Abstract
Aims
Small, short studies suggest metformin influences the glucagon-like peptide (GLP)-1 axis in individuals with and without type 2 diabetes (T2DM). In the Carotid Atherosclerosis: Metformin for insulin ResistAnce (CAMERA) trial (NCT00723307) we investigated whether this effect is sustained and related to changes in glycaemia or weight. In the cross-sectional DIabetes REsearCh on patient straTification (DIRECT) study, we investigated basal and post-meal GLP-1 levels in diabetic patients.
Materials and Methods
CAMERA was a double-blinded randomized placebo-controlled trial of metformin in 173 participants without diabetes. Using six-monthly fasted total GLP-1 levels over 18 months, we evaluated metformin’s effect on total GLP-1 with repeated-measures and ANCOVA analyses. In DIRECT, we examined active and total fasting and 60-minute post-meal GLP-1 levels in 775 patients recently diagnosed with T2DM treated with metformin or diet, using Student’s T-tests and linear regression.
Results
In CAMERA, metformin increased total GLP-1 at 6 (+20.7%, [95% confidence intervals 4.7-39.0%]), 12 (+26.7% [10.3-45.6%]) and 18 months (+18.7% [3.8-35.7%]), an overall increase of 23.4% (11.2-36.9%; p<0.0001) versus placebo. Adjustment for changes in glycaemia and adiposity, individually or combined, did not attenuate this effect. In DIRECT, metformin was associated with higher fasting active (39.1% [21.3-56.4%]) and total GLP-1 (14.1% [1.2-25.9%]) but not post-meal incremental GLP-1. These changes were independent of potential confounders including age, sex, adiposity and HbA1c.
Conclusions
In non-diabetic individuals, metformin increases total GLP-1 in a sustained manner and independently of changes in weight or glycaemia. Metformin-treated diabetic patients also have higher fasted GLP-1 independent of weight and glycaemia.
Keywords: antidiabetic drug, GLP-1, metformin
Introduction
Metformin is recommended as first-line therapy for the majority of individuals with type 2 diabetes mellitus (T2DM)1. This is based on evidence of cardiovascular benefit and also its capacity to maintain or reduce weight. In the United Kingdom Prospective Diabetes Study, metformin monotherapy led to a 39% reduction in the risk of myocardial infarction compared to conventional dietary therapy over 10 years, a finding not explained by the drug’s effect on glycaemia2. Metformin has also been shown to reduce the risk of developing T2DM. In the Diabetes Prevention Program, metformin therapy reduced new-onset T2DM by 31% and also led to 2.1kg weight loss compared to placebo over 2.8 years3,4.
The glucagon-like peptide 1 (GLP-1) axis remains at the forefront of T2DM and cardiovascular research. Major outcomes trials of dipeptidyl peptidase-4 (DPP-4) inhibitors and the first completed outcome trial of a GLP-1 receptor agonist in T2DM patients indicated cardiovascular safety, though not benefit5–8. However, it was recently reported that the potent GLP-1 receptor agonist, liraglutide, has demonstrated cardiovascular benefit9. Furthermore, it has been reported that another GLP-1 receptor agonist, semaglutide, has also provided cardiovascular benefit in a major trial10. This is supported by recently published results from a Mendelian randomization study of a GLP-1 genetic variant (Ala316Thr; rs10305492) strongly associated with lower fasting glucose levels which demonstrated a lower risk of cardiovascular disease11, supporting the concept that GLP-1 may indeed be protective against cardiovascular disease. In addition, GLP-1 receptor agonists can yield modest weight loss12 and blood pressure reduction, important goals in the management of T2DM.
It is unclear whether some of metformin’s benefits may be mediated via GLP-1. To explore this, it is important to robustly establish the effect of metformin on GLP-1, and whether any effect is mediated by changes in related parameters such as weight or glycaemia. Various small studies of short duration have investigated the effect of metformin therapy on circulating GLP-1 levels in individuals with and without T2DM13–20. While results have been inconsistent, some have shown increases in active GLP-1 and total GLP-1 in both the fasting and post-prandial states. To date, however, no suitable studies have been conducted to robustly investigate whether metformin therapy influences circulating GLP-1 levels in individuals with and without T2DM, whether any observed effect is sustained in the longer term (i.e. beyond a few weeks), and whether any effect is related to changes in other variables which metformin is known to impact on, such as weight and glycaemia. To address these questions, we performed complementary studies namely an ancillary study using data from a randomized placebo-controlled repeated measures study with 18 months follow-up, the Carotid Atherosclerosis: Metformin for insulin ResistAnce (CAMERA)21 and a cross-sectional study from the DIabetes Research on Patient StraTification (DIRECT) consortium22.
Materials and Methods
CAMERA was a randomized double-blinded placebo-controlled trial designed to investigate the effect of metformin on surrogate markers of cardiovascular disease in patients without diabetes, aged 35 to 75, with established coronary heart disease and a large waist circumference (≥ 94cm in men, ≥80 cm in women) (NCT00723307). This single-centre trial enrolled 173 adults who were followed up for 18 months each. Patients attended the research centre every 6 months in a fasted state. A detailed description of the trial and its results has been published previously21. Participants were randomized 1:1 to 850mg metformin or matched placebo twice daily with meals though they could reduce the dose to once daily based on side-effects for the duration of the trial. Weight was measured in light clothing using a bio-impedance scale. While bio-impedance body fat results were available from the trial, we opted to measure circulating leptin levels as a better marker of body fat.
The DIRECT (DIabetes Research on Patient StraTification) study (www.direct-diabetes.org) is part of a European Union Innovative Medicines Initiative project, with the overarching aim to discover and validate biomarkers of rapid diabetes development, progression and drug response22. It involves four industrial partners and 21 academic institutes within Europe. As part of Work Package 2 that aimed to identify predictive biomarkers of glycaemic deterioration, deep phenotyping and biochemical assays were performed in 836 people recently diagnosed with T2D who had been on either metformin or life-style therapy alone at baseline. The 18 months follow up data are being collected. For this study, complete cross-sectional data were analysed from the baseline visit in 775 participants from all six clinical centres.
Sample assays
In CAMERA, participants attended six monthly visits after overnight fasts and before taking their morning dose of metformin. Blood samples collected during the trial were centrifuged at 4 degrees Celsius soon after sampling, separated and stored at -80°C at the Western Infirmary’s Clinical Research Facility, Glasgow, for subsequent analyses. Six monthly fasting plasma glucose, fasting insulin and HbA1c were analysed as previously described21. We calculated the Homeostasis Model Assessment for Insulin Resistance (HOMA2-IR) index using the HOMA Calculator (v2.2.3, https://www.dtu.ox.ac.uk/homacalculator/). Using available stored EDTA plasma samples, six monthly total GLP-1 levels (Meso scale discovery, Maryland, USA) were measured with commercially available electrochemiluminescence assay (Meso scale discovery, Maryland, USA). Leptin levels were measured with a commercially available enzyme-linked immunosorbent assay (R&D systems Oxon, UK). For total GLP-1, the mean inter-assay and intra-assay coefficients of variation (CVs) were 2.6% and 17.3% respectively. For leptin, the mean inter-assay and intra-assay CVs were 10.1% and 6.3%. All time points for an individual participant were run on the same plate, blinded to treatment arm.
For the DIRECT study, blood samples were collected in the morning after a 10 hour overnight fast. Metformin was stopped for the 24 hours preceding the study visit and restarted immediately thereafter. For a Mixed Meal Test (MMT), participants drank 250mL Fortisip liquid drink (18.4g carbohydrate/100mL) over 2-5 minutes. Blood samples were taken immediately prior to the drink (time 0) and then every 30 minutes up to 120 minutes. Samples for GLP-1 measurement were collected using P800 (for active GLP-1) and EDTA tubes (for total GLP-1) (Becton Dickenson, UK) at 0 and 60 minutes. The same commercial kits were used to measure GLP-1 levels as in CAMERA. In DIRECT, the mean intra- and inter-assay CVs for active GLP-1 were 9% and 10%, respectively. For total GLP-1, these CVs were 6% and 9%, respectively.
Ethics and consent
All participants provided written informed consent for participation in both studies. For the CAMERA study, this included permission for biochemical assays that were not planned at the time of the trial. The CAMERA trial was approved by the Medicines and Healthcare Products Regulatory Agency and West Glasgow Research Ethics Committee. In DIRECT, each partner clinical centre obtained approval from their respective research ethics review boards.
Statistics
Normality was assessed for all variables and non-normally distributed data were transformed using the natural log value where relevant (specifically for active GLP-1, total GLP-1, leptin and HOMA2-IR).
In the CAMERA study, analyses were performed for the modified intention-to-treat population (i.e. participants with a baseline total GLP-1 and at least one subsequent total GLP-1 result). The effect of metformin on total GLP-1 was investigated using two different approaches. First, repeated-measures analysis was carried out, allowing a comparison of metformin-treated and placebo-treated participants over the entire trial (assuming a general covariance structure). Repeated-measures analyses were only performed after demonstrating that there was no significant treatment-by-visit interaction (i.e. that any observed effect was stable over the trial). Secondly, analyses of covariance (ANCOVA) were carried out to determine the effect of metformin versus placebo on total GLP-1 at 6, 12 and 18 months respectively. Additional on-treatment analyses were performed to assess whether any change in total GLP-1 due to metformin was related to simultaneous changes in weight, HOMA2-IR, HbA1c, leptin or all four variables combined by adding these as cofactors.
In DIRECT, fasting active and total GLP-1, and 60-minute post-meal total GLP-1 levels were compared between metformin and lifestyle groups using Student’s T-tests. Anthropometric measures (age, sex, waist to hip ratio [WHR], BMI), lifestyle factors (smoking and alcohol use), HbA1c, fasting glucose and centre were investigated regarding any influence of metformin on GLP-1 levels using linear regression models.
Due to the natural log transformation for GLP-1 measures, results are presented as the percentage differences in geometric means of GLP-1 measures on metformin vs. placebo or metformin vs. lifestyle to aid interpretation. The same approach was taken to present leptin results. Statistical analyses were carried out using the statistical packages SPSS (version 22, SPSS Inc., Chicago, Ill) and R (version 3.0.1). A two-sided p-value of 0.05 was used as the threshold for statistical significance.
Results
Baseline characteristics for the CAMERA and DIRECT participants are summarized in Supplementary Table 1 and Table 1, respectively. It has previously been reported that metformin led to falls in HbA1c (1.4mmol/mol), fasting insulin (21%), Homeostasis Model Assessment of Insulin Resistance (HOMA-IR; 26%) and weight (3.2kg) compared to placebo over 1.5 years in CAMERA.
Table 1.
Characteristics of DIRECT participants
| Characteristics | Metformin (n=270) | Lifestyle (n=505) | P |
|---|---|---|---|
| Males (%) | 151 (55.9%) | 295 (58.4%) | 0.70 |
| Age (years)* | 63 (35-75) | 64 (35-75) | 0.064 |
| Duration of diabetes (years) | 1.18 (0.82) | 1.25 (0.75) | 0.10 |
| Weight (kg) | 88.9 (16.7) | 89.7 (17.0) | 0.53 |
| BMI (kg/m2) | 30.1 (4.7) | 30.7 (5.1) | 0.11 |
| WHR | 0.97 (0.08) | 0.96 (0.08) | 0.045 |
| HbA1c (%) | 6.40 (0.60) | 6.34 (0.59) | 0.20 |
| HbA1c (mmol/mol) | 46.44 (6.57) | 45.81 (6.39) | 0.20 |
| Fasting plasma glucose (mmol/L) | 7.49 (1.47) | 6.94 (1.37) | <0.001 |
| Fasting active GLP-1 (pg/ml) † | 0.45 (0.40-0.51) | 0.36 (0.33-0.39) | <0.001 |
| Fasting total GLP-1 (pg/ml) † | 7.9 (7.2-8.5) | 6.9 (6.4-7.3) | 0.0097 |
| 60-min total GLP-1 (pg/ml) † | 16.0 (14.9 – 17.3) | 14.3 (13.3-15.3) | 0.031 |
Data presented as mean (SD) or n (%) except where indicated (*median [range]; †geometric mean [95% CI])
In DIRECT there was no significant difference in age, sex, BMI, duration of diabetes or HbA1c between the metformin and non-metformin treated groups. Metformin treated individuals had a higher fasting glucose (<0.001) and a slightly higher WHR than those on no treatment (p=0.045) in DIRECT.
CAMERA results: metformin increases fasting total GLP-1 over 18 months
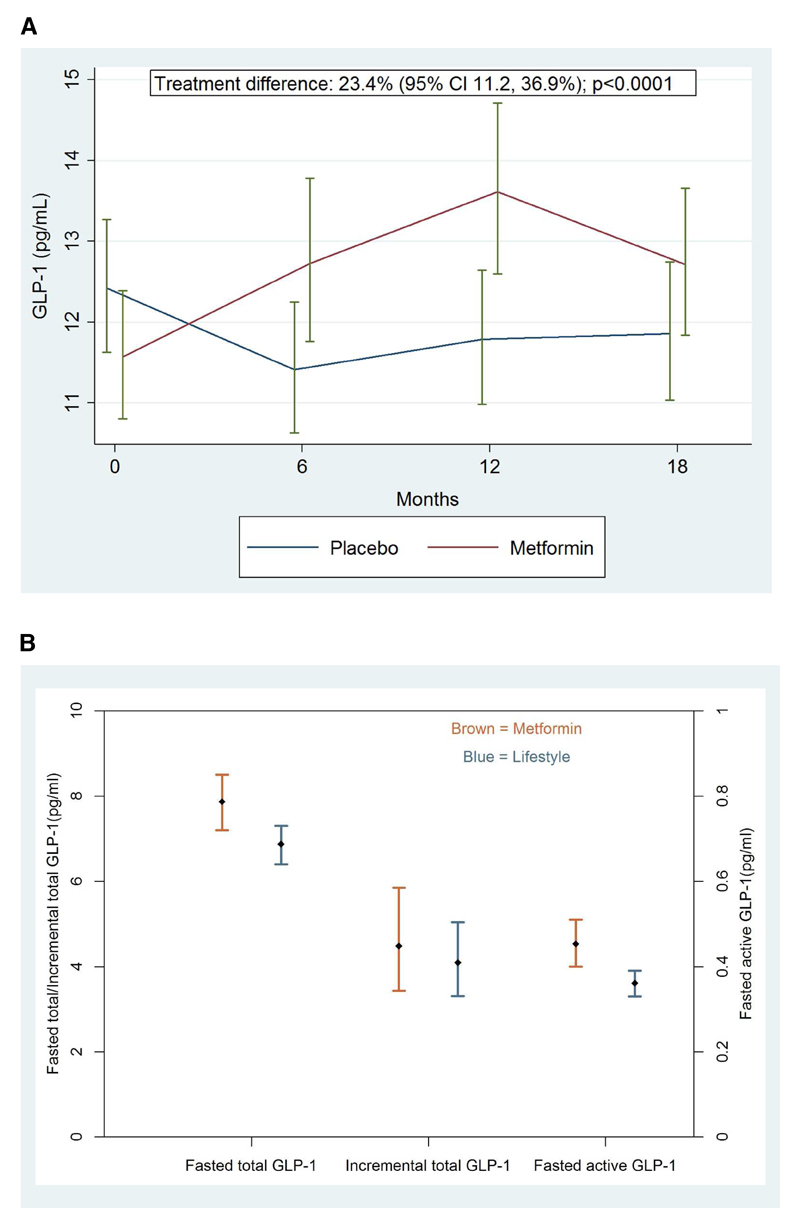
The geometric mean for total GLP-1 was 11.6pg/mL in metformin recipients and 12.4pg/mL in placebo recipients at baseline. Metformin therapy led to significant increases in fasting total GLP-1 compared to placebo at each of the 6, 12 and 18-month study visits (see Table 2 and Figure 1A). The increases in total GLP-1 at these visits were 21% (p=0.010), 27% (p=0.001) and 19% (p=0.012) respectively. In repeated-measures analysis, metformin increased total GLP-1 by 23.4% (p<0.0001) across the entire duration of the 18 month follow-up with no evidence of heterogeneity between study visits (p=0.74).
Table 2.
Change in GLP-1 and leptin levels on metformin vs. placebo over 18 months in CAMERA
| Visit (nr of paired samples) | Metformin vs. Placebo * | Average treatment effect (Metformin – Placebo) † | p-value for interaction across visits | ||
|---|---|---|---|---|---|
| Effect (95% CI) | p-value | ||||
| GLP-1 (natural log units) | 6 months (n=150) | 0.188 (0.046, 0.329) | 0.210 (0.106, 0.314) | <0.0001 | 0.74 |
| 12 months (n=146) | 0.237 (0.098, 0.376) | ||||
| 18 months (n=157) | 0.172 (0.038, 0.305) | ||||
| GLP-1 (%) ‡ | 6 months (n=150) | 20.7% (4.7, 39.0%) | 23.4% (11.2, 36.9%) | ||
| 12 months (n=146) | 26.7% (10.3, 45.6%) | ||||
| 18 months (n=157) | 18.7 (3.8, 35.7%) | ||||
| Leptin (natural log units) | 6 months (n=152) | -0.262 (-0.403, -0.120) | -0.286 (-0.419; -0.153) | <0.0001 | 0.80 |
| 12 months (n=146) | -0.293 (-0.467, -0.118) | ||||
| 18 months (n=157) | -0.237 (-0.405, -0.069) | ||||
| Leptin (%) c | 6 months (n=152) | -23.1% (-33.2, -11.3%) | -24.9% (-34.2, -14.2%) | ||
| 12 months (n=146) | -25.4% (-37.3, -11.1%) | ||||
| 18 months (n=157) | -21.1% (-33.3, -6.7%) | ||||
ANCOVA analysis for visits at 6, 12 and 18 months respectively
Repeated measures analysis for the overall treatment effect over 18 months
Percentage difference in geometric means
Figure 1.
Metformin and GLP-1 (A) total GLP-1 levels on metformin vs. placebo over 18 months in the CAMERA study (B) The association of metformin therapy vs. lifestyle treatment with fasting active GLP-1, fasting total GLP-1 and incremental total GLP-1 in DIRECT
Footnote: Data displayed as geometric mean (1SE) (A) and geometric mean (95% CI) (B)
Leptin levels fell with metformin treatment in keeping with a reduction in body fat (see Table 2). Overall, metformin therapy reduced leptin by 25% (p<0.0001) compared to placebo with similar changes observed at each visit.
Adjustment for the observed changes in weight, HOMA2-IR, HbA1c and leptin at each visit, whether individually or combined, did not attenuate metformin’s effect on total GLP-1 (see Table 3). Adjusted comparisons (for all four parameters) at 6, 12 and 18 months showed increased in total GLP-1 of 32% (p=0.001), 35% (p≤0.001) and 26% (p=0.002) respectively for metformin compared to placebo therapy.
Table 3.
Effects of metformin on total GLP-1 without and with on-treatment adjustments for changes in key variables
| Variable | Adjustment | Metformin-Placebo Mean % change (95%CI)a | P- value |
|---|---|---|---|
| GLP-1, 6 months | No adjustment | 20.7 (4.7, 39.0) | 0.010 |
| Weight | 25.0 (7.6, 45.3) | 0.004 | |
| HOMA2-IR | 24.6 (8.0, 43.7) | 0.003 | |
| HbA1c | 26.1 (8.4, 46.8) | 0.003 | |
| Leptin | 22.9 (6.4, 41.9) | 0.005 | |
| Combined† | 32.4 (13.0, 55.1) | 0.001 | |
| GLP-1, 12 months | No adjustment | 26.7 (10.3, 45.6) | 0.001 |
| Weight | 35.0 (15.9, 57.3) | <0.001 | |
| HOMA2-IR | 27.2 (10.4, 46.7) | 0.001 | |
| HbA1c | 28.7 (11.0, 49.2) | 0.001 | |
| Leptin | 33.0 (15.7, 53.0) | <0.001 | |
| Combined † | 35.4 (15.8, 58.1) | <0.001 | |
| GLP-1, 18 months | No adjustment | 18.7 (3.8, 35.7) | 0.012 |
| Weight | 23.5 (7.0, 42.5) | 0.004 | |
| HOMA2-IR | 21.4 (6.2, 39.0) | 0.005 | |
| HbA1c | 20.9 (5.3, 38.8) | 0.007 | |
| Leptin | 20.8 (5.6, 38.2) | 0.006 | |
| Combined † | 26.0 (9.1, 45.6) | 0.002 |
The unadjusted result at each time point is provided, followed by the result adjusted for changes in weight, HOMA2-IR, HbA1c and leptin respectively; this is followed by the result adjusted for all these variables combined (indicated by †)
HOMA2-IR: Homeostasis Model Assessment of Insulin Resistance
Results displayed as percentage change in geometric mean (95% CI)
DIRECT results: the association of metformin with fasting and post-meal GLP-1
The geometric mean for total fasted GLP-1 was 7.9pg/mL in metformin recipients and 6.9pg/mL in lifestyle-treated patients. Metformin users had higher basal fasted active GLP-1 (+25.5% [95%CI 17.0-35.5%], p<0.001) and fasted total GLP-1 (+14.5% [95%CI 8.4-21.0%], p=0.0097) than individuals who were on lifestyle therapy (see Table 1 and Figure 1B). These differences persisted after controlling for anthropometric measures (age, sex, waist to hip ratio, BMI), lifestyle factors (smoking and alcohol), study centre and HbA1c for both fasted active and fasted total GLP-1 (+39.1% [21.3-56.4%]; p=1.35e-05 and +14.1% [1.2-25.9%] respectively; p=0.03). Replacing HbA1c with fasting glucose in these models did not materially alter these results. There was no difference in the 60 minute total GLP-1 concentration between metformin users and non-metformin users after adjusting for these covariates and baseline total GLP-1 (4.4% [95%CI -0.5-9.4%]; p=0.27).
Discussion
In these complementary studies we sought further information regarding the relationship between metformin therapy and circulating GLP-1. We demonstrate that daily metformin therapy for 18 months led to a 25% increase in circulating total GLP-1 levels in individuals without diabetes but with elevated waist circumferences, and this increase was sustained across the entire duration of the study and did not appear to be related to any changes in glycaemia or adiposity. In recently diagnosed T2D individuals, metformin treatment was associated with higher fasted active and fasted total, but not incremental, GLP-1 levels. In both studies, these differences in GLP-1 levels occurred despite the previous dose of metformin having been taken the day before each visit (>24 hours in DIRECT), suggesting that circulating GLP-1 levels probably remain consistently elevated in patients on metformin therapy.
Active GLP-1 is secreted by gastro-intestinal L cells in response to the presence of nutrients in the small intestine leading to an increase in glucose-stimulated insulin secretion and suppressed glucagon secretion. GLP-1 also delays gastric emptying and promotes satiety. This bioactive form of the hormone is rapidly metabolized by the enzyme DPP-4 with the result that its half-life in the circulation is less than two minutes. Understanding the incretin pathway led to the development of related medications, namely GLP-1 receptor agonists (incretin mimetics) and DPP-4 inhibitors (incretin enhancers)23, which are designed to directly or indirectly increase the in vivo activity of GLP-1.
Previous small studies with various designs have produced mixed, often null, results but with some suggesting that metformin therapy increases circulating GLP-1 levels by various mechanisms24 (see Supplementary Table 2). In a study of 10 obese participants without diabetes and 10 controls who were given metformin 2.55g/d for two weeks, GLP-1 levels at 30 and 60 minutes after a glucose load were increased though baseline GLP-1 levels (and leptin) were unchanged on metformin13. An uncontrolled study of metformin therapy (2g/d) in 40 women with polycystic ovarian syndrome over 8 months, albeit with substantial loss to follow up with only 22 women completing metformin therapy, produced similar findings to our own with a 25% increase in area-under-the-curve GLP-1 levels over 180 minutes during oral glucose loading compared to baseline14. A crossover study of 10 individuals with T2DM given three single dose interventions on three different days a week apart (either metformin 1g plus placebo subcutaneous injection; or placebo tablet plus subcutaneous GLP-1; or metformin 1g plus subcutaneous GLP-1)15. Glucose was infused to achieve a concentration of approximately 15mmol/L. Analyses showed that metformin therapy inhibited DPP-4 activity and also increased active GLP-1 levels. In a further crossover study conducted in 20 participants with T2DM who were treated for 6 days with each of four respective regimens (placebo or metformin or sitagliptin or the combination) with washout periods in between interventions, metformin therapy led to an increase in fasted and post-challenge total GLP-1 levels though no change in intact GLP-1 levels16. And in a crossover study of 12 participants with T2DM treated with placebo or metformin for seven days respectively and then investigated during intraduodenal catheter infusion of glucose, DPP-4 activity fell modestly while intact and total GLP-1 levels rose at baseline and during the infusion after metformin17. By contrast, a crossover study of 16 participants with T2DM treated for 4 weeks respectively with placebo, metformin, sitagliptin and combined metformin / sitagliptin yielded no increase in active GLP-1 on metformin18. Other studies have suggested no effect on DPP-4 activity. In a study of eight drug-naïve participants with T2DM treated with metformin for three months, the area-under-the-curve for active GLP-1 over 6 hours following a standard mixed meal rose though DPP-4 activity was unchanged19. It is therefore apparent that most studies in this area have been limited by small sample size (and therefore reduced power) and that most have focused on the acute effect of metformin therapy as opposed to its longer term effects. Animal studies have produced similarly mixed results including evidence of an acute increase in GLP-1 with metformin treatment and of DPP-4 inhibition in some studies but not all25–27. In contrast our results have examined the relationship of metformin with circulating GLP-1 in large cohorts with and without type 2 diabetes and addressed long term effects of metformin over 18 months in non-diabetic individuals.
In individuals without diabetes, our finding that the increase in GLP-1 was not related to the observed 3.2kg decrease in weight or the 25% improvement in insulin sensitivity is in keeping with a direct effect of metformin on the incretin axis. The sustained nature of the GLP-1 increase suggests the possibility that metformin may in part provide cardio-metabolic benefit, even in a non-diabetic population and beyond reducing the risk of developing T2DM, by increasing exposure of treated individuals to GLP-1 in the longer term. This is supported by findings from both recently completed outcomes trials of GLP-1 receptor agonists9,10 and from a Mendelian11 randomization study of a GLP-1 receptor variant associated with lower fasting glucose which was also associated with lower risk of coronary heart disease. It also provides further rationale to test these potential benefits of metformin in a population without diabetes. The Glucose Lowering In Non-diabetic hyperglycaemia Trial (GLINT; ISRCTN34875079) is studying whether metformin reduces cardiovascular risk as well as cancer and other outcomes in non-diabetic participants.
Our study has numerous strengths. The CAMERA study is by far the largest and longest trial to address the question of metformin’s impact on circulating GLP-1 levels and its randomized design minimises the possibility of unmeasured or unaccounted for confounding. Though cross-sectional and therefore unable to directly address causality, DIRECT is the largest study to investigate the association of metformin with GLP-1 levels in T2DM individuals, and was able to adjust for a range of potential confounding factors. The CAMERA trial was specifically conducted in participants without T2DM (though with elevated waist circumferences) which enabled us to avoid the potential effects of other glucose-lowering agents and also to provide novel data on a group at high risk of T2DM in whom metformin is being investigated in a major trial, GLINT. Samples were available at 6 month intervals, providing data on the sustained effect of metformin on GLP-1 levels. An important weakness of the CAMERA trial was that we did not have access to suitably prepared samples to allow the measurement of active GLP-1 levels and only fasted samples were available. However, in the DIRECT study in which we had access to both active and total fasted GLP-1 levels, metformin recipients demonstrated clearly higher levels of both, in particular active GLP-1. Notably, however, in both studies, higher GLP-1 levels were noted despite the last metformin dose having been taken the day before blood sampling which, in the context of the limited bioavailability of metformin (less than 60%), suggests that some of this effect may reflect the impact of the drug in the distal small intestine and colon as highlighted in other studies28. Consistent with this, the apparent impact of metformin in DIRECT was on fasting GLP-1 rather than meal stimulated GLP-1. In addition, the fact that some CAMERA participants reduced their metformin dose and, in some cases, stopped trial medication suggests that our results are likely to be an underestimation of the true effect of metformin on fasting total GLP-1 in this population.
Further studies are needed to determine the longitudinal effect of metformin on GLP-1 levels in diabetic individuals. Additional research on the mechanism by which metformin increases GLP-1 would also be useful, including whether this effect is largely a direct of metformin on L-cells or is mediated indirectly via metformin’s many other effects on the gastro-intestinal tract such as altering the microbiome or decreasing bile acid reabsorption29.
In summary, we report evidence from two major studies demonstrating that metformin treatment leads to a sustained and long term increase in circulating total GLP-1 levels in non-diabetic individuals independent of changes in weight and glycaemia, while metformin therapy is also associated with higher fasted total and active GLP-1 in diabetic patients, independent of weight and glycaemia. These complementary findings support a potential direct role for the incretin axis on the cardiometabolic benefits of metformin.
Supplementary Materials
Acknowledgements
DP is guarantor for CAMERA data and ERP for DIRECT data. CAMERA was funded by the Chief Scientist Office, Scotland (CZB/4/613). We are grateful to Sara Jane Duffus and Elaine Butler for analysing GLP-1 on the CAMERA samples. The work leading to this publication has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n°115317 (DIRECT), resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution. ERP holds a Wellcome Trust New Investigator Award (ref 102820/Z/13/Z).
Footnotes
Contributors: DP had the idea for and designed the analysis, analysed and interpreted data, wrote the first draft and revised later drafts of the article. AD analysed the DIRECT data and contributed to the manuscript. ERP, PWF, AJ and MW participated in the design and sample collection of the DIRECT diabetes progression study, interpreted the data and revised the manuscript. PW co-ordinated the laboratory work for GLP-1 and leptin measurements, interpreted data and revised the article. CS analysed leptin, analysed and interpreted the data and revised the article. RRH interpreted the data and revised the article. NS had the idea for and designed the analysis, interpreted data, revised the article, and supervised the analysis. DIRECT collaborators are listed in Supplementary Table 3.
Conflicts of interest: DP, PW, CS, AJ, THH, JD, and RK report no conflicts of interest. RRH has received research support from Amylin, Bayer, Merck, and Novartis; participated in advisory boards for Amylin, Lilly, Merck, Novartis, and Novo Nordisk; and received compensation for lectures from Bayer, Lilly, Merck, and Merck Serono. ERP has received lecture fees from Eli Lilly, Novo Nordisk, Astra Zeneca and Sanofi. NS has consulted for Eli Lilly, Bristol-Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Sanofi, and Boehringer Ingelheim; and received research support from Merck. PWF has received consulting honoraria from Sanofi Aventis and Eli Lilly Inc, and research support from Novo Nordisk.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55(6):1577–96. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 2.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65. [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes Prevention Program Research G. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35(4):731–7. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 6.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 7.Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373(3):232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373(23):2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 9.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016 doi: 10.1056/NEJMoa1607141. (online ahead of print) [DOI] [PubMed] [Google Scholar]
- 11.Scott RA, Freitag DF, Li L, et al. A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease. Sci Transl Med. 2016;8(341):341ra76. doi: 10.1126/scitranslmed.aad3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potts JE, Gray LJ, Brady EM, Khunti K, Davies MJ, Bodicoat DH. The Effect of Glucagon-Like Peptide 1 Receptor Agonists on Weight Loss in Type 2 Diabetes: A Systematic Review and Mixed Treatment Comparison Meta-Analysis. PLoS One. 2015;10(6):e0126769. doi: 10.1371/journal.pone.0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannucci E, Ognibene A, Cremasco F, et al. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care. 2001;24(3):489–94. doi: 10.2337/diacare.24.3.489. [DOI] [PubMed] [Google Scholar]
- 14.Svendsen PF, Nilas L, Madsbad S, Holst JJ. Incretin hormone secretion in women with polycystic ovary syndrome: roles of obesity, insulin sensitivity, and treatment with metformin. Metabolism. 2009;58(5):586–93. doi: 10.1016/j.metabol.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Cuthbertson J, Patterson S, O'Harte FP, Bell PM. Addition of metformin to exogenous glucagon-like peptide-1 results in increased serum glucagon-like peptide-1 concentrations and greater glucose lowering in type 2 diabetes mellitus. Metabolism. 2011;60(1):52–6. doi: 10.1016/j.metabol.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Vardarli I, Arndt E, Deacon CF, Holst JJ, Nauck MA. Effects of sitagliptin and metformin treatment on incretin hormone and insulin secretory responses to oral and “isoglycemic” intravenous glucose. Diabetes. 2014;63(2):663–74. doi: 10.2337/db13-0805. [DOI] [PubMed] [Google Scholar]
- 17.Wu T, Thazhath SS, Bound MJ, Jones KL, Horowitz M, Rayner CK. Mechanism of increase in plasma intact GLP-1 by metformin in type 2 diabetes: stimulation of GLP-1 secretion or reduction in plasma DPP-4 activity? Diabetes Res Clin Pract. 2014;106(1):e3–6. doi: 10.1016/j.diabres.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Solis-Herrera C, Triplitt C, Garduno-Garcia Jde J, Adams J, DeFronzo RA, Cersosimo E. Mechanisms of glucose lowering of dipeptidyl peptidase-4 inhibitor sitagliptin when used alone or with metformin in type 2 diabetes: a double-tracer study. Diabetes Care. 2013;36(9):2756–62. doi: 10.2337/dc12-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thondam SK, Cross A, Cuthbertson DJ, Wilding JP, Daousi C. Effects of chronic treatment with metformin on dipeptidyl peptidase-4 activity, glucagon-like peptide 1 and ghrelin in obese patients with Type 2 diabetes mellitus. Diabet Med. 2012;29(8):e205–10. doi: 10.1111/j.1464-5491.2012.03675.x. [DOI] [PubMed] [Google Scholar]
- 20.Migoya EM, Bergeron R, Miller JL, et al. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clin Pharmacol Ther. 2010;88(6):801–8. doi: 10.1038/clpt.2010.184. [DOI] [PubMed] [Google Scholar]
- 21.Preiss D, Lloyd SM, Ford I, et al. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2(2):116–24. doi: 10.1016/S2213-8587(13)70152-9. [DOI] [PubMed] [Google Scholar]
- 22.Koivula RW, Heggie A, Barnett A, et al. Discovery of biomarkers for glycaemic deterioration before and after the onset of type 2 diabetes: rationale and design of the epidemiological studies within the IMI DIRECT Consortium. Diabetologia. 2014;57(6):1132–42. doi: 10.1007/s00125-014-3216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahren B, Schmitz O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res. 2004;36(11–12):867–76. doi: 10.1055/s-2004-826178. [DOI] [PubMed] [Google Scholar]
- 24.Bahne E, Hansen M, Bronden A, Sonne DP, Vilsboll T, Knop FK. Involvement of glucagon-like peptide-1 in the glucose-lowering effect of metformin. Diabetes Obes Metab. 2016;18(10):955–61. doi: 10.1111/dom.12697. [DOI] [PubMed] [Google Scholar]
- 25.Maida A, Lamont BJ, Cao X, Drucker DJ. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-alpha in mice. Diabetologia. 2011;54(2):339–49. doi: 10.1007/s00125-010-1937-z. [DOI] [PubMed] [Google Scholar]
- 26.Mulherin AJ, Oh AH, Kim H, Grieco A, Lauffer LM, Brubaker PL. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology. 2011;152(12):4610–9. doi: 10.1210/en.2011-1485. [DOI] [PubMed] [Google Scholar]
- 27.Green BD, Irwin N, Duffy NA, Gault VA, O'Harte FP, Flatt PR. Inhibition of dipeptidyl peptidase-IV activity by metformin enhances the antidiabetic effects of glucagon-like peptide-1. Eur J Pharmacol. 2006;547(1–3):192–9. doi: 10.1016/j.ejphar.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 28.Buse JB, DeFronzo RA, Rosenstock J, et al. The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation: Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes Care. 2016;39(2):198–205. doi: 10.2337/dc15-0488. [DOI] [PubMed] [Google Scholar]
- 29.McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59(3):426–35. doi: 10.1007/s00125-015-3844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.