Abstract
Due to the close relation between oxidative stress and a plethora of inflammatory diseases, antioxidants have received an increased attention for incorporation into dermatological products. Their use and absorption is however limited by their low solubility in water-rich formulations. Herein, a set of novel cholinium-based salts, namely dicholinium ellagate and cholinium caffeate, syringate, vanillate, gallate and salicylate were synthetized and characterized. Their melting and decomposition temperatures, water solubility, and toxicological, antioxidant, cytotoxicity and pro-/anti-inflammatory activities were addressed. These new salts, exclusively composed of ions derived from natural sources, display a high thermal stability – up to 150 ºC. The synthesized compounds are significantly more soluble in water (in average, 3 orders of magnitude higher) than the corresponding phenolic acids. Furthermore, they present not only similar but even higher antioxidant and anti-inflammatory activities, as well as comparable cytotoxicity and lower ecotoxicity profiles than their acidic precursors. Amongst all the investigated salts, dicholinium ellagate is the most promising synthesized salt when considering the respective antioxidant and anti-inflammatory activities. Since all the synthesized salts are based on the cholinium cation, they can further be envisaged as essential nutrients to be used in oral drugs.
Keywords: Antioxidant salts, ecotoxicity, cytotoxicity, solubility, anti-inflammatory activity
Introduction
The human skin is constantly exposed to both endogenous and environmental pro-oxidant agents, leading to the formation of highly noxious reactive oxygen species (ROS). ROS-mediated oxidative damage includes a wide variety of pathological effects, such as DNA modification, lipid peroxidation, as well as the activation of inflammatory pathways. To minimize these deleterious effects, mammalian skin cells have antioxidant defense mechanisms, which comprise enzymatic and non-enzymatic antioxidant agents.1–2 However, these systems may not be enough to ensure the skin barrier integrity.3 In this context, antioxidants have found an increased interest as constituents of dermatological pharmaceutical formulations and skin care products.4–5 In both of these products there is a preference for antioxidants from natural rather than synthetic sources.6 Phenolic compounds are the most abundant secondary metabolites of plants, being recognized by their antioxidant and anti-inflammatory properties. These chemical compounds have one or more aromatic rings, with one or more hydroxyl groups directly bonded, and which can donate a hydrogen atom or an electron to a free radical, being thus ideal structures for free radical scavenging. Naturally available phenolic acids include gallic, caffeic, syringic and vanillic acids, typically present in sources such as fruits and vegetables.7–8 Nevertheless, the limited aqueous solubility of some of these phenol-based antioxidants represents a major drawback when envisaging their incorporation into water-rich dermatological formulations or for their absorption and transport in body fluids. To overcome this limitation, cholinium-based salts appear as promising candidates if based in compounds with antioxidant features aiming at enhancing their water solubility. The pioneering synthesis of cholinium salicylate ([Chol][Sal]) led to an increase in the water solubility (when compared with the salicylic acid precursor), while maintaining its anti-inflammatory, analgesic and antipyretic properties.9–10 Actually, [Chol][Sal] is an active pharmaceutical ingredient currently used in various medicinal products, namely Bonjela, Arthropan and Bucagel®.11
Cholinium chloride, an essential nutrient, has been receiving considerable attention due to its biocompatible and “non-toxic” nature.12–15 A significant number of cholinium salts has been reported coupled with a wide range of anions, such as amino acid-,16–20 carboxylic acid-,21–25 and good’s-buffers-based anions.26 In fact, the anion selection has been carried out according to specific tasks for which cholinium-based salts can be used and have demonstrated an enhanced potential. Distinct applications have been suggested, namely in catalysis,16 in photodynamic therapy,27 in electrical and pH-sensitive drug delivery systems,28 as crosslinking agents for collagen-based materials,23 as major solvents in the pre-treatment and dissolution of biomass,29 as co-substrates for microorganisms in the degradation of dyes,13 and as self-buffering compounds for the extraction and purification of biologically active molecules.26 Nevertheless, to the best of our knowledge, cholinium-based salts with remarkable antioxidant activities have not been reported hitherto.
In this work, a series of new cholinium-based salts with antioxidant and anti-inflammatory features were synthetized and characterized. Five anions with antioxidant and anti-inflammatory characteristics, namely gallate, caffeate, vanillate, syringate and ellagate, were conjugated with the cholinium (2-hydroxyethyl)trimethylammonium) cation. Additionally, [Chol][Sal] was also synthetized by neutralization to compare its antioxidant performance with the new cholinium-based salts studied in this work. The antioxidant activity of these compounds was investigated using the 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH) radical scavenging assay and compared with the archetypal ascorbic acid - a well-known antioxidant.30–32 Their physicochemical properties, namely melting point, decomposition temperature and water solubility were also assessed, as well as their impact towards Vibrio fischeri, a standard marine luminescent bacteria (Microtox® assay).33 Finally, the impact of these novel antioxidant salts on mammalian cells was evaluated. For that purpose, the three cholinium-based salts which have shown a better performance on the DPPH radical scavenging assay were chosen (cholinium gallate, cholinium caffeate and cholinium ellagate), and their cytotoxicity and pro-/anti-inflammatory activities were evaluated in Raw 264.7 and HaCaT mammalian cell lines.
Experimental Section
Materials
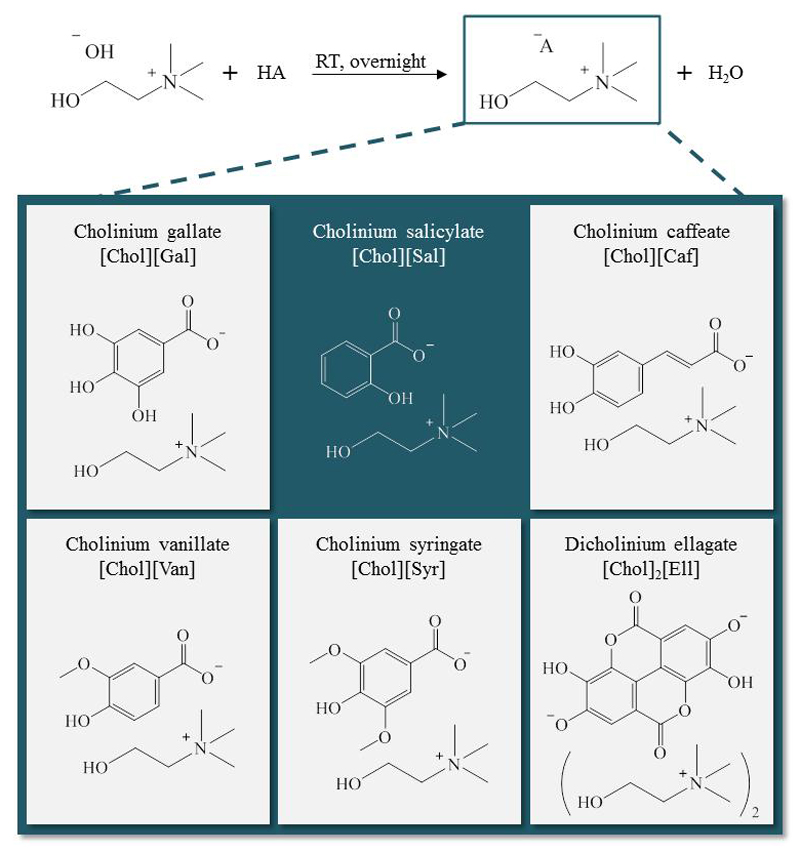
Six cholinium-based salts with antioxidant and/or anti-inflammatory properties were synthetized, namely [Chol][Gal], (2-hydroxyethyl) trimethylammonium 3,4,5-trihydroxybenzoate; [Chol][Sal], (2-hydroxyethyl) trimethylammonium 2-hydroxybenzoate; [Chol][Caf], (2-hydroxyethyl) trimethylammonium (E)-3-(3,4-dihydroxyphenyl)acrylate; [Chol][Van], (2-hydroxyethyl) trimethylammonium 4-hydroxy-3-methoxybenzoate, [Chol][Syr], (2-hydroxyethyl) trimethylammonium 4-hydroxy-3,5-dimethoxybenzoate; [Chol]2[Ell], di((2-hydroxyethyl) trimethylammonium) 3,8-dihydroxy-5,10-dioxo-5,10-dihydrochromeno[5,4,3-cde]chromene-2,7-bis(olate). Their full name, acronym and chemical structure are depicted in Scheme 1. Cholinium hydroxide ([Chol]OH, in methanol solution at 45 wt%), vanillic acid (97 wt% of purity) and 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH) were acquired from Sigma-Aldrich®. Syringic (98 wt% of purity) and ellagic (97 wt% of purity) acids were from Alfa Aesar®. Salicylic (99 wt% of purity), gallic (99.5 wt% of purity) and caffeic (99 wt% of purity) acids were from Acofarma, Merck® and Acros Organics, respectively. Methanol (HPLC grade), acetone (99.9 wt% of purity), and ethyl acetate (99 wt% of purity) were from VWR. The water used was double distilled, passed by a reverse osmosis system and further treated with a Milli-Q plus 185 water purification apparatus. The human keratinocyte cell line HaCaT, obtained from DKFZ (Heidelberg), was kindly supplied by Doctor Eugénia Carvalho (Centre for Neuroscience and Cell Biology, University of Coimbra, Portugal). Raw 264.7 (ATCC number: TIB-71), a mouse macrophage cell line, was kindly supplied by Doctor Otília Vieira (Centre for Neuroscience and Cell Biology, University of Coimbra, Portugal).
Scheme 1.
Synthesis scheme and chemical structure of the cholinium-based salts prepared.
Synthesis and characterization of cholinium salts
Six cholinium-based salts were synthetized by the neutralization of [Chol]OH with the respective acid, with a well-known antioxidant/anti-inflammatory character, namely the gallic, vanillic, caffeic, salicylic, syringic, and ellagic acids (Scheme 1).17–18, 23 The synthesis of [Chol]2[Ell] and [Chol][Gal] has already been reported in literature, however the synthetic route here proposed is more simple.34–35 [Chol]OH (1 equivalent, 45 wt% in a methanol solution) was added drop wise to the acidic solution in methanol, with a molar excess of 1.1 equivalents, at 0 ºC, under nitrogen atmosphere. Regarding the [Chol]2[Ell] synthesis, the [Chol]OH was added to the ellagic acid solution in methanol, with a molar ratio of 2:1. The reaction mixture was stirred at room temperature, under nitrogen atmosphere, and protected from light overnight, producing the cholinium salt and water as the by-product. The solvent and water were then removed under reduced pressure. Moreover, in the synthesis of [Chol][Van], [Chol][Syr] and [Chol][Caf], the unreacted antioxidant acid accumulated in the prepared IL was eliminated with acetone (3 x 20 mL), followed by filtration to remove the cholinium salt (which is in the solid state). The same procedure was adopted for [Chol][Gal], only replacing acetone by methanol. In the synthesis of [Chol][Sal], the remaining salicylic acid was accumulated as a viscous liquid, being removed by a liquid-liquid extraction with ethyl acetate (3 x 20 mL).24 Finally, the residual solvent was removed under reduced pressure and the obtained compound was dried under high vacuum for at least 48 h. The structure of all compounds synthesized was confirmed by 1H and 13C NMR, IR spectroscopy and elemental analysis, showing the high purity level of all the ionic structures after their synthesis, as reported in the Supporting Information.
Thermogravimetric analysis
The decomposition temperature was determined by thermogravimetric analysis (TGA). TGA was conducted on a Setsys Evolution 1750 (SETARAM) instrument. The sample was heated in an alumina pan, under a nitrogen atmosphere, over a temperature range of 25 - 800 ºC, and with a heating rate of 10 ºC.min-1.
Differential scanning calorimetry
Temperatures of melting transition temperature were measured in a power compensation differential scanning calorimeter, PERKIN ELMER model Pyris Diamond DSC, using hermetically sealed aluminum crucibles with a constant flow of nitrogen (50 mL.min-1). Samples of about 15 mg were used in each experiment. The temperature and heat flux scales of the power compensation DSC were calibrated by measuring the temperature and the enthalpy of fusion of reference materials namely benzoic acid, 4-metoxibenzoic acid, triphenylene, naphthalene, anthracene, 1,3,5-triphenylbenzene, diphenylacetic acid, perylene, o-terphenyl and 9,10-diphenylanthracene, at the scanning rate of 2 K.min-1 and flow of nitrogen. Temperatures of the thermal transitions and melting temperature were taken as the onset temperatures.
Water solubility
The water solubility of cholinium-based salts and of the corresponding antioxidant acids was determined from a saturated aqueous solution. An excess of each compound was added to pure water (≈ 1 mL), and allowed to equilibrate at constant temperature (25.0 ± 0.5 ºC), under constant agitation (750 rpm) for 72 hours using an Eppendorf Thermomixer Comfort equipment. After the equilibration time, properly optimized in this work, all samples were centrifuged at (25.0 ± 0.5) ºC in a Hettich Mikro 120 centrifuge during 20 minutes at 4500 rpm. Then, all samples were placed in an air bath equipped with a Pt 100 probe and PID controller at the aforementioned temperature in equilibrium assays during 2 hours. To determine the concentration of each cholinium salt and acid, a sample of the aqueous liquid phase was carefully collected, diluted in ultra-pure water, and quantified through UV-spectroscopy, using a SHIMADZU UV-1700, Pharma-Spec Spectrometer., at each λmax in the UV region. Their values, as well as the respective calibration curves, are reported in the Supporting Information, Table S1. Triplicate measurements were carried out.
DPPH radical scavenging assay
The antioxidant activities of cholinium-based salts and the respective acids, were determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay.30–32 The principle of the assay is based on the color change of the DPPH solution from purple to yellow, as the radical is quenched by the antioxidant. When a solution of DPPH is mixed with a substance that can donate a hydrogen, the reduced form of DPPH is obtained, and the solution which started to be violet turns to be yellow. This change in color was monitored by Visible (Vis) spectroscopy at 517 nm. Briefly, 250 μL of a DPPH solution (0.91 mmol.L-1) in methanol was mixed with different volumes (20, 30, 40, 50, 60, 70 and 80 μL) of a stock solution (with a well-known concentration) of each compound and then methanol was added to complete 4 mL (final volume). The samples were kept in the dark for 30, 90 and 120 minutes at room temperature and then the decrease in the absorbance at 517 nm was measured. The absorbance of the DPPH solution in the absence of the compounds under analysis was also measured as control. Ascorbic acid was used as positive control. DPPH radical scavenging activity - AA(%) - was expressed using equation (1):
| (1) |
where A0 is the absorbance of the control and A1 is the absorbance of the sample at 517 nm. DPPH scavenging activity is defined by the IC50 value - the concentration of the antioxidant needed to scavenge 50% of the DPPH present in the test solution. IC50 values were determined from the equations reported in the Supporting Information (Table S2) derived from the graphical representation of the scavenging activity against the sample concentration. Triplicate measurements were carried out.
Microtox® assay
To evaluate the ecotoxicity of the cholinium salts synthetized, as well as of the corresponding acids, the Standard Microtox® liquid-phase assay was applied. Microtox® is a bioluminescence inhibition method based on the bacterium Vibrio fischeri (strain NRRL B-11177) luminescence after its exposure to each sample solution at 15 ºC. In this work, the standard 81.9% test protocol was followed.33 The microorganism was exposed to a range of diluted aqueous solutions of each compound (from 0 to 81.9 wt%), where 100% corresponds to a previously prepared stock solution, with a known concentration. After 5, 15, and 30 minutes of exposure to each aqueous solution, the bioluminescence emission of Vibrio fischeri was measured and compared with the bioluminescence emission of a blank control sample. Thus, the corresponding 5 min-, 15 min- and 30 min-EC50 values (EC50 being the estimated concentration yielding a 50% of inhibition effect), plus the corresponding 95% confidence intervals, were estimated for each compound tested by non-linear regression, using the least-squares method to fit the data to the logistic equation. Previously to Microtox® testing, the amount of water was determined by Karl Fischer (KF) titration using a Metrohom 831 KF coulometric titrator. On the basis of this parameter, the real concentration of each stock solution was corrected, thus obtaining EC50 values with higher accuracy.
Evaluation of cytotoxicity
Human keratinocyte cell line HaCaT and Raw 264.7
Keratinocytes were cultured in a Dulbecco’s Modified Eagle Medium (high glucose) supplemented with 4 mM of glutamine, 10% heated inactivated fetal bovine serum, penicillin (100 U.mL-1) and streptomycin (100 μg.mL-1), at 37 °C, in a humidified atmosphere of 95% of air and 5% of CO2. Raw 264.7 was cultured in an Iscove’s Modified Dulbecco’s Eagle Medium supplemented with 10% of non-inactivated fetal bovine serum, penicillin (100 U.mL-1), and streptomycin (100 μg.mL-1), at 37 °C, in a humidified atmosphere of 95% of air and 5% of CO2. Along the experiments, the cells were periodically monitored by microscope observations in order to detect any morphological change imposed to the cells.
Cytotoxicity tests of cholinium-based salts and the respective acids
The cytotoxicity of the cholinium salts and respective acids was determined by exposing HaCaT and Raw 264.7 cells to distinct and increased concentrations of [Chol][Gal], [Chol][Caf], [Chol]2[Ell], gallic acid, caffeic acid and ellagic acid (in a range of concentrations between 1 µM and 5000 µM). The cells were seeded in 96-well plates and incubated during 24 hours to allow attachment, thus enabling high-throughput screening. cholinium salts samples, formulated at various dilutions in full-complement media, were added to the cells. A resazurin solution (10% v/v) was added to the cells during the last 2 and 1 hour(s) of incubation for HaCat and Raw 264.7 cells, respectively. After incubation, the absorbance of resorufin (the product of the resazurin reduction) was measured at 570 and 600 nm in a standard spectrophotometer MultiSkan Go (Thermo Fisher Scientific, Waltham, MA, USA). The treated cells were normalized regarding the control (untreated cells). To calculate the EC50 values, dose–response curves were fitted with the non-linear least squares method using a linear logistic model. The data reported correspond to the average of three biological independent experiments conducted in triplicate for each compound.
Nitric oxide (NO) measurement
The pro- or anti-inflammatory activity of [Chol][Gal], [Chol][Caf], [Chol]2[Ell], gallic acid, caffeic acid and ellagic acid, was evaluated in the mouse macrophage cell line Raw 264.7. The production of NO was measured by the accumulation of nitrite in the culture supernatants, using a colorimetric reaction with the Griess reagent. The cells were plated at 3 × 105 cells/well in 48-well culture plates, allowed to stabilize for 12 hours, and then incubated with the culture medium (control), or stimulated with 1 μg.mL-1 of lipopolysaccharide (LPS), or with 1 μg.mL-1 of LPS in presence of three concentrations (100 μM, 50 μM and 10 μM) of [Chol][Gal], [Chol][Caf], [Chol]2[Ell], ellagic acid, gallic acid and caffeic acid, for 24 hours. Briefly, 100 µL of culture supernatants were collected and diluted with equal volume of the Griess reagent [0.1% (w/v) N-(1-naphthyl) ethylenediamine dihydrochloride and 1% (w/v) sulfanilamide containing 5% (w/v) H3PO4] during 30 minutes, in the dark. The absorbance at 550 nm was measured using a standard spectrophotometer MultiSkan Go (Thermo Fisher Scientific, Waltham, MA, USA). Comparisons between multiple groups were performed by One-Way ANOVA analysis, with a Bonferroni´s Multiple Comparison post-test. Statistical analysis was performed using GraphPad Prism, version 5.02 (GraphPad Software, San Diego, CA, USA). Significance levels are as follows: *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001.
Results and Discussion
A set of new cholinium-based salts with antioxidant features were synthetized by the neutralization of [Chol]OH with five distinct acids with antioxidant and anti-inflammatory characteristics, namely the gallic, vanillic, caffeic, syringic, and ellagic acids. By way of comparison, [Chol][Sal] was also prepared. Their full name, acronym and chemical structure are depicted in Scheme 1. All cholinium salts were obtained with high purity levels and yield – cf. Experimental Section.
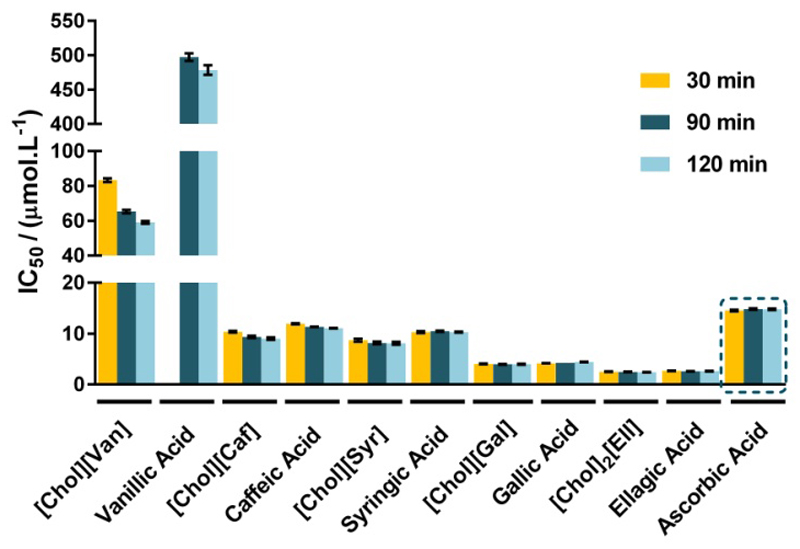
The antioxidant activity of all novel cholinium-based salts, as well as of their respective precursors, the phenolic acids, was investigated using the 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH) radical scavenging assay and compared with ascorbic acid, a well-known prototypic antioxidant. DPPH scavenging activity is usually evaluated by the IC50 value output, defined as the concentration of a given compound needed to scavenge 50% of DPPH present in the test solution. Taking into account the IC50 definition, a lower IC50 value reflects a better DPPH radical scavenging activity. The values depicted in Figure 1, expressed in μmol.L-1, reveal that all the synthesized cholinium-based salts present a higher antioxidant activity when compared with the respective acidic precursor. This trend is particularly visible with [Chol][Van] that displays a significantly higher DPPH radical scavenging activity than vanillic acid. Therefore, these novel compounds appear as promising antioxidant candidates since lower amounts of the cholinium salts are required to reach the same antioxidant activity when compared with the respective and traditional phenolic acids currently used. Moreover, since they are coupled to the cholinium cation, they can also be envisaged as a source of essential nutrients within the vitamin B complex – an outstanding characteristic for use either in dermatological formulations or as oral drugs.
Figure 1.
IC50 values (μmol.L-1) and respective standard deviations, after 30, 90 and 120 minutes of exposure to DPPH.
The antioxidant activity of [Chol][Sal] and salicylic acid was tested up to a concentration of 1 mg.mL-1, being impossible to determine the IC50 value for any of these two compounds. Vanillic acid also requires more time to exert its antioxidant activity, being the IC50 only reached after 90 and 120 minutes of exposure to DPPH. The IC50 data (in μg.mL-1), as well as the respective standard deviations, are provided in the Supporting Information, Table S3.
In general, for the cholinium-based salts synthetized, their antioxidant activity increases in the following order:
with [Chol]2[Ell] presenting the highest antioxidant activity.
The physicochemical properties of the antioxidant cholinium salts and respective acids, namely melting point, decomposition temperature and water solubility were additionally addressed. The melting temperatures (Tfus), dehydration temperature for the [Chol][Gal], glass transition temperature and cold crystallization temperatures were measured by differential scanning calorimetry (DSC) data and are presented in Table 1. The onset temperatures of decomposition (Td) were further evaluated by thermogravimetric analysis (TGA), and are reported in Table 1.
Table 1.
Thermal properties of the synthetized cholinium-based salts, namely the melting temperature (Tfus) and temperature of decomposition (Td).
| [Chol] [Van] |
[Chol] [Caf] |
[Chol] [Syr] |
[Chol] [Gal]a |
[Chol]2 [Ell] |
[Chol] [Sal]b |
Van. Acid | Caf. Acid |
Syr. Acid |
Gal. Acid |
Ell. Acid |
Sal. Acid |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tfus / ºC | 169 | 155 | 150 | 179 | 259 | 38 | 210e | 191d | 207f | 262c | 287d | 158c |
| Td / ºC | 186.8 | 155.0 | 178.9 | 185.3 | 265.0 | 226.0 | 233.7 | 218.3 | 256.4 | 262.5 | 472.6 | 183.9 |
Dehydration temperature = 140 ºC.
Glass transition temperature = – 56 ºC; Cold crystallization temperature = -14 ºC.
Mota et al.37
Estimated using a group-contribution method.38
Sigma database (http://www.sigmaaldrich.com/portugal.html).
Queimada et al.39
From the TGA profiles of the cholinium-based salts prepared, and of the corresponding acids (shown in the Supporting Information, Figure S1), as well as from the Td values reported in Table 1, it is possible to conclude that all compounds studied present a high thermal stability – at least up to 150 ºC. However, [Chol][Caf] and gallic acid decompose immediately after their melting temperatures are reached. Amongst all the investigated cholinium-based salts, [Chol]2[Ell] is the salt with the highest thermal stability (265 ºC). Cholinium-based salts also display a slightly lower thermal stability than the respective acids. [Chol][Sal] appears as an exception to this pattern since it presents a higher decomposition temperature compared to salicylic acid. Taking into account that the decomposition of cholinium chloride occurs at circa 305ºC,36 the obtained results show that the anion plays a crucial role in the thermal stability of cholinium salts. Actually, the trend observed in the thermal stability of phenolic acids is similar to that corresponding to cholinium-based salts. In general, the increase in the number of substituents at the benzene ring leads to a decrease of the thermal stability, particularly by the introduction of a methoxy group.
It is well-established that cholinium-based salts display, in general, a high solubility in water.40–41 The water solubility of the antioxidant-cholinium salts was also determined and compared with the water solubility of their corresponding acids at 25 ºC. The solubility data and the respective standard deviations are reported in Table 2. The values obtained for the phenolic acids are in close agreement with literature.37, 39 The results obtained for the cholinium-based salts demonstrate that these new antioxidant compounds display a solubility in water three orders of magnitude (in average) higher than the respective acidic precursors. This feature is certainly a great advantage afforded by these antioxidant salts to be incorporated into more formulations and for a widespread range of applications for which their high water solubility is relevant.
Table 2.
Water solubility of the synthetized salts and of the corresponding acids (mmol.L-1) at 25 ºC. The respective standard deviations (std) are also presented.
| (Water solubility ± std) / (mmol.L-1) |
||
|---|---|---|
| Y | [Chol][Y] | HY |
| Van | 3181.40 ± 118.17 | 10.43 ± 0.30 |
| Caf | 2722.47 ± 63.30 | 3.84 ± 0.04 |
| Syr | 2793.71 ± 17.98 | 7.40 ± 0.02 |
| Gal | 2402.27 ± 6.09 | 71.33 ± 2.70 |
| Ella | 622.97 ± 36.98 | 1.26E-02 ± 3.31E-04b |
| Sal | Completely miscible | 15.66 ± 0.14 |
[Chol]2[Ell].
From Queimada et al.39
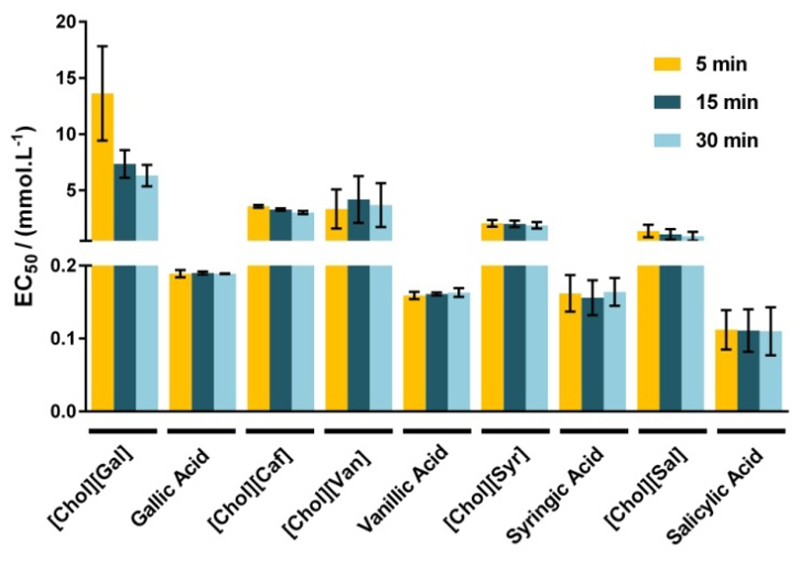
Albeit a high water solubility of the new ionic compounds can be valuable for their incorporation into dermatological and pharmaceutical formulations, on the other hand, these may lead to an increase on their potential release into aquatic ecosystems. The legislation concerning the (eco)toxicological hazards of several chemical compounds is nowadays more stringent in Europe, and all the new substances should be evaluated a priori by REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) before any industrial-scale application.42 Standard assays using the luminescent marine bacteria Vibrio fischeri are one of the most widespread toxicological bioassays used.43–46 The ecotoxicological impact of these cholinium-based salts was evaluated using the standard Microtox® acute assay. EC50 values (mg.L-1), the estimated concentration yielding a 50% of inhibition effect in the microorganism luminescence, were determined for the cholinium salts and the simple acids after 5, 15 and 30 minutes of exposure to the bacteria Vibrio fischeri - values reported in the Supporting Information file, Table S4. According to the obtained results (EC50 values at 30 min of exposure time), it is possible to categorize these compounds as belonging to the following Category: Acute III according to the European Classification; as (1) “practically harmless” ([Chol][Caf], [Chol][Syr] and [Chol][Sal], with 100 mg.L-1 < EC50 < 1000 mg.L-1); and as (2) “harmless” ([Chol][Van] and [Chol][Gal] with EC50 > 1000 mg.L-1).47 On the opposite, all antioxidant acidic precursors are “moderately toxic” (with 10 mg.L-1 < EC50 < 100 mg.L-1), according to Passino’s classification.47 Figure 2 depicts the EC50 data in mmol.L-1. Their ecotoxicity increases as follows:
being [Chol][Gal] the less toxic and [Chol][Sal] the most toxic cholinium-based salts, respectively. The EC50 values of [Chol][Van] and [Chol][Syr] suggest that the incorporation of methoxy groups into the aromatic ring increases their ecotoxicity. Even though, in general, all the cholinium-based salts with antioxidant features also display a remarkably lower ecotoxicological impact than their precursors, which further supports their potential use at large-scale applications.
Figure 2.
EC50 values (mmol.L-1) determined after 5, 15 and 30 minutes of Vibrio fischeri exposure. The error bars correspond to 95 % confidence level limits.
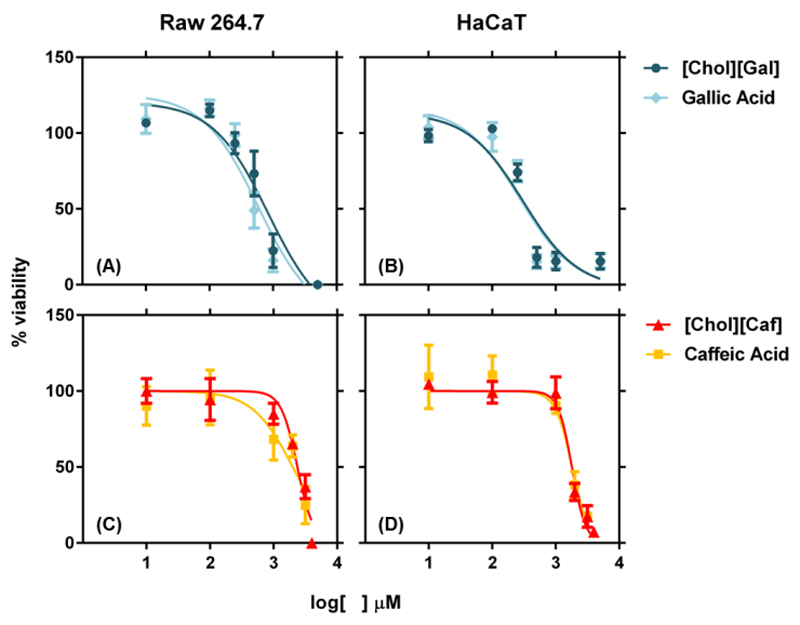
Taking into consideration the high antioxidant activity and/or low ecotoxicity of [Chol][Gal], [Chol][Caf] and [Chol]2[Ell], these cholinium salts were chosen to further evaluate their in vitro cytotoxicity and anti-inflammatory features, by addressing their impact, as well as of the corresponding acid counterparts, on the capacity of Raw 264.7 (macrophages) and HaCaT (keratinocytes) to metabolize the dye resazurin. Figure 3 depicts the EC50 data, which represents the concentration of each compound that, for 24 hours of exposure, induces a 50% decrease in the cell viability.
Figure 3.
Viability of Raw 264.7 and HaCaT cells assessed as the normalized response of treated cells to untreated controls, measured by the metabolic conversion of resazurin. The data shown represents the dose-response curves of Raw 264.7 cells to: (A) [Chol][Gal] EC50: 835.8 µM and Gallic Acid EC50: 590.7 µM; and (C) [Chol][Caf] EC50: 2336 µM and Caffeic Acid EC50: 1996 µM; and HaCaT cells to (B) [Chol][Gal] EC50: 303.5 µM and Gallic Acid EC50: 267.1 µM; and (D) [Chol][Caf] EC50: 1794 µM and Caffeic Acid EC50: 1803 µM.
[Chol][Gal] and gallic acid have similar cytotoxic profiles against Raw 264.7 (EC50: 835.8 µM and EC50: 590.7 µM, respectively) and HaCaT (EC50: 303.5 µM and EC50: 267.1 µM, respectively) cell lines. [Chol][Caf] causes a 50% decrease in cell viability at 2336 µM towards the macrophage cells and at 1794 µM for the keratinocytes. These values are of similar magnitude with those found for caffeic acid in macrophage cells (EC50: 1996 µM) and keratinocytes (EC50: 1803 µM). For [Chol]2[Ell] and ellagic acid it was not possible to accurately determine the EC50 cytotoxic values due to restrictions regarding their solubility limits in cell culture medium, given that the presence of salts in the cells medium leads to the cholinium salt and acid precipitation. Although the [Chol]2[Ell] presents a high water solubility, its precipitation was also observed with the addiction of osmotic solution during the Microtox® test. For the maximum concentration of [Chol]2[Ell] achieved (125 µM), it was not observed a decrease in the cell viability.
For all the compounds investigated, the cells survival rate decreases with the increase on the cholinium salt concentration. Overall, the obtained results demonstrate that the antioxidant cholinium-based salts possess cytotoxicity profiles over mammalian cells similar to their parent acids, allowing therefore their safe utilization in products for human healthcare.
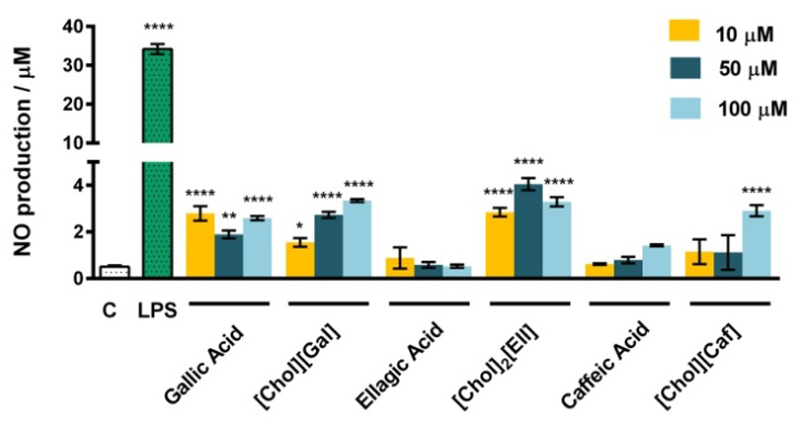
In addition to the evaluation of the toxicity profile of the novel cholinium-based salts, it is crucial to ensure that they do not present immunostimulatory abilities when envisaged as novel products for the formulation of human care products. With this goal in mind, we further analyzed the effects of [Cho][Gal], [Chol][Caf], [Chol]2[Ell] and the respective counterpart acids in the production of nitric oxide (NO) by macrophages. The production of NO results from the activation of macrophages and consequent increased expression of nitric oxide synthase, a strong pro-inflammatory mediator closely associated with numerous inflammatory diseases. As shown in Figure 4, the three concentrations tested for each compound (100 μM, 50 μM and 10 μM) barely induce the production of NO in macrophages when compared to a classical pro-inflammatory stimulus, such as bacterial lipopolysaccharide (LPS). Additionally, the investigated cholinium salts do not present a significant pro-inflammatory activity when compared with the respective acids, indicating thus that the synthesis pathway used in the present work represents a biologically safe modification.
Figure 4.
Effect of cholinium-based salts and their respective acids on the NO production in macrophages for concentrations of 10 μM, 50 μM and 100 μM. The results are expressed as the amount of NO produced by the control cells maintained in a culture medium. LPS at a concentration of 1 μg.mL-1 was used as a positive control. Each value represents the average value and the respective standard deviation obtained from 3 independent experiments (*p <0.05, **p <0.01, ****p <0.0001).
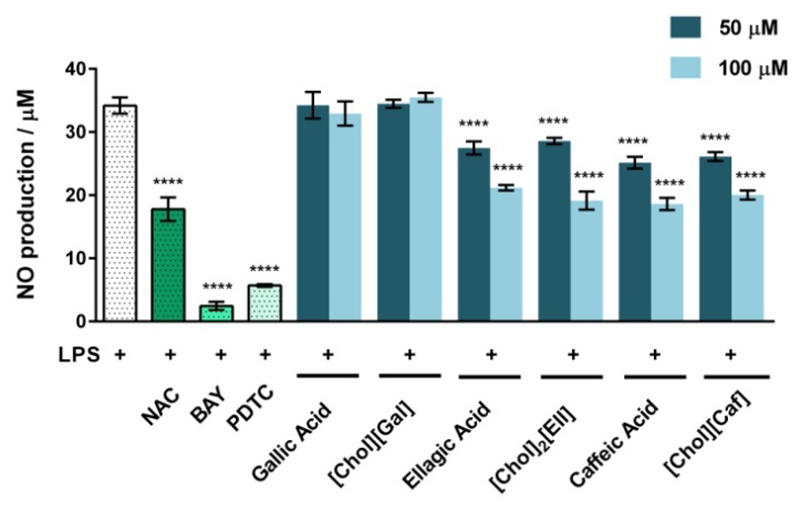
As some antioxidant compounds display also anti-inflammatory activity, we finally addressed whether the synthesized cholinium salts and respective acids can be used in parallel as anti-inflammatory drugs. To this end, an in vitro inflammatory model consisting of Raw 264.7 macrophages stimulated with LPS was used. Cells were pre-treated for 1 hour with 100 μM and 50 μM of the cholinium-based salts or their respective acids and then exposed to the strong inflammation activator LPS. The potential anti-inflammatory activities of the studied compounds were evaluated as the effect over the LPS-induced NO production (Figure 5). The prototypical anti-inflammatory N-Acetyl-Cysteine (NAC) compound, as well as the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) inhibitors, BAY and pyrrolidine dithiocarbamate (PTDC), were also used as positive controls.
Figure 5.
Effect of NAC, BAY, PDTC, cholinium-based salts and their respective acids (at 100 μM and 50 μM) on the inhibition of LPS-induced NO production in macrophages. The results are expressed as the amount of NO produced relatively to cells treated with 1 μg.mL-1 of LPS. Each value represents the average value and the respective standard deviation obtained from 3 independent experiments (****p<0.0001: LPS vs LPS + treatment).
The capacity of the synthesized cholinium-based salts to inhibit the NO production is identical to their parent acids. The compounds with higher anti-inflammatory activity are [Chol]2[Ell] and [Chol][Caf] which significantly inhibit the LPS-induced NO increase. Their anti-inflammatory effects are however smaller than BAY and PDTC; yet, of the same order of magnitude of NAC, a well-known antioxidant and anti-inflammatory molecule. It should be highlighted that although the NO production is a common readout in high-throughput screening for anti-inflammatory compounds, it represents only a single parameter that not completely resumes an inflammation pattern. In addition to the NO inhibition, caffeic acid strongly inhibits the production of prostaglandin E2, leukotrienes and pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-648–50, while the anti-inflammatory activity of ellagic acid was shown to rely on the decrease of NO, IL-6, TNF-α, IFN-γ and COX-2.51–52 Therefore, it is expected that the correspondent cholinium-based salts are also able to maintain these biological effects. On the other hand, we observed that neither [Chol][Gal] nor gallic acid decrease the LPS-induced NO production by macrophages. These results are in accordance with previous reports, where 3,4,5-trihydroxybenzoic acid (gallic acid) was shown to display a limited anti-inflammatory ability, while being more effective as an antibacterial and anticancer agent.53–55
Conclusions
Antioxidant cholinium-based salts with outstanding water-solubility, and anti-inflammatory activities, exclusively composed of ions derived from natural sources, were synthesized and characterized for the first time. All these compounds present a good thermal stability, at least up to 150 ºC, with [Chol]2[Ell] being the cholinium salt with the highest thermal stability (265 ºC). The data obtained further reveal that these new cholinium-based salts present not only similar or even higher antioxidant and anti-inflammatory activities, as well as comparable cytotoxicity and lower ecotoxicity profiles than their respective acidic precursors. Considering the [Chol][Sal] and salicylic acid, and although their anti-inflammatory profiles are well reported in the literature, their antioxidant activity was tested up to a concentration of 1 mg.mL-1, being impossible to determine the IC50 value for any of these two compounds by DPPH. Then, the five new cholinium-based salts display a significant added-value in terms of antioxidant activity compared with [Cho][Sal]. Furthermore, the synthesized compounds are significantly more soluble in water (in average, 3 orders of magnitude higher) than the corresponding acids, rendering thus these new antioxidant and anti-inflammatory cholinium salts as more valuable candidates in the formulation of pharmaceutical/cosmetic products. Finally, [Chol]2[Ell] seems to be one of the most promising cholinium salts here synthetized in terms of antioxidant and anti-inflammatory activities. Since all synthetized compounds are based on the cholinium cation, they can also be foreseen as essential nutrients for use in dermatological formulations and oral drugs.
Supplementary Material
Synthesis and characterization of cholinium-based salts. Calibration curves used to determine the water solubility of the cholinium-based salts and of the respective acids. Equations derived from the graphical representation of scavenging activity against the sample concentration. IC50 (μg.mL-1) values determined for the cholinium-based salts under study and for the respective acids. TGA curves of the synthesized cholinium-based salts and of the respective acidic species. EC50 values (mg.L-1) for the antioxidant cholinium salts under study and for the corresponding acids, after exposure to the Vibrio fischeri. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
This work was developed in the scope of the project CICECO - Aveiro Institute of Materials (Ref. FCT UID/CTM/50011/2013), financed by national funds through the FCT/MEC and when applicable, co-financed by FEDER under the PT2020 Partnership Agreement. The authors are grateful for financial support from FCT for the doctoral grant SFRH/BD/85871/2012 of T.E. Sintra. and post-doctoral grant SFRH/BPD/79263/2011 of S. P. M. Ventura. M. G. Freire acknowledges the European Research Council (ERC) for the Starting Grant ERC 2013-StG-337753.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Kovacic P, Somanathan R. Dermal Toxicity and Environmental Contamination: Electron Transfer, Reactive Oxygen Species, Oxidative Stress, Cell Signaling, and Protection by Antioxidants. In: Whitacre DM, editor. Reviews of Environmental Contamination and Toxicology. Vol. 203. Springer; New York: 2010. pp. 119–138. [DOI] [PubMed] [Google Scholar]
- 2.Lin CB, Southall MD. New insights on the regulation of extracellular matrix proteins during skin aging. Skin Aging: New Research. 2013:23–41. [Google Scholar]
- 3.Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol. 2003;17(6):663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 4.Podda M, Grundmann-Kollmann M. Low molecular weight antioxidants and their role in skin ageing. Clin Exp Dermatol. 2001;26(7):578–582. doi: 10.1046/j.1365-2230.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs J. Potentials and limitations of the natural antioxidants RRR-alpha-tocopherol, l-ascorbic acid and β-carotene in cutaneous photoprotection. Free Radic Biol Med. 1998;25(7):848–873. doi: 10.1016/s0891-5849(98)00161-0. [DOI] [PubMed] [Google Scholar]
- 6.Qi H, Zhang Q, Zhao T, Chen R, Zhang H, Niu X, Li Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromolec. 2005;37(4):195–199. doi: 10.1016/j.ijbiomac.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Škrovánková S, Mišurcová L, Machu L. Chapter three - Antioxidant activity and protecting health effects of common medicinal plants. In: Jeyakumar H, editor. Advances in Food and Nutrition Research. Vol. 67. Academic Press; 2012. pp. 75–139. [DOI] [PubMed] [Google Scholar]
- 9.Richard SB, Lorz E. Process for the preparation of choline salicylate. Patent US3141035 A. 1964
- 10.Broh-Kahn RH, Sasmor EJ. Choline salicylate composition and methods of use. Patent US3069321 A. 1962
- 11.Bonjela. A topical salicylate gel for control of pain and inflammation in dentistry and oral medicine. The Lamp. 1972;29(1):24. [PubMed] [Google Scholar]
- 12.Petkovic M, Ferguson JL, Gunaratne HQN, Ferreira R, Leitao MC, Seddon KR, Rebelo LPN, Pereira CS. Novel biocompatible cholinium-based ionic liquids-toxicity and biodegradability. Green Chem. 2010;12(4):643–649. [Google Scholar]
- 13.Sekar S, Surianarayanan M, Ranganathan V, MacFarlane DR, Mandal AB. Choline-based ionic liquids-enhanced biodegradation of azo dyes. Environ Sci Technol. 2012;46(9):4902–4908. doi: 10.1021/es204489h. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Liu X, Pei Y, Wang J, He M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012;14(10):2941–2950. [Google Scholar]
- 15.Gorke J, Srienc F, Kazlauskas R. Toward advanced ionic liquids. Polar, enzyme-friendly solvents for biocatalysis. Biotechnol Bioprocess Eng. 2010;15(1):40–53. doi: 10.1007/s12257-009-3079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S, Jiang T, Zhang Z, Zhu A, Han B, Song J, Xie Y, Li W. Functional ionic liquid from biorenewable materials: synthesis and application as a catalyst in direct aldol reactions. Tetrahedron Lett. 2007;48(32):5613–5617. [Google Scholar]
- 17.García-Suárez EJ, Menéndez-Vázquez C, García AB. Chemical stability of choline-based ionic liquids supported on carbon materials. J Mol Liq. 2012;169:37–42. [Google Scholar]
- 18.Moriel P, García-Suárez EJ, Martínez M, García AB, Montes-Morán MA, Calvino-Casilda V, Bañares MA. Synthesis, characterization, and catalytic activity of ionic liquids based on biosources. Tetrahedron Lett. 2010;51(37):4877–4881. [Google Scholar]
- 19.Gouveia W, Jorge TF, Martins S, Meireles M, Carolino M, Cruz C, Almeida TV, Araújo MEM. Toxicity of ionic liquids prepared from biomaterials. Chemosphere. 2014;104:51–56. doi: 10.1016/j.chemosphere.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 20.Hou X-D, Li N, Zong M-H. Facile and simple pretreatment of sugar cane bagasse without size reduction using renewable ionic liquids–water mixtures. ACS Sustainable Chem Eng. 2013;1(5):519–526. [Google Scholar]
- 21.Fukaya Y, Iizuka Y, Sekikawa K, Ohno H. Bio ionic liquids: room temperature ionic liquids composed wholly of biomaterials. Green Chem. 2007;9(11):1155–1157. [Google Scholar]
- 22.Muhammad N, Hossain MI, Man Z, El-Harbawi M, Bustam MA, Noaman YA, Mohamed Alitheen NB, Ng MK, Hefter G, Yin C-Y. Synthesis and physical properties of choline carboxylate ionic liquids. J Chem Eng Data. 2012;57(8):2191–2196. [Google Scholar]
- 23.Vijayaraghavan R, Thompson BC, MacFarlane DR, Kumar R, Surianarayanan M, Aishwarya S, Sehgal PK. Biocompatibility of choline salts as crosslinking agents for collagen based biomaterials. Chem Commun. 2010;46(2):294–296. doi: 10.1039/b910601d. [DOI] [PubMed] [Google Scholar]
- 24.Yu Y, Lu X, Zhou Q, Dong K, Yao H, Zhang S. Biodegradable naphthenic acid ionic liquids: synthesis, characterization, and quantitative structure–biodegradation relationship. Chem Eur J. 2008;14(35):11174–11182. doi: 10.1002/chem.200800620. [DOI] [PubMed] [Google Scholar]
- 25.Mourão T, Tomé LC, Florindo C, Rebelo LPN, Marrucho IM. Understanding the role of cholinium carboxylate ionic liquids in PEG-based aqueous biphasic systems. ACS Sustainable Chem Eng. 2014;2(10):2426–2434. [Google Scholar]
- 26.Taha M, Almeida MR, e Silva FA, Domingues P, Ventura SPM, Coutinho JAP, Freire MG. Novel biocompatible and self-buffering ionic liquids for biopharmaceutical applications. Chem Eur J. 2015;21(12):4781–4788. doi: 10.1002/chem.201405693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demberelnyamba D, Ariunaa M, Shim Y. Newly synthesized water soluble cholinium-purpurin photosensitizers and their stabilized gold nanoparticles as promising anticancer agents. Int J Mol Sci. 2008;9(5):864–871. doi: 10.3390/ijms9050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dias AMA, Cortez AR, Barsan MM, Santos JB, Brett CMA, de Sousa HC. Development of greener multi-responsive chitosan biomaterials doped with biocompatible ammonium ionic liquids. ACS Sustainable Chem Eng. 2013;1(11):1480–1492. [Google Scholar]
- 29.Garcia H, Ferreira R, Petkovic M, Ferguson JL, Leitao MC, Gunaratne HQN, Seddon KR, Rebelo LPN, Silva Pereira C. Dissolution of cork biopolymers in biocompatible ionic liquids. Green Chem. 2010;12(3):367–369. [Google Scholar]
- 30.Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26:211–219. [Google Scholar]
- 31.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1985;181:1199–1200. [Google Scholar]
- 32.Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21(2):143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AZUR Environmental, MicrotoxOmniTM Software for Windows® 95/98/NT. Carlsbad, CA U.S.A: 1999. [Google Scholar]
- 34.Hochstein FA. Water soluble salts of ellagic acid. Patent US3576007 A. 1971
- 35.John K. Choline gallate and its preparation. Patent US2589707 A. 1952
- 36.Lide DR, Milne GWA. Handbook of data on organic compounds. 3rd ed. Vol. 1. CRC Press; Boca Raton Fla: 1994. pp V3: 2594. [Google Scholar]
- 37.Mota FL, Queimada AJ, Pinho SP, Macedo EA. Aqueous solubility of some natural phenolic compounds. Ind Eng Chem Res. 2008;47(15):5182–5189. [Google Scholar]
- 38.Marrero J, Gani R. Group-contribution based estimation of pure component properties. Fluid Phase Equilib. 2001;183–184:183–208. [Google Scholar]
- 39.Queimada AJ, Mota FL, Pinho SP, Macedo EA. Solubilities of biologically active phenolic compounds: measurements and modeling. J Phys Chem B. 2009;113(11):3469–3476. doi: 10.1021/jp808683y. [DOI] [PubMed] [Google Scholar]
- 40.Klein R, Kellermeier M, Touraud D, Müller E, Kunz W. Choline alkylsulfates – New promising green surfactants. J Colloid Interface Sci. 2013;392:274–280. doi: 10.1016/j.jcis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Klein R, Tiddy GJT, Maurer E, Touraud D, Esquena J, Tache O, Kunz W. Aqueous phase behaviour of choline carboxylate surfactants-exceptional variety and extent of cubic phases. Soft Matter. 2011;7(15):6973–6983. [Google Scholar]
- 42.EC 2007. http://ec.europa.eu/environment/chemicals/reach.
- 43.Ventura SPM, e Silva FA, Gonçalves AMM, Pereira JL, Gonçalves F, Coutinho JAP. Ecotoxicity analysis of cholinium-based ionic liquids to Vibrio fischeri marine bacteria. Ecotoxicol Environ Saf. 2014;102:48–54. doi: 10.1016/j.ecoenv.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Ventura SPM, Marques CS, Rosatella AA, Afonso CAM, Gonçalves F, Coutinho JAP. Toxicity assessment of various ionic liquid families towards Vibrio fischeri marine bacteria. Ecotoxicol Environ Saf. 2012;76(1):162–168. doi: 10.1016/j.ecoenv.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Hernández-Fernández FJ, Bayo J, Pérez de los Ríos A, Vicente MA, Bernal FJ, Quesada-Medina J. Discovering less toxic ionic liquids by using the Microtox® toxicity test. Ecotoxicol Environ Saf. 2015;116:29–33. doi: 10.1016/j.ecoenv.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 46.Parvez S, Venkataraman C, Mukherji S. A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ Int. 2006;32(2):265–268. doi: 10.1016/j.envint.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 47.Passino DRM, Smith SB. Acute bioassays and hazard evaluation of representative contaminants detected in great lakes fish. Environ Toxicol Chem. 1987;6(11):901–907. [Google Scholar]
- 48.Koshihara Y, Neichi T, Murota S, Lao A, Fujimoto Y, Tatsuno T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. BBA-Lipid Lipid Met. 1984;792(1):92–97. [PubMed] [Google Scholar]
- 49.Yang WS, Jeong D, Yi Y-S, Park JG, Seo H, Moh SH, Hong S, Cho JY. IRAK1/4-Targeted anti-inflammatory action of caffeic acid. Mediators Inflamm. 2013;2013:12. doi: 10.1155/2013/518183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Búfalo MC, Ferreira I, Costa G, Francisco V, Liberal J, Cruz MT, Lopes MC, Batista MT, Sforcin JM. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J Ethnopharmacol. 2013;149(1):84–92. doi: 10.1016/j.jep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Hseu Y-C, Chou C-W, Senthil Kumar KJ, Fu K-T, Wang H-M, Hsu L-S, Kuo Y-H, Wu C-R, Chen S-C, Yang H-L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol. 2012;50(5):1245–1255. doi: 10.1016/j.fct.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 52.Chao PC, Hsu CC, Yin MC. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab. 2009;6:33. doi: 10.1186/1743-7075-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:21. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borges A, Ferreira C, Saavedra MJ, Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist. 2013;19(4):256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 55.Wang K, Zhu X, Zhang K, Zhu L, Zhou F. Investigation of gallic acid induced anticancer effect in human breast carcinoma MCF-7 Cells. J Biochem Mol Toxic. 2014;28(9):387–393. doi: 10.1002/jbt.21575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.