Abstract
Some genetically modified (GM) plants have transgenes that confer tolerance to abiotic stressors. Meanwhile, other transgenes may interact with abiotic stressors, causing pleiotropic effects that will affect the plant physiology. Thus, physiological alteration might have an impact on the product safety. However, routine risk assessment (RA) analyses do not evaluate the response of GM plants exposed to different environmental conditions. Therefore, we here present a proteome profile of herbicide-tolerant maize, including the levels of phytohormones and related compounds, compared to its near-isogenic non-GM variety under drought and herbicide stresses. Twenty differentially abundant proteins were detected between GM and non-GM hybrids under different water deficiency conditions and herbicide sprays. Pathway enrichment analysis showed that most of these proteins are assigned to energetic/carbohydrate metabolic processes. Among phytohormones and related compounds, different levels of ABA, CA, JA, MeJA and SA were detected in the maize varieties and stress conditions analysed. In pathway and proteome analyses, environment was found to be the major source of variation followed by the genetic transformation factor. Nonetheless, differences were detected in the levels of JA, MeJA and CA and in the abundance of 11 proteins when comparing the GM plant and its non-GM near-isogenic variety under the same environmental conditions. Thus, these findings do support molecular studies in GM plants Risk Assessment analyses.
1. Introduction
Recurrent advances in molecular techniques and scientific knowledge have resulted in the ability to genetically manipulate many organisms, especially the development of genetically modified (GM) plants. And because GM plants are regulated in most countries, risk assessment (RA) and equivalence criteria to assess the safety of GM crops have been conducted during the past few years [1–7]. The concept of ‘substantial equivalence’ can be defined as the close nutritional and elemental similarity between a GM crop and a non-GM near-isogenic variety. It has often been used to claim that GM crops are as safe and nutritious as currently consumed plant-derived foods [8]. However, the parameters, criteria and analyses used to declare a GMO substantially equivalent are unclear and reduced to a limited set of variables, such as the total amounts of carbohydrates, proteins and minerals. In addition, pesticide residues can become part of the composition of a herbicide-tolerant GM plant, and they may add toxic properties to the final plant product, either alone or by affecting plant metabolism [9]. Nonetheless, the identification of such pesticide residues is overlooked in compositional analyses [10]. Therefore, one of the most reliable ways to detect unintended consequences of genetic modification is through a more detailed survey of molecular components [11].
Herbicide use has been steadily increasing, particularly after the release of herbicide-tolerant GM crops [12] which encompass approximately 80% of GM crops, and are able to accumulate residues without dying in order to facilitate weed management [13]. By definition, herbicides are chemicals that impair plant development and, therefore, are used to eliminate many different weeds [14]. Herbicide application is considered one of the major abiotic stressors of both weed and herbicide-tolerant GM plants, leading to stress response pathways in exposed plants, thus affecting gene expression and physiology [15–17]. Stress is defined as any external abiotic or biotic constraint that reduces a plant’s ability to convert energy into biomass [18]. In most cases, low crop productivity can be attributed to various abiotic stresses, including high salinity, drought, cold, and heat, all of which negatively influence the survival, biomass production, and yield of major crops [19–21]. Specifically, drought stress can be defined as any reduction in the amount of water available for the crop, regardless of irrigation, which reduces its productivity below expected yields under adequate water supply. Drought is a common recurring phenomenon and its effects are extremely complex [22, 23]. Water shortage leads to reduced growth for the entire plant, especially in the shoots, as well as alterations on the regulation of gene expression products, such as hormones, transcripts and proteins [24–27].
Changes at transcript level are not necessarily reflected by corresponding changes in plant proteome [28,29]. Thus, thorough investigations regarding changes in plant proteome submitted to stress conditions are crucial, since they directly indicate plant stress responses. Unlike the genome, which is expected to be constant for an organism, the proteome is highly dependent on environmental influences, such as abiotic stresses [4]. Profiling tools (e.g. proteomics) applied to the molecular characterization step can assist on the identification of potentially hazardous products, such as toxins, allergens and antinutrients. In addition, with the emergence of new GMO types, broader use of molecular profiling tools are required for reliable pre-market RA [30]. Moreover, plant hormones play central roles in the ability of plants to adapt to changing environments and the resultant abiotic stress. Phytohormones can also rapidly alter gene expression by inducing or preventing the degradation of transcriptional regulators via the ubiquitin-proteasome system [31]. Furthermore, some of these plant hormones are the main mediators of plant defense mechanisms against stress, such as plant growth regulators (e.g. abscisic acid (ABA), ethylene, cytokinins (CK), auxin (IAA), gibberellin (GA), jasmonate (JA), salicylic acid (SA), among others) [31].
Generally, RA analyses do not include information on the response of GM plants exposed to different environmental conditions. Water deficit has been common in major growing regions where, at the same time, the application of herbicides is a common occurrence. Thus, it seems reasonable to assess the response of GM plants to adverse conditions, especially in light of climate change times. Therefore, to test our hypothesis that abiotic stress conditions lead to off-target effects in protein and metabolite levels in GM plants, we have performed a proteomic and metabolomic characterization of the herbicide-tolerant NK603 GM maize and its non-GM near-isogenic line, under distinct stress conditions, including drought and herbicide application.
2. Material and methods
2.1. Plant material and experimental design
In order to elucidate the responses of a genetically modified maize plant to water stress and herbicide application, two independent experiments were performed. Both experiments were carried out under identical growing conditions, except for the application of Roundup herbicide in the second experiment.
Experiment 1 aimed at comparing the herbicide-tolerant GM maize DKB 245 RR2 (transformation event NK 603; unique identifier MON-ØØ6Ø3-6 from Monsanto Company, glyphosate-based herbicide tolerance, Dekalb Brand Corn) and its near-isogenic non-GM counterpart DKB 245 under water stress. Seeds of these hybrids were previously germinated in a growth chamber (Eletrolab™ Model 202/3) set to 16 h light period and 25°C (± 2°C). Seedlings were placed in 8 L plastic pots filled with Plantmax HT substrate (Buschle & Lepper S.A), transferred to a greenhouse and watered daily, keeping substrate moisture at 125% (1.25 g H2O / g of soil) controlled by a soil tensiometer (WaterMeter MOD WS-76). The plants were kept in these conditions until they reached five leaves fully emerged (stage V5), then a subset of both varieties (GM and non-GM) were stressed by withholding irrigation, according to Vicent et al. [16] with modifications. The drought stress period was maintained until substrate moisture reached 25% (0.25 g H20 / g of soil), approximately 15 days after water restriction. At this time, leaf samples were collected. Studies on plant response to water deprivation report a significant decrease in leaf conductance from the seventh or eighth day of water stress, indicating that the stress is being perceived by the plant [32, 33]. For this reason, plants in Experiment 1 were subjected to 15 days of water stress, allowing the opportunity for direct response to the stress condition.
Experiment 2 aimed at testing the influence of herbicide application on the herbicide-tolerant GM maize hybrid (NK603). This experiment was carried out similarly to Experiment 1, except that two sequential applications of the commercial herbicide Roundup Original (Monsanto do Brasil LTDA) were done in a subset of twelve plants. Herbicide application was performed following the manufacturer’s recommendation. The first application took place 15 days after plant emergence, and the second application was performed 30 days after emergence, both at doses of 2.0 L / ha in a concentration of 960 g of glyphosate (isopropylamine salt) per ha. Similar to Experiment 1, the drought stress period was maintained until substrate moisture reached 25%.
For both experiments, 12 plants per treatment were randomly sampled and separated into three groups of four plants each. The four plants of each group were pooled and were considered as one biological replicate (sample) of each treatment for the proteomic and plant hormone analysis. Then, leaf pieces were cut from each biological replicate, weighed, immediately placed in 3.8 ml cryogenic tubes, and frozen in liquid nitrogen. The samples were kept at -80°C until protein and hormone extraction.
2.2. Proteomic analysis
2.2.1. Protein extraction and fluorescence hybridization
For two-dimensional (2D) Difference gel electrophoresis (DIGE) proteomic analysis, we followed the methodology of Agapito-Tenfen et al. [1]. Briefly, approximately 100 mg of each sample were ground in a mortar with liquid nitrogen and subsequently extracted and precipitated using phenol and methanol/ammonium acetate. Pellets were re suspended in a urea/thiourea buffer compatible with further fluorescent labelling (4% CHAPS (w/v), 5 mM PMSF, 7 M urea, 2 M thiourea and 30 mM Tris-base). Protein quantification was performed with the 2-D Quant Kit (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Eighty μg of each protein sample pool were labelled with 400 ρmol/μl of CyDye DIGE fluors (Cy3 and Cy5; GE Healthcare) and subsequently stored at -80°C. An internal standard labelled with Cy2 was used in every run for normalization. The internal standard was a mixture of equal amounts of each plant variety sample. After protein-fluor hybridization, lysine (10 mM) was used to stop the reaction and then mixed together for 2D-DIGE. Sample pairs were randomly selected for bi-dimensional electrophoresis runs.
2.2.2. 2-D DIGE conditions
After protein labelling, samples were prepared for the isoelectric focusing (IEF) step. Strip gels of 24 cm and a linear pH range of 4–7 (GE Healthcare) were used. Strips were initially rehydrated with labelled protein samples (7 M urea, 2 M thiourea, 2% CHAPS (w/v), 0.5% IPG buffer (v/v) (GE Healthcare), 2% DTT). Strips were then processed using an Ettan IPGPhor IEF system (GE Healthcare) in a total of 35,000 Volts.h-1 and, subsequently, reduced and alkylated for 30 min under slow agitation in Tris-HCl solution (75 mM), pH 8.8, containing 2% SDS (w/v), 29.3% glycerol (v/v), 6 M urea, 1% DTT (w/v) and 2.5% iodocetamide (w/v). The 2D- DIGE conditions were performed as described by Weiss and Görg [32]. Gels were immediately scanned with a FLA-9000 modular image scanner (Fujifilm Lifescience, Dusseldorf, Germany). To ensure maximum pixel intensity between 60,000 and 90,000 pixels for the three dyes, all gels were scanned at a 100 μm resolution and the photomultiplier tube (PMT) voltage was set between 500 and 700 V. As described by Agapito-Tenfen et al. [2], we performed preparative gels for each plant variety in order to extract relevant spots. These were performed with a 450 μg load of total protein pools in 24 cm gels from each variety, separately, and stained with colloidal Coomassie Brilliant Blue G-250 (MS/MS compatible).
2.2.3. Image analysis
The scanned gel images were transferred to the ImageQuant V8.1 software package (GE Healthcare) and appropriately cropped. Afterwards, the images were exported to ImageMasterTM 2D Platinum 7.0, version 7.06 (GE Healthcare), for comparative analysis. Each 2D-DIGE gel is loaded with treatment and control samples plus an internal standard control (equal mix of all samples of the experiment) and leabeled with a different fluorescence fluor. Therefore, the DIGE software uses a co-detection algorithm, which is capable of realizing simultaneous detection of protein spots labelled in different images, but present on the same gel and normalize it with the internal control. Thus, this co-detection significantly increases the accuracy of a comparative proteomic study, especially compared to traditional 2D-PAGE using Comassie Brilliant Blue. Thus, the simultaneous electropheresis of treatment and control samples, followed by normalization with the corresponding internal standard greatly minimizes technical variation and thus does not require technical replicate gels. In fact, technical replicates are represented by the internal standard control which is present in every DIGE gel.
2.2.4. In-gel digestion and protein identification by MS/MS
Spots from preparative gels were excised and sent to the Proteomic Platform Laboratory at the University of Tromsø (Norway) for processing and analysis. They were subjected to in-gel reduction, alkylation, and tryptic digestion using 2–10 ng/μl trypsin (V511A; Promega) [34]. Peptide mixtures containing 0.5% formic acid were loaded onto a nano ACQUITY Ultra Performance LC System (Waters, Milford, MA, USA), containing a 5-μm Symmetry C18 Trap column (180 μm × 20 mm; Waters) in front of a 1.7-μm BEH130 C18 analytical column (100 μm × 100 mm; Waters). Peptides were separated with a gradient of 5–95% acetonitrile, 0.1% formic acid, with a flow rate of 0.4 μl/min eluted to a Quadrupole Time-of-Flight (Q-TOF) Ultima mass spectrometer (Micromass; Waters). The samples were run in data- dependent tandem MS mode. Peak lists were generated from MS/MS by Protein Lynx Global server software (version 2.2; Waters). The resulting ‘pkl’ files were searched against the NCBI protein sequence databases using Mascot Distiller, ver. 2.5.1.0 (Matrix Sciences; http://matrixscience.com). Data were searched against the NCBInr database, ver. 20151109, with Viridiplantae taxonomy (Green Plants; 3334509 sequences) and against ‘all entries’ and ‘contaminants’ (76068736 sequences; 27658295194 residues) for contamination verification. The following parameters were adopted for database searches: complete carbamidomethylation of cysteines and partial oxidation of methionines; peptide mass tolerance ± 100 ppm; fragment mass tolerance ± 0.1 Da; missed cleavages 1; and significance threshold level (P < 0.05) for Mascot scores (-10 Log (P)). Even though high Mascot scores could be obtained with significant values, a combination of automated database searches and manual interpretation of peptide fragmentation spectra was used to validate protein assignments. Molecular functions and cellular components of proteins were searched against the Encyclopedia of Genes and Genomes (KEGG) Orthology system database, release 69.0 2014 (http://kegg.jp/kegg/ko.html).
2.2.5. Pathway enrichment analysis
Differentially abundant proteins were submitted to enrichment analysis by the online tool agriGO, v1.2 [35], using the Single Enrichment Analysis (SEA) category, with the following parameters: 1) Selected species: Zea mays ssp; 2) Statistical test method: Hypergeometric; 3) Multi-test adjustment method: Hochberg (FDR); 4) Significance level of 0.05; 5) Minimum number of 5 mapping entries; and 6) Gene ontology type: Plant GO Slim. Next, the online tool REVIGO [34] was used to remove the redundant Gene Ontology (GO) terms. Only significant GO terms (False Discovery Rate (FDR) values < 0.05) were used with the following parameters: 1) Allowed similarity: medium (0.7); 2) Database with GO term sizes: Zea mays; and 3) Semantic similarity measure: SimRel.
2.3. Plant hormone and related compounds
2.3.1. Compound extraction
Samples were polled up to 40 mg of freeze-dried leaf tissue (biological replicates) for the extraction of phytohormones and related compounds. The leaf tissue was ground in liquid nitrogen until total spraying, according to Pan et al. [36]. Subsequently, 500 μl of extraction solution consisting of isopropanol, H2O and HCl (2:1:0.002, v/v/v) were added in each tube. Tubes were then placed on a shaker at 100 rpm for 30 min at 4°C. After stirring, 1 ml of dichloromethane solvent and 10 μl of 2-acetamidophenol (10 ppm) (internal standard) were added to each tube and stirred at 100 rpm for 30 min at 4°C. Samples were centrifuged at 13,000 g for 5 min at 4°C. After centrifugation, 900 μl of solvent present in the lower phase were transferred into a new tube using a Pasteur pipette. The solvent mixture was concentrated (not completely dried) using a nitrogen evaporator with nitrogen flow and then dissolved in 100 μl of methanol. Samples were kept at -20°C until analysis.
2.3.2. Liquid chromatography—Tandem mass spectrometry
The quantitative analysis of phytohormones and related compounds was conducted at the Metabolomic Centre of the Department of Biochemistry (North-West University, South Africa). Quantification of phytohormones and related compounds, which belong to the main classes involved in plant defense-related metabolic pathways during stress, was performed for Salicylic Acid (SA), Jasmonic acid (JA), Methyl Jasmonate (MeJA), Abscisic acid (ABA), Indoleacetic acid (IAA) and Cinnamic acid (CA). Quantification was determined using reverse-phase liquid chromatography-tandem mass spectrometry with multiple reaction monitoring [36]. An Agilent 1290 Liquid chromatograph coupled to an Agilent 6460 triple quadrupole mass spectrometer was used. Chromatography was performed with an Agilent Zorbax Eclipse plus C18 (2.1 x 100 mm, 1.8 μm). The chromatographic separation started with 5% solvent B (methanol) and 95% solvent A (water and 0.1% formic acid). The percentage of solvent B was increased to 90% over 7 min. From 7 to 9 min, solvent B was maintained at 90% and then decreased after 9 min to reach 5% solvent B at 12 min. A post-run of 4 min was used to ensure column re-equilibration. A constant flow rate of 0.2 ml/min and a column temperature of 50°C were used.
The ESI source with Agilent Jet Stream technology was set in either positive or negative ionization for each of the two methods used. A gas temperature of 325°C, gas flow of 10 L/min and nebulizer pressure of 45 psi were used in both methods. Multiple reactions monitoring (MRM) transition of each analyte was optimized (Additional file 1). Three runs (technical replicates) were conducted for each biological pool, to inform the technical variation.
2.4. Statistical analysis
Multiple Co-Inertia Analysis (MCIA) was performed in order to explore the experimental quality and the main sources of variation in proteomic and plant hormone datasets simultaneously. For the proteomics experiment, one-way ANOVA was used to investigate differences at individual protein levels. Tukey test at P < 0.05 was used to compare the multiple means in the dataset. The calculations were performed on normalized spot volume ratios based on the total intensity of valid spots in a single gel. Differences at P < 0.05 were considered statistically significant. Statistical analyses were performed using ImageMasterTM 2D Platinum ver. 7.06 (GE Healthcare). For plant hormone quantitative analysis, one-way ANOVA and Tukey test were also used at statistical significance of P < 0.05. Because of non-normal distribution, the data were natural logarithm (LN) transformed. Fold changes are also presented in logarithm base 2 (Log2FC). Log2FC is the most widely used alternative transformation of the ratio since it has the advantage of producing a continuous spectrum of values and representing up- and downregulated compound values in a similar and reader friendly fashion [37].
3. Results and discussion
Proteomic profile and key metabolic compounds were analysed in comparison to the non-GM near-isogenic variety under the same experimental conditions aiming at exploring molecular responses of GM maize variety to drought and herbicide stress conditions. To facilitate the presentation of results, we have named each plant variety and its corresponding growing conditions as follows: Experiment 1—non-GM D (conventional hybrid under drought stress); non-GM (Conventional hybrid under normal growth conditions); GM D (GM hybrid under drought stress); GM (GM hybrid under normal growth conditions). Experiment 2—GM DH (GM under drought stress and herbicide application); GM D (GM hybrid under drought stress) (Table 1).
Table 1. Commercial maize varieties and treatments used in this study.
| Variety commercial name | GM event name | Transgenes | Stress condition | Study designation |
|---|---|---|---|---|
| DKB 245 | non-GM | - | Drought | non-GM D |
| DKB 245 | non-GM | - | Control | non-GM |
| DKB 245 RR2 | NK603 | CP4EPSPS | Drought | GM D |
| DKB 245 RR2 | NK603 | CP4EPSPS | Control | GM |
| DKB 245 RR2 | NK603 | CP4EPSPS | Drought + Herbicide | GM DH |
3.1. Experimental quality assessment using MCIA
MCIA has been recognized as an excellent tool for integrating the results of different "omics" techniques. It is an exploratory data analysis method that is able to provide a simple graphical representation that identifies the concordance between these multiple datasets [38]. It is noteworthy that one component of MCIA can be used to highlight the absence or presence of co-structure between the datasets, thereby permitting the choice of the strongest elements for the following analysis [38], and based on these merits, MCIA was applied to this exploratory analysis of proteomic and metabolic studies in both experiments.
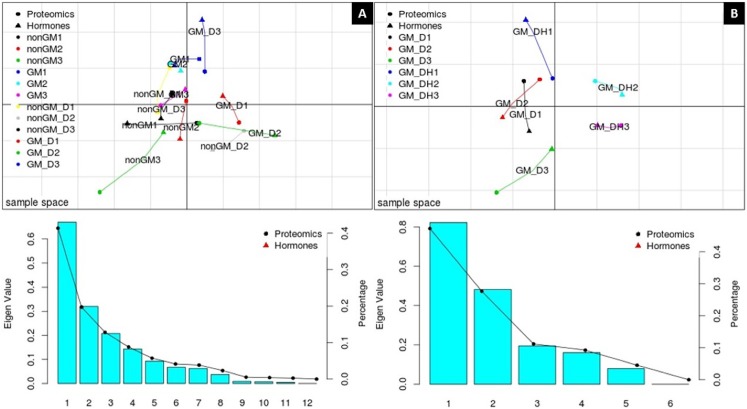
The coordinates of each pool of biological replicates for each treatment are connected by lines, the length of which indicates the divergence (the shorter the line, the higher the level of concordance) between the hormone (and related compounds) and protein relative abundance levels for a particular replicate. For the Experiment 1 dataset (Fig 1A), the MCIA plot shows the two principal components (PC1 x PC2). PC1 separated the biological replicates of maize varieties submitted to drought stress from those of non-stressed varieties, accounting for 41.35% of the total variation in the dataset. PC2 separated GM from non-GM varieties, which accounted for 19.73% of the total variation. Fig 1B shows the MCIA plot for Experiment 2. In this case, the MCIA plot shows similar trends in proteome and metabolic profiles, indicating that the sources of biological information for most variants were similar. The MCIA plot also showed that both PC1 and PC2 separated samples with herbicide application from those whithout application. PC1 accounted for 47.29% of the variation in the dataset, while PC2 accounted for 27.70% of the variation. The combination of both PCs correspond to ~75% of the variation in the experiment, representing a high percentage of the total variation. Based on these results, we conclude that the main source of quantitative variation in our study originated from the environment (factor stress), followed by the hybrids (genetic modification factor). As expected for different growing conditions, these results agree with those of previous studies [2,4,39,40]. Mesnage et al. [13] used the same MCIA approach to determine the relationship of proteome and metabolome profiles of non-GM and GM (NK603) maize seeds. Overall, the authors showed that the GM transformation process was the major contributor to variation in the protein and metabolite profiles, followed by the spraying of herbicide [13]. Other multivariate analyses, such as Principal Component Analysis (PCA), have been used to demonstrate similarities in the quantity of proteins and metabolites between different conditions in multi-omics studies. However, unlike MCIA analysis, PCA has to be done separately for each different “omic” approach [1–3]. Therefore, when conducting gene, protein and metabolite expression analyses in the different maize hybrids MCIA could be a more reliable integrative tool in this case than PCA.
Fig 1. MCIA projection plot.
(A) MCIA projection plot for Experiment 1, representing the proteomic and metabolomic datasets: PC1 = 41.35% and PC2 = 19.73%. (B) MCIA projection plot for Experiment 2, representing the proteomic and metabolomic datasets: PC1 = 47.29% and PC2 = 27.7%. PC1 is represented by the first axis (horizontal), and PC2 is represented by the second axis (vertical). Eigenvalue and Percentage graphics show the amount of variation in the dataset corresponding to each PC. Different symbols represent the respective “omics” analysis and are connected by lines where the length is proportional to the divergence between the data from a same replicate. Lines are joined by a common point, representing the reference structure, which maximizes covariance derived from the MCIA synthetic analysis. Colors represent the biological replicates.
3.2. Differential proteomic analysis
Total protein content mean was 4.50 ± 0.14 mg.g-1 (fresh weight) of leaf material, which is considered a sufficient quantity for 2D-DIGE analysis. No significant difference was observed in the one-way ANOVA (P < 0.05) for the protein content mean between different growing conditions in Experiments 1 and 2. The number of spots in a two-dimensional protein gel is one of the most important components for a comparative proteomics analysis. Essentially, the number of spots present in the gels of each treatment correlates with comparative efficiency. Here, the average number of spots detected was 643 and, no statistically significant difference was found between or within treatments (plant varieties and growing conditions) in either experiment (one-way ANOVA, P < 0.05; data not shown). Comparative proteomic studies using 2D-DIGE analysis in plant leaves have shown an average number of detected spots ranging from 500 to 900, which agrees with our results [41–43].
Overall comparative analysis revealed a total of 20 different proteins that were present, absent, or up/downregulated in one of the treatments at statistically significant level (P < 0.05) (Tables 2 and 3). Proteins that were not detected in this study were below the detection limit of ± 1 ng and were therefore considered absent in the sample. All 20 different proteins were identified using Quadrupole Time-of-Flight (Q-TOF) tandem mass spectrometry analysis (MS/MS) (P < 0.05). Table 2 shows the Log2 fold-change variation of identified protein for each comparison. Table 3 shows a description of each of these proteins, including the associated biological process and molecular function according to the Uniprot database.
Table 2. Fold-change (Log2FC) of differentially abundant proteins in herbicide-tolerant GM maize NK603 and its non-GM near-isogenic variety grown under suboptimal conditions in Experiment 1 and 2.
A positive value indicates that the protein showed up-regulation for the first treatment of the comparison. A negative value means that the protein showed downregulation for the first treatment of the comparison. ‘ns’ indicates that no significant difference (ANOVA P < 0.05) was found for the relative abundance of this protein on that specific comparison.
| Protein | UniProt ID | Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|---|---|
| Factor 1 (Stress) | Factor 2 (Genetic Modification) | Unifactorial | ||||
| non-GM D x non-GM | GM D x GM | GM x non-GM | GM D x non-GM D | GM DH x GM D | ||
| ATP synthase subunit gamma, chloroplastic precursor (Zea mays) | P0C1M0 | -1.34 | -1.19 | ns | ns | 1.33 |
| Ferredoxin-NADP reductase (Zea mays) | B6T9S5 | 0.57 | -0.75 | ns | -0.93 | 0.72 |
| Photosystem I reaction center subunit II (Zea mays) | B6SKI1 | 0.71 | ns | ns | -0.80 | ns |
| Photosystem I reaction center subunit IV A (Zea mays) | B6SPC1 | 1.21 | ns | ns | ns | ns |
| ATP synthase CF1 alpha subunit (Saccharum hybrid cultivar SP-80-3280) | A0A0U2U1F5 | ns | -0.95 | ns | ns | ns |
| ATP synthase CF1 beta subunit (chloroplast) (Zea mays) | P00827 | ns | 0.76 | ns | ns | ns |
| NAD-dependent epimerase/dehydratase (Zea mays) | B4FH62 | ns | -0.77 | ns | ns | ns |
| Photosystem I subunit VII (chloroplast) (Zea mays) | P11601 | ns | -0.81 | ns | -0.84 | ns |
| Oxygen-evolving enhancer protein 2, chloroplastic (Zea mays) | A0A096QKN7 | ns | -0.85 | ns | -0.67 | -0.44 |
| Phosphoglycerate kinase (Zea mays) | C0PDB0 | ns | -0.96 | ns | 1.42 | ns |
| Cytochrome b6-f complex iron-sulfur subunit (Zea mays) | B4FTU7 | ns | 0.63 | ns | ns | ns |
| Inorganic pyrophosphatase (Zea mays) | B6SQQ0 | ns | -0.65 | ns | ns | 0.57 |
| psbP-like protein 2, chloroplastic (Zea mays) | A0A096TZ12 | ns | ns | 0.88 | ns | ns |
| 3-phosphoshikimate-1-carboxyvinyltransferase (Glycine max) | Q71LY8 | ns | ns | 1.26 | 1.17 | Exclusive GM D |
| Ribulose bisphosphate carboxylase/oxygenase activase, chloroplastic precursor (Zea mays) | Q9ZT00 | ns | ns | -0.80 | ns | ns |
| Ribulose bisphosphate carboxylase large subunit (Zea mays) | A0A059Q6R9 | ns | ns | -0.86 | ns | ns |
| Chain A, The Crystal Structure of Psbp (Zea mays) | A0A096Q0E5 | ns | ns | 0.49 | ns | ns |
| Malate dehydrogenase (Zea mays) | B4FZU8 | 1.15 | ns | ns | ns | ns |
| Fructose-bisphosphate aldolase (Zea mays) | C0PD30 | ns | ns | 0.64 | ns | ns |
| Uncharacterized protein LOC100273394 (Zea mays) | B4FV82 | ns | -1.06 | ns | ns | ns |
Table 3. Description of differentially expressed proteins found in this study.
Proteins were considered differentially modulated at statistically significant difference in normalized volume in each comparison between the treatments (GM; non-GM; GM D; non-GM D; GM DH) at ANOVA P < 0.05 according to Table 2. Proteins were classified in functional categories based on the KEGG Orthology databases and on careful literature evaluation. The table reports spot number (Match ID), accession number and protein name, together with Mascot score, sequence coverage, number of matched peptides, theoretical and experimental molecular weight (MW) and isoelectric point (pI).
| Spot ID | Accession | Protein Name | Mascot Score | Sequence Coverage (%) | Peptides | Theor. Mass (kDa) | Theor. pI (pH) | Exp. Mass (kDa) | Exp. pI (pH) | Biological Process / Molecular Function |
|---|---|---|---|---|---|---|---|---|---|---|
| 1689 | NP_001150872 | ATP synthase subunit gamma, chloroplastic precursor (Zea mays) | 520 | 31 | 12 | 54 | 5.31 | 52 | 5.4 | Energy metabolism / Stress response (ATP synthesis coupled proton transport) |
| 1576 | ACG33858 | Ferredoxin-NADP reductase, (Zea mays) | 536 | 44 | 19 | 40 | 8.56 | 44 | 8.2 | Energy metabolism (oxidoreductase; nucleotide binding) |
| 1364 | ACG25364 | Photosystem I reaction center subunit II (Zea mays) | 220 | 24 | 5 | 21 | 9.77 | 20 | 9.1 | Energy metabolism (photosynthesis) |
| 1329 | NP_001149700 | Photosystem I reaction center subunit IV A (Zea mays) | 204 | 47 | 6 | 14 | 9.79 | 15 | 9.1 | Energy metabolism (photosynthesis) |
| 1881 | YP_024377 | ATP synthase CF1 alpha subunit (Saccharum hybrid cultivar SP-80-3280) | 842 | 40 | 17 | 55 | 5.87 | 55 | 5.8 | Energy metabolism (ATP hydrolysis-coupled proton transport) |
| 1826 | NP_043032 | ATP synthase CF1 beta subunit (chloroplast) (Zea mays) | 237 | 16 | 5 | 54 | 5.31 | 55 | 5.4 | Energy metabolism (ATP hydrolysis-coupled proton transport) |
| 1511 | ACG31416 | NAD-dependent epimerase/dehydratase (Zea mays) | 264 | 35 | 7 | 31 | 9.11 | 30 | 9.1 | Carbohydrate metabolism (catalytic activity) |
| 1261 | NP_039445 | Photosystem I subunit VII (chloroplast) (Zea mays) | 110 | 46 | 3 | 8 | 6.51 | 8 | 6.2 | Energy metabolism (photosynthetic electron transport in photosystem I; oxidoreductase) |
| 1429 | XP_008652004 | Oxygen-evolving enhancer protein 2, chloroplastic (Zea mays) | 345 | 37 | 8 | 31 | 9.50 | 33 | 9.1 | Energy metabolism (photosynthesis; calcium ion binding; photosystem I) |
| 1756 | NP_001147628 | Phosphoglycerate kinase (Zea mays) | 413 | 23 | 10 | 50 | 6.07 | 48 | 5.8 | Carbohydrate metabolism (glycolytic process; phosphorylation) |
| 1365 | ACG28186 | Cytochrome b6-f complex iron-sulfur subunit (Zea mays) | 204 | 28 | 5 | 24 | 8.52 | 21 | 8.4 | Energy metabolism (photosynthesis; oxidoreductase activity; electron transport) |
| 1529 | ACG27183 | Inorganic pyrophosphatase (Zea mays) | 744 | 49 | 19 | 31 | 5.79 | 30 | 5.9 | Carbohydrate metabolism (phosphate-containing compound metabolic process; magnesium ion binding) |
| 1341 | XP_008654301 | psbP-like protein 2, chloroplastic (Zea mays) | 301 | 36 | 8 | 25 | 8.34 | 25 | 7.5 | Energy metabolism (photosynthesis; calcium ion binding; photosystem II) |
| 1763 | AAL67577 | 3-phosphoshikimate-1-carboxyvinyltransferase (Glycine max) | 944 | 50 | 19 | 47 | 5.13 | 49 | 5.2 | Energy metabolism (chorismate biosynthetic process; shikimate pathway); |
| 1743 | NP_001104921 | Ribulose bisphosphate carboxylase/oxygenase activase, chloroplastic precursor (Zea mays) | 487 | 31 | 12 | 47 | 6.29 | 49 | 6.4 | Energy metabolism (Calvin cycle; ATP binding; activation of RuBisCO) |
| 1848 | NP_043033 | Ribulose bisphosphate carboxylase large subunit (Zea mays) | 521 | 27 | 12 | 52 | 6.33 | 52 | 6.3 | Energy metabolism (photosynthesis; carbon fixation) |
| 1449 | 4RTH_A | Chain A, The Crystal Structure of Psbp (Zea mays) | 272 | 44 | 6 | 20 | 5.96 | 21 | 6.0 | Energy metabolism (photosynthesis; calcium ion binding) |
| 1638 | NP_001142100 | Malate dehydrogenase (Zea mays) | 110 | 22 | 4 | 35 | 8.23 | 34 | 8.2 | Carbohydrate metabolism (oxidoreductase) |
| 1617 | ACG33017 | Fructose-bisphosphate aldolase (Zea mays) | 522 | 27 | 10 | 40 | 5.39 | 39 | 6.1 | Carbohydrate metabolism (glycolysis; carbohydrate degradation) |
| 1581 | NP_001141303 | Uncharacterized protein LOC100273394 (Zea mays) | 109 | 11 | 3 | 31 | 5.57 | 31 | 5.4 | Unidentified |
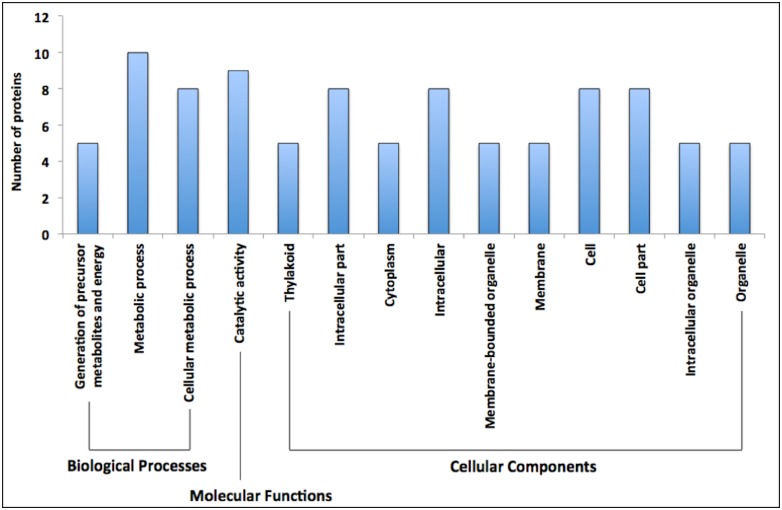
We performed a pathway enrichment analysis in order to rank associations between the set of differentially regulated proteins representing metabolic pathways and the respective statistical probability. This analysis allows us to identify the relevant results of potentially altered pathways. All significantly enriched GO terms (FDR < 0.05) are shown in Fig 2. The altered biological processes with the highest number of assigned proteins were metabolic process (GO:0008152), catalytic activity (GO:0003824) and cellular metabolic process (GO:0044237). The differentially abundant proteins related to each different biological process will be discussed separately in the following sections.
Fig 2. Enrichment analysis of 20 differentially expressed proteins of herbicide-tolerant GM maize variety (NK603) and its non-GM near-isogenic counterpart under control and stress conditions (drought and herbicide application).
The analysis was performed using the online tool agriGO v1.2, using Single Enrichment Analysis (SEA) with the following parameters: 1) Selected species: Zea mays ssp; 2) Statistical test method: Hypergeometric; 3) Multi-test adjustment method: Hochberg (FDR); 4) Significance level of 0.05; 5) Minimum number of 5 mapping entries; and 6) Gene ontology type: Plant GO Slim.
3.2.1. Experiment 1—Effects of drought stress
Two separate pairwise analyses were carried out to assess the effects of drought stress in GM and non-GM plants: GM D x GM and non-GM D x non-GM. A total of 14 differentially abundant proteins were observed in the two comparisons. Five of these proteins were differentially abundant in control only, with log2 fold-change values (log2FC) ranging from -1.34 to 1.15 (Table 2). Nevertheless, 4 of these 5 proteins were upregulated in the stress treatment, except for ATP synthase subunit gamma (chloroplastic precursor), which was downregulated in stress. These 5 proteins are involved in molecular functions belonging to energy metabolism and stress response biological processes (Table 3). The comparison between GM D x GM revealed 11 differentially abundant proteins with log2FC ranging from -1.19 to 0.76, and most of the proteins were downregulated in the GM D samples. These 11 proteins are not only involved in energy metabolism, but also in primary reactions of carbohydrate metabolism, such as glycolysis and phosphorylation (Table 3).
As expected, some of the proteins were found differentially abundant in both comparisons, but with distinct fold-changes. For instance, ATP synthase subunit gamma (chloroplastic precursor) was downregulated in the GM D samples (log2FC = -1.19) and in non-GM D samples (log2FC = -1.34), when compared to the control under optimal conditions. ATP synthase is an enzyme that modulates the electrochemical gradient across the thylakoid membrane and directly influences the rate of photosynthetic electron transport, thus representing a key factor in the regulation of photosynthetic energy conversion in chloroplasts [44]. Kohzuma et al. [45] demonstrated that thylakoid membranes of wild watermelon under drought conditions were drained of chloroplast ATP synthase e-subunit. Later, the same authors reported the downregulation of ATP synthase as a natural response of plants to drought stress [46]. In addition, Western blotting revealed that ATP synthase content decreased significantly in wild watermelon plants under drought stress. Therefore, these authors [46] also showed that plant responses to drought stress influence the regulatory ‘set point’ of photosynthesis by changing the expression of chloroplast ATP synthase and its activity. Thus, as an adaptive response, decreasing ATP synthase content leads to a more sensitive ‘set point’ for downregulation of the antenna, tending toward photoprotection over photosynthetic efficiency under stress such that the accessibility of light is likely to far transcend that for different resources needed to store photosynthetic vitality, e.g., water or CO2 [46]. Our results endorse these findings and suggest that quantitative control of ATP synthase is a consequence of plant response to the regulation of photosynthetic energy conversion under adverse environmental conditions.
On the other hand, Ferredoxin-NADP reductase showed different regulation in GM D samples (log2FC = -0.75) and non-GM D samples (FC = 0.57) when compared with the same varieties under normal growth conditions (Table 2). This protein is also located at the thylakoid membranes that participate in the ferredoxin reductase pathway responsible for NADPH production, which, in turn, is primarily used in the Calvin cycle [47]. Lehtimäki et al. [48] reported that the total expression level of the Ferredoxin-NADP+-oxidoreductase genes increased upon drought stress in Arabidopsis thaliana. In contrast, a decrease in Ferredoxin-NADP reductase was reported in Populus cathayana and wheat in response to drought stress [49], which seems to be the same case for the non-GM hybrid under drought stress. Most other differentially abundant proteins in the GM D x GM comparison were downregulated in the GM D treatment, such as ATP synthase CF1 alpha subunit, NAD-dependent epimerase/dehydratase, Photosystem I subunit VII (chloroplast), Oxygen-envolving enhancer protein 2 (chloroplastic), Phosphoglycerate kinase, Inorganic pyrophosphatase, and Uncharacterized protein LOC100273394. They are all involved in energetic metabolism and processes, including transport activities in photosynthesis, oxidoreductase, photosynthetic carbon metabolism and catalytic activities. Previous proteomic studies have reported similar results, showing that drought stress leads to an overall reduction of the photosynthetic transport chain [50], which consequently induces oxidative stress [51].
During the past few years, several reviews have discussed how drought constrains photosynthesis [52,53]. Although some controversy on the subject remains, decreased CO2 diffusion from the atmosphere to the site of carboxylation is generally considered the main cause for decreased photosynthesis under mild to moderate water limitation [52,54,55]. Corroborating our findings, other studies also recognize energetic metabolism as a key component of cellular function under limited supply of CO2 as a consequence of drought stress [53,56]. For instance, under these stress conditions, Tezara et al. [57] found impaired ATP production and, thus, ribulose bisphosphate regeneration. More recently, reactive oxygen species generated under highly reduced conditions in the chloroplast were shown to damage ATP synthase [53].
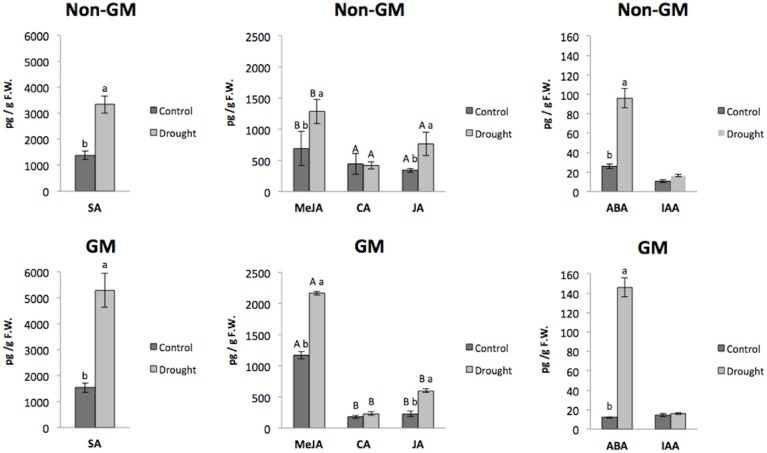
Regarding to the phytohormone and related compounds analysis, our results showed statistically significant difference in four (SA, MeJA, JA and ABA) of the six phytohormones and related compounds quantified (Fig 3). All four hormones revealed average levels significantly higher in the treatments under drought stress (non-GM D and GM-D) compared to control treatments (non-GM and GM), showing log2FC values between 0.89 (MeJA) and 3.60 (ABA) (Table 4). These results were expected since these four plant growth regulators have all been previously associated with plant response to environmental changes via cell signaling pathways [58].
Fig 3. Levels of plant hormones and related compounds for Experiment 1.
Levels of SA, MeJA, CA, JA, ABA and AIA in leaves of herbicide-tolerant GM maize (NK603) variety and its non-GM counterpart under control and drought stress conditions. Compound levels were considered significantly different at ANOVA P < 0.05. When significant, lowercase letters represent the horizontal comparisons in each compound from the Stress factor (non-GM D x non-GM; GM D x GM). Uppercase letters represent the vertical comparisons from the Genetic Modification factor (GM x non-GM; GM D x non-GM D)
Table 4. Fold-change (Log2FC) for plant hormones and related compounds levels in herbicide-tolerant GM maize variety (NK603) and its non-GM near-isogenic variety in different comparisons of Experiment 1 and 2.
A positive value indicates that the compound showed upregulation for the first treatment of the comparison. A negative value indicates that the compound showed downregulation for the first treatment of the comparison. ‘ns’ indicates that no significant difference was found (ANOVA P < 0.05) for the levels of this compound on that specific comparison.
| Hormone | PubChem ID | Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|---|---|
| Factor 1 (Stress) | Factor 2 (Genetic Modification) | Unifactorial | ||||
| non-GM D x non-GM | GM D x GM | GM x non-GM | GM D x non-GM D | GM DH x GM D | ||
| Salicylic Acid (SA) | 338 | 1.27 | 1.78 | ns | ns | -1.35 |
| Methyl Jasmonate (MeJA) | 5367719 | 0.89 | 0.89 | 0.75 | 0.75 | ns |
| Cinnamic Acid (CA) | 444539 | ns | ns | -1.28 | -0.85 | ns |
| Jasmonic Acid (JA) | 5281166 | 1.15 | 1.37 | -0.56 | -0.34 | 1.01 |
| Abscisic Acid (ABA) | 5280896 | 1.88 | 3.60 | ns | ns | 2.92 |
| Indole-3-acetic acid (IAA) | 801 | ns | ns | ns | ns | ns |
Water deficit is widely known to affect biosynthesis, accumulation, and redistribution of major plant hormones and other related compounds. ABA, which is synthesized either in leaves or in roots, plays a major role in commanding stomatal closure and, therefore, photosynthetic carbon uptake under conditions of water deficit [59,60]. In addition, ABA synthesis is one of the fastest responses of plants to drought stress, which triggers ABA-inducible gene expression [61], causing stomatal closure, thereby reducing water loss via transpiration [62] and eventually restricting cellular growth. Nevertheless, recent studies have shown evidence of crosstalk between different hormones and other compounds, such as auxin, cytokinin, ethylene, brassinosteroids, jasmonates, and salicylic acid, resulting in synergistic or antagonistic interactions and coordinated regulation of hormone biosynthetic pathways, which play crucial roles in the response of plants to abiotic stress [31,63–65].
Acharya and Assman [66] suggested that other hormones, such as JA, SA and IAA, are also involved in stomatal function. Whereas ABA, SA, JA and other related compounds induce stomatal closure, IAA, together with citokinin, promotes stomatal opening. These hormones act to modulate the expression of different drought-related genes [67,68]. A wide range of cellular biochemical events has also been associated with the regulation of stomatal guard cells, such as the activation of G-proteins, the production of reactive oxygen species (ROS) (ABA-stimulated), protein phosphorylation/dephosphorylation, and reorganization of the cytoskeleton [66]. Under drought stress, our results showed a significant increase in ABA levels in GM plants, as well as a significant downregulation of proteins involved in phosphorylation and dephosphorylation, such as Phosphoglycerate kinase and Inorganic pyrophosphatase. Phosphoglycerate kinase catalyses the phosphorylation of 3-PG, producing 1,3-BPG and ADP, as part of the reactions that regenerate ribulose-1,5-bisphosphate in the Calvin cycle. On the other hand, inorganic pyrophosphatases are important enzymes that generate the thermodynamic driving force for some cellular biosynthetic reactions by catalysing the hydrolysis of inorganic pyrophosphate (PPi) to inorganic orthophosphate (Pi) [69]. Pyrophosphatases not only play a crucial role in ATP production, but are also important for biopolymer synthesis in the construction of new cell walls and membranes [70,71]. Similar to our results, Fukuda and Tanaka [72] showed that ABA mediates the expression of genes responsible for regulating pyrophosphatases in response to osmotic stresses resulting from environmental changes, e.g., salt and drought stress.
3.2.2. Experiment 1—Effects of genetic modification
The comparative analysis between genetically modified and unmodified varieties: GM x non-GM and GM D x non-GM D, which was performed to assess the effects of genetic modification, revealed a total of 11 differentially regulated proteins. The GM x non-GM comparison showed six differentially abundant proteins, with four of them upregulated in the GM variety, and these four are involved in carbohydrate and energy metabolism: psbP-like protein 2 (chloroplastic), EPSPS, Chain A, and Fructose-bisphosphate aldolase (Table 2). Proteins involved in carbohydrate and energy metabolism have also been detected in other proteomic studies comparing GM varieties and their near-isogenic conventional counterparts [1–4,73–76]. These results suggest that GM crops have a higher energy demand in comparison to conventional plants which do not bear the necessity of calling upon energy sources to synthetize transgenic proteins. In addition, previous findings suggested that the strong and constitutive promoters, e.g., CaMV-35S, used for genetic modification could lead to this high energetic cost in transgenic plants [77,78]. If so, additional studies should be carried out to identify which metabolic pathways will be more affected by the reduced amount of available energy.
The other two proteins detected, Ribulose bisphosphate carboxylase/oxygenase activase and Ribulose bisphosphate carboxylase large subunit, showed downregulation in the GM variety. These are two similar proteins, located in the chloroplast, and they play major roles in the activation of RuBisCO activity. The drop in Rubisco activase is presumably a key factor in slowing down Rubisco activity [53]. Downregulation of key enzymes involved in RuBisCO activity might result in a reduction of photosynthetic rates of the GM plants (NK603). Since none of these six differential proteins from the GM x non-GM comparison was differentially abundant for factor 1 (stress) comparative analysis, such reduction in photosynthetic rates could be a result of genetic modification. Again, it remains to be scrutinized if this reduction of photosynthetic rates will impose additional burden on plant development.
The GM D x non-GM D comparison also showed six differentially abundant proteins, with four downregulated in the GM D treatment. Again, these are proteins that play crucial roles, including, for example, electron transport and oxidoreductase activity, in photosynthesis, acting mainly in Photosystem I. On the other hand, drought stress leads to a reduction of the photosynthetic rates, but in the present study, GM plants have shown a significant downregulation of these photosynthesis-related proteins compared to their non-GM near-isogenic variety under the same environmental stresses.
MeJA, CA and JA showed significantly different levels between the GM and its non-GM near-isogenic variety under both control and stress conditions (Fig 3). MeJA levels increased in the GM and GM D samples, while significantly lower levels were observed for CA and JA. Opposite regulation of JA and MeJA was an unexpected result since they are synthesized in the same main octadecanoid pathway. Moreover, these compounds play important roles together to activate plant defense mechanisms in response to biotic and abiotic stresses, such as drought, low temperature, and salinity [79].
The aromatic amino acid products of the shikimate pathway (phenylalanine, tyrosine and tryptophan) are not only essential components of protein synthesis, but also serve as precursors for a wide range of secondary metabolites that are important for plant growth. Sasaki-Sekimoto et al. [80] reported that the Arabidopsis gene encoding IGPS of Tryptophan biosynthesis is regulated by JA. Since the shikimate pathway is upregulated in GM plants by the insertion of a new EPSPS, our results suggest that JA levels in herbicide-tolerant GM plants are higher from the possible upregulation of the shikimate pathway to increase tryptophan production.
CA is also a key intermediate in the shikimate pathway, and it is formed through deamination of L-phenylalanine, which is one of the primary aromatic amino acid products of the shikimate pathway [81]. Since the NK603 GM event was modified to produce a glyphosate-based herbicide-tolerant form of EPSPS enzyme, which is also involved in the biosynthesis of phenylalanine, tryptophan and tyrosin in the shikimate pathway, our findings suggest a possible influence of the genetic transformation on the synthesis of secondary nontarget metabolites as result of new pleiotropic effects by the presence of the integrated transgene.
Overall, the differences in the protein profile and metabolite levels between the GM maize NK603 event and its near-isogenic non-GM variety can affect many physiological processes and biochemical pathways, such as photosynthesis-related pathways. Taken together, our results demonstrate that these two hybrids may not behave similarly at the molecular level, thus precluding the assumption that they are substantially equivalent.
3.2.3. Experiment 2—Combined effects of drought and herbicide stress
In the second experiment, we tested the influence of herbicide application on plants under drought stress, and we compared GM DH x GM D samples. The comparative proteomic analysis revealed four differentially abundant proteins differentially, three of which were shown to be upregulated in the GM DH samples (Table 2). These proteins were associated with increased energy metabolism, such as ATP synthase subunit gamma, Ferredoxin-NADP reductase and Inorganic pyrophosphatase, suggesting that an increased amount of energy is required to adapt to cumulative stress. Meanwhile, the downregulation of Oxygen-envolving enchancer protein 2 (chloroplastic) might be a result of photosynthesis reduction in these plants under drought and herbicide stress, resulting from involvement in the photosynthesis process. Ahsan et al. [82] found similar results when analysing the proteomic profile of rice leaves under two concentrations of arsenate. An increased activity of several proteins associated with energy metabolism was found, confirming the assumption that energy is required for stress adaptation. Glyphosate-based herbicide was also applied to rice leaves as a stress factor, and it resulted in increased accumulation of antioxidant enzymes, such as ascorbate peroxidase, glutathione S-transferase, thioredoxin h-type, nucleoside diphosphate kinase 1, peroxiredoxin and a superoxide dismutase [Cu—Zn] chloroplast precursor. Therefore, the authors suggest that glyphosate-based herbicides might induce oxidative stress in plants [13, 83].
Furthermore, the comparative analysis showed exclusive expression of the EPSPS synthase protein in plants without herbicide application (GM D), which was expected since native EPSPS synthase is inhibited by the glyphosate-based herbicide. This is an important enzyme involved in the shikimate pathway, which is primordial in one step of chorismate synthesis, preceding the synthesis of some essential aromatic amino acids, such as phenylalanine, tyrosine and tryptophan [84]. In addition, this pathway inhibited by the herbicide based on Glyphosate (N-(phosphonomethyl)glycine) is also involved in the synthesis of lignin, flavonoids, phenolic compounds, and other secondary metabolites that can account for as much as 35% of a plant’s biomass [85].
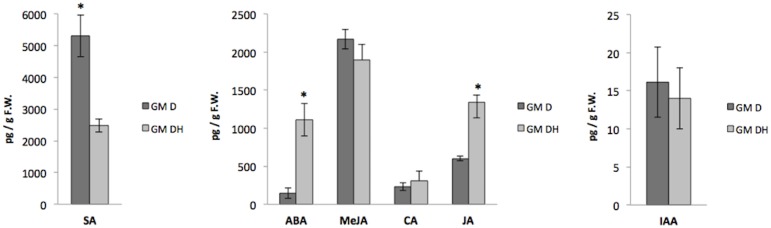
Comparison of GM D x GM HD revealed that sequential applications of glyphosate-based herbicide resulted in different levels of SA, ABA and JA (Fig 4). While SA levels were significantly lower in the GM HD samples, ABA and JA were detected at higher levels in the same treatment, when compared to the absence of the herbicide. Plants that received herbicide application showed levels of ABA and JA up to 8 times greater (log2FC = 2.9) than those without herbicide (Table 4). Interestingly, proteomic analysis showed that an ATP synthase subunit gamma protein was upregulated in the GM DH samples. This protein mediates plant perception in stress through the induction of volatile, phenylpropanoid and protease inhibitor defenses, such as JA and SA [86].
Fig 4. Levels of plant hormones and related compounds for Experiment 2.
Levels of SA, ABA, MeJA, CA, JA, and AIA in leaves of herbicide-tolerant GM maize NK603 under drought stress conditions and herbicide application. (*) Compound level was considered significantly different at ANOVA P < 0.05.
Doğramacı et al. [87] studied the impact of glyphosate-based herbicide on the vegetative growth of Leafy spurge (Euphorbia esula), and although abundance of jasmonic acid (JA) was not quantified, significant increase of transcripts homologous to Arabidopsis genes involved in biosynthesis of JA was also observed in plants treated with the herbicide. The authors also reported a significant increase in other important bioactive auxins (IAA, IBA) in glyphosate-based, herbicide-treated plants.
Reduced amounts of EPSPS caused by the glyphosate-based herbicide led to reductions in aromatic amino acids and indole-3-acetic acid (IAA), which is an important signaling hormone for plant growth and development, ultimately causing the plant to weaken and die [88]. Although not statistically significant, a decrease of IAA levels was verified in the GM samples treated with herbicide (GM DH). This was not expected since these plants constantly synthesize a substantial equivalent EPSPS enzyme. Although these plants were genetically modified to produce a glyphosate-tolerant form of EPSPS, the herbicide is somehow affecting the secondary metabolism of these herbicide-tolerant plants. Consequently, the synthesis of other compounds, such as lignin, flavonoids, phenolic compounds, and other secondary metabolites, is also involved in the shikimate pathway and may also be altered as an unintended effect. In addition, Bohn et al. [9] reported that herbicide-tolerant GM soy may have high residue levels of glyphosate and aminomethylphosphonic acid (AMPA) and that different agricultural practices may result in a markedly different nutritional composition of soybeans. Thus, in addition to presenting a markedly different nutritional composition and gene expression, secondary metabolism could be affected by the presence of the transgene and/or Roundup herbicide.
Moreover, it is important to mention that the NK603 event carries two gene cassettes, both containing the CP4 EPSPS coding sequence, but under the regulation of different promoters. While the first one contains the 5'-end of the rice actin sequence (ract1) promoter and the first intron upstream of the CTP sequence, the second CP4 EPSPS gene is under the control of the enhanced CaMV 35S promoter fused to the 0.8 kb intron sequence from the gene of maize heat shock 70 protein [89]. Castan et al. [90] recently reported a study in which the NK603 construct was sequenced, with the aim of examining its genetic stability. The authors consistently detected two nucleotide insertions in all sequenced samples of NK603 × MON810 (varieties 631RR2/Bt and DKC 26–79 progeny), as well as the certified reference material, by comparing the sequenced construct NK603 to the published patent sequence [91]. Since the detected nucleotide insertions were located in a promoter region, the authors highlighted the possibility of an influence on promoter activity and, hence, the expression rate of the transgene. However, determining whether one of the cassettes was the major source of the impacts affecting proteins and hormones in unexpected ways is outside the scope of the present paper, but we could consider this hypothesis for future specific studies.
3.3. Contributions to risk assessment
Our findings challenge the general presumption of molecular stability of commercially approved GM plants compared to their non-GM near-isogenic varieties, in particular when considering environmental variables. Although no consensus has been reached on the overall utility of omics techniques, they have been acknowledged as a potentially valuable tool in RA [92–95]. Trough the use of omics analysis, our study has shown that proteins assigned to important biological processes, such as energy/carbohydrate metabolism, have had their relative abundance level altered in GM plants under normal conditions and abiotic stresses and this might have a safety relevance. The applicability and usefulness of proteomics and metabolomics to the molecular characterization step of premarket biosafety RA of GMOs is still an evolving view, many studies, including the present one, demonstrate the feasibility and the necessity of such characterization studies for any GM event. In addition, as new kinds of GMOs are developed, wide use of molecular profiling should be added to the required criteria for risk assessment.
Even though studies have assessed unintended effects of GM plants derived from genetic modification based on “omics” approaches, most of them still evaluate these plants under controlled or invariable growth conditions [1,73,95]. The aim of the present study was to broaden knowledge about potential variations in the proteome and secondary metabolism of GM plants that result from genetic transformation, environmental variables, or the interaction between these factors. So far, no similar study has integrated proteomics and metabolomics approaches to explore plant molecular response of GM plants to abiotic stresses in a manner equal to that of the present work.
It is anticipated that the present work will pave the way for collaborative studies for the development of gene, protein, and metabolite databases of important crops grown across a range of environmentally variable conditions, thus providing an important and useful benchmark for safety assessment [92]. It is also hoped that this work will set a precedent whereby newly developed GM plants are subjected to experimentation under different environmental conditions as a part of risk assessment before approval by regulatory agencies.
4. Conclusions
Significant differences in protein relative abundance and levels of plant hormones and related compounds of the herbicide-tolerant GM NK603 maize variety under optimal and stress conditions were observed. The protein profile of GM maize under different environmental conditions revealed 20 different proteins, which were classified into three major categories: metabolic process, catalytic activity and cellular metabolic process, with most of the detected proteins assigned to energy/carbohydrate metabolic processes. Our findings suggest that environmental factors, such as drought and herbicide application, are major sources of quantitative variation in protein relative abundance and phytohormones/related compound levels, followed by the genetic modification. Some differences in protein relative abundance (11 proteins) and some compound levels (JA, MeJA and CA) were found between the GM plant and its non-GM near-isogenic variety under the same environmental conditions, suggesting that genetic modification itself may also play an important role in the appearance of pleiotropic effects. Taking into account the consequences of differences in protein profile and metabolite levels, including hormones and related compounds, the results found in this study do not support the substantial equivalence between the tested GM maize (NK603) and its non-GM near-isogenic variety.
Therefore, based on environmental variables, especially those exerting stress, it was our goal to show that proteomic profiling can assist in the detection of unintended changes that cannot otherwise be detected using a comparison with field-grown plants under suboptimal conditions. Our research findings could be applied to the GMO RA step right away; therefore, they are likely to be of great interest to scientists, researchers, regulators, industrial leaders, and other private organizations involved in the development and/or evaluation of GMO safety.
Supporting information
Optimization of each analyte for Multiple Reactions Monitoring (MRM) transition and overlaid multiple reaction monitoring chromatograms of both positive and negative ionization mode.
(DOCX)
Sequences of all peptides from the differentially abundant proteins identified.
(XLSX)
Acknowledgments
The authors would like to thank CAPES for scholarships provided to R.B. and CNPq for the scholarship to V.V. and R.O.N. Financial support was provided by The Norwegian Agency for Development Cooperation (Ministry of Foreign Affairs, Norway) under the GenØk South-America Research Hub grant FAPEU 077/2012. The authors also thank the Metabolomic Centre of the Department of Biochemistry (North-West University, South Africa) for quantitative analysis of phytohormones and related compounds. This was a joint project between UFSC and GenØk—Centre for Biosafety.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors would like to thank CAPES for scholarships provided to RB and CNPq for the scholarship to VV and RON. Financial support was provided by The Norwegian Agency for Development Cooperation (Ministry of Foreign Affairs, Norway) under the GenØk South-America Research Hub grant FAPEU 077/2012. The authors also thank the Metabolomic Centre of the Department of Biochemistry (North-West University, South Africa) for quantitative analysis of phytohormones and related compounds. This was a joint project between UFSC and GenØk – Center for Biosafety.
References
- 1.Agapito-Tenfen SZ, Vilperte V, Benevenuto RF, Rover CM, Traavik TI, Nodari RO. Effect of stacking insecticidal cry and herbicide tolerance epsps transgenes on transgenic maize proteome. BMC plant biology. 2014. December 10;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agapito-Tenfen SZ, Guerra MP, Wikmark OG, Nodari RO. Comparative proteomic analysis of genetically modified maize grown under different agroecosystems conditions in Brazil. Proteome science. 2013. December 4;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barros E, Lezar S, Anttonen MJ, Van Dijk JP, Röhlig RM, Kok EJ, Engel KH. Comparison of two GM maize varieties with a near-isogenic non-GM variety using transcriptomics, proteomics and metabolomics. Plant Biotechnology Journal. 2010. May 1;8(4):436–51. 10.1111/j.1467-7652.2009.00487.x [DOI] [PubMed] [Google Scholar]
- 4.Zolla L, Rinalducci S, Antonioli P, Righetti PG. Proteomics as a complementary tool for identifying unintended side effects occurring in transgenic maize seeds as a result of genetic modifications. Journal of Proteome Research. 2008. April 5;7(5):1850–61. 10.1021/pr0705082 [DOI] [PubMed] [Google Scholar]
- 5.Rosi-Marshall EJ, Tank JL, Royer TV, Whiles MR, Evans-White M, Chambers C, Griffiths NA, Pokelsek J, Stephen ML. Toxins in transgenic crop byproducts may affect headwater stream ecosystems. Proceedings of the National Academy of Sciences. 2007. October 9;104(41):16204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Séralini GE, Cellier D, De Vendomois JS. New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Archives of environmental contamination and toxicology. 2007. May 1;52(4):596–602. 10.1007/s00244-006-0149-5 [DOI] [PubMed] [Google Scholar]
- 7.Sagstad A, Sanden M, Haugland Ø, Hansen AC, Olsvik PA, Hemre GI. Evaluation of stress-and immune-response biomarkers in Atlantic salmon, Salmo salar L., fed different levels of genetically modified maize (Bt maize), compared with its near-isogenic parental line and a commercial suprex maize. Journal of fish diseases. 2007. April 1;30(4):201–12. 10.1111/j.1365-2761.2007.00808.x [DOI] [PubMed] [Google Scholar]
- 8.Aumaitre LA. New feeds from genetically modified plants: substantial equivalence, nutritional equivalence and safety for animals and animal products. Productions Animales. 2002. May 1;15:97–108. [Google Scholar]
- 9.Bøhn T, Cuhra M, Traavik T, Sanden M, Fagan J, Primicerio R. Compositional differences in soybeans on the market: Glyphosate accumulates in Roundup Ready GM soybeans. Food Chemistry. 2014. June 15;153:207–15. 10.1016/j.foodchem.2013.12.054 [DOI] [PubMed] [Google Scholar]
- 10.Cuhra M. Review of GMO safety assessment studies: glyphosate residues in Roundup Ready crops is an ignored issue. Environmental Sciences Europe 27, 1–14 (2015). [Google Scholar]
- 11.AHTEG. Guidance Document on Risk Assessment of Living Modified Organisms http://www.cbd.int/doc/meetings/bs/mop-05/official/mop-05-12-en.pdf; 2010. United Nations Environment Programme Convention for Biodiversity.
- 12.Benbrook CM. Impacts of genetically engineered crops on pesticide use in the US—the first sixteen years. Environmental Sciences Europe. 2012. September 28;24(24):2190–4715. [Google Scholar]
- 13.Mesnage R, Agapito-Tenfen S, Vilperte V, Renney G, Ward M, Séralini GE, Nodari N, Antoniou MN. An integrated multi-omics analysis of the NK603 Roundup-tolerant GM maize reveals metabolism disturbances caused by the transformation process. Scientific Reports, 2016; 6:37855 10.1038/srep37855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosová K, Vítámvás P, Prášil IT, Renaut J. Plant proteome changes under abiotic stress—contribution of proteomics studies to understanding plant stress response. Journal of proteomics. 2011. August 12;74(8):1301–22. 10.1016/j.jprot.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 15.Duhoux A, Délye C. Reference genes to study herbicide stress response in Lolium sp.: up-regulation of P450 genes in plants resistant to acetolactate-synthase inhibitors. PloS one. 2013. May 16;8(5):e63576 10.1371/journal.pone.0063576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Délye C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: a major challenge for weed science in the forthcoming decade. Pest management science. 2013. February 1;69(2):176–87. 10.1002/ps.3318 [DOI] [PubMed] [Google Scholar]
- 17.Castro AJ, Carapito C, Zorn N, Magné C, Leize E, Van Dorsselaer A, Clément C. Proteomic analysis of grapevine (Vitis vinifera L.) tissues subjected to herbicide stress. Journal of Experimental Botany. 2005. November 1;56(421):2783–95. 10.1093/jxb/eri271 [DOI] [PubMed] [Google Scholar]
- 18.Grime JP. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American naturalist. 1977. November 1:1169–94. [Google Scholar]
- 19.Vorasoot N, Songsri P, Akkasaeng C, Jogloy S, Patanothai A. Effect of water stress on yield and agronomic characters of peanut (Arachis hypogaea L.). Songklanakarin J. Sci. Technol. 2003. January 1;25(3):283–8. [Google Scholar]
- 20.Ahmad P, Kumar A, Ashraf M, Akram NA. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). African Journal of Biotechnology. 2012. February 7;11(11):2694. [Google Scholar]
- 21.Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Critical reviews in biotechnology. 2010. September 1;30(3):161–75. 10.3109/07388550903524243 [DOI] [PubMed] [Google Scholar]
- 22.Wilhite DA, Svoboda MD, Hayes MJ. Understanding the complex impacts of drought: a key to enhancing drought mitigation and preparedness. Water Resources Management. 2007. May 1;21(5):763–74. [Google Scholar]
- 23.Gurian-Sherman D. High and dry: Why genetic engineering is not solving agriculture’s drought problem in a thirsty world. Cambridge, MA, Union of Concerned Scientists; 2012. [Google Scholar]
- 24.Vicent D., Lapierre C., Pollet B., Cornic G., Negroni L., Zivy M.. Water deficits affect caffeate O-methyltransferase, lignification, and related enzymes in maize leaves. A proteomic investigation. Plant Physiology 2005. 137:949–960. 10.1104/pp.104.050815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishitani M, Nakamura T, Han SY, Takabe T. Expression of the betaine aldehyde dehydrogenase gene in barley in response to osmotic stress and abscisic acid. Plant molecular biology. 1995. January 1;27(2):307–15. [DOI] [PubMed] [Google Scholar]
- 26.Guerrero FD, Jones JT, Mullet JE. Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted. Sequence and expression of three inducible genes. Plant molecular biology. 1990. July 1;15(1):11–26. [DOI] [PubMed] [Google Scholar]
- 27.FAROOQ M., WAHID A., KOBAYASHI N., FUJITA D., BASRA S.M.A., 2009. Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev., 29: 185–212. [Google Scholar]
- 28.Bogeat-Triboulot MB, Brosché M, Renaut J, Jouve L, Le Thiec D, Fayyaz P, Vinocur B, Witters E, Laukens K, Teichmann T, Altman A. Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus euphratica, a poplar growing in arid regions. Plant physiology. 2007. February 1;143(2):876–92. 10.1104/pp.106.088708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Molecular and cellular biology. 1999. March 1;19(3):1720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinemann JA, Kurenbach B, Quist D: Molecular profiling—a tool for addressing emerging gaps in the comparative risk assessment of GMOs. Environ Int. 2011, 37: 1285–1293 10.1016/j.envint.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 31.Santner A, Estelle M. Recent advances and emerging trends in plant hormone signalling. Nature. 2009. June 25;459(7250):1071–8. 10.1038/nature08122 [DOI] [PubMed] [Google Scholar]
- 32.Alvarez S, Marsh EL, Schroeder SG, Schachtman DP. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant, Cell & Environment. 2008. March 1;31(3):325–40. [DOI] [PubMed] [Google Scholar]
- 33.Goodger JQ, Sharp RE, Marsh EL, Schachtman DP. Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sap sampling techniques. Journal of experimental botany. 2005. September 1;56(419):2389–400. 10.1093/jxb/eri231 [DOI] [PubMed] [Google Scholar]
- 34.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Analytical chemistry. 1996. March 1;68(5):850–8. [DOI] [PubMed] [Google Scholar]
- 35.Du Z, Zhou X, Ling Y, Zhang Z, Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic acids research. 2010. April 30:gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan X, Welti R, Wang X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography—mass spectrometry. Nature protocols. 2010. June 1;5(6):986–92. 10.1038/nprot.2010.37 [DOI] [PubMed] [Google Scholar]
- 37.Quackenbush J. Microarray data normalization and transformation. Nature genetics. 2002. December 1;32:496–501. 10.1038/ng1032 [DOI] [PubMed] [Google Scholar]
- 38.Meng C, Kuster B, Culhane AC, Gholami AM. A multivariate approach to the integration of multi-omics datasets. BMC bioinformatics. 2014. May 29;15(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anttonen MJ, Lehesranta S, Auriola S, Röhlig RM, Engel KH, Kärenlampi SO. Genetic and environmental influence on maize kernel proteome. Journal of proteome research. 2010. October 22;9(12):6160–8. 10.1021/pr100251p [DOI] [PubMed] [Google Scholar]
- 40.Ruebelt MC, Lipp M, Reynolds TL, Schmuke JJ, Astwood JD, DellaPenna D, Engel KH, Jany KD. Application of two-dimensional gel electrophoresis to interrogate alterations in the proteome of gentically modified crops. 3. Assessing unintended effects. Journal of Agricultural and Food Chemistry. 2006. March 22;54(6):2169–77. 10.1021/jf052358q [DOI] [PubMed] [Google Scholar]
- 41.Fang Y, Lu H, Chen S, Zhu K, Song H, Qian H. Leaf proteome analysis provides insights into the molecular mechanisms of bentazon detoxification in rice. Pesticide biochemistry and physiology. 2015. November 30;125:45–52. 10.1016/j.pestbp.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 42.Vera-Estrella R, Barkla BJ, Pantoja O. Comparative 2D-DIGE analysis of salinity responsive microsomal proteins from leaves of salt-sensitive Arabidopsis thaliana and salt-tolerant Thellungiella salsuginea. Journal of proteomics. 2014. December 5;111:113–27. 10.1016/j.jprot.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 43.Nilo R, Saffie C, Lilley K, Baeza-Yates R, Cambiazo V, Campos-Vargas R, González M, Meisel LA, Retamales J, Silva H, Orellana A. Proteomic analysis of peach fruit mesocarp softening and chilling injury using difference gel electrophoresis (DIGE). BMC genomics. 2010. January 18;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kramer DM, Cruz JA, Kanazawa A. Balancing the central roles of the thylakoid proton gradient. Trends in plant science. 2003. January 31;8(1):27–32. [DOI] [PubMed] [Google Scholar]
- 45.Kohzuma K, Akashi K, Munekage Y, Sanda S, Hisabori T, Yokota A. A novel behavior of e-subunit of chloroplast ATP synthase. Proceedings of the 14th International Congress of Photosynthesis, Fundamental Aspects to Global Perspectives. 2005. 1, 8–9. [Google Scholar]
- 46.Kohzuma K, Cruz JA, Akashi K, Hoshiyasu S, Munekage YN, Yokota A, Kramer DM. The long-term responses of the photosynthetic proton circuit to drought. Plant, cell & environment. 2009. March 1;32(3):209–19. [DOI] [PubMed] [Google Scholar]
- 47.Sparla F, Zaffagnini M, Wedel N, Scheibe R, Pupillo P, Trost P. Regulation of photosynthetic GAPDH dissected by mutants. Plant physiology. 2005. August 1;138(4):2210–9. 10.1104/pp.105.062117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehtimäki N, Lintala M, Allahverdiyeva Y, Aro EM, Mulo P. Drought stress- induced upregulation of components involved in ferredoxin-dependent cyclic electron transfer. Journal of plant physiology. 2010. August 15;167(12):1018–22. 10.1016/j.jplph.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 49.Budak H, Akpinar BA, Unver T, Turktas M. Proteome changes in wild and modern wheat leaves upon drought stress by two-dimensional electrophoresis and nanoLC-ESI—MS/MS. Plant Mol Biol 2013;83:89–103. 10.1007/s11103-013-0024-5 [DOI] [PubMed] [Google Scholar]
- 50.Osmond CB, Grace SC. Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis?. Journal of Experimental Botany. 1995. September 1;46(special issue):1351–62. [Google Scholar]
- 51.Borsani O, Dıaz P, Agius MF, Valpuesta V, Monza J. Water stress generates an oxidative stress through the induction of a specific Cu/Zn superoxide dismutase in Lotus corniculatus leaves. Plant Science. 2001. September 30;161(4):757–63. [Google Scholar]
- 52.Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of botany. 2009. February 1;103(4):551–60. 10.1093/aob/mcn125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawlor DW, Tezara W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of botany. 2009. January 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grassi G, Magnani F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell & Environment. 2005. July 1;28(7):834–49. [Google Scholar]
- 55.Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology. 2004. May 1;6(3):269–79. 10.1055/s-2004-820867 [DOI] [PubMed] [Google Scholar]
- 56.Pfannschmidt T, Bräutigam K, Wagner R, Dietzel L, Schröter Y, Steiner S, Nykytenko A. Potential regulation of gene expression in photosynthetic cells by redox and energy state: approaches towards better understanding. Annals of Botany. 2009. February 1;103(4):599–607. 10.1093/aob/mcn081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature. 1999. October 28;401(6756):914–7. [Google Scholar]
- 58.Peleg Z, Blumwald E. Hormone balance and abiotic stress tolerance in crop plants. Current opinion in plant biology. 2011. June 30;14(3):290–5. 10.1016/j.pbi.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 59.Hirayama T, Shinozaki K. Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends in plant science. 2007. August 31;12(8):343–51. 10.1016/j.tplants.2007.06.013 [DOI] [PubMed] [Google Scholar]
- 60.Dodd IC. Hormonal interactions and stomatal responses. Journal of plant growth regulation. 2003. March 1;22(1):32–46. [Google Scholar]
- 61.Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006. June 2;57:781–803. 10.1146/annurev.arplant.57.032905.105444 [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson S, Davies WJ. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, cell & environment. 2010. April 1;33(4):510–25. [DOI] [PubMed] [Google Scholar]
- 63.Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant molecular biology. 2009. March 1;69(4):473–88. 10.1007/s11103-008-9435-0 [DOI] [PubMed] [Google Scholar]
- 64.Wolters H, Jurgens G. Survival of the flexible: hormonal growth control and adaptation in plant development. Nature Reviews Genetics 2009, 10:305–317. 10.1038/nrg2558 [DOI] [PubMed] [Google Scholar]
- 65.Zhang G, Chen M, Li L, Xu Z, Chen X, Guo J, Ma Y. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. Journal of experimental botany. 2009:erp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Acharya B, Assmann S. Hormone interactions in stomatal function. Plant Molecular Biology 2009, 69:451–462. 10.1007/s11103-008-9427-0 [DOI] [PubMed] [Google Scholar]
- 67.Huang D, Wu W, Abrams SR, Cutler AJ. The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. Journal of experimental Botany. 2008. August 1;59(11):2991–3007. 10.1093/jxb/ern155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. Journal of experimental botany. 2007;58(2):221–7. 10.1093/jxb/erl164 [DOI] [PubMed] [Google Scholar]
- 69.De Graaf BH, Rudd JJ, Wheeler MJ, Perry RM, Bell EM, Osman K, Franklin FC, Franklin-Tong VE. Self-incompatibility in Papaver targets soluble inorganic pyrophosphatases in pollen. Nature. 2006. November 23;444(7118):490–3. 10.1038/nature05311 [DOI] [PubMed] [Google Scholar]
- 70.George GM, Van Der Merwe MJ, Nunes-Nesi A, Bauer R, Fernie AR, Kossmann J, Lloyd JR. Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduces drought stress tolerance in Nicotiana benthamiana. Plant physiology. 2010. September 1;154(1):55–66. 10.1104/pp.110.157776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rudd JJ, Franklin-Tong VE. Signals and targets of the self-incompatibility response in pollen of Papaver rhoeas. Journal of Experimental Botany. 2003. January 1;54(380):141–8. [DOI] [PubMed] [Google Scholar]
- 72.Fukuda A, Tanaka Y. Effects of ABA, auxin, and gibberellin on the expression of genes for vacuolar H+-inorganic pyrophosphatase, H+-ATPase subunit A, and Na+/H+ antiporter in barley. Plant Physiology and Biochemistry. 2006. June 30;44(5):351–8. [DOI] [PubMed] [Google Scholar]
- 73.Balsamo GM, Cangahuala-Inocente GC, Bertoldo JB, Terenzi H, Arisi AC. Proteomic analysis of four Brazilian MON810 maize varieties and their four non- genetically-modified isogenic varieties. Journal of agricultural and food chemistry. 2011. October 11;59(21):11553–9. 10.1021/jf202635r [DOI] [PubMed] [Google Scholar]
- 74.Coll A, Nadal A, Rossignol M, Puigdomènech P, Pla M. Proteomic analysis of MON810 and comparable non-GM maize varieties grown in agricultural fields. Transgenic research. 2011. August 1;20(4):939–49. 10.1007/s11248-010-9453-y [DOI] [PubMed] [Google Scholar]
- 75.Coll A, Nadal A, Collado R, Capellades G, Kubista M, Messeguer J, Pla M. Natural variation explains most transcriptomic changes among maize plants of MON810 and comparable non-GM varieties subjected to two N-fertilization farming practices. Plant molecular biology. 2010. June 1;73(3):349–62. 10.1007/s11103-010-9624-5 [DOI] [PubMed] [Google Scholar]
- 76.Coll A, Nadal A, Palaudelmas M, Messeguer J, Melé E, Puigdomenech P, Pla M. Lack of repeatable differential expression patterns between MON810 and comparable commercial varieties of maize. Plant molecular biology. 2008. September 1;68(1–2):105–17. 10.1007/s11103-008-9355-z [DOI] [PubMed] [Google Scholar]
- 77.Muñoz-Mayor A, Pineda B, Garcia-Abellán JO, Garcia-Sogo B, Moyano E, Atares A, Vicente-Agulló F, Serrano R, Moreno V, Bolarin MC. The HAL1 function on Na+ homeostasis is maintained over time in salt-treated transgenic tomato plants, but the high reduction of Na+ in leaf is not associated with salt tolerance. Physiologia plantarum. 2008. June 1;133(2):288–97. 10.1111/j.1399-3054.2008.01060.x [DOI] [PubMed] [Google Scholar]
- 78.Grover WH, Skelley AM, Liu CN, Lagally ET, Mathies RA. Monolithic membrane valves and diaphragm pumps for practical large-scale integration into glass microfluidic devices. Sensors and Actuators B: Chemical. 2003. April 1;89(3):315–23. [Google Scholar]
- 79.Cheong JJ, Choi YD. Methyl jasmonate as a vital substance in plants. Trends Genet 2003, 19:409–413. 10.1016/S0168-9525(03)00138-0 [DOI] [PubMed] [Google Scholar]
- 80.Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hirai MY, Noji M, Saito K, Masuda T. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. The Plant Journal. 2005. November 1;44(4):653–68. 10.1111/j.1365-313X.2005.02560.x [DOI] [PubMed] [Google Scholar]
- 81.Marasco EK, Schmidt-Dannert C. Biosynthesis of plant natural products and characterization of plant biosynthetic pathways in recombinant microorganisms InApplications of Plant Metabolic Engineering 2007. (pp. 1–43). Springer; Netherlands. [Google Scholar]
- 82.Ahsan N, Lee DG, Kim KH, Alam I, Lee SH, Lee KW, Lee H, Lee BH. Analysis of arsenic stress-induced differentially expressed proteins in rice leaves by two-dimensional gel electrophoresis coupled with mass spectrometry. Chemosphere. 2010. January 31;78(3):224–31. 10.1016/j.chemosphere.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 83.Ahsan N, Lee DG, Lee KW, Alam I, Lee SH, Bahk JD, Lee BH. Glyphosate- induced oxidative stress in rice leaves revealed by proteomic approach. Plant Physiology and Biochemistry. 2008. December 31;46(12):1062–70. 10.1016/j.plaphy.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 84.Zablotowicz RM, Reddy KN. Impact of glyphosate on the symbiosis with glyphosate-resistant transgenic soybean. Journal of Environmental Quality. 2004. May 1;33(3):825–31. [DOI] [PubMed] [Google Scholar]
- 85.Franz JE, Mao MK, Sikorski JA. Glyphosate: a unique global herbicide. American Chemical Society; 1997. [Google Scholar]
- 86.Schmelz EA, Carroll MJ, LeClere S, Phipps SM, Meredith J, Chourey PS, Alborn HT, Teal PE. Fragments of ATP synthase mediate plant perception of insect attack. Proceedings of the National Academy of Sciences. 2006. June 6;103(23):8894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dogğramacı M, Foley ME, Horvath DP, Hernandez AG, Khetani RS, Fields CJ, Keating KM, Mikel MA, Anderson JV. Glyphosate’s impact on vegetative growth in leafy spurge identifies molecular processes and hormone cross-talk associated with increased branching. BMC genomics. 2015. May 19;16(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Funke T, Han H, Healy-Fried ML, Fischer M, Schönbrunn E. Molecular basis for the herbicide resistance of Roundup Ready crops. Proceedings of the National Academy of Sciences. 2006. August 29;103(35):13010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.CERA-GMC GM Crop Database. (n.d.). Retrieved May 30, 2016, http://www.cera-gmc.org/GmCropDatabaseEvent/NK603/short/print [Google Scholar]
- 90.Castan M, Ali SE, Hochegger R, Ruppitsch W, Haslberger AG, Brandes C. Analysis of the genetic stability of event NK603 in stacked corn varieties using high- resolution melting (HRM) analysis and Sanger sequencing. European Food Research and Technology. 2016:1–3. [Google Scholar]
- 91.Behr C, Heck G, Hironaka C, You J, inventors; Behr Carl F, Heck Gregory R, assignee. Corn event PV-ZMGT32 (nk603) and compositions and methods for detection thereof. United States patent application US 10/919,423. 2004 Aug 16.
- 92.Davies H. A role for “omics” technologies in food safety assessment. Food Control. 2010. December 31;21(12):1601–10. [Google Scholar]
- 93.van Dijk JP, Leifert C, Barros E, Kok EJ. Gene expression profiling for food safety assessment: examples in potato and maize. Regulatory Toxicology and Pharmacology. 2010. December 1;58(3):S21–5. [DOI] [PubMed] [Google Scholar]
- 94.Kok EJ, Keijer J, Kleter GA, Kuiper HA. Comparative safety assessment of plant-derived foods. Regulatory Toxicology and Pharmacology. 2008. February 29;50(1):98–113. 10.1016/j.yrtph.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 95.Ruebelt MC, Lipp M, Reynolds TL, Astwood JD, Engel KH, Jany KD. Application of two-dimensional gel electrophoresis to interrogate alterations in the proteome of genetically modified crops. 2. Assessing natural variability. Journal of agricultural and food chemistry. 2006. March 22;54(6):2162–8. 10.1021/jf052357y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Optimization of each analyte for Multiple Reactions Monitoring (MRM) transition and overlaid multiple reaction monitoring chromatograms of both positive and negative ionization mode.
(DOCX)
Sequences of all peptides from the differentially abundant proteins identified.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.