Abstract
Background
The relationships between obsessive-compulsive symptoms and distinct forms of impulsivity and compulsivity are unclear. Such examination would be relevant in terms of how best to classify psychiatric disorders and in understanding candidate ‘latent traits’ that extend across a continuum between normalcy and clinical disorders.
Method
515 young adults (aged 18-29 years) completed the Padua Inventory and undertook detailed clinical and neurocognitive assessments. Relationships between obsessive-compulsive symptoms and distinct types of impulsivity and compulsivity were evaluated using linear regression modelling.
Results
Obsessive-compulsive symptoms were significantly predicted by female gender, lower quality of life, psychiatric disorders in general (but not impulse control disorders), and worse extra-dimensional set-shifting. Obsessive-compulsive symptoms were not significantly predicted by alcohol/nicotine consumption, stop-signal reaction times, or decision-making abilities.
Conclusion
These data indicate that obsessive-compulsive symptoms are more related to certain forms of compulsivity than to impulsivity. These findings have important implications for diagnostic conceptualizations and neurobiological models.
Keywords: impulsive, compulsive, cognition, substance use, obsessive
1.0. Introduction
While psychiatry traditionally focused on overt symptomatology, it is increasingly recognized that it is necessary to identify intermediate markers, or latent traits, that predispose towards a range of overt psychiatric pathologies.1 One potential ‘latent’ type of trait, of relevance not just at a clinical but also population level, is that of a tendency towards obsessive and compulsive thoughts and behaviors (hereafter referred to as ‘obsessive-compulsive symptoms’). Questionnaire-based self-report measures of obsessive-compulsive symptoms are ideally suited for large-scale studies in the population: they are relatively cheap and rapid to administer, and provide a broad or over-arching composite measure of compulsivity. The Padua Inventory2 is such a questionnaire, developed to study obsessive-compulsive thoughts and behaviors both at a subsyndromal level and in clinical populations, including patients with obsessive-compulsive disorder (OCD).3
Are obsessive-compulsive symptoms impulsive or compulsive? Behaviors described as ‘impulsive’ or ‘compulsive’ cut across multiple psychiatric disorders, and also exist in the general population to variable degrees. Broadly speaking, compulsivity refers to thoughts and behaviors that are repetitive, and performed in a stereotyped or habitual fashion. One conceptualization holds that impulsivity (tendency towards premature, poorly thought out acts)4 is diametrically opposed to compulsivity, with impulsivity and compulsivity representing opposing ends of a behavioral spectrum.5 Alternatively, the two terms may be seen as overlapping, in that they both imply underlying problems with top-down inhibitory control.6–7 Furthermore, certain disorders formally regarded as impulsive (for example, trichotillomania) are now included in the same diagnostic category as OCD in DSM-5, suggesting that the consensus view holds them to be ‘compulsive’ rather than ‘impulsive’. Considerable research has focused on fractionating impulsivity,8–9 such as in terms of cognitive tests and disorders; while the concept of compulsivity is perhaps less fully developed.
Impulsivity and compulsivity can be considered at the level of syndrome, behavior, or cognition. Studies exploring the inter-relationships between potentially separable forms of compulsivity have focused mainly at the level of clinical disorder.10 Patients with OCD show elevated occurrence of other disorders traditionally regarded as impulse control disorders, such as compulsive gambling, hair pulling disorder, and skin picking disorder; and vice versa.11–12 This phenomenological and comorbid overlap was originally recognized some years ago, but more recently resulted in OCD being clustered under the umbrella category of ‘Obsessive-Compulsive and Related Disorders’ in the Diagnostic and Statistical Manual Version 5,13 alongside hair pulling disorder, and skin picking disorder, as noted above. However, important differences exist between these disorders.14 For example, OCD and these disorders differ both at the level of neuropsychological test performance,7 and in terms of treatment responsiveness.15–16
From a neurobiological perspective, compulsivity has mostly been considered in terms of the ‘archetypal’ disorder of compulsivity, namely OCD. OCD has been associated with structural and functional abnormalities of fronto-striatal circuitry, in part, responsible for habit learning and top-down control. Notable implicated brain regions include the ventral and dorsal striatum (accumbens, and caudate/putamen), and frontal cortex sectors (orbitofrontal and dorsolateral).17 Consistent with involvement of these regions in the pathophysiology of compulsivity, OCD patients often exhibit deficits on neurocognitive tasks versus healthy controls, particularly those cognitive domains involving top-down control over flexible responding, such as the Wisconsin Card Sorting Test (WCST) or conceptually related Intra-Dimensional/Extra-Dimensional set-shift test.18–20 One key caveat in this approach to understanding the neurobiology of compulsivity is that OCD represents the end point of complex brain pathologies. Thus, focusing on OCD alone provides limited insights into the chain of pathogenesis, and the relationship between compulsivity in the broader population and clinical manifestations of compulsivity in other disorders.
Relatively little is known about whether compulsivity-relevant cognitive impairment extends along a continuum, beyond just those people with formal psychiatric disorders. Impairments on tests of flexible responding appear to extend into the remission phase of OCD,21 and to exist in clinically asymptomatic first-degree relatives of OCD patients.22–26 These data suggest that neurocognitive measures of compulsivity may exist not only at the categorical level of OCD, but also in people at elevated risk of OCD. However, these approaches in themselves (in the absence of twin adoption studies) cannot rule out influences of potential confounds: for example, OCD patients and their relatives may share environmental commonalities (e.g. family environment in childhood) that differ from controls without a family history of OCD, which confound neuropsychological parameters.
Only a handful of studies have explored the relationships between dimensional composite measures of obsessive-compulsiveness, indexed by questionnaires, and other discrete impulsive and compulsive measures. Some data suggest that high compulsive subclinical individuals show similar personality and clinical features to those with clinically diagnosed OCD.27–29 In terms of cognition, several studies have found significant associations between elevated questionnaire-based compulsivity and impaired cognitive flexibility, indexed using the Wisconsin Card Sorting Test (WCST)28,30 while another study was negative on this paradigm.31
1.1. Aims of the Study
The aim of the current study was to explore relationships between obsessive-compulsive symptoms (Padua Inventory total scores) and distinct measures of impulsivity and compulsivity in young adults. We predicted that obsessive-compulsive symptoms would be significantly associated with worse flexible responding (impaired set-shifting) and the presence of one or more impulse control disorders.
2.0. Material and Methods
2.1. Subjects
Study participants comprised 515 young adults aged 18-29 years, recruited consecutively using media advertisements in two US cities. The only inclusion criterion was gambling at least five times in the preceding year (gambling was defined as playing any game of chance for the possibility of winning money, and this was used a proxy for a minimal baseline level of impulsive or compulsive tendencies). The age and minimal gambling criteria were a consequence of the funding source for this study, as funding was supplied for a broader project focusing on the development of gambling-related behaviors over time. As such, our sample can be viewed as being somewhat enriched for gambling tendencies. Exclusion criteria were an inability to understand/undertake the assessments, and failure to provide written informed consent. The study was undertaken in accordance with the Declaration of Helsinki.
2.2. Assessments
Assessments comprised completion of the Padua Inventory, detailed psychiatric interview, and neuropsychological testing. All were undertaken in a quiet environment. The Padua Inventory is a 60-item questionnaire originally developed to study obsessive-compulsive thoughts and behaviors in the general population.2 It yields a total score, derived on the basis of factor analysis. Occurrence of mainstream psychiatric conditions was evaluated using the Mini International Neuropsychiatric Inventory (MINI) (English Version 5.0.0),32 while the presence of impulse control disorders was detected using the Minnesota Impulsive Disorders Interview (MIDI)33. Both of these instruments (MINI, MIDI) represent structured, gold-standard psychiatric assessments. The MINI screens for anxiety, mood, eating, psychotic, and alcohol and substance use disorders. Although the formal category of impulse control disorders from DSM-IV has been modified by DSM-5, we believe the disorders captured by the MIDI reflect an array of behaviors which have impulsivity as a cardinal feature, regardless of DSM categorization.12,33 Information was collected in relation to frequency of alcohol use, and equivalent numbers of cigarette packs smoked per day. Quality of life was measured using the Quality of Life Inventory (QOLI).34
Cognitive testing was completed using a selected range of paradigms from the computerized Cambridge Neuropsychological Test Automated Battery (CANTABeclipse, version 3, Cambridge Cognition Ltd, UK), which utilizes a touch-screen computer interface. Tests were selected on the basis of cognitive domains often found to be impaired in compulsive disorders including obsessive-compulsive disorder (OCD) and gambling disorder,17–19,35 specifically set-shifting, response inhibition, and decision-making.
Set-shifting was measured using the Intra-dimensional/Extra-dimensional task (IED). This paradigm decomposes different aspects of rule learning and flexible responding, and was derived from the Wisconsin Card Sorting Test. Participants view a series of trials, each involving presentation of two stimuli on-screen, and attempt to work out an underlying ‘rule’ about which stimuli is correct. For each trial, the participant chooses one stimuli or the other, and then receives feedback as to whether the choice was correct or incorrect. Through trial and error, the individual works out the underlying rule, that is then changed by the computer in order to explore different aspects of behavior. The main measure of compulsivity on the task is the number of errors made on the extra-dimensional set-shift stage, which requires shifting attention away from a previously relevant stimulus dimension (such as the number of lines), and onto a previously irrelevant stimulus dimension (such as the appearance of a polygonal shape).
Response inhibition was measured using the Stop-Signal Task (SST). On the SST, a series of directional arrows are presented on the computer screen one per time, and volunteers make quick responses (left button for a left-facing arrow, and vice versa). On a subset of trials, a ‘stop’ signal (beep) occurs after presentation of the arrow, and the participant attempts to withhold their response just for the given trial. By varying the time between presentation of the arrow and the stop-signal, the task calculates a measure of the time taken by the subject to suppress a response that would normally be made, referred to as the stop-signal reaction time (SSRT). Longer SSRTs reflect an inability to suppress motor responses, classically believed to reflect impulsivity (for example, often found in attention-deficit hyperactivity disorder, although results have been less consistent in other impulsive disorders such as gambling).
Decision-making was measured using the Cambridge Gamble Task (CGT). On each trial, ten boxes are shown on the computer screen, some blue and some red, with a token having been hidden behind one of these by the computer. For a given trial, the participant selects the color (red or blue) they think the token is hidden behind, and then choose how many of their cumulative ‘points’ to gamble on having made the correct decision. The hidden token is then revealed, and the participant receives feedback (gain or loss of points). Over the course of the exercise, the aim is to acquire as many points as possible. The main measures of decision-making on the task are the proportion of points gambled overall, the proportion of rational decisions made (proportion of trials when the volunteer opted for the color that was in the majority), and risk adjustment (the extent to which subjects modulated the amount gambled depending on the probability of making correct choices) (36).
2.3. Data Analysis
Salient demographic, clinical, and cognitive measures were tabulated across the whole sample. In primary analysis, the relationship between Padua Inventory total scores and these predictor variables was explored using linear regression (method ‘enter’, criterion for entry p=0.05 and for removal p=0.10). Two sets of secondary analyses were then conducted, again using linear regression, to explore: (i) relationships between individual MINI and MIDI psychiatric disorders and Padua total scores; and (ii) relationships between significantly predictive variables identified in the primary analysis and subscale total scores for Padua (contamination obsessions & washing compulsions; dressing/grooming compulsions; checking compulsions; obsessional thoughts of harming self/others; and impulses to harm self/others). Statistical significance was defined as p<0.05 uncorrected two-tailed. All statistical analyses were undertaken using SPSS version 22.
3.0. Results
515 subjects were enrolled into the study, and completed clinical and cognitive assessment. The mean (SD) Padua total scores of the sample was 17.8 (17.3). Demographic, clinical, and cognitive characteristics of the sample are presented in Table 1.
Table 1. Demographic and Clinical Variables.
| Variable | Controls (N=515) | |
|---|---|---|
| Mean | SD | |
| Demographic and clinical measures | ||
| Age, years | 22.2 | 3.5 |
| Gender, male [%] | 341 [66.2%] | |
| Education level # | 3.2 | 0.9 |
| Number of times alcohol consumed per week | 1.4 | 1.4 |
| Nicotine consumption, packs per day equivalent | 0.13 | 0.30 |
| One or more mainstream psychiatric disorder, MINI, N[%] @ | ||
| Any mood disorder | 23 [4.5%] | |
| Any anxiety disorder | 63 [12.2%] | |
| Any alcohol or substance use disorder | 130 [25.3%] | |
| Any psychotic disorder | 3 [0.6%] | |
| Any eating disorder | 12 [2.3%] | |
| One or more of the above (any MINI) | 186 [36.1%] | |
| One or more Impulse Control Disorder, MIDI, N [%] | ||
| Gambling disorder | 44 [8.5%] | |
| Kleptomania | 3 [0.6%] | |
| Pyromania | 1 [0.2%] | |
| Trichotillomania | 2 [0.4%] | |
| Skin picking disorder | 38 [7.4%] | |
| Intermittent explosive disorder | 9 [1.7%] | |
| Compulsive buying | 25 [4.9%] | |
| Compulsive sexual behavior | 15 [2.9%] | |
| Binge eating disorder | 7 [1.4%] | |
| One or more of the above (any MIDI) | 134 [26.0%] | |
| Quality of Life Score | 31.3 | 22.9 |
| Cognitive measures | ||
| SST stop-signal reaction time, ms | 182.3 | 62.0 |
| IED ED errors | 10.1 | 9.9 |
| CGT overall proportion bet | 0.54 | 0.14 |
| CGT quality of decision-making | 0.95 | 0.09 |
| CGT risk adjustment | 1.59 | 1.22 |
MINI = Mini International Neuropsychiatric Inventory, MIDI = Minnesota Impulse Disorder Inventory, SST = Stop-Signal Task, IED = Intra-Dimensional/Extra-Dimensional Shift Task, CGT = Cambridge Gamble Task. # education scores: 1 = did not complete high school, 2 = high school graduate, 3 = some college, 4 = college graduate, 5 = beyond college level education.
Primary analysis
Linear regression identified a statistically significant model (F=7.161, p<0.001), which accounted for 15.7% of the variance in Padua total scores, based on variables listed in Table 1. The contribution of each variable to the model is displayed in Table 2. It can be seen that higher Padua scores were significantly associated with female gender (male gender was coded positive), occurrence of one or more MINI psychiatric disorders, lower quality of life, and more extra-dimensional set-shifting errors. Padua total scores were not significantly predicted by age, education levels, alcohol/nicotine consumption, presence of one or more impulse control disorders on the MIDI, stop-signal reaction times, or decision-making performance.
Table 2. Results of the linear regression model, with Padua total scores as the dependent variable.
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | ||
|---|---|---|---|---|---|---|
| B | Std. Error | Beta | ||||
| (Constant) | 36.327 | 11.084 | 3.277 | .001 | ||
| Age (years) | .219 | .251 | .045 | .871 | .384 | |
| Gender | -3.729 | 1.608 | -.102 | -2.319 | .021 | |
| Education scores | -1.694 | .954 | -.084 | -1.775 | .076 | |
| Drink Frequency (times per week) | -.710 | .528 | -.059 | -1.346 | .179 | |
| Nicotine Quantity (packs per day) | -1.218 | 2.592 | -.021 | -.470 | .639 | |
| Presence of one or more MINI psychiatric disorders | 5.050 | 1.583 | .140 | 3.189 | .002 | |
| Presence of one or more MIDI psychiatric disorders | 3.036 | 2.678 | .049 | 1.134 | .257 | |
| Quality of life | -.107 | .033 | -.142 | -3.242 | .001 | |
| SST SSRT | .003 | .012 | .012 | .293 | .770 | |
| IED Errors | .209 | .075 | .119 | 2.781 | .006 | |
| CGT Overall proportion bet | .971 | 6.075 | .008 | .160 | .873 | |
| CGT Quality of decision making | -14.738 | 9.614 | -.074 | -1.533 | .126 | |
| CGT Risk adjustment | -1.479 | .832 | -.104 | -1.777 | .076 | |
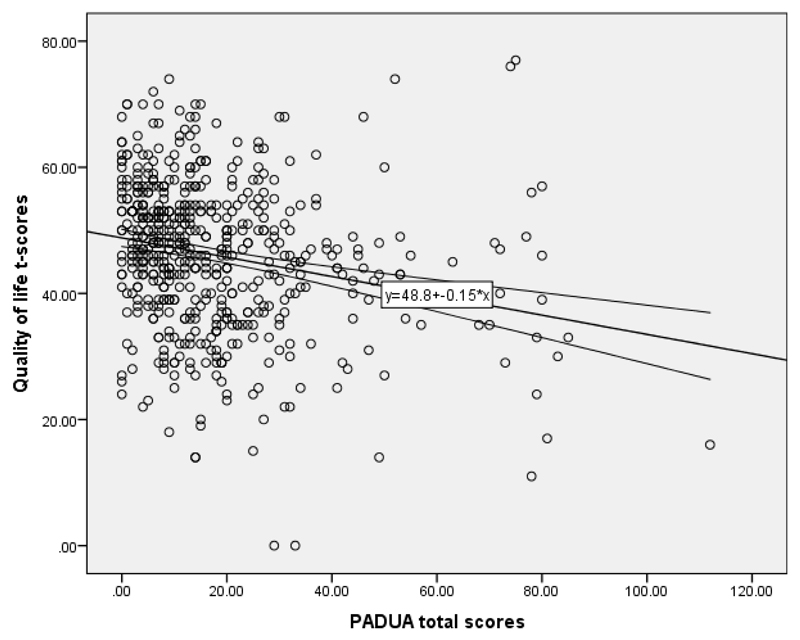
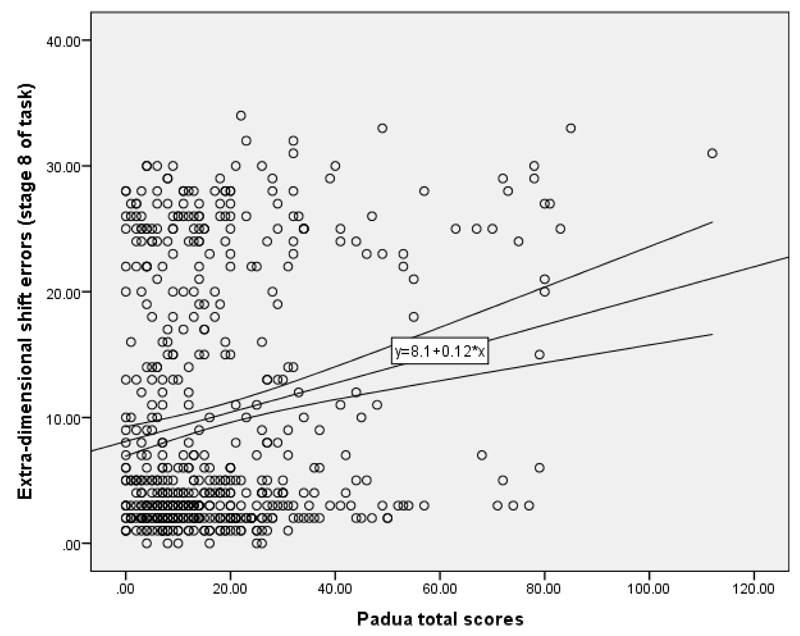
The mean (+/- SD) Padua total score was 21.4 (21.6) in females and 16.2 (14.2) in males. The Mean (+/- SD) Padua total score for those without any MINI disorders was 15.3 (15.2), while in those with one or more MINI disorders the mean was 22.5 (19.6). Plot of Padua total scores (x-axes) against significant predictive variables (y-axes) are shown below (Figure 1: quality of life; Figure 2: ED shift errors).
Secondary analyses
Results from the regression exploring individual MINI disorders as possible predictors for Padua total scores are provided in the supplementary online file; a significant model was found (F=4.142, p<0.001), accounting for 9.6% of the variance in Padua total scores. Disorders significantly contributing to the model (higher Padua scores = higher occurrence of disorders) were: social phobia, OCD, PTSD, panic disorder, and antisocial personality disorder.
When presence of individual MIDI disorders were used as possible predictors for Padua total scores, the overall model was not significant (F=1.426, p=0.210); nor was any individual MIDI disorder significantly predictive in its own right (all p>0.05).
Results of the regression analyses for Padua subscale scores are provided in the supplementary online section. Findings were qualitatively similar to those seen for Padua total scores, except that no significant model was identified for the ‘Padua impulses to harm self/others’ subscale.
4.0. Discussion
In this study, we assessed relationships between obsessive-compulsive tendencies (Padua Inventory total scores), and distinct forms of impulsivity and compulsivity, in young non-treatment seeking adults recruited from the general population. The main finding was that higher obsessive-compulsive symptoms were significantly predicted by presence of psychiatric disorders (but not impulse control disorders), lower quality of life, female gender, and more extra-dimensional set-shifting errors.
4.1. Demographic and clinical correlates of obsessive-compulsiveness
Examination of questionnaire-based obsessive-compulsive scores resulted in several important findings that may deserve further examination. First, our study found an association between higher obsessive-compulsive symptoms and female gender. Associations between female gender and higher Padua Inventory total scores have been observed in several previous studies conducted in young adult populations using the same instrument,2,37 and also other obsessive-compulsive questionnaires.38 One explanation could be that higher obsessive-compulsive symptoms reflect a disposition towards harm avoidance type temperament. Meta-analyses have demonstrated that harm avoidance is more common in women,39 and also that harm avoidance links OCD, panic disorder, and social anxiety disorder.40
Obsessive-compulsiveness was associated with psychiatric disorders as assessed by the MINI, although contrary to our hypothesis, was not significantly associated with occurrence of impulse control disorders as indexed by the MIDI. Arguably these findings fit closer with the previous rather than revised DSM conceptualization. In DSM-IV, OCD was classified as an anxiety disorder, and impulse control disorders as a separate group. In DSM-5, OCD and certain former impulse control disorders are clustered together as OC Related Disorders. Alternatively, it may be that obsessive-compulsiveness is related only to certain impulse control disorders, rather than to impulse control disorders in general.
4.2. Cognitive correlates of obsessive-compulsiveness
It was observed that higher questionnaire-based obsessive-compulsiveness was associated with worse set-shifting (higher errors on the extra-dimensional shift stage of the IED task), which accords well with the existing literature. This type of task fractionates different components of behavioral flexibility, and the extra-dimensional shift stage corresponds to the ability to inhibit attention away from a previously relevant stimulus dimension, onto a different stimulus dimension that was previously irrelevant. Extra-dimensional shifting represents a relatively high level of flexible responding, contingent on the integrity of the dorsolateral prefrontal cortices.41 In previous work, patients with OCD showed extra-dimensional set-shift deficits on this same task, compared to controls, in most7,26,42–44 but not all45 studies. Meta-analysis across OCD studies confirmed impaired cognitive flexibility using various tasks.46 Extra-dimensional shift impairment on the IED task extends to clinically asymptomatic first-degree relatives of OCD patients.26 Our data extend upon this previous work with the task, showing for the first time that IED impairment exists along a continuum in relation to obsessive-compulsiveness. This is consistent with two previous studies of high compulsive nonclinical subjects, which identified impaired performance compared to controls on the WCST;28,30 although a third study was negative.31 The current study, using subjects recruited from the background population, viewed alongside the majority of the previous data, supports impaired set-shifting as an important underpinning feature of compulsivity at a dimensional level, rather than just at the level of formal clinical diagnoses.
In terms of cognitive functions besides set-shifting, there was no evidence for a significant relationship between questionnaire-based obsessive-compulsiveness and prepotent response inhibition (Stop-Signal Task), nor decision-making measures. This may be taken to imply that set-shifting is particularly robustly associated with obsessive-compulsive tendencies.
4.3. Limitations
Although this is the first study to explore relationships between questionnaire-based obsessive-compulsive tendencies and a broad range of other facets of impulsivity and compulsivity, there are several limitations that should be considered. We did not exclude people with psychiatric disorders, because we wished to study relationships between Padua total scores and occurrence of general psychiatric disorders, and impulse control disorders. The study deliberately focused on the global composite measure from the Padua Inventory in the primary analysis, rather than individual items, because the aim was to evaluate ‘obsessive-compulsiveness’ viewed as a broad or ‘latent’ trait, rather than specific thoughts or behaviors that are likely to be more individualistic. Total Padua Inventory scores in our whole sample were somewhat lower than those reported in other studies,2,47 but our sample was recruited using a different approach to these other studies, with different inclusion criteria. In the interests of time and convenience for participants, only a limited range of cognitive functions was examined: we focused on tests of impulsivity (stop-signal task, decision-making task) and compulsivity (set-shifting task). Other types of task measuring impulsivity and compulsivity are available in the literature, or are in the process of being developed, and could be included in more comprehensive future work. It should be noted that we did not screen for tic disorders, binge-eating disorder, or body dysmorphic disorder: these disorders merit study in their own right, in relation to the “impulsive versus compulsive” debate. We did not evaluate any medications the subjects were taking; some medications could contribute to impulsivity or compulsivity, either in terms of causing it, or treating it. As this was a non-treatment seeking sample, it is highly unlikely that medications significantly accounted for the overall findings. In addition, although this was a non-treatment seeking sample, the sample was enhanced for a predisposition to gambling and this may result in findings less generalizable to the population at large. It is also of course possible that our measurements of impulsivity (the use of the DSM-OV impulse control disorders and the SST) are of limited utility. Although the impulsivity assessments in this study are commonly used, including a wider range of behaviors, disorders, and neurocognitive tests would facilitate a more understanding of impulsivity. We have addressed only the neuropsychological features of compulsivity and impulsivity, and the lack of assessments of the symptom/behavioral aspects of compulsivity and impulsivity are an additional limitation of this study. Lastly, it should be noted that there are other questionnaire-based measures of obsessive-compulsiveness that we did not include in our study, e.g. Obsessive Compulsive Inventory Revised (OCI-R).48 It would be useful to include these in future studies in this area.
4.4. Concluding Remarks
In summary, obsessive-compulsive symptoms were significantly associated with female gender, worse set-shifting, lower quality of life, and psychiatric disorders besides impulse control disorders. The findings may militate against obsessive-compulsive symptoms being primarily ‘impulsive’ as opposed to ‘compulsive’.
Supplementary Material
Figure 1.
Raw quality of life t-scores (y-axis) against raw PADUA total scores (x-axis). The correlation was statistically significant (Pearson’s r=-0.208, p<0.001). Line indicates linear line-of-best fit with accompanying 95% confidence intervals.
Figure 2.
Raw quality of life t-scores (y-axis) against raw PADUA total scores (x-axis). The correlation was statistically significant (Spearman’s r=0.202, p<0.001). Line indicates linear line-of-best fit with accompanying 95% confidence intervals.
Acknowledgements
This research was supported by a grant from the National Center for Responsible Gaming to Dr. Grant. Dr. Chamberlain’s involvement in this work was funded by a grant from the Academy of Medical Sciences, UK. Dr. Grant has received research grants from NIMH, National Center for Responsible Gaming, and Forest and Roche Pharmaceuticals Dr. Grant receives yearly compensation from Springer Publishing for acting as Editor-in-Chief of the Journal of Gambling Studies and has received royalties from Oxford University Press, American Psychiatric Publishing, Inc., Norton Press, and McGraw Hill. Dr. Chamberlain consults for Cambridge Cognition.
Footnotes
Declarations of Interest
The other authors have no disclosures.
References
- 1.Cutherbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanavio E. Obsessions and compulsions: the Padua Inventory. Behav Res Ther. 1988;26:169–77. doi: 10.1016/0005-7967(88)90116-7. [DOI] [PubMed] [Google Scholar]
- 3.Anholt GE, Van Oppen P, Emmelkamp PM, et al. Measuring obsessive-compulsive symptoms: Padua Inventory-Revised vs. Yale-Brown Obsessive Compulsive Scale. J Anxiety Disord. 2009;23:830–5. doi: 10.1016/j.janxdis.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Durana JH, Barnes PA. A neurodevelopmental view of impulsivity and its relationship to the superfactors of personality. In: Mccown WG, Johnson JL, Shure MB, editors. The Impulsive Client; Theory, Research and Treatment. American Psychological Association; Washington, D.C.: 1993. [Google Scholar]
- 5.Hollander E, Cohen LJ. Impulsivity and Compulsivity. American Psychiatric Press Inc.; Washington, D.C.: 1996. [Google Scholar]
- 6.Fineberg NA, Chamberlain SR, Goudriaan AE, et al. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. 2014;19:69–89. doi: 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry. 2006;163:1282–4. doi: 10.1176/ajp.2006.163.7.1282. [DOI] [PubMed] [Google Scholar]
- 8.Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 9.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Grant JE, Potenza MN. Compulsive aspects of impulse-control disorders. Psychiatr Clin North Am. 2006;29:539–51. doi: 10.1016/j.psc.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollander E, Kim S, Braun A, Simeon D, Zohar J. Cross-cutting issues and future directions for the OCD spectrum. Psychiatry Res. 2009;170:3–6. doi: 10.1016/j.psychres.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Fontenelle LF, Oostermeijer S, Harrison BJ, Pantelis C, Yucel M. Obsessive-compulsive disorder, impulse control disorders and drug addiction: common features and potential treatments. Drugs. 2011;71:827–40. doi: 10.2165/11591790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Asscoaition. Diagnostic and Statistical Manual of Mental Disorders (5th ed.) (DSM-5) American Psychiatric Publishing; Arlington,VA: 2013. [Google Scholar]
- 14.Pallanti S, Hollander E. Obsessive-compulsive disorder spectrum as a scientific “metaphor”. CNS Spectr. 2008;13:6–15. doi: 10.1017/s1092852900026882. [DOI] [PubMed] [Google Scholar]
- 15.Fineberg NA, Brown A, Reghunandanans S, Pampaloni I. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2012;15:1173–91. doi: 10.1017/S1461145711001829. [DOI] [PubMed] [Google Scholar]
- 16.Rothbart R, Amos T, Siegfried N, et al. Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev. 2013;11:CD007662. doi: 10.1002/14651858.CD007662.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65:185–236. doi: 10.1016/j.biopsycho.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain SR, Blaackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Baaldacchino A, Balfour D, Passetti F, Humphris G, Matthews K. Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci Biobehav Rev. 2012;36:2056–68. doi: 10.1016/j.neubiorev.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Rao NP, Reddy YC, Kumar KJ, Kandavel T, Chandrashekar CR. Are neuropsychological deficits trait markers in OCD? Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1574–9. doi: 10.1016/j.pnpbp.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Rajender G, Bhatia MS, Kanwal K, Malhotra S, Singh TB, Chaudhary D. Study of neurocognitive endophenotypes in drug-naïve obsessive-compulsive disorder patients, their first-degree relatives and healthy controls. Acta Psychiatr Scand. 2011;124:152–61. doi: 10.1111/j.1600-0447.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- 23.Viswanath B, Reddy YC, Kumar KJ, Kandavel T, Chandrashekar CR. Cognitive endophenotypes in OCD: a study of unaffected siblings of probands with familial OCD. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:610–5. doi: 10.1016/j.pnpbp.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain SR, Menzies L, Hampshire A, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–2. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 25.Menzies L, Achard S, Chamberlain SR, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–36. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain SR, Fineberg NA, Menzies LA, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:335–8. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frost RO, Steketee G, Cohn L, Griess K. Personality traits in subclinical and non-obsessive-compulsive volunteers and their parents. Behav Res Ther. 1994;32:47–56. doi: 10.1016/0005-7967(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 28.Gershuny BS, Sher KJ. Compulsive checking and anxiety in a nonclinical sample: differences in cognition, behavior, personality, and affect. J Psychopath Behav Assess. 1995;17:19–38. [Google Scholar]
- 29.Mataix-Cols D, Jungue C, Vallejo J, Sanchez-Turet M, Verger K, Barrios M. Hemispheric functional imbalance in a sub-clinical obsessive-compulsive sample assessed by the Continuous Performance Test, Identical Pairs version. Psychiatry Res. 1997;72:115–26. doi: 10.1016/s0165-1781(97)00074-7. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin AH, Sher KJ. Deficits in set-shifting ability in nonclinical compulsive checkers. J Psychopath Behav Assess. 1992;14:81–92. [Google Scholar]
- 31.Mataix-Cols D, Junque C, Sanchez-Turet M, Vallejo J, Verger K, Barrios M. Neuropsychological functioning in a subclinical obsessive-compulsive sample. Biol Psychiatry. 1999;45:898–904. doi: 10.1016/s0006-3223(98)00260-1. [DOI] [PubMed] [Google Scholar]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 33.Grant JE. Impulse Control Disorders: A Clinician's Guide to Understanding and Treating Behavioral Addictions. WW Norton and Company; New York, NY: 2008. [Google Scholar]
- 34.Frisch MB, Cornell JMV. Clinical validation of the Quality of Life Inventory: a measure of life satisfaction for use in treatment planning and outcome assessment. Psychol Assess. 1993;4:92–101. [Google Scholar]
- 35.Lipszyc J, Schachar R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J Int Neuropsychol Soc. 2010;16:1064–76. doi: 10.1017/S1355617710000895. [DOI] [PubMed] [Google Scholar]
- 36.Newcombe VF, Outtrim JG, Chatfield DA, et al. Parcellating the neuroanatomical basis of impaired decision-making in traumatic brain injury. Brain. 2011;134:759–68. doi: 10.1093/brain/awq388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancini F, Gragnani A, Orazi F, Pietrangeli MG. Obsessions and compulsions: normative data on the Padua Inventory from an Italian non-clinical adolescent sample. Behav Res Ther. 1999;37:919–25. doi: 10.1016/s0005-7967(98)00195-8. [DOI] [PubMed] [Google Scholar]
- 38.Vivanade S, Rodrigues L, Wendt G, Bicca MG, Braga DT, Cordioli AV. Obsessive-compulsive symptoms and obsessive-compulsive disorder in adolescents: a population-based study. Rev Bras Psiquiatr. 2014;36:111–8. doi: 10.1590/1516-4446-2013-1113. [DOI] [PubMed] [Google Scholar]
- 39.Miettunen J, Veijola J, Lauronen E, Kantojarvi L, Joukamaa M. Sex differences in Cloninger's temperament dimensions--a meta-analysis. Compr Psychiatry. 2007;48:161–9. doi: 10.1016/j.comppsych.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Kampman OI, Viikki M, Jarventausta K, Leinonen E. Meta-analysis of anxiety disorders and temperament. Neuropsychobiology. 2014;69:175–86. doi: 10.1159/000360738. [DOI] [PubMed] [Google Scholar]
- 41.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- 42.Watkins LH, Sahakian BJ, Robertson MM, et al. Executive function in Tourette's syndrome and obsessive-compulsive disorder. Psychol Med. 2005;35:571–82. doi: 10.1017/s0033291704003691. [DOI] [PubMed] [Google Scholar]
- 43.Veale DM, Sahakian BJ, Owen AM, Marks IM. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive-compulsive disorder. Psychol Med. 1996;26:1261–9. doi: 10.1017/s0033291700035984. [DOI] [PubMed] [Google Scholar]
- 44.Morein-Zamir S, Papmeyer M, Pertusa A, et al. The profile of executive function in OCD hoarders and hoarding disorder. Psychiatry Res. 2014;215:659–67. doi: 10.1016/j.psychres.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological deficits in obsessive-compulsive disorder: a comparison with unipolar depression, panic disorder, and normal controls. Arch Gen Psychiatry. 1998;55:415–23. doi: 10.1001/archpsyc.55.5.415. [DOI] [PubMed] [Google Scholar]
- 46.Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin Psychol Rev. 2013;33:1163–71. doi: 10.1016/j.cpr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Sternberger LG, Burns GL. Obsessions and compulsions: psychometric properties of the Padua Inventory with an American college population. Behav Res Ther. 1990;28:341–5. doi: 10.1016/0005-7967(90)90087-y. [DOI] [PubMed] [Google Scholar]
- 48.Foa EB, Huppert JD, Leiberg S, et al. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess. 2002;14:485–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.