Abstract
Vertebrate eggs arrest at metaphase of the second meiotic division before fertilization under the effect of a cytostatic factor (CSF). This arrest is established during oocyte maturation by the MAPK kinase module, comprised of Mos, MEK, MAPKs and p90Rsk. Maintenance of CSF arrest at metaphase requires inhibitors of the anaphase-promoting complex (APC) like Emi1, which sequesters the APC activator Cdc20. Although it was proposed that the Mos pathway and Emi1 act independently, neither one alone is sufficient to entirely reproduce CSF arrest. Herein we demonstrate that p90Rsk2 associates with and phosphorylates Emi1 upstream of the binding region for Cdc20, thus stabilizing their interaction. Experiments in transfected cells and two-cell embryos indicate that Emi1 and p90Rsk2 cooperate to induce the metaphase arrest. Moreover, oocyte maturation was impaired by interfering with the interaction between p90Rsk2 and Emi1 or by RNA interference of Emi1. Our results indicate that p90Rsk2 and Emi1 functionally interact during oocyte maturation and that the Mos pathway establishes CSF activity through stabilization of an APC-inhibitory complex composed by Emi1 and Cdc20 before fertilization.
Keywords: Cdc20, CSF, fertilization, metaphase arrest, mouse oocytes
Introduction
In vertebrate eggs, a cytostatic factor (CSF) is responsible for the stabilization of the cyclinB/cdc2 complex known as maturation-promoting factor (MPF) (Masui and Markert, 1971). CSF prevents the transition to anaphase until fertilization occurs and can induce cytostatic arrest when injected into the cytoplasm of mitotically dividing blastomeres (reviewed in Masui, 2001). A breakthrough in the molecular characterization of CSF came with the identification of Mos, a serine-threonine kinase newly synthesized during oocyte maturation and capable of inducing metaphase arrest in injected blastomeres (Sagata et al, 1988). Mos translation during oocyte maturation establishes CSF arrest (Masui, 2001), whereas Mos depletion by RNAi prevents its establishment (Dupré et al, 2002). Moreover, the mitogen-activated protein kinase (MAPK) pathway is necessary for the biological activity of Mos in both Xenopus and mouse eggs (Haccard et al, 1993; Kosako et al, 1994; Verlhac et al, 2000a). The downstream effector of the Mos-dependent MAPK pathway in Xenopus eggs is p90Rsk. It was demonstrated that p90Rsk2 is the predominant Rsk isoform expressed in Xenopus eggs and embryos and that immunodepletion of p90Rsk2 from cycling egg extracts completely abolished the CSF activity of Mos (Bhatt and Ferrell, 1999; 2000). Furthermore, in the absence of Mos, a CSF effect could also be obtained by microinjection of a constitutively active p90Rsk1 isoform (Gross et al, 1999), confirming that a p90Rsk isoform acts downstream of Mos. The role of Mos in the establishment of cytostatic activity appears to be evolutionarily conserved in vertebrate eggs because mice with homozygous deletion of the mos gene ovulate oocytes that do not arrest at metaphase and undergo spontaneous parthenogenetic activation (Colledge et al, 1994; Hashimoto et al, 1994). In addition, both MAPK and p90Rsk are similarly activated during meiotic maturation of mouse oocytes (Kalab et al, 1996; Verlhac et al, 1996), indicating conservation of the whole pathway.
Most of the data available on the molecular mechanisms of CSF arrest have been gathered using a constitutively active p90Rsk1. Gross et al (2000) demonstrated that this protein inhibits the ubiquitin–ligase complex known as anaphase-promoting complex (APC), thus preventing the complete destruction of cyclin B at anaphase I and promoting its rapid accumulation during meiosis II. Recent evidence suggests that the Mos pathway inhibits the APC through activation of the spindle checkpoint (Tunquist and Maller, 2003). The kinase Bub1 is phosphorylated and activated by the constitutively active p90Rsk1 in vitro and in a MAPK-dependent manner during meiotic maturation (Schwab et al, 2001), and other components of this checkpoint, like Mad1 and Mad2, are required for the establishment of the Mos-dependent cytostatic arrest in Xenopus eggs (Tunquist et al, 2002; 2003). However, the function of the spindle checkpoint in CSF maintenance must be distinguishable from that exerted in mitotic cells, because the checkpoint appears active even though in metaphase-arrested oocytes chromosomes are correctly attached with their kinetochores to microtubules of the spindle. Moreover, in eggs, only Mad1 is required for maintenance of CSF, whereas Mad2, the direct inhibitor of Cdc20, is dispensable at metaphase. Thus, the connection between the endogenous p90Rsk and Mad1 in CSF arrest is still unknown (Tunquist et al, 2003).
One puzzling feature of the elusive CSF of vertebrate eggs is that while the Mos/MAPK/p90Rsk pathway participates in its establishment during meiosis II, it becomes dispensable afterwards. Indeed, immunodepletion of p90Rsk2, or of Bub1 kinase, from Xenopus egg extracts is not sufficient to release them from the metaphase arrest (Bhatt and Ferrell, 1999; Tunquist et al, 2002). A possible explanation is that these kinases act upstream of the real effectors of the cytostatic activity. In this regard, it has been proposed that a novel regulator of the APC, Emi1, is directly responsible of the metaphase arrest of Xenopus eggs (Reimann and Jackson, 2002). Emi1 interacts with the substrate-binding region of Cdc20 and stabilizes mitotic cyclins in Xenopus egg extracts (Reimann et al, 2001a; 2001b). Moreover, immunodepletion of Emi1 from these extracts caused degradation of MPF and release from the CSF arrest, whereas an excess of Emi1 blocked release of CSF arrest by Ca++ or activation of CamKII (Reimann and Jackson, 2002), events that mimic egg activation at fertilization (Lorca et al, 1993; Markoulaki et al, 2003). Thus, since Emi1 appeared both necessary and sufficient to produce CSF activity in Xenopus egg extracts, it was proposed that this protein was the long-sought CSF responsible for the metaphase II arrest of vertebrate eggs (Reimann and Jackson, 2002).
Surprisingly, the activity of Emi1 in egg extracts did not require the Mos/MAPK/p90Rsk pathway, suggesting that Emi1 acts independently (Reimann and Jackson, 2002). However, it remains unclear why in the absence of Mos the cytostatic activity of vertebrate eggs does not develop (Colledge et al, 1994; Hashimoto et al, 1994; Dupré et al, 2002) and why mitotic cells, which express Emi1 in the G2 phase like the oocytes, do not arrest at metaphase (Hsu et al, 2002). Emi1 accumulates in the S and G2 phases of mitotic cycles and its destruction at the onset of the M phase allows progression through mitosis. Phosphorylation of Emi1 by Cdc2 in prometaphase promotes its interaction with the substrate adaptor protein βTrCP and its degradation by the SCF (Skp1/Cullin/F-box) ubiquitin–ligase complex (Margottin-Goguet et al, 2003), dictating the timing of entry into mitosis. However, in meiosis, Emi1 is stable throughout maturation of Xenopus oocytes (Reimann and Jackson, 2002) but cyclin B stabilization and metaphase arrest occur only in the second division, after full activation of the Mos pathway. Mos being the only component of CSF selectively expressed during the meiotic divisions (Masui, 2001), it is possible that its pathway functionally interacts with Emi1 during maturation when CSF appears.
Herein we provide evidence that phosphorylation of Emi1 by p90Rsk2 stabilizes its interaction with the APC activator Cdc20 and that the two proteins cooperate to establish CSF arrest during mouse oocyte maturation. Our studies provide a direct link between components involved in the establishment and maintenance of CSF activity before fertilization.
Results
p90Rsk2 directly interacts with Emi1
Since p90Rsk2 and Emi1 are both involved in cytostatic activity in Xenopus eggs, we initially investigated whether they are also present in mouse eggs. RT–PCR analysis from metaphase-arrested oocytes revealed that p90Rsk2 and Emi1 are both present at the mRNA level (data not shown). Both proteins were detected in mouse oocytes by Western blot (Figure 1A) and immunofluorescence analyses showed that they were diffuse in the cytoplasm (Figure 1B). Moreover, both Emi1 and p90Rsk2 seemed to decorate the meiotic spindle (Figure 1B, insets), suggesting that they could partially colocalize.
Figure 1.
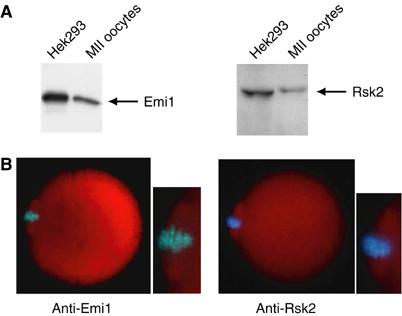
Emi1 and p90Rsk2 are expressed in mouse oocytes. (A) Western blot analysis of Emi1 and p90Rsk2 expression in mouse oocytes. Hek293 cell extracts (2 μg) or mouse oocyte extracts (300 oocytes) were loaded on a 10% SDS–PAGE and stained with either rabbit polyclonal anti-Emi1 antibody (1:500) or goat polyclonal anti-p90Rsk2 antibody (1:500). Hek293 cell extracts were loaded as positive control of protein expression. Relevant bands are indicated by arrows on the right. (B) Immunofluorescence analysis of Emi1 and p90Rsk2 in mouse oocytes. Double staining with either anti-Emi1 (1:200) or anti-p90Rsk2 (1:200) antibodies (red) and Hoechst for DNA staining (blue). Both proteins localize to the cytoplasm and the meiotic spindle (see insets).
Next, we investigated whether Emi1 and p90Rsk2 physically interact. The mouse Emi1 homologue was cloned by RT–PCR from a 13 days postcoitum (dpc) embryo library and its identity was verified by direct sequencing. HA-tagged p90Rsk2 and myc-tagged Emi1 were expressed in Hek293 cells either alone or in combination. When cell extracts were immunoprecipitated with anti-myc antibody, p90Rsk2 was specifically co-immunoprecipitated with myc-Emi1 (Figure 2A). Interestingly, we found that Emi1 also co-immunoprecipitated with the endogenous APC activator Cdc20, and that coexpression of p90Rsk2 increased this association (Figure 2A).
Figure 2.
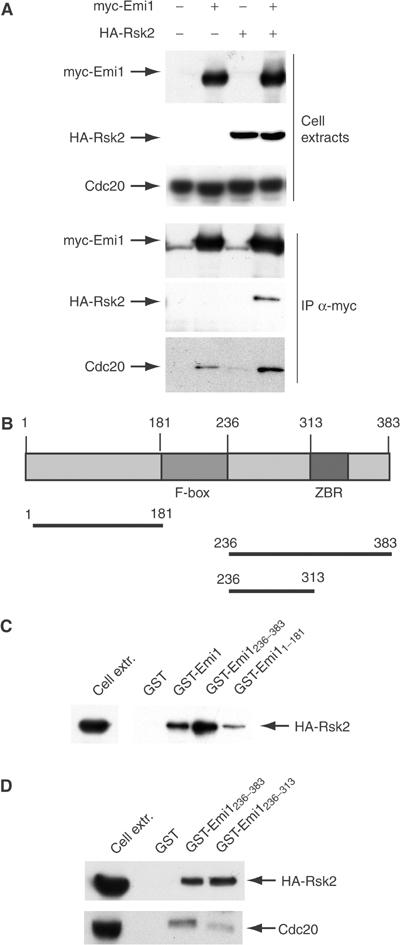
Emi1 associates with p90Rsk2. (A) Emi1 and p90Rsk2 specifically co-immunoprecipitate. Western blot analyses of proteins in cell extracts (first three rows) or co-immunoprecipitating with myc-Emi1 (three bottom rows). Hek293 cells were transfected with empty vectors or myc-Emi1 and HA-p90Rsk2 either alone or in combination. A 20 μg portion of total extracts was loaded in each lane (first three rows) and 500 μg of total proteins was immunoprecipitated for each sample (last three rows). Blots were stained with anti-myc for myc-Emi1, anti-HA for HA- p90Rsk2, and anti-Cdc20. (C, D) Cdc20 and p90Rsk2 bind to different sites in the C-terminus of Emi1. Pull-down experiments using purified GST fusion proteins of Emi1 (elucidated in the scheme in B) and extracts of Hek293 cells transfected with HA-p90Rsk2 (500 μg). Bound proteins were revealed by Western blot analyses using either anti-HA or anti-Cdc20 antibodies as described in the figure.
Hence, we analysed the regions of Emi1 required for this interaction using pull-down experiments with purified GST-Emi1 proteins. Emi1 contains an N-terminal region (aa 1–181 in mouse Emi1) upstream of the degradation box (F-box, aa 182–236) and a C-terminal region that contains a zinc-binding motif rich in cysteines (aa 313–383). Mitotic cyclins bind to the N-terminal region of Xenopus Emi1, whereas both the N-terminal and the C-terminal regions of Emi1 interact with Cdc20 (Reimann et al, 2001a). Remarkably, only the C-terminal region of Emi1 acts as a Cdc20 inhibitor and is necessary and sufficient for the cytostatic activity of Xenopus eggs (Reimann et al, 2001a; 2001b; Reimann and Jackson, 2002). We found that p90Rsk2 bound to full-length Emi1 and to both GST-Emi11–181 and GST-Emi1236–383, albeit interaction with the C-terminal region was stronger (Figure 2C). Interestingly, while the cysteine-rich motif of mouse Emi1 was required for efficient binding to Cdc20, its deletion did not affect the interaction with p90Rsk2 (see GST-Emi1236–313 in Figure 2D), indicating that the kinase binds a different site in the C-terminus of Emi1.
p90Rsk2 phosphorylates Emi1 and promotes its interaction with Cdc20
To test whether Emi1 was a substrate for p90Rsk2, we incubated a purified active form of p90Rsk2 with purified GST-Emi1 in vitro. As shown in Figure 3A, GST-Emi1, but not GST alone, was readily phosphorylated by the kinase. Moreover, we found that p90Rsk2 phosphorylated GST-Emi1236–383 much more efficiently than GST-Emi11–181, and that the region of Emi1 upstream of the zinc-finger motif was sufficient for phosphorylation (GST-Emi1236–313 in Figure 3B). An analysis of the known Emi1 gene sequences revealed that there are only four serine/threonine residues that are conserved between amino acids 236 and 313: ser246, thr251, thr304 and ser310 (Figure 3C). Single substitutions of these residues with alanine showed that only ser246 and thr251 are substrates for p90Rsk2 (Figure 3D). Moreover, p90Rsk2 could still phosphorylate a GST-Emi1236–302 fusion protein but not GST-Emi1294–383 (Figure 3B). However, even when a double mutation ser246ala/thr251ala (ST/AA) was produced, phosphorylation of GST-Emi1236–313 was decreased but not abolished (Figure 3D), indicating that additional, nonconserved residues between amino acids 236 and 302 of Emi1 are substrates for p90Rsk2 in vitro.
Figure 3.
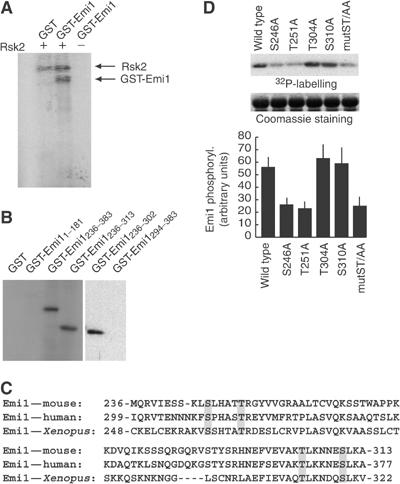
Emi1 is phosphorylated by p90Rsk2. (A) In vitro kinase assay using 5 U of purified p90Rsk2 (Upstate Biotechnology) and 1 μg of GST or GST-Emi1 as substrates. Proteins were incubated for 20 min at 30°C, separated on SDS–PAGE and analysed by autoradiography. (B) Emi1236–313 is sufficient for phosphorylation by p90Rsk2. In vitro kinase assay as described in (A) using purified p90Rsk2 and 1 μg of the purified subregions of Emi1 described in the text of the figure. (C) Alignment of the region of homology in the 236–313 region of mouse Emi1 (NP_080271.1) reveals that only four serine/threonine residues are conserved from Xenopus (AAK62272) to human (AAH18905.1). (D) Site-directed mutagenesis shows that ser246 and thr251 are in vitro substrates for p90Rsk2. The upper panel shows an in vitro kinase assay using purified p90Rsk2 and GST-Emi1236–313 wild type or mutant forms. The middle panel shows the corresponding Coomassie blue staining to verify that equal amounts of GST-Emi1 proteins were used for the assay. The lower panel shows a densitometric analysis from three separate phosphorylation experiments performed as described above.
Since p90Rsk2 interacts with and phosphorylates Emi1 upstream of the binding site for Cdc20, we asked whether it influenced their interaction. GST-Emi1 full length was bound to GSH-agarose beads and incubated for 30 min in the absence or presence of purified p90Rsk2 to obtain nonphosphorylated or phosphorylated sources of the protein (see Figure 3A). At the end of the incubation, beads were washed and the kinase removed, as demonstrated by Western blot analysis (third panel). Hence, beads were incubated with extracts of proliferating Hek293 cells, which express high levels of Cdc20. As expected, GST-Emi1 was able to bind to Cdc20; however, phosphorylation by p90Rsk2 strongly increased this interaction (approximately four-fold; Figure 4A). The same effect of phosphorylation by p90Rsk2 was observed when a GST-Emi1236–383 was used (Figure 4B), confirming that the action of p90Rsk2 is exerted on the region of Emi1 that is necessary and sufficient for its cytostatic activity (Reimann et al, 2001a). Remarkably, the effect of phosphorylation by p90Rsk2 was completely suppressed when the GST-Emi1236–383ST/AA mutant (ser246ala/thr251ala) was used for Cdc20 binding assays (Figure 4C). These results suggest that phosphorylation by p90Rsk2 stabilizes the interaction of Emi1 with Cdc20.
Figure 4.
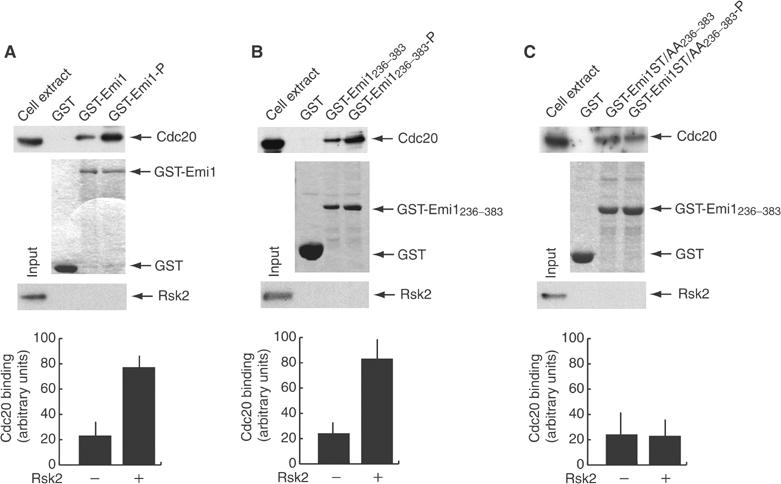
Phosphorylation of Emi1 by p90Rsk2 potentiates its binding to Cdc20. GST-Emi1 full length (A) or either wild type (B) or ST/AA mutant (C) C-terminus, aa 236–383, was purified on GSH-agarose beads and incubated for 30 min in the absence or presence of purified active form of p90Rsk2 to obtain a source of nonphosphorylated or phosphorylated protein. After washes to remove the kinase (the corresponding Western blot is shown in the bottom panels of each experiment), GST fusion proteins were used in pull-down experiments with extracts from Hek293 cells (500 μg of total proteins) as a source of endogenous Cdc20. The first panels in (A–C) represent anti-Cdc20 Western blot, and the second panels represent Coomassie blue staining of the pull-down experiments. The third panels display the lack of residual kinase on beads before incubation with extracts. The results from three different experiments were quantitated by optical densitometry and the data are shown as average±standard deviation in the panels at the bottom of the figure.
Emi1 and p90Rsk2 cooperate to induce the metaphase arrest of Hek293 cells
Since phosphorylation of Emi1 by p90Rsk2 increases its ability to interact with Cdc20 and this interaction prevents activation of the APC, we asked if p90Rsk2 could augment the cytostatic activity of Emi1 in vivo. To this end, we transfected Hek293 cells with constructs for p90Rsk2 and Emi1 either alone or in combination. In addition, cells were cotransfected with a GFP construct (1:10) to identify transfected cells. After 24 h from transfection, cells were fixed, stained with Hoechst and analysed for the percentage of mitotic cells in the GFP-positive population. Control cells transfected with empty vectors displayed 5% of GFP-positive cells in either prometaphase or metaphase (Figure 5A and B); overexpression of Emi1 caused an increase of mitotic cells to 12%, similar to that observed previously for Xenopus Emi1 (Reimann et al, 2001a). Interestingly, we observed that coexpression of Emi1 with p90Rsk2 led to a further increase in mitotic cells (24%). This effect is similar to that obtained with a stabilized Emi1 mutant (Reimann et al, 2001a) and suggests that the two proteins cooperate to delay or arrest Hek293 cells in mitosis. Overexpression of p90Rsk2 alone also caused a small increase in mitotic index (9 versus 5% in control cells), which could be due to an effect of the kinase on endogenous Emi1.
Figure 5.
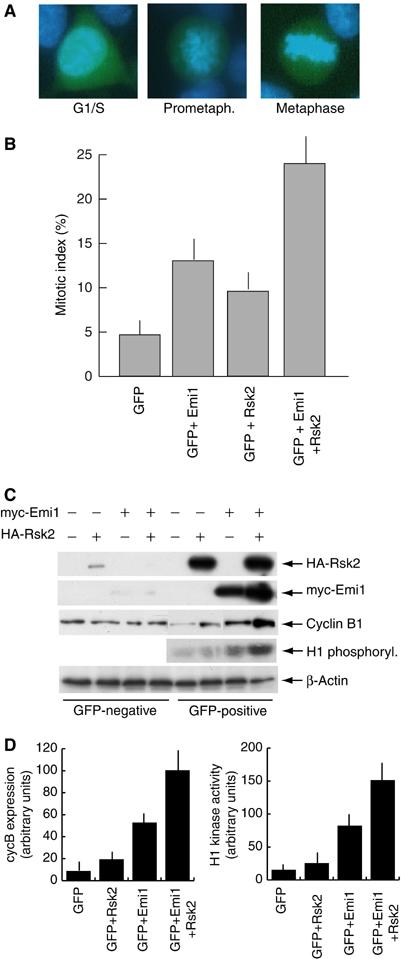
Emi1 and p90Rsk2 cooperate to induce cytostatic arrest in mitotic cells. Cells were transfected with either empty vector (−) or the indicated expression vector and with a GFP construct (1:10) to be able to identify transfected cells. At 24 h after transfection, cells were fixed, stained for DNA using Hoechst dye (1 μg/ml) and analysed under a fluorescence microscope to determine the percentage of mitotic cells (prometaphase and metaphase in (A) in the GFP-positive population. (B) Summary of the mitotic indexes obtained in three different experiments. In each experiment, 300 GFP-positive cells were counted in five separate fields. Data are shown as average±standard deviation. (C) Cells transfected as described above were sorted by FACS to separate the GFP-positive population from the GFP-negative population. Cell extracts (10 μg) obtained from these populations were analysed by Western blot for HA-p90Rsk2, myc-Emi1, cyclin B1 and β-actin. The first and second panels demonstrate that recombinant proteins were almost exclusively expressed in the GFP-positive population, assessing the quality of the sorting procedure. MPF activity was measured in GFP-positive cell extracts (10 μg) using H1 (1 μg) as substrate. (D) The results from three different experiments for cyclin B expression and H1 kinase activity were quantitated by optical densitometry and the data are shown as average±standard deviation.
To confirm that overexpression of Emi1 and p90Rsk2 caused a mitotic arrest in Hek293 cells, we analysed cyclin B1 expression levels and H1 kinase activity. GFP-positive and GFP-negative cells were sorted by FACS, and protein expression was tested in the two populations. Overexpression of Emi1 stabilized cyclin B1 and p90Rsk2 further increased the levels of cyclin B1 (Figure 5C and D). Stabilization of cyclin B1 was already appreciated when p90Rsk2 was transfected alone, further suggesting an effect of p90Rsk2 on endogenous Emi1. Moreover, cyclin B1 increase in cells cotransfected with Emi1 and p90Rsk2 was accompanied by MPF activation (Figure 5C, fourth panel, and Figure 5D). These results support the hypothesis of cooperation between these proteins to induce the metaphase arrest.
Emi1 and p90Rsk2 cooperate to induce metaphase arrest of early blastomeres
CSF activity has been defined by the ability of purified proteins and/or extracts to induce cell cycle arrest when microinjected into dividing blastomeres. To examine the cooperation between p90Rsk2 and Emi1 by this classical bioassay, we microinjected these purified proteins into one blastomere of mouse two-cell embryos (Masui, 2001). We found that microinjection of GST-Emi1236–383 did not exert a cytostatic effect in mouse embryos, which developed to the four-cell stage as control (not shown) or GST-injected embryos (Figure 6B). On the other hand, microinjection of an active form of p90Rsk2 was able to induce the typical three-cell stage arrest in 42% of microinjected embryos (Figure 6A and B). Remarkably, when p90Rsk2 was coinjected with GST-Emi1236–383, the cytostatic effect was strongly increased (66% arrest at the three-cell stage), indicating cooperation between the two proteins in vivo. By contrast, GST-Emi1236–383ST/AA did not reinforce the kinase action, indicating that phosphorylation on the conserved ser/thr residues of Emi1 is important for the cooperative effect (Figure 6B).
Figure 6.
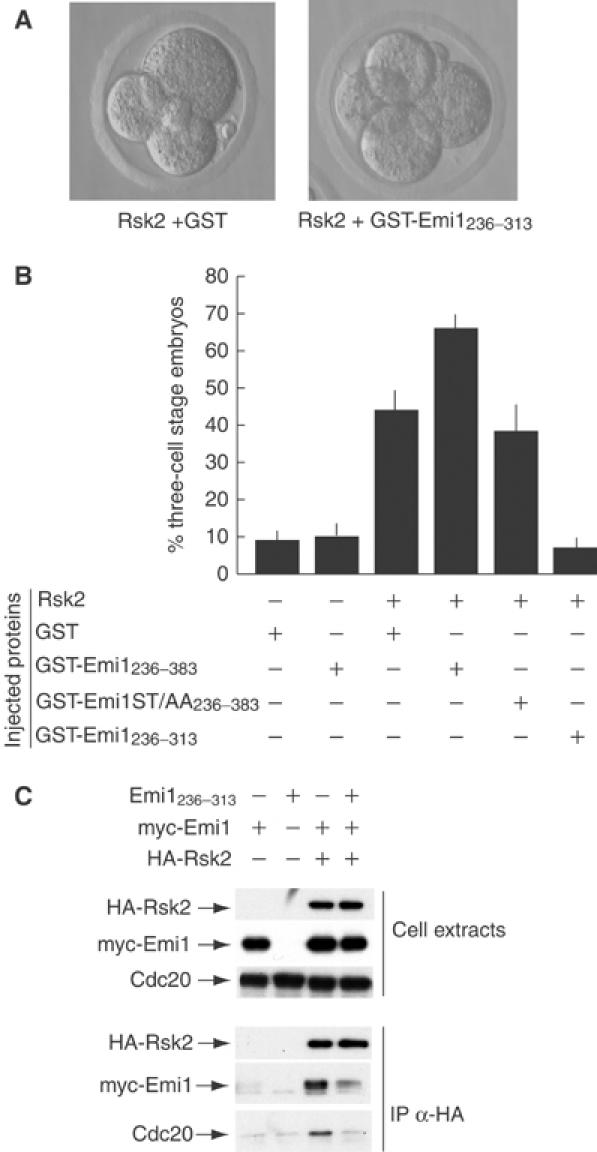
Emi1 potentiates the cytostatic activity of p90Rsk2 in mouse blastomeres. Purified GST fusion proteins (5–8 pl of 1 mg/ml solution of GST, GST-Emi1236–383 or GST-Emi1236–313) were injected either alone or with purified active p90Rsk2 in one blastomere of two-cell mouse embryos (45–50 h after hCG injection). At 12–15 h after microinjection, embryos were scored for cell cycle progression to the four-cell stage (no cytostatic arrest) or to the three-cell stage (cytostatic arrest). Representative examples of arrested and not arrested embryos are shown in (A). (B) Summary of the results obtained. Each bar corresponds to four separate experiments in which we analysed a total of at least 35 embryos. Data represent average±standard deviation from these experiments. (C) Western blot analyses of proteins in cell extracts (first three rows) or co-immunoprecipitating with HA-Rsk2 (three bottom rows). Hek293 cells were transfected with empty vectors or myc-Emi1, Emi1236–313 and HA-p90Rsk2 either alone or in combination. A 20 μg portion of total extracts was loaded in each lane (first three rows) and 500 μg of total proteins was immunoprecipitated for each sample (last three rows).
Since we found that GST-Emi1236–313 is phosphorylated by p90Rsk2 and is sufficient for interaction with the kinase but does not interact with Cdc20, we reasoned that this portion could interfere with binding of endogenous Emi1 in vivo and could compete as substrate for p90Rsk2. Indeed, in transfected cells, coexpression of Emi1236–313 not only competed with the interaction between full-length Emi1 and p90Rsk2 but also prevented association of Cdc20 with the kinase (Figure 6C), suggesting that it blocks the formation of a stable complex between Emi1/Cdc20 and p90Rsk2. Moreover, we found that coinjection of GST-Emi1236–313 in two-cell embryos inhibited the cytostatic effect exerted by p90Rsk2, with only 8% of injected embryos blocked at the three-cell stage (Figure 6A and B).
Interaction between p90Rsk2 and Emi1 is required during oocyte maturation
Since GST-Emi1236–313 acts as an inhibitor of the functional interaction between p90Rsk2 and Emi1 in vivo, we set out to determine the role of this interaction during oocyte maturation, when cytostatic activity is physiologically established. Oocytes at the germinal vesicle stage were allowed to undergo germinal vesicle breakdown (GVBD) in culture. After GVBD, oocytes were microinjected with either GST as control or GST-Emi1236–313 and incubated for additional 12–14 h to complete maturation. Control or GST-injected oocytes reached the metaphase II arrest typical of ovulated oocytes; the chromosomes were aligned at the equator of the meiotic spindle, which was located at the periphery of the cell (Figure 7A, Ba, b and C). On the contrary, microinjection of GST-Emi1236–313 caused abnormal maturation, with 30% of the oocytes extruding a second polar body (Figure 7A and C). Interestingly, in some oocytes, the polar body was much larger than normal (Figure 7A), indicating a defect in asymmetric division similar to that observed in mos−/− oocytes (Choi et al, 1996; Verlhac et al, 2000b). Immunofluorescence analysis of GST-Emi1236–313-injected oocytes revealed either abnormal spindles with misaligned chromosomes (Figure 7Bc and d) or the absence of a meiotic spindle and completion of anaphase (Figure 7Be). On the other hand, the GST-Emi1236–313ST/AA mutant did not cause alterations of meiotic progression (Figure 7Bf and C), suggesting that it was unable to interfere with Emi1 phosphorylation in vivo. These results highlight the lack of a normal metaphase II arrest in oocytes injected with wild-type GST-Emi1236–313 and indicate that the functional interaction between Emi1 and p90Rsk2 is required for normal meiotic progression.
Figure 7.
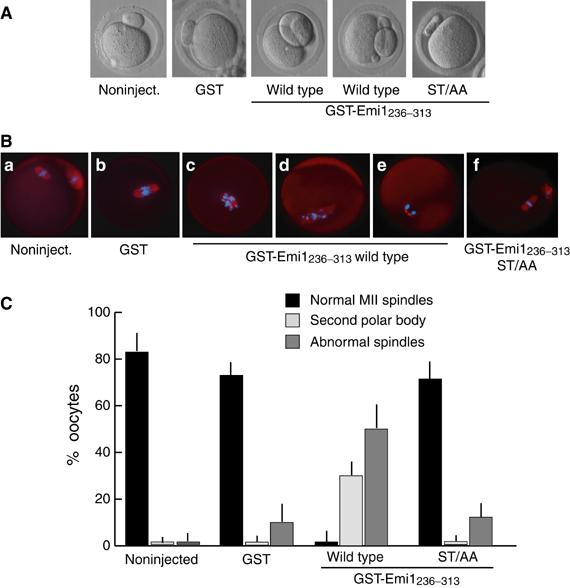
GST-Emi1236–313 interferes with oocyte maturation and cytostatic arrest. Mouse oocytes were collected from PMSG-primed mice and let undergo GVBD in culture. Immediately after GVBD, oocytes were microinjected with 5–8 pl of GST (1 mg/ml) or either wild-type or ST/AA mutant GST-Emi1236–313 (1 mg/ml) and cultured for additional 14–16 h. (A) Representative phase contrast images of control or microinjected oocytes, showing that GST-Emi1236–313 induces either abnormal maturation or extrusion of the second polar body. (B) Immunofluorescence analysis of the meiotic spindle in microinjected oocytes. Oocytes were fixed and stained with anti-tubulin antibody and Hoechst dye for DNA. Control or GST-injected oocytes displayed a metaphase plate with a normal spindle. GST-Emi1236–313-injected oocytes displayed either abnormal spindle (c, d) or decondensed chromatin in anaphase position (e), whereas GST-Emi1ST/AA236–313-injected oocytes displayed a normal maturation with an MII spindle (f). (C) Summary of the results obtained is shown. Abnormal spindles are considered oocytes as in (Bc) or (Bd). Each bar corresponds to average±standard deviation from four separate experiments in which we analysed a total of at least 35 oocytes.
RNAi of Emi1 interferes with mouse oocyte maturation
To test whether the morphological defects observed in maturing oocytes injected with the GST-Emi1236–313 were due to alterations of Emi1 function, we set out to interfere with RNA expression in oocytes by RNAi. It was shown that microinjection of 300–500 bp double-stranded RNA (dsRNA) sequences in maturing mouse oocytes is capable of specifically depleting endogenous mRNAs (Svoboda et al, 2000). Thus, we in vitro synthesized dsRNAs comprising the 3′ region of mouse Emi1 mRNA, or of GFP mRNA as control, and microinjected them into GV oocytes. Oocytes were allowed to mature and Emi1 mRNA levels were measured in GFPdsRNA- or Emi1dsRNA-injected oocytes. As shown in Figure 8A, Emi1 mRNA was readily detected in GFPdsRNA-injected oocytes, whereas it was absent in Emi1dsRNA-injected oocytes. Interestingly, depletion of Emi1 caused similar morphological defects as those observed by injection of GST-Emi1236–313. We observed that 30% of Emi1dsRNA-injected oocytes extruded a second polar body (Figure 8C and Bb) or a much larger polar body indicating the lack of asymmetric division as reported for mos−/− oocytes (Figure 8Bc and d), whereas 60% of the oocytes displayed abnormal spindles with scattered chromosomes or decondensed chromatin (Figure 8Bc, d and C). By contrast, GFPdsRNA-injected oocytes underwent normal maturation and arrested with a metaphase II spindle (Figure 8Ba and C). These results indicate that oocyte maturation was severely impaired by selectively interfering with Emi1 expression in mouse oocytes.
Figure 8.
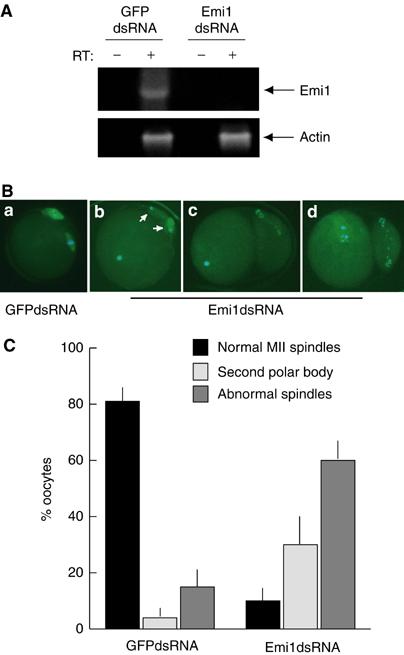
Emi1 RNAi interferes with oocyte maturation and cytostatic arrest. Mouse oocytes were collected from PMSG-primed mice at the GV stage and microinjected with 5–8 pl of either GFPdsRNA (0.5 mg/ml) or Emi1dsRNA (0.5 mg/ml) and cultured for additional 16–20 h to allow maturation. At the end of the incubation, oocytes were scored for the second polar body emission and either frozen in Trizol for RT–PCR analysis or fixed in 4% paraformaldehyde for immunofluorescence analysis. (A) RT–PCR analysis of Emi1 and actin RNA expression in oocytes microinjected with either GFPdsRNA or Emi1dsRNA. A pool of 15 oocytes was used for each sample. Oligonucleotides amplifying a 543 bp sequence in the 5′ region of mouse Emi1 (see Materials and methods, oligos for Emi11–181) were used. (B) Immunofluorescence analysis of the meiotic spindle in microinjected oocytes. Oocytes were fixed and stained with a fluorescein-conjugated anti-tubulin antibody (green) and Hoechst dye for DNA (blue). GFPdsRNA-injected oocytes displayed a metaphase plate with a normal spindle (a). Emi1dsRNA-injected oocytes displayed either decondensed chromatin (b, c) and two polar bodies (white arrows in (b)) or scattered chromosomes and abnormal spindle (d) and they often extruded a larger polar body (c, d). (C) A summary of the results obtained is shown; the score was performed using the criteria described in Figure 7. Each bar corresponds to average±standard deviation from four separate experiments in which we analysed a total of at least 40 oocytes.
Discussion
The CSF activity present in vertebrate oocytes requires activation of the Mos/MAPK/p90Rsk2 pathway during oocyte maturation and APCCdc20 inhibitory regulators, such as Emi1 and Mad1, to be maintained once it is established (Reimann and Jackson, 2002; Tunquist et al, 2003). However, the connection between the factors required for the establishment and the maintenance of CSF is still unclear. Herein, we report that p90Rsk2, the effector of the Mos pathway, directly interacts with and phosphorylates Emi1, an essential inhibitor of APC at metaphase (Reimann and Jackson, 2002). Moreover, we show that functional interaction between p90Rsk2 and Emi1 potentiates the ability of Emi1 to bind to Cdc20 and to induce cytostatic arrest both in mouse blastomeres and in transfected cells. Thus, our results establish a first direct connection between proteins involved in the establishment and in the maintenance of cytostatic activity in vertebrate eggs.
In vitro binding experiments, supported also by co-immunoprecipitation experiments, indicate that phosphorylation of the C-terminal region of Emi1 by p90Rsk2 potentiates four-fold its ability to bind Cdc20. Notably, Reimann and Jackson (2002) showed that cytostatic arrest was released when recombinant Cdc20 was added to Xenopus egg extracts at 3 μM concentration, but not at 1 μM concentration, and that it was prevented by addition of equimolar amounts of Emi1. Their results indicate that CSF arrest is maintained by a strict balance between Emi1 and Cdc20 concentrations, and that changing this balance by as little as three-fold triggers metaphase-to-anaphase transition. Thus, we propose that one of the functions of the Mos pathway during oocyte maturation is to reinforce the cytostatic activity of Emi1 through phosphorylation by p90Rsk2 (Figure 9). Our conclusion is supported by the observations that coinjection of Emi1 with p90Rsk2 in mouse blastomeres exerts a stronger cytostatic effect than either protein alone. In addition, substitution of the evolutionarily conserved ser246/thr251 residues, which are phosphorylated by p90Rsk2, suppresses the cooperation between the two proteins in vivo and the ability to stabilize the Cdc20/Emi1 interaction in vitro. Our results also show that injection of GST-Emi1236–313, which interacts with p90Rsk2 and is efficiently phosphorylated by the kinase but is unable to bind Cdc20 efficiently, blocks the cytostatic activity of constitutively active p90Rsk2 in two-cell embryos and it interferes with meiosis II progression and cytostatic arrest in maturing mouse oocytes. Since similar defects were observed when Emi1 expression was impaired by RNAi, it seems that the effects are due to alterations of Emi1 function. These results suggest that GST-Emi1236–313 competes for the interaction of p90Rsk2 with endogenous Emi1 and that this interaction is necessary for the establishment of cytostatic arrest.
Figure 9.
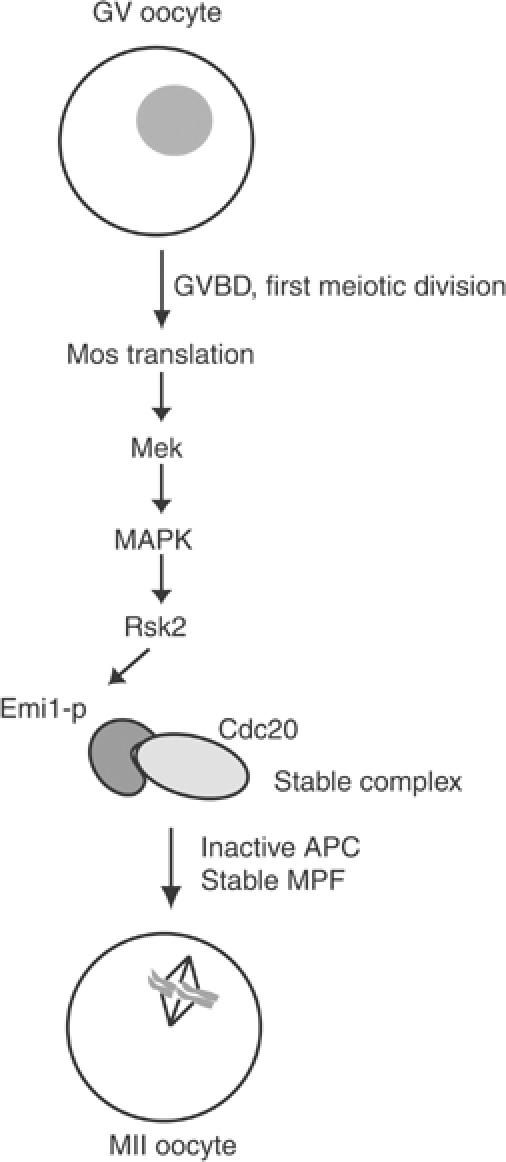
Hypothetical model of CSF establishment in mouse oocytes. Schematic representation of the model hypothesized on the basis of data in the literature and the new findings of this work. See description in the text for a detailed explanation.
The morphological examination of oocytes matured in the presence of GST-Emi1236–313, or where Emi1 expression was decreased by RNAi, revealed that they closely resemble mos−/− oocytes (Choi et al, 1996; Verlhac et al, 2000a; 2000b), with some oocytes extruding a second polar body and others with abnormal spindles and misaligned chromosomes or abnormal cytokinesis. Beside stabilization of MPF activity through inhibition of the APCCdc20, the Mos/MAPK/p90Rsk pathway is also important for stabilization of the spindle through post-translational modifications of microtubule-associating proteins like MISS and DOC1R (Verlhac et al, 1996; Lefebvre et al, 2002; Terret et al, 2003). The spindle abnormalities in oocytes injected with GST-Emi1236–313 or Emi1dsRNA suggest that the functional interaction between Emi1 and p90Rsk2 contributes also to this aspect of meiosis II. Interestingly, it has been shown that MISS protein is stable only in metaphase II-arrested oocytes, once the cytostatic activity has been established. Since the spindle defects observed after in vivo depletion of MISS (Lefebvre et al, 2002) closely resemble those obtained by interference with Emi1, it would be interesting to determine whether MISS is a substrate for the APCCdc20 and whether Emi1 prevents its degradation in meiosis.
The only proteins shown to be required for maintenance of the cytostatic arrest in eggs are the APCCdc20 inhibitors Emi1 and Mad1 (Reimann and Jackson, 2002; Tunquist et al, 2003). In both cases, the crucial experiments employed an immunodepletion approach from Xenopus egg extracts, which are typically arrested at metaphase with high MPF and MAPK activity. Depletion of either Mad1 or Emi1 led to activation of the APC, cyclin B degradation and mitotic exit. Since addition of either recombinant Mad1 or Emi1 was sufficient to block these events, in both works it was concluded that these proteins were necessary and sufficient to maintain the CSF arrest of Xenopus eggs. One possibility to reconcile this apparent paradox is that Mad1 and Emi1 associate in a complex and depletion of either one causes mitotic exit because of depletion of the inhibitory complex. However, it was not checked whether or not Emi1 co-immunoprecipitates with Mad1 in the immunodepletion experiments (Reimann and Jackson, 2002; Tunquist et al, 2003). Because of the limiting volume of mouse oocytes, such approaches cannot be applied to our system. Nevertheless, our in vivo competition and/or depletion approaches suggest that Emi1 participates in cytostatic arrest also in mouse oocytes.
Our observations suggest that the metaphase-to-anaphase transition in vertebrate eggs is controlled by a relay of phosphorylation events that fine-tune the reciprocal affinity of regulatory components of the APC. In our model, p90Rsk2 is activated by the Mos pathway during oocyte maturation and phosphorylates Emi1, increasing its affinity for Cdc20 and preventing activation of the APC (Figure 9). After metaphase is reached, the Mos pathway becomes dispensable (Tunquist and Maller, 2003), possibly because phosphorylated Emi1 is stable in the oocyte environment. This might be due to the absence of a counteracting phosphatase or to inaccessibility of Emi1 when complexed to Cdc20. In agreement with this second hypothesis, it was noted that Emi1 has a stronger affinity for the APC activator Cdh1 than for Cdc20, and it is rapidly degraded when Cdh1 disappears (Reimann et al, 2001b; Hsu et al, 2002). Since only Cdc20 is present in oocytes (Lorca et al, 1998), it is possible that phosphorylation by p90Rsk2 prolongs Emi1 life in meiosis through the stabilization of the normally loose interaction with Cdc20. At fertilization, activation of CaMKII triggers activation of APCCdc20 and degradation of cyclin B (Lorca et al, 1993). It remains to be established whether CaMKII acts directly through phosphorylation of Emi1 or Cdc20 and whether phosphorylation causes dissociation of the complex. In this regard, our preliminary data suggest that at least Emi1 is not a direct in vitro substrate for CamKII (MP Paronetto and C Sette, unpublished observation).
In conclusion, our results indicate that p90Rsk2 functionally interacts with Emi1 in the establishment of CSF activity in mouse eggs. Our model offers a reconciling view on the connection between two components of CSF that were previously considered independent, leaving puzzling doubts on the relation between the establishment and the maintenance of CSF arrest (Duesbery and Vande Woude, 2002). Moreover, since activation of the MAPK pathway is required also in the response to the spindle- and DNA-damage checkpoints in mitotic cells (Chung and Chen, 2003; Panta et al, 2004), our results may provide a direct link between this pathway and cell cycle arrest through the inhibition of the APC.
Materials and methods
Plasmid construction
Mouse Emi1 was amplified by PCR using a 13 dpc embryo cDNA library and oligonucleotides 5′-AGGAATTCATGAAGTGTTTTAATTGCAACCCT G-3′ (forward) and 5′-GGTCGACTCACAATCTTTGTAAGTTCTTTTTA C-3′ (reverse) and subcloned into the EcoRI and SalI sites of either pCDNA3-myc or pGEX4T1 expression vectors for myc- or GST-tagged Emi1, respectively. Oligonucleotides were derived from the mouse Emi1 homologue deposited in the NCBI database (Fbxo5: NM_025995). Additional oligonucleotides used were as follows: 5-GGTCGACTCATAGGTGCTCCAGGCCCAT-3′ (reverse) for GST-Emi11–181, 5′-AGGAATTCATGCAGCGAGTCATTGAAAGC-3′ (forward) for Emi1236–383, 5′-GGTCGACTCAGGCTTTGAGGCTTTCGTTG-3′ (reverse) for Emi1236–313. Point mutations in Emi1 were introduced by using mutated oligonucleotides and PCR amplification. Pfu polymerase (Stratagene) was used for all amplifications and constructs sequences were verified by direct sequencing. The vector pMT2-HAp90Rsk2 was a generous gift of Dr Mortin Frodin.
Expression and purification of GST fusion proteins Plasmids (pGEX-) containing GST fusion proteins were transformed into the Escherichia coli BL21 strain, and grown at 30°C in LB medium to an OD600=0.6 before induction with 0.5 mM isopropyl-β-thiogalactopyranoside (IPTG, Sigma-Aldrich) for 3 h. GST fusion proteins were purified from bacterial lysates on glutathione-agarose (Sigma-Aldrich) as previously described (Sette et al, 1998) and analysed by SDS–PAGE and Coomassie blue staining to test purity and integrity.
Cell culture and transfections Hek293 cells were maintained in Dulbecco's medium supplemented with 10% fetal bovine serum (FBS) (Gibco BRL) in 90 mm dishes. Subconfluent monolayers were processed for CaPO4 transfection with 1–10 μg of the appropriate plasmids or by Fugene (Stratagene) with 0.2–2 μg of the appropriate plasmids as previously described (Sette et al, 2002). At 24–48 h after transfection, cells were harvested in lysis buffer (50 mM Hepes, pH 7.5, 75 mM NaCl, 10 mM β-glycerophosphate, 2 mM EGTA, 15 mM MgCl2, 0.1 mM sodium orthovanadate, 1 mM DTT, 0.5% Triton X-100, protease inhibitor cocktail (Sigma-Aldrich)) and incubated for 10 min on ice. Lysates were centrifuged for 10 min at 10 000 g at 4°C and used for further analysis. Protein concentration was determined using a protein assay kit (Bio-Rad) following the manufacturer's instructions.
FACS sorting Transfected cells were separated based on size (forward scatter) and green fluorescence (GFP-positive) using a FACSVantage cell sorter (Beckton and Dickinson). Purity of GFP-positive and -negative populations was >98%. Sorted cells were used for Western blot analysis as described below.
Pull-down assays Cell extracts (500 μg of total proteins) were added to 2 μg of GST fusion protein adsorbed on glutathione-agarose (Sigma-Aldrich) in 250 μl (final volume) of lysis buffer supplemented with 0.05% bovine serum albumin (BSA). After incubation for 90 min at 4°C under constant shaking, beads were washed three times with lysis buffer without Triton X-100, and absorbed proteins were eluted in SDS sample buffer (62.5 mM Tris–HCl, pH 6.8, 10% glycerol, 2% (wt/vol) SDS, 0.7 M 2-mercaptoethanol and 0.0025% (wt/vol) bromophenol blue) and resolved on a 10% SDS–PAGE for subsequent Western blot analysis.
Immunoprecipitation assay Cell extracts (500 μg of total proteins) were incubated with 1 μg of anti-myc antibody for 2 h at 4°C under constant shaking. Protein A–Sepharose or protein G–Sepharose (Sigma-Aldrich) was preadsorbed with 0.05% BSA before incubation with the immunocomplexes for an additional hour. Hence, beads were washed three times with lysis buffer and absorbed proteins were eluted in SDS sample buffer for Western blot analysis.
Kinase assays For p90Rsk2 assays, 1 μg of each GST-Emi1 fusion protein was incubated at 30°C for 20 min with the purified active form of the kinase (5 U, Upstate Biotechnology) in reaction buffer: 50 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM EGTA, 1 mM DTT, 10 mM β-glycerophosphate, 0.5 mM NaVO4, 50 μM ATP and 5 μCi of 32P-γ-ATP. In some experiments, GST fusion proteins were phosphorylated while still bound to the GSH-agarose beads and at the end of the incubation the kinases were washed by rinsing three times with an excess of kinase buffer (without label) before using the proteins for pull-down assays. H1 kinase assays were performed on cell extracts of GFP-positive Hek293 cells as previously described (Bhatt and Ferrell, 1999).
Western blot analysis Cell extracts or immunoprecipitated proteins were diluted in SDS sample buffer as described above and boiled for 5 min. For oocyte extracts, 300 metaphase II oocytes/sample were collected and immediately frozen in sample buffer. After thawing, oocytes were sonicated and boiled before loading. Proteins were separated on 10% SDS–PAGE gels and transferred to polyvinylidene fluoride Immobilon-P membranes (Millipore) using a semidry blotting apparatus (Bio-Rad). The membranes were saturated with 5% nonfat dry milk in PBS containing 0.1% Tween 20 for 1 h at room temperature, and incubated with the following primary antibodies (1:1000 dilution) overnight at 4°C: mouse anti-HA (for HA-p90Rsk2, from BabCO Berkeley antibody company); rabbit anti-actin (Sigma-Aldrich); mouse anti-Myc (for myc-Emi1); rabbit anti-Emi1 (Gentaur); goat anti-p90Rsk2, rabbit anti-Cdc20, rabbit anti-cyclin A2, mouse anti-cyclin-B1. Primary antibodies were all from SantaCruz Biotechnology, unless specified otherwise. Secondary anti-mouse or anti-rabbit IgGs conjugated to horseradish peroxidase (Amersham) were incubated with the membranes for 1 h at room temperature at a 1:10 000 dilution in PBS containing 0.1% Tween 20. Immunostained bands were detected by chemiluminescent method (SantaCruz Biotechnology).
Immunofluorescence analysis Oocytes were processed for immunofluorescence analysis using anti-tubulin antibody (1:100, Sigma-Aldrich) or anti-Emi1 antibody (1:200, Gentaur) or anti-p90Rsk2 antibody (1:200, SantaCruz Biotechnology) as previously documented (Sette et al, 2002).
Oocyte collection, microinjection and in vitro culture
Two-cell embryos and GV oocytes were collected from hormonally primed 6- to 7-week-old CD1 female mice (Charles River Italia) and cultured in M16 medium under mineral oil as previously described (Hogan et al, 1994). Oocytes were allowed to undergo GVBD by incubation in the absence of an exogenous cAMP source and used for microinjection either immediately (for dsRNAi) or after GVBD (approximately 2 h after collection). Before injection, oocytes and embryos were washed in M2 medium and then transferred to 50 μl drops of the same medium under mineral oil. Microinjection manipulations were performed as previously described (Sette et al, 1998). Briefly, into the cytoplasm of one blastomere of a two-cell embryo we injected 2–5 pl of a purified p90Rsk2 (1–5 U, Upstate Biotechnology) together with either GST or GST-Emi1 diluted to a protein concentration of 1 mg/ml in injection buffer (20 mM Hepes, pH 7.4, 120 mM KCl, 100 μM EGTA, 10 mM β-glycerophosphate, 1 mM DTT, 10 μg/ml leupeptin, 10 μg/ml pepstatin). Microinjections were performed using an Olympus invertoscope (Olympus) equipped with Hoffman modulation contrast optics (Modulation optics Inc., Greenvale, NY) and two Leitz mechanical micromanipulators (Leica AG, Heerbrugg, Switzerland). After microinjections, embryos or oocytes were returned to M16 medium drops and cultured at 37°C under a humidified atmosphere of 5% CO2 in air. At 12–14 h after injection, cells were scored for mitotic or meiotic divisions or processed for immunofluorescence analysis.
dsRNA preparation
To generate templates for dsRNA synthesis, we employed forward and reverse oligonucleotides containing at the 5′ end a T7 promoter sequence. For Emi1 amplification, the following primers were designed: forward 5′-GTAATACGACTCACTACTATAGGGCATGCAGC GAGTCA-3′; reverse 5′-GTAATACGACTCACTACTATAGGGCTCACAAT CTTTGT. These oligonucleotides amplify a region of 447 bp from base 945 to 1392 at the 3′ end of mouse Emi1 (AK011820). For GFP amplification, the following primers were designed: forward 5′-GTAATACGACTCACTACTATAGGGCATGCATA AAGGAG-3′; reverse 5′-GTAATACGACTCACTACTATAGGGCTCAATGC ATTAGTTC-3′. These oligonucleotides amplify a region of 600 bp of the GFP sequence. pCDNA3-mycEmi1 and pCMV5-GFP expression vectors were used as templates for PCR amplification. Amplified bands were gel-purified and used as templates (1 μg) for in vitro RNA transcription in order to obtain sense and antisense RNA sequences as previously described (Svoboda et al, 2000). RNA was extracted and precipitated by standard procedures and dissolved in RNAse-free H2O. Equimolar amounts of sense and antisense RNA were annealed in DEPC-water supplemented with 1 U/μl of RNasin (Invitrogen) and 4 μg of RNA was boiled for 1 min and allowed to cool down at room temperature before phenol/chloroform extraction and ethanol precipitation. dsRNA was resuspended in H2O, assayed by agarose electrophoresis and stored at −80°C prior to use.
Acknowledgments
We thank Drs Francesco Cenci and Federica Capolunghi for their help with FACS analysis, Drs Manuela Pellegrini and Susanna Dolci for critically reading the manuscript and Dr Mortin Frodin for the gift of the pMT2-HA-p90Rsk2 vector. This work was supported by MIUR Cofin 2002 and 2003.
References
- Bhatt RR, Ferrell JE Jr (1999) The protein kinase p90 rsk as an essential mediator of cytostatic factor activity. Science 286: 1362–1365 [DOI] [PubMed] [Google Scholar]
- Bhatt RR, Ferrell JE Jr (2000) Cloning and characterization of Xenopus Rsk2, the predominant p90 Rsk isozyme in oocytes and eggs. J Biol Chem 275: 32983–32990 [DOI] [PubMed] [Google Scholar]
- Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF (1996) The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci USA 93: 7032–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Chen RH (2003) Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat Cell Biol 5: 748–753 [DOI] [PubMed] [Google Scholar]
- Colledge WH, Carlton MB, Udy GB, Evans MJ (1994) Disruption of c-mos causes parthenogenetic development of unfertilised mouse eggs. Nature 370: 65–68 [DOI] [PubMed] [Google Scholar]
- Duesbery NS, Vande Woude GF (2002) Developmental biology: an arresting activity. Nature 416: 804–805 [DOI] [PubMed] [Google Scholar]
- Dupré A, Jessus C, Ozon R, Haccard O (2002) Mos is not required for the initiation of meiotic maturation in Xenopus oocytes. EMBO J 21: 4026–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Lewellyn AL, Maller JL (1999) Induction of metaphase arrest in cleaving Xenopus embryos by the protein kinase p90Rsk. Science 286: 1365–1367 [DOI] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL (2000) The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk). Curr Biol 10: 430–438 [DOI] [PubMed] [Google Scholar]
- Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL (1993) Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science 262: 1262–1265 [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, Aizaway S (1994) Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature 370: 68–71 [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E (1994) Manipulating the Mouse Embryo. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Hsu JY, Reimann JD, Sorensen CS, Lukas J, Jackson PK (2002) E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat Cell Biol 4: 358–366 [DOI] [PubMed] [Google Scholar]
- Kalab P, Kubiak JZ, Verlhac MH, Colledge WH, Maro B (1996) Activation of p90rsk during meiotic maturation and first mitosis in mouse oocytes and eggs: MAP kinase-independent and -dependent activation. Development 122: 1957–1964 [DOI] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E (1994) Mitogen-activated protein kinase kinase is required for the mos-induced metaphase arrest. J Biol Chem 269: 28354–28358 [PubMed] [Google Scholar]
- Lefebvre C, Terret ME, Djiane A, Rassinier P, Maro B, Verlhac MH (2002) Meiotic spindle stability depends on MAPK-interacting and spindle-stabilizing protein (MISS), a new MAPK substrate. J Cell Biol 157: 603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T, Castro A, Martinez AM, Vigneron S, Morin N, Sigrist S, Lehner C, Doree M, Labbe JC (1998) Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J 17: 3565–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T, Cruzalegui FH, Fesquet D, Cavadore JC, Mery J, Means A, Doree M (1993) Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature 366: 270–273 [DOI] [PubMed] [Google Scholar]
- Margottin-Goguet F, Hsu JY, Koktev A, Hsieh HM, Reimann JDR, Jackson PK (2003) Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev Cell 4: 813–826 [DOI] [PubMed] [Google Scholar]
- Markoulaki S, Matson S, Abbott AL, Ducibella T (2003) Oscillatory CaMKII activity in mouse egg activation. Dev Biol 258: 464–474 [DOI] [PubMed] [Google Scholar]
- Masui Y (2001) From oocyte maturation to the in vitro cell cycle: the history of discoveries of maturation-promoting factor (MPF) and cytostatic factor (CSF). Differentiation 69: 1–17 [DOI] [PubMed] [Google Scholar]
- Masui Y, Markert CL (1971) Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 177: 129–145 [DOI] [PubMed] [Google Scholar]
- Panta GR, Kaur S, Cavin LG, Cortes ML, Mercurio F, Lothstein L, Sweatman TW, Israel M, Arsura M (2004) ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol Cell Biol 24: 1823–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK (2001a) Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105: 645–655 [DOI] [PubMed] [Google Scholar]
- Reimann JD, Gardner BE, Margottin-Goguet F, Jackson PK (2001b) Emi1 regulates the anaphase-promoting complex by a different mechanism than Mad2 proteins. Genes Dev 15: 3278–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann JDR, Jackson PK (2002) Emi1 is required for cytostatic factor arrest in vertebrate eggs. Nature 416: 850–854 [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF (1988) Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature 335: 519–525 [DOI] [PubMed] [Google Scholar]
- Schwab MS, Roberts BT, Gross SD, Tunquist BJ, Taieb FE, Lewellin AL, Maller JL (2001) Bub1 is activated by the protein kinase p90(Rsk) during Xenopus oocyte maturation. Curr Biol 11: 141–150 [DOI] [PubMed] [Google Scholar]
- Sette C, Bevilacqua A, Geremia R, Rossi P (1998) Involvement of phospholipase Cgamma1 in mouse egg activation induced by a truncated form of the C-kit tyrosine kinase present in spermatozoa. J Cell Biol 142: 1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette C, Paronetto MP, Barchi M, Bevilacqua A, Geremia R, Rossi P (2002) Tr-kit-induced resumption of the cell cycle in mouse eggs requires activation of a Src-like kinase. EMBO J 21: 5386–5395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Hayashi H, Schultz RM (2000) Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development 127: 4147–4156 [DOI] [PubMed] [Google Scholar]
- Terret ME, Lefebvre C, Djiane A, Rassinier P, Moreau J, Maro B, Verlhac MH (2003) DOC1R: a MAP kinase substrate that control microtubule organization of metaphase II mouse oocytes. Development 130: 5169–5177 [DOI] [PubMed] [Google Scholar]
- Tunquist BJ, Eyers PA, Chen LG, Lewellyn AL, Maller JL (2003) Spindle checkpoint proteins Mad1 and Mad2 are required for cytostatic factor-mediated metaphase arrest. J Cell Biol 163: 1231–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunquist BJ, Maller JL (2003) Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev 17: 683–710 [DOI] [PubMed] [Google Scholar]
- Tunquist BJ, Schwab MS, Chen LG, Maller JL (2002) The spindle checkpoint kinase bub1 and cyclin e/cdk2 both contribute to the establishment of meiotic metaphase arrest by cytostatic factor. Curr Biol 12: 1027–1033 [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Weber M, Geraud G, Colledge WH, Evans MJ, Maro B (1996) Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development 122: 815–822 [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Lefebvre C, Guillaud P, Rassinier P, Maro B (2000b) Asymmetric division in mouse oocytes: with or without Mos. Curr Biol 10: 1303–1306 [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Lefebvre C, Kubiak JZ, Umbhauer M, Rassinier P, Colledge W, Maro B (2000a) Mos activates MAP kinase in mouse oocytes through two opposite pathways. EMBO J 19: 6065–6074 [DOI] [PMC free article] [PubMed] [Google Scholar]


