Abstract
The purpose of this study was to examine the oxygen uptake () kinetics and the energy systems’ contribution at 97.5, 100 and 102.5% of the maximal lactate steady state (MLSS) swimming intensity. Ten elite female swimmers performed three-to-five 30 min submaximal constant swimming bouts at imposed paces for the determination of the swimming velocity (v) at 100%MLSS based on a 7 x 200 m intermittent incremental protocol until voluntary exhaustion to find the v associated at the individual anaerobic threshold. kinetics (cardiodynamic, primary and slow component phases) and the aerobic and anaerobic energy contributions were assessed during the continuous exercises, which the former was studied for the beginning and second phase of exercise. Subjects showed similar time delay (TD) (mean = 11.5–14.3 s) and time constant (τp) (mean = 13.8–16.3 s) as a function of v, but reduced amplitude of the primary component for 97.5% (35.7 ± 7.3 mL.kg.min-1) compared to 100 and 102.5%MLSS (41.0 ± 7.0 and 41.3 ± 5.4 mL.kg.min-1, respectively), and τp decreased (mean = 9.6–10.8 s) during the second phase of exercise. Despite the slow component did not occur for all swimmers at all swim intensities, when observed it tended to increase as a function of v. Moreover, the total energy contribution was almost exclusively aerobic (98–99%) at 97.5, 100 and 102.5%MLSS. We suggest that well-trained endurance swimmers with a fast TD and τp values may be able to adjust faster the physiological requirements to minimize the amplitude of the slow component appearance, parameter associated with the fatigue delay and increase in exhaustion time during performance, however, these fast adjustments were not able to control the progressive fatigue occurred slightly above MLSS, and most of swimmers reached exhaustion before 30min swam.
Introduction
An important aspect of aerobic endurance performance is the ability to sustain the highest percentage of maximal oxygen uptake () as long as possible. In this sense, coaches and swimmers have used the in different submaximal intensities to control, prescribe and improve sports training [1]. Additionally, scientists have shown that the kinetics analysis may help to understand the physiological adjustments produced over time by the athletes in several sports, allowing them to maintain a high in a physiological steady-state during aerobic endurance performance [2–4].
Meanwhile, the scientific community has mainly described the kinetics in three different intensity domains during continuous exercise. First, the moderate domain is described as the exercise intensities in which a state steady for is achieved within 3 min of constant exercise [5]. Subsequently, the heavy domain is described as the exercise intensities in which slow component should be evident, causing a delay on the achievement of the steady-state during exercise [2]. Last, the severe domain is described as the exercise intensities in which is elevated compared to rest values and continue to increase over time, leading to attain the [6, 7].
Maximal lactate steady state (MLSS) is considered one of the main relevant parameters for prescription and improvement of aerobic endurance performance, once it has been assumed as the limit intensity at which, during prolonged and submaximal exercise, the metabolic energy is produced mainly by the aerobic metabolism of pyruvate and glycolysis [8, 9]. Moreover, MLSS is identified as the maximal intensity that can be maintained over time without the lactate production exceeding removal more than 1 mmol.L-1, and considered gold-standard method for the evaluation of aerobic capacity [10–12].
Once maximal velocity where a steady-state is found represents a fundamental physiological border, subtle changes in this intensity could likely modify kinetics response. For instance, when the exercise is performed at intensities slightly below MLSS, a physiological steady state is sustained for both blood lactate concentration [La-] and as a function of time [6, 7, 13]. On the other hand, at intensities above the MLSS, a significant increase in [La-] and is likely to be observed throughout time [3, 7, 8, 12], leading to fatigue and voluntary exhaustion [3, 4, 14]. Moreover, the swimming MLSS determination needs a short time of interruption for the blood collection during the 10th minute of exercise for the analysis of [La-], and then, a resumption of exercise to complete the test. Thus, it seems to be fundamental to examine the behavior of kinetics not only the beginning of exercise, but too after the resumption of exercise throughout exercise to better understanding of the entire process of the swimmer physiological response along the exercise.
kinetics has been studied in different sports over the last decades [2, 6, 15], and there are relevant number of researches based on [La-] and gas exchange at intensities related to MLSS [8, 13, 14]. However, no study has evaluated kinetics at (and around) the MLSS intensity. Thus, our purpose was to examine kinetics and the energy systems’ contribution at 97.5, 100 and 102.5%MLSS in swimming. It was hypothesized that at 97.5%MLSS, kinetics adjustments may not be so evident such as 100 and 102.5%MLSS. It was further hypothesized that even at the 100%MLSS intensity, swimmers may also have to adjust kinetics during the exercise, once this intensity would lead to voluntary exhaustion over time. On the other hand, at the intensity of 102.5%MLSS, kinetics may be compromised by fatigue, requiring faster time adjustments for time delay and time constant, and higher amplitudes either for primary or slow components compared to lower exercise intensities. We further intended to assess kinetics of the second phase of exercise, starting after the collection of [La-] and resumption of exercise (from 10th min to the exercise end—final exercise), hypothesizing that these parameters could be faster than without previous exercise. Moreover, as MLSS may be maintained for long time period without continuous [La-] accumulation, as well as a submaximal exercise, the energy supply should be mainly supported through the aerobic system for the swimming intensities of ± 2.5% around MLSS.
Material and methods
Ten elite female swimmers volunteered and gave written informed consent (or parent/guardian when subjects were under 18yrs) to participate in the present study, which was approved by the Ethics Committee of Faculty of Sport from the University of Porto and performed according to the Declaration of Helsinki. The swimmers were (mean ± SD) 17.6 ± 1.9 years of age, 1.70 ± 0.05 m height, 61.3 ± 5.8 kg body mass, 15.5 ± 2.9% body fat mass, and 54.9 ± 6.7 mL.kg.min-1 , specialized in middle- and long-distance swimming events. The subjects had, at the least, seven years of experience as competitive swimmers and their mean performance over a 400m freestyle swim was 88.0 ± 3.4% of the short course word record.
The test sessions were performed in a 25 m indoor swimming pool. Air humidity was maintained nominally between 40–60%, and pool water temperature between 27–28°C. Swimmers were advised to refrain from intense training at least 24 h before the experimental sessions. The tests were conducted within a seven day period, at the same time of the day (± 2 h), minimizing the circadian rhythm effects. Previously to the test sessions, swimmers performed a 1000 m warm-up at low/moderate intensity. The tests were performed in front crawl, with in-water starts and open turns, without relevant underwater glides. A 24 h interval was imposed between all tests.
Initially, swimmers performed an intermittent incremental protocol until voluntary exhaustion to find the velocity (v) corresponding to the individual anaerobic threshold (IAnT). The distance covered in each step was 200 m, with v increases of 0.05 m.s-1 and 30 s rest intervals between each swim [16]. According to these authors, the predetermined v of the last step was defined as the currently best expected performance for the subjects’ 400 m front crawl, and then used to define all the v steps for the incremental test. The IAnT was assessed by the relationship between [La-] and v using a curve fitting method, and considered the interception point between linear and exponential regressions to determine the accurate v where [La-] increased exponentially [16, 17].
Subsequently, each swimmer performed three-to-five 30 min submaximal constant swimming bouts at imposed paces to determine the highest v where a MLSS was achieved (100%MLSS). The first trial was performed at the v corresponding to IAnT; and, if a steady state or a decrease in [La-] was observed, further subsequent trials with 2.5% higher velocities were performed until no [La-] steady state could be maintained [14]. Following this study, if the first trial resulted in a clearly identifiable increase of the [La-], and/or could not be sustained due to exhaustion, further trials were conducted with reduced velocities. MLSS was defined as the [La-] that increased by no more than 1 mmol.l-1 between the 10th and 30th min of the test [9].
Earlobe capillary blood samples (5 μL) were collected: (a) at rest and in the first 30 s after each step of the incremental test, immediately after exhaustion, and at each 2 min of recovery (until the [La-] recovery peak was found); and (b) at rest, 10 and 30th min (or voluntary exhaustion) of each continuous bout (Lactate Pro, Arkray, Inc., Kyoto, Japan).
The v was set and maintained using a visual underwater pacer (GBK-Pacer, GBK Electronics, Aveiro, Portugal), with lights located each 2.5 m apart by a light strip on the bottom of the pool. Swimmers followed the flashing lights to maintain the predetermined velocities. and were instructed to keep their heads above each visual signal. Exhaustion was defined when the swimmers remained 5 m behind the lights.
was measured by a telemetric portable gas analyzer (K4b2, Cosmed, Italy) in both tests, connected to the swimmer by a low hydrodynamic resistance respiratory snorkel and valve system (New AquaTrainer®, Cosmed, Italy). This system has been previously validated [18] and used in similar studies [15]. The device was calibrated for minute ventilation () with a calibrated syringe (3 L) and the O2 and CO2 analyzers with standard calibration gases (16% O2 and 5% CO2) before each test. In all tests, data were analyzed and errant breaths occurred by swallow water and/or saliva, sighs and coughs were excluded. Afterwards, values were measured in mean ± 3 SD and outside values were removed. Subsequently, the breath-by-breath data were linearly interpolated to provide five-by-five s values, and smoothed using three breath averages [15, 19]. Heart rate (HR) was monitored and registered continuously by a HR monitor system (Polar Vantage NV, Polar electro Oy, Kempele, Finland) and transferred in real time, through a telemetric signal, to the K4b2 device. The HR values were also averaged every 5 s intervals.
The average values were analyzed by a nonlinear least squares algorithm to fit the data through MatLab 7.0 Software (MathWorks, Natick, MA). The mathematical model consisted of two (cardiodynamic and primary components) or three (cardiodynamic, primary and slow components) exponential models. An F-Test (p < 0.05) was used to evaluate whether the two or three exponentials models provided the best fit to each data set.
where (t) represents the absolute at time, is the in resting baseline period, Ac and τc are the amplitude and the time constant of the cardiodynamic component; Ap, TDp and τp are the amplitude, the time delay and the time constant of the primary component; As, TDs and τs are the amplitude, the time delay and the time constant of the slow component. The mean response time (MRT) was applied to represent the overall pulmonary kinetics response, which was determined as the sum of TDp and τp [15]. The kinetics was assessed during the beginning of exercise until the break (at the 10th min) of swim for collection of [La-] (initial exercise), and the second phase of exercise, starting after the collection of [La-] and resumption of exercise (final exercise).
The energy systems’ contribution has been assessed by the total energy expenditure (). The was obtained by the addition of the aerobic energy expenditure calculated by the difference between the exercise () and baseline () (mL.kg-1.min-1), and by the anaerobic energy expenditure that was calculated by the net [La-] values transformed into O2 equivalents using the constant value of 2.7 mLO2.kg-1.mM-1 [15, 20] during continuous exercises.
Data are presented as mean and standard deviation (± SD). Normality and sphericity of data were checked with the Shapiro-Wilk’s W and Mauchley Sphericity tests. When the assumption of sphericity was not attained, Greenhouse-Geisser or the Huynh-Feld adjusted univariate tests for repeated measures were used. The partial Eta square (ήp2) was used to measure the effect size, defined as small, medium and large for values of 0.01, 0.06 and 0.14, respectively [21]. The comparisons of kinetics (cardiodynamic and primary components) and energy systems’ contribution (aerobic and anaerobic energy expenditure) were performed using multivariate ANOVA and examined by the intensity and previous exercise effects. The v and [La-] values were performed using the univariate ANOVA. All analyses were conducted for repeated measures, complemented with the Bonferroni correction post-hoc test with a significance level of p < 0.05.
Results
All swimmers performed 30 min when swimming at 97.5 and 100%MLSS, but eight swimmers were not able to maintain the predetermined v during 30 min at 102.5%MLSS, reaching voluntary exhaustion at 19.3 ± 4.9 min. The average v and values were different in between the three swim intensities, with 97.5%MLSS slowest and lowest, and 102.5%MLSS fastest and highest (F2,18 = 2560.200, p < 0.001, ήp2 = 0.996; F2,18 = 15.538, p < 0.001, ήp2 = 0.633, respectively) (Table 1). [La-] and HR values for the three swim intensities are also shown in Table 1 with a higher values at 102.5%MLSS compared to 97.5 and 100%MLSS for [La-] (F2,18 = 18.123, p < 0.001, ήp2 = 0.668), and at 102.5%MLSS compared to 97.5%MLSS for HR (F2,18 = 7.222, p < 0.005, ήp2 = 0.445).
Table 1. Mean (SD) values of swimming velocity (v), blood lactate concentrations ([La-]), heart rate (HR), and percentage of maximal oxygen uptake () are shown at 97.5, 100 and 102.5% of the maximal lactate steady state (MLSS) (N = 10).
| 97.5%MLSS | 100%MLSS | 102.5%MLSS | |
|---|---|---|---|
| v (m.s-1) | 1.21 (0.07) | 1.24 (0.07)1 | 1.27 (0.07)1,2 |
| [La-] (mmol.L-1) | 1.48 (0.39) | 1.89 (0.77) | 2.97 (0.87)1,2 |
| HR (beats.min-1) | 167.1 (15.0) | 173.6 (9.7) | 179.3 (9.2)1 |
| (%) | 78.9 (8.7) | 84.7 (3.8)1 | 90.9 (4.6)1,2 |
1,2 Values different from 97.5 and 100%MLSS, respectively (p < 0.05).
kinetics parameters are presented in Table 2. Ap tended to increase with the swimming intensity (v) during the initial exercise, but differences were only noticed comparing 100 and 102.5%MLSS to 97.5%MLSS (F2,18 = 8.249, p < 0.05, ήp2 = 0.478). Meanwhile, Ap was similar at final exercise for the three swim conditions (F2,18 = 1.167, p = 0.334, ήp2 = 0.115). On the other hand, Ap decreased as a function of previous exercise for the three swims bouts. TDp, τp and MRT were similar as function of v at initial exercise and final exercise during the three swimming conditions. However, when analyzed the swim bouts as a function of previous exercise, TDp decreased for the 97.5%MLSS, but the values remained similar for 100 and 102.5%MLSS; τp decreased for all swim intensities, and MRT decreased for the 97.5 and 102.5%MLSS, but remained similar for 100%MLSS.
Table 2. Mean (SD) values of kinetics parameters at velocities of 97.5, 100 and 102.5% of the maximal lactate steady state (MLSS) for the beginning of exercise until the break of swim for blood collection (initial exercise), and the second phase of exercise, starting after blood collection (final exercise) (N = 10).
| 97.5%MLSS | 100%MLSS | 102.5%MLSS | ||||
|---|---|---|---|---|---|---|
| Initial exercise | Final exercise | Initial exercise | Final exercise | Initial exercise | Final exercise | |
| VO2 baseline (mL.kg-1.min-1) | 7.2 (2.1) | 16.0 (5.3)a | 6.0 (1.0) | 17.4 (5.7)a | 6.4 (0.8) | 18.8 (5.8)a |
| Ac (mL.kg-1.min-1) | 16.4 (5.9) | 10.4 (4.9)a | 16.1 (7.1) | 14.2 (5.4) | 15.1 (6.5) | 14.9 (5.7) |
| Ap (mL.kg-1.min-1) | 35.7 (7.3) | 26.3 (7.4)a | 41.0 (7.0)1 | 28.3 (5.2)a | 41.3 (5.4)1 | 29.8 (5.5)a |
| TDp (s) | 14.3 (5.5) | 12.0 (5.3)a | 12.4 (8.1) | 11.9 (4.9) | 11.5 (6.8) | 11.1 (4.7) |
| τp (s) | 16.3 (5.4) | 10.8 (4.7)a | 13.8 (4.5) | 9.7 (4.5)a | 16.0 (5.8) | 9.6 (5.3)a |
| MRT (s) | 30.6 (5.2) | 22.8 (5.4)a | 26.2 (6.8) | 21.6 (4.6) | 27.4 (8.5) | 20.7 (5.2)a |
Statistical analyses were described by intensity and previous exercise effect.
1 Values different from 97.5%MLSS for initial exercise.
a Values different from initial exercise (p < 0.05).
The both measured at initial exercise (F2,18 = 2.389, p = 0.120, ήp2 = 0.210) and final exercise (F2,18 = 1.034, p = 0.376, ήp2 = 0.103) were similar in between the three swim conditions, but increased as a function of previous exercise (initial to final exercise) for all continuous intensities (F1,9 = 68.311, p < 0.001, ήp2 = 0.884). Ac was similar as a function of v for both initial exercise (F2,18 = 0.134, p = 0.876, ήp2 = 0.015) and final exercise (F2,18 = 1.974, p = 0.168, ήp2 = 0.180). Moreover, at 97.5%MLSS, Ac was lower comparing initial and final exercise, but values remained similar for 100 and 102.5%MLSS.
As of kinetics was observed for all tested swimming intensities and testing phases (initial and final exercise) only in two out of ten subjects. In one subject As was not observed. The As was observed for 6 swimmers during initial exercise and 8 swimmers during final exercise at 97.5%MLSS, for 6 swimmers during initial exercise and 7 swimmers during final exercise at 100%MLSS, and for 9 swimmers during initial exercise and 5 swimmers during final exercise at 102.5%MLSS. The As values are presented in Table 3. As tended to increase with swimming intensity during initial exercise, but keeping constant during final exercise whatever the intensity considered; however no statistical analysis was applied, once the occurrence of the As was apparently chaotic among swimmers both considering swimming intensities and phases of testing (initial and final exercise).
Table 3. Individual and mean (SD) values of the amplitude of slow component (As) at velocities of 97.5, 100 and 102.5% of the maximal lactate steady state (MLSS) for the beginning of exercise until the break of swim for blood collection (initial exercise), and the second phase of exercise, starting after blood collection (final exercise) (N = 10).
| As (mL.kg-1.min-1) | ||||||
|---|---|---|---|---|---|---|
| 97.5%MLSS | 100%MLSS | 102.5%MLSS | ||||
| swimmer | Initial exercise | Final exercise | Initial exercise | Final exercise | Initial exercise | Final exercise |
| 1 | 1.7 | 2.9 | 2.3 | 3.8 | 4.5 | 1.6 |
| 2 | 2.3 | 0.7 | 4.4 | 0 | 3.7 | 0 |
| 3 | 1.1 | 0 | 2.6 | 0 | 4.4 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 4.2 | 0.9 | 2.8 | 0.8 | 2.9 | 0 |
| 6 | 0 | 0.8 | 0 | 0.8 | 1.9 | 2.2 |
| 7 | 0 | 1.2 | 0 | 0.9 | 7.2 | 1.1 |
| 8 | 0 | 1.7 | 2.8 | 1.1 | 4.5 | 0 |
| 9 | 2.5 | 1.3 | 2.6 | 1.5 | 6.1 | 0.8 |
| 10 | 1.4 | 0 | 0 | 1.5 | 5.1 | 1.8 |
| Mean (SD) | 2.2 (1.1) | 1.4 (0.8) | 2.9 (0.8) | 1.5 (1.1) | 4.5 (1.6) | 1.5 (0.6) |
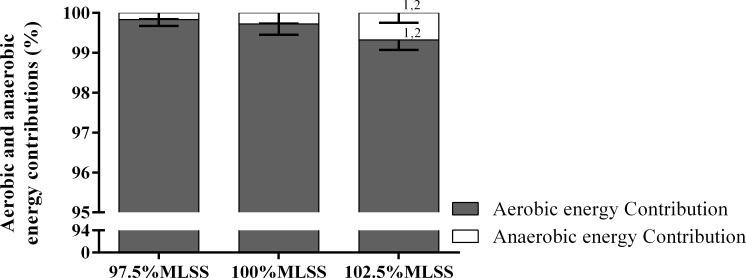
The relative energy contribution for each one of the three swim intensity bouts is shown in Fig 1. The aerobic energy contribution decreased (F2,18 = 15.254, p < 0.001, ήp2 = 0.629) and the anaerobic energy increased (F2,18 = 15.254, p < 0.001, ήp2 = 0.629) at 102.5%MLSS compared to 97.5 and 100%MLSS.
Fig 1. Mean ± SD of aerobic and anaerobic energy relative contribution values at velocities corresponding to 97.5, 100 and 102.5% of the maximal lactate steady state (MLSS).
Discussion
The purposes of this study were to examine the kinetics responses during constant-velocity swims at intensities of 97.5, 100 and 102.5%MLSS, the effect of previous exercise on the parameters of kinetics, and the contribution of the energetic systems at the three conditions. The main original findings were that increasing exercise intensity resulted in greater primary amplitude of the kinetics, in accordance with previous results in running [22]. As demonstrated by other studies [23–25], the previous exercise may increase the amplitude of the primary component and accelerate kinetics (i.e., MRT) during the subsequent exercise. There was a significant increase in the anaerobic contribution when swimming above MLSS. However, the aerobic energetic system contribution corresponded to ~99% of the total energy demand of the exercise in all exercise conditions analyzed in this study.
In sports science, kinetics have added the understanding of physiological adjustments over time [2–4], such as muscle metabolism and systemic oxygen transport [26]. Moreover, one of the most relevant exercise intensities in swimming for aerobic training, prescription and evaluation is the v at which MLSS is obtained, being considered the direct and gold-standard method for the evaluation of aerobic capacity [8, 10–12, 14]. Thus, both aspects ( kinetics and MLSS) are decisive for the understanding of energy supply and oxidative metabolism supporting muscular exercise. Therefore, our purpose was to examine the amplitude and time adjustments of kinetics during swims at intensities of 97.5, 100 and 102.5%MLSS, exploring the effects of small prescriptions variations on swimming oxidative physiology.
The main findings were: (a) Ap tended to increase with swimming v for the initial phase of exercise, despite differences were only noticed comparing 100 and 102.5%MLSS to 97.5%MLSS. Meanwhile, Ap was similar at the final phase of exercise during the three swim conditions. However, Ap decreased as a function of previous exercise for the three swim intensities; (b) TDp, τp and MRT were similar irrespective of v both at initial and final exercise; (c) regarding the effect of previous exercise comparing initial and final exercise for the three swimming intensities, TDp decreased for the 97.5%MLSS, but was similar for 100 and 102.5%MLSS, τp decreased for all swim intensities, and MRT decreased for the 97.5 and 102.5%MLSS, but was similar for 100%MLSS; (d) although As was not evident for all swimmers during the three swimming conditions, it tended to increase with intensity during initial exercise, remaining constant during final exercise; (e) Ac was similar both for the initial and final exercise comparing the three swim intensities, but was lower during final exercise compared to initial exercise at 97.5%MLSS, and was similar at 100 and 102.5%MLSS; (f) aerobic and anaerobic energy contributions were different at 102.5%MLSS compared to lower swim velocities; (g) at the three swim intensities, the aerobic contribution values were higher than 98% of the total energy input.
The values in the present study were directly measured breath-by-breath throughout time for the three swim intensities. Subsequently, the data were fitted through mathematical modelling as previously applied in swimming for maximal and submaximal exercises [15, 19, 27–30]. Some studies have reported kinetics at intensities near the maximal v where a steady state in swimming is found (MLSS) [27–29], however we are unaware of a study that has evaluated and compared kinetics at or around the MLSS in swimming. Most of previous studies reported in sports science [2, 15, 19, 27–31] have studied kinetics at maximal and submaximal intensities, demonstrating the fundamental role of kinetics to understand the physiological mechanisms underpinning the dynamics of the aerobic response at different exercise intensities. Thus, the understanding of the kinetics throughout time may aid the evaluation of aerobic capacity and prescription of specific training sets during these fundamental training intensities around MLSS.
The 100%MLSS v values reported in this study are in accordance with those reported in previous ones [13, 14, 32], in spite of the fact that most of the swimmers examined in the previous studies were male when compared with the female subjects of the present study. Despite higher v values at a given relative intensity are expected to be higher for male than female counterparts of similar training level [33], the sex similitude comparing our results with literature could likely be explained by a higher technical and biomechanical proficiency of our female swimmers when compared to the male swimmers of the previous studies. Indeed, the at 100%MLSS (85 ± 4%) observed in the present study for women is similar to previously reported data for men (86.1% ) [34], suggesting similar levels of aerobic capacity development, even the being higher in the previous study (mean = ~83 mL.kg-1.min-1) when compared with our results (54.9 ± 6.7 mL.kg-1.min-1). Meanwhile, the mean HR value at 100%MLSS was 174 ± 10 beats.min-1 in the present study, values which were similar to the previous reported in literature [32, 34], as expected by the comparable age of samples.
Moreover, the [La-] at 100%MLSS (1.89 ± 0.77 mmol.L-1) in the present study were lower when compared to swimming literature (2.8–3.3 mmol.L-1) [14, 34, 35]. These lower [La-] values may be explained by sex differences for similar levels of aerobic capacity development, with expected lower values for women due to lower body mass and lean muscle mass compared to men [36]. Furthermore, women have showed lower testosterone concentration compared to men [37] during aerobic endurance exercise [33, 36], suggesting different metabolic contributions between carbohydrates and fat during long-distance exercise [33, 38], and supporting comparable lower [La-].
Since the early research on kinetics [39] until up to date, the time constant (τ) has been studied in sports science in the attempt to comprehend the physiological adjustments during the non-steady state period at the beginning of exercise due to the increase of metabolic demand. In the present study, the τp values were similar between intensity levels for the initial exercise phase (mean = 15.4 ± 5.2 s) and final exercise phase (mean = 10.0 ± 4.7 s), but the values decreased with previous exercise for the three swim conditions. This is particularly relevant for training practice, underlining the influence of previous exercise on the subsequent metabolic dynamics. In all studied exercise intensities, the τp in the present study showed similar values compared than those previously reported in swimming (~15–20 s) [27–29], cycling [40, 41], rowing [42], and running [43, 44]. Thus, those values reported for intensities up to and above the MLSS seem to behave similarly as expected, based on the previous knowledge on the kinetics during different intensity domains for well-trained athletes. Indeed, a faster attainment of a steady state and a reduction in the oxygen deficit are associated to the fatigue delay and increase in exhaustion time, being well trained athletes able to perform at higher intensities with lower requirements of anaerobic energy during the transition from rest to exercise [5]. Hence, the lower τp values reported in this study when compared to previously published ones regarding physiological adaptations induced by aerobic endurance training confirm the highly endurance training status and specialization (endurance athletes) of our swimmers [5, 44].
Partially in contrast with previous literature that showed the existence of the As at these exercise intensities [2, 4, 5], in the present study it has shown to occur chaotically during the three swimming conditions, with very diverse individual occurrence profiles; however, observing the sample data a tendency to As increase as a function of intensity was observed (2.2 ± 1.1, 2.9 ± 0.8 and 4.5 ± 1.6 mL.kg-1.min-1, respectively for 97.5, 100 and 102.5%MLSS), but only during initial exercise, not during the final phase after metabolic adaptation already occurred. Besides, only two swimmers showed As occurrence in all trials both at the initial and final exercise phases, and one swimmer did not show any As during all the swimming efforts and phases. It is worthy to emphasize the curiosity of that particular swimmer being a national record holder (800 and 1500m) and the best endurance swimmer of the sample. These partially contradictory findings could be explained, at least in part, by the specific physiological adaptations occurred through the highly endurance training status for our swimmers, such as faster τp [44], possible increase in the mitochondrial content of the cell [45], beyond also possible alterations in the mitochondrial sensitivity to the respiration regulators [46], and the fact of these endurance athletes might have mainly type I muscle fibers [45]. Thus, our endurance swimmers with fast kinetics would be able to adjust faster the physiological requirements for aerobic performance during the high intensity aerobic exercises, minimizing the As demand. In addition, the appearance of the As is normally explained by a phenomena that may be attenuated in our very specialized sample, namely the recruitment of type II fibers with fatigue [47], after which the magnitude of As has been correlated with the rise of [La-] [2, 45]. Thereby, the absence of significant As in the present study may be likely explained by the high-level of endurance training of the sample [48].
Moreover, to reinforce the predominance of aerobic energy system during the three swim conditions around MLSS, the present study determined the total energy contribution at each one of the studied exercise intensities. At all swimming intensities up to and above MLSS, the aerobic energy contribution was higher than 98% of the total energy contribution; however there were significant differences between the highest and the lower v regarding aerobic and anaerobic energy contributions. This study was the first study to show the energy contribution during intensities at and around MLSS directly measured breath-by-breath in swimming, which highlights that even at intensities above MLSS; the total energy contribution was mainly and almost exclusively controlled by the oxidative bioenergetics system.
Conclusions
The present study showed that well-trained endurance swimmers with a fast component of kinetics, i.e. an abrupt and fast increase in response, and low [La-] may be able to adjust faster the physiological requirements during intensities up to and slightly above MLSS to minimize the appearance of the slow component of and reduce the oxygen deficit, both parameters are associated to the fatigue delay and the increase in exhaustion time, key factors to endurance performance. however, these fast adjustments were not able to control the progressive fatigue occurred slightly above MLSS, and most of swimmers reached exhaustion before 30min swam. Moreover, the data shows that the aerobic energy contribution at intensities up to and slightly above MLSS plays a fundamental role controlling almost exclusively the required energy supply.
Supporting information
(PDF)
(PDF)
Acknowledgments
This investigation was supported by grants of the Capes Foundation, Ministry of Education of Brazil (BEX: 0536/10-5), and project PTDC/DES/101224/2008 (FCOMP-01-0124-FEDER-009577).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This investigation was supported by grants of the Capes Foundation, Ministry of Education of Brazil (BEX: 0536/10-5): JGP and project PTDC/DES/101224/2008 (FCOMP-01-0124-FEDER-009577): JPVB RF.
References
- 1.Bosquet L, Leger L, Legros P. Methods to determine aerobic endurance. Sports Med. 2002;32(11):675–700. [DOI] [PubMed] [Google Scholar]
- 2.Gaesser GA, Poole DC. The slow component of oxygen uptake kinetics in humans. Exerc Sport Sci Rev. 1996;24(1):35–71. [PubMed] [Google Scholar]
- 3.Poole DC, Ward SA, Gardner GW, Whipp BJ. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics. 1988;31(9):1265–79. Epub 1988/09/01. 10.1080/00140138808966766 [DOI] [PubMed] [Google Scholar]
- 4.Poole DC, Richardson RS. Determinants of oxygen uptake. Implications for exercise testing. Sports Med. 1997;24(5):308–20. Epub 1997/11/22. [DOI] [PubMed] [Google Scholar]
- 5.Burnley M, Jones A. Oxygen uptake kinetics as a determinant of sports performance. European Journal of Sport Science. 2007;7(2):63–9. [Google Scholar]
- 6.Whipp BJ, Wasserman K. Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol. 1972;33(3):351–6. Epub 1972/09/01. [DOI] [PubMed] [Google Scholar]
- 7.Whipp BJ, Ward SA. Physiological determinants of pulmonary gas exchange kinetics during exercise. Med Sci Sports Exerc. 1990;22(1):62–71. Epub 1990/02/01. [PubMed] [Google Scholar]
- 8.Beneke R. Maximal lactate steady state concentration (MLSS): experimental and modelling approaches. Eur J Appl Physiol. 2003;88(4–5):361–9. 10.1007/s00421-002-0713-2 [DOI] [PubMed] [Google Scholar]
- 9.Heck H, Mader A, Hess G, Mucke S, Muller R, Hollmann W. Justification of the 4-mmol/l lactate threshold. Int J Sports Med. 1985;6(3):117–30. 10.1055/s-2008-1025824 [DOI] [PubMed] [Google Scholar]
- 10.Beneke R, von Duvillard SP. Determination of maximal lactate steady state response in selected sports events. Med Sci Sports Exerc. 1996;28(2):241–6. [DOI] [PubMed] [Google Scholar]
- 11.Faude O, Kindermann W, Meyer T. Lactate threshold concepts: how valid are they? Sports Med. 2009;39(6):469–90. 10.2165/00007256-200939060-00003 [DOI] [PubMed] [Google Scholar]
- 12.Billat VL, Sirvent P, Py G, Koralsztein JP, Mercier J. The concept of maximal lactate steady state: a bridge between biochemistry, physiology and sport science. Sports Med. 2003;33(6):407–26. [DOI] [PubMed] [Google Scholar]
- 13.Baron B, Dekerle J, Depretz S, Lefevre T, Pelayo P. Self selected speed and maximal lactate steady state speed in swimming. J Sports Med Phys Fitness. 2005;45(1):1–6. [PubMed] [Google Scholar]
- 14.Pelarigo JG, Denadai BS, Greco CC. Stroke phases responses around maximal lactate steady state in front crawl. J Sci Med Sport. 2011;14(2):168 e1–e5. Epub 2010/10/12. [DOI] [PubMed] [Google Scholar]
- 15.Sousa AC, Vilas-Boas JP, Fernandes RJ. VO2 Kinetics and Metabolic Contributions Whilst Swimming at 95, 100, and 105% of the Velocity at VO2max. Biomed Res Int. 2014;1(1):1–9. Epub 2014/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes RJ, Billat VL, Cruz AC, Colaco PJ, Cardoso CS, Vilas-Boas JP. Does net energy cost of swimming affect time to exhaustion at the individual's maximal oxygen consumption velocity? J Sports Med Phys Fitness. 2006;46(3):373–80. [PubMed] [Google Scholar]
- 17.Machado L, Almeida M, Morais P, Fernandes R, Vilas-Boas JP, editors. Assessing the individual anaerobic threshold: the mathematical model. Xth International Symposium of Biomechanics and Medicine in Swimming; 2006; Porto, Portugal2006.
- 18.Baldari C, Fernandes RJ, Meucci M, Ribeiro J, Vilas-Boas JP, Guidetti L. Is the new AquaTrainer® snorkel valid for VO2 assessment in swimming? Int J Sports Med. 2013;34(4):336–44. 10.1055/s-0032-1321804 [DOI] [PubMed] [Google Scholar]
- 19.Fernandes RJ, de Jesus K, Baldari C, Sousa AC, Vilas-Boas JP, Guidetti L. Different VO2max time-averaging intervals in swimming. Int J Sports Med. 2012;33(12):1010–5. 10.1055/s-0032-1316362 [DOI] [PubMed] [Google Scholar]
- 20.di Prampero PE. Energetics of muscular exercise. Rev Physiol Biochem Pharmacol. 1981;89(1):143–222. Epub 1981/01/01. [DOI] [PubMed] [Google Scholar]
- 21.Stevens JP. Applied multivariate statistics for the social sciences Fourth edition ed. Mahwah: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- 22.Carter H, Pringle JS, Jones AM, Doust JH. Oxygen uptake kinetics during treadmill running across exercise intensity domains. Eur J Appl Physiol. 2002;86(4):347–54. [DOI] [PubMed] [Google Scholar]
- 23.Burnley M, Doust JH, Carter H, Jones AM. Effects of prior exercise and recovery duration on oxygen uptake kinetics during heavy exercise in humans. Exp Physiol. 2001;86(3):417–25. [DOI] [PubMed] [Google Scholar]
- 24.Sousa A, Ribeiro J, Sousa M, Vilas-Boas JP, Fernandes RJ. Influence of Prior Exercise on VO2 Kinetics Subsequent Exhaustive Rowing Performance. PLoS One. 2014;9(1):0084208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones AM, Koppo K, Burnley M. Effects of prior exercise on metabolic and gas exchange responses to exercise. Sports Med. 2003;33(13):949–71. [DOI] [PubMed] [Google Scholar]
- 26.Xu F, Rhodes EC. Oxygen uptake kinetics during exercise. Sports Med. 1999;27(5):313–27. [DOI] [PubMed] [Google Scholar]
- 27.Reis JF, Alves FB, Bruno PM, Vleck V, Millet GP. Effects of aerobic fitness on oxygen uptake kinetics in heavy intensity swimming. Eur J Appl Physiol. 2012;112(5):1689–97. 10.1007/s00421-011-2126-6 [DOI] [PubMed] [Google Scholar]
- 28.Reis JF, Alves FB, Bruno PM, Vleck V, Millet GP. Oxygen uptake kinetics and middle distance swimming performance. J Sci Med Sport. 2012;15(1):58–63. Epub 2011/08/02. 10.1016/j.jsams.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 29.Pessoa Filho DM, Alves FB, Reis JF, Greco CC, Denadai BS. VO2 kinetics during heavy and severe exercise in swimming. Int J Sports Med. 2012;33(9):744–8. 10.1055/s-0031-1299753 [DOI] [PubMed] [Google Scholar]
- 30.Reis VM, Marinho DA, Policarpo FB, Carneiro AL, Baldari C, Silva AJ. Examining the accumulated oxygen deficit method in front crawl swimming. Int J Sports Med. 2010;31(6):421–7. 10.1055/s-0030-1248286 [DOI] [PubMed] [Google Scholar]
- 31.Jones AM, Burnley M. Oxygen uptake kinetics: an underappreciated determinant of exercise performance. Int J Sports Physiol Perform. 2009;4(4):524–32. [DOI] [PubMed] [Google Scholar]
- 32.Dekerle J, Pelayo P, Clipet B, Depretz S, Lefevre T, Sidney M. Critical swimming speed does not represent the speed at maximal lactate steady state. Int J Sports Med. 2005;26(7):524–30. 10.1055/s-2004-821227 [DOI] [PubMed] [Google Scholar]
- 33.Greco CC, Pelarigo JG, Figueira TR, Denadai BS. Effects of gender on stroke rates, critical speed and velocity of a 30-min swim in young swimmers. J Sports Sci Med. 2007;6(4):441–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Dekerle J, Nesi X, Lefevre T, Depretz S, Sidney M, Marchand FH, et al. Stroking parameters in front crawl swimming and maximal lactate steady state speed. Int J Sports Med. 2005;26(1):53–8. 10.1055/s-2004-817854 [DOI] [PubMed] [Google Scholar]
- 35.Figueiredo P, Nazário R, Sousa M, Pelarigo JG, Vilas-Boas JP, Fernandes R. Kinematical Analysis along Maximal Lactate Steady State Swimming Intensity. J Sports Sci Med. 2014;13(3):610–5. [PMC free article] [PubMed] [Google Scholar]
- 36.Crewther B, Cronin J, Keogh J. Possible stimuli for strength and power adaptation: acute metabolic responses. Sports Med. 2006;36(1):65–78. [DOI] [PubMed] [Google Scholar]
- 37.Deschenes MR, Kraemer WJ. Performance and physiologic adaptations to resistance training. Am J Phys Med Rehabil. 2002;81(11 Suppl):S3–16. Epub 2002/11/01. 10.1097/01.PHM.0000029722.06777.E9 [DOI] [PubMed] [Google Scholar]
- 38.Tarnopolsky MA, Atkinson SA, Phillips SM, MacDougall JD. Carbohydrate loading and metabolism during exercise in men and women. J Appl Physiol. 1995;78(4):1360–8. Epub 1995/04/01. [DOI] [PubMed] [Google Scholar]
- 39.Margaria R, Edwards HT, Dill DB. The possible mechanisms of contracting and paying the oxygen debt and the rôle of lactic acid in muscular contraction1933 1933-11-30 00:00:00. 689–715 p.
- 40.Berger NJ, Jones AM. Pulmonary O2 uptake on-kinetics in sprint- and endurance-trained athletes. Appl Physiol Nutr Metab. 2007;32(3):383–93. Epub 2007/05/19. 10.1139/H06-109 [DOI] [PubMed] [Google Scholar]
- 41.Koppo K, Bouckaert J, Jones AM. Effects of training status and exercise intensity on phase II VO2 kinetics. Med Sci Sports Exerc. 2004;36(2):225–32. Epub 2004/02/10. 10.1249/01.MSS.0000113473.48220.20 [DOI] [PubMed] [Google Scholar]
- 42.Ingham SA, Carter H, Whyte GP, Doust JH. Comparison of the oxygen uptake kinetics of club and olympic champion rowers. Med Sci Sports Exerc. 2007;39(5):865–71. Epub 2007/05/01. 10.1249/mss.0b013e31803350c7 [DOI] [PubMed] [Google Scholar]
- 43.Borrani F, Candau R, Millet GY, Perrey S, Fuchslocher J, Rouillon JD. Is the VO2 slow component dependent on progressive recruitment of fast-twitch fibers in trained runners? J Appl Physiol. 2001;90(6):2212–20. [DOI] [PubMed] [Google Scholar]
- 44.Carter H, Jones AM, Barstow TJ, Burnley M, Williams C, Doust JH. Effect of endurance training on oxygen uptake kinetics during treadmill running. J Appl Physiol. 2000;89(5):1744–52. [DOI] [PubMed] [Google Scholar]
- 45.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(4):831–8. Epub 1984/04/01. [DOI] [PubMed] [Google Scholar]
- 46.Dudley GA, Tullson PC, Terjung RL. Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem. 1987;262(19):9109–14. Epub 1987/07/05. [PubMed] [Google Scholar]
- 47.Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, et al. Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol. 1991;71(4):1245–60. Epub 1991/10/01. [DOI] [PubMed] [Google Scholar]
- 48.Billat V, Binsse V, Petit B, Koralsztein JP. High level runners are able to maintain a VO2 steady-state below VO2max in an all-out run over their critical velocity. Arch Physiol Biochem. 1998;106(1):38–45. Epub 1998/10/23. 10.1076/apab.106.1.38.4396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.