clostridium septicum sepsis and meningitis as a complication of the hemolytic-uremic syndrome. Clin Pediatr (Phila). 1993;32(12):750–752 [DOI] [PubMed] [Google Scholar]
- 11.Chiang V, Adelson PD, Poussaint TY, Hand M, Churchwell KB. Brain abscesses caused by Clostridium septicum as a complication of hemolytic-uremic syndrome. Pediatr Infect Dis J. 1995;14(1):72–74 [DOI] [PubMed] [Google Scholar]
- 12.Riccio JA, Oberkircher OR. Clostridium septicum sepsis and cerebritis: a rare complication of the hemolytic-uremic syndrome. Pediatr Infect Dis J. 1988;7(5):342–345 [DOI] [PubMed] [Google Scholar]
- 13.Randall JM, Hall K, Coulthard MG. Diffuse pneumocephalus due to Clostridium septicum cerebritis in haemolytic uraemic syndrome: CT demonstration. Neuroradiology. 1993;35(3):218–220 [DOI] [PubMed] [Google Scholar]
- 14.Sadarangani SP, Batdorf R, Buchhalter LC, et al. Clostridium septicum brain abscesses in a premature neonate. Pediatr Infect Dis J. 2014;33(5):538–540 [DOI] [PubMed] [Google Scholar]
- 15.Beyers R, Baldwin M, Dalabih S, Dalabih A.. Clostridium sordellii as a cause of fatal septic shock in a child with hemolytic uremic syndrome. Case Rep Pediatr. 2014;2014:237674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiel N, Ho V, Pascoe A. A case of gas gangrene in an immunosuppressed Crohn’s patient. World J Gastroenterol. 2011;17(33):3856–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics Clostridial myonecrosis. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015:297–298 [Google Scholar]
- 18.Freedman SB, Xie J, Neufeld MS, Hamilton WL, Hartling L, Tarr PI; Alberta Provincial Pediatric Enteric Infection Team (APPETITE) . Shiga toxin-producing Escherichia coli infection, antibiotics, and risk of developing hemolytic uremic syndrome: a meta-analysis. Clin Infect Dis. 2016;62(10):1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dien Bard J, McElvania TeKippe E. Diagnosis of bloodstream infections in children. J Clin Microbiol. 2016;54(6):1418–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidi AK, Knaut AL, Mirrett S, Reller LB. Value of routine anaerobic blood cultures for pediatric patients. J Pediatr. 1995;127(2):263–268 [DOI] [PubMed] [Google Scholar]
- 21.Kothari A, Morgan M, Haake DA. Emerging technologies for rapid identification of bloodstream pathogens. Clin Infect Dis. 2014;59(2):272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malcomson C, Ng K, Hughes S, et al. Impact of matrix-assisted laser desorption and ionization time-of-flight and antimicrobial stewardship intervention on treatment of bloodstream infections in hospitalized children [published online ahead of print June 23, 2016]. J Pediactric Infect Dis Soc. 10.1093/jpids/piw033 [DOI] [PubMed] [Google Scholar]