Abstract
Antibiotic resistance increases the likelihood of death from infection by common pathogens such as Escherichia coli and Klebsiella pneumoniae in developed and developing countries alike. Most important modern antibiotic resistance genes spread between such species on self-transmissible (conjugative) plasmids. These plasmids are traditionally grouped on the basis of replicon incompatibility (Inc), which prevents coexistence of related plasmids in the same cell. These plasmids also use post-segregational killing (‘addiction’) systems, which poison any bacterial cells that lose the addictive plasmid, to guarantee their own survival. This study demonstrates that plasmid incompatibilities and addiction systems can be exploited to achieve the safe and complete eradication of antibiotic resistance from bacteria in vitro and in the mouse gut. Conjugative ‘interference plasmids’ were constructed by specifically deleting toxin and antibiotic resistance genes from target plasmids. These interference plasmids efficiently cured the corresponding antibiotic resistant target plasmid from different Enterobacteriaceae in vitro and restored antibiotic susceptibility in vivo to all bacterial populations into which plasmid-mediated resistance had spread. This approach might allow eradication of emergent or established populations of resistance plasmids in individuals at risk of severe sepsis, enabling subsequent use of less toxic and/or more effective antibiotics than would otherwise be possible, if sepsis develops. The generalisability of this approach and its potential applications in bioremediation of animal and environmental microbiomes should now be systematically explored.
Introduction
The efficacy of antibiotics used for decades to treat serious infections is increasingly threatened by large, low-copy, self-transmitting resistance plasmids in the Enterobacteriaceae. These conjugative plasmids are arguably the most important vectors of modern antibiotic resistance, and are directly linked to major outbreaks of antibiotic resistant infection [1–4]. Modern resistance plasmids may spread through a range of different member species of the Enterobacteriaceae as a plasmid epidemic, but host strain contributions to plasmid-encoded antibiotic resistance phenotypes [4] further complicate surveillance and control [1, 4]. A conjugative resistance plasmid in the microflora directly increases risk of therapeutic failure [5], and may spread resistance to others. Antibiotic resistance carried on large conjugative plasmids may also persist for months even in the absence of ongoing specific selection [6]. Even when spread of a particular resistance plasmid is defined early enough for implementation of containment strategies, the only available option is to use an antibiotic to which the plasmid does not confer resistance, in an attempt to entirely eliminate all bacterial populations that carry it.
Older antibiotics and those not used as primary therapy in severe sepsis may provide options for killing bacterial populations harbouring dangerous plasmids. Indeed, non-absorbable antibiotics such as colistin and neomycin have long been used to ‘selectively decontaminate’ the gut. The consequences of this ablative approach are not fully defined and is not widely adopted despite promising results in clinical trials [7, 8], due to clinician concerns about development of antibiotic resistance [9, 10]. Approaches that specifically eradicate problem plasmids and the phenotypes they encode without destroying host bacterial populations or other resident plasmids is the ideal next step toward microbial husbandry.
Multiple plasmids commonly coexist in the same bacterial cell but cross-interference between plasmid replication systems ensures that the most closely related plasmids are incompatible and cannot stably persist together [11, 12]. Entry exclusion systems (EES) also inhibit conjugation of a plasmid into a cell that already has a resident plasmid of the same ‘exclusion group’ by ten- [13] to more than a thousand-fold under certain conditions [14]. Strong selection for a plasmid entering a bacterial population therefore normally results in displacement of any resident incompatible plasmids that are not selected, allowing the incoming plasmid to take over the ecological niche.
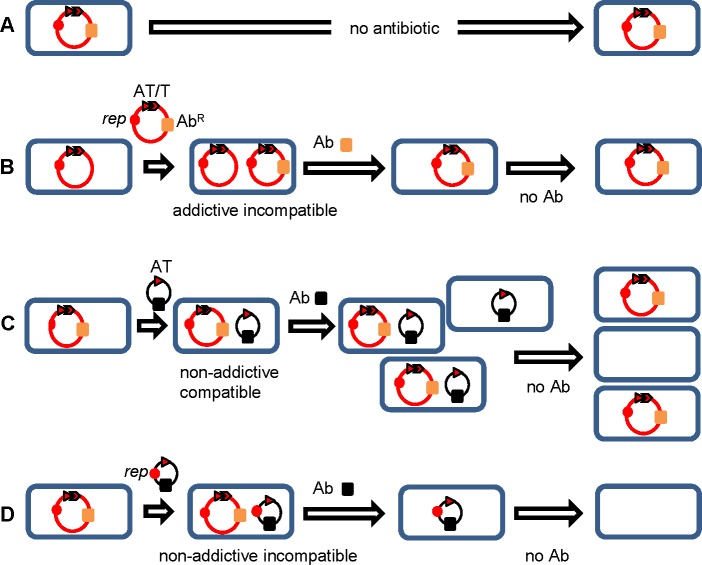
Large conjugative low-copy number plasmids have also acquired specific ‘addiction’ mechanisms that are important for their long term persistence (Fig 1). Small protein toxins that help regulate bacterial death under stress conditions were first identified as part of post-segregational killing (PSK) / ‘addiction’ systems [15] in plasmids. A typical addiction system includes a long-lived toxin and cognate short-lived antitoxin, with the unopposed toxin killing any bacteria that have lost the plasmid, and thus the encoded antitoxin, during cell division [16–19] (Fig 1).
Fig 1. Addictive antibiotic resistance plasmids.
The replicon (rep, solid circle), antitoxin (AT, arrowhead) and toxin (T, arrow) genes of a PSK/addiction system, an antibiotic resistance gene (AbR) and corresponding antibiotic (Ab, solid blocks) are shown. (A) An addictive plasmid is stable in the absence of antibiotic selection. (B) An addictive plasmid can be displaced by an incompatible plasmid. (C) A compatible plasmid providing specific antitoxin (non-addictive compatible) leads to loss of addictive resistance plasmids from some cells. (D) An incompatible non-addictive interference plasmid providing specific antitoxin (non-addictive incompatible) ensures that all bacterial cells are ultimately free of both plasmid types.
T/A systems are generally classified by mode of action with the largest numbers and diversity in types I and II. In type I systems, e.g. pndBCA found in IncI1 plasmids, toxin (PndA) translation is inhibited by an unstable antisense RNA (pndB) transcribed in reverse orientation at the toxin gene locus [20–22]. In type II systems e.g. pemIK, first described in the IncFII plasmid R1 as Kis-Kid [23] and common in IncL/M and IncF plasmids, the protein antitoxin (PemI) is stable only when complexed with the cognate toxin PemK [24, 25].
The combined effects of addiction, incompatibility and repeated antibiotic exposure favour the emergence of a few lineages of resistance plasmids in populations of antibiotic resistant Enterobacteriaceae [26], possibly at the expense of diverse antibiotic-susceptible indigenous plasmids that have evolved over thousands of years. Targeting of incompatibility and addiction systems to selectively remove plasmids from bacterial populations has been proposed as part of an optimal ecological/evolutionary approach [27] that had been previously demonstrated in vitro with IncP and IncF plasmids [28]. Conjugative addictive plasmids (including plasmids with multiple addiction systems) can also be eliminated in vitro by blocking replication, with [28] or without [29, 30] blocking addiction, by using intercalating dyes and drugs such as quinolones [31], small molecule replication inhibitors [32, 33] and even by applying heat stress [34], while fatty acids or tanzawaic acids can be used to inhibit spread by conjugation [35, 36] but no such approach has ever been demonstrated in vivo.
We postulated that antibiotic susceptibility could be restored to an entire gut microbiome by the introduction of an appropriately-designed, orally administered conjugative ‘interference’ plasmid without the gain of any new antibiotic resistance and without the loss of the original bacterial populations or of any bystander plasmid populations. Plasmids encoding resistance to carbapenems and/or third-generation cephalosporins, which often also carry aminoglycoside resistance determinants, are the highest priority for elimination. Two locally-endemic plasmids carrying different β-lactamase genes and with different replicons and different addiction systems were therefore chosen for an experimental proof of principle.
Materials and methods
Bacteria, culture conditions, primers and plasmids
Tables 1 and 2 and S1 Table list plasmids, bacterial strains and primers, respectively. Bacteria were grown in Luria-Bertani (LB) broth (Invitrogen, CA, USA) and plated on CHROMagar Orientation (CHROMagar, Paris, France). Ampicillin (100 μg/mL), tetracycline (10 μg/mL), gentamicin (8 μg/mL), chloramphenicol (20 μg/mL), cefotaxime (8 μg/mL), fosfomycin (200 μg/mL), rifampicin (100 μg/mL), and/or sodium azide (100 μg/mL) were added as indicated. Chemical transformation and electroporation were carried out using standard protocols. Conjugations were performed by filter mating [37], with overnight or 2 h incubation on filters before antibiotic selection of specific transconjugants. Conjugation efficiency was calculated as the number of transconjugants per donor cell.
Table 1. Plasmids used in this study.
| Plasmid | Characteristics | Source/Reference |
|---|---|---|
| pBCSK+ | High copy phagemid cloning vector (CHLR) | Stratagene, USA; |
| pGEM-T Easy | Cloning vector for direct cloning of PCR products (AMPR) | Promega, USA |
| pBAD18 | Arabinose inducible expression vector (AMPR) | [38] |
| pKM200 | Plasmid carrying the lambda-Red recombinase system (CHLR) | Addgene, USA |
| pEl1573 | Naturally occurring conjugative IncL/M plasmid carrying blaIMP-4 from clinical isolate E. cloacae El1573 (JX101693) | [39, 40] |
| pJIBE401 | Naturally occurring conjugative IncL/M plasmid from clinical isolate K. pneumoniae Kp1239; identical to pEl1573 | [39] |
| pJIE512b | Naturally occurring conjugative IncI1 plasmid carrying blaCMY-2 from clinical isolate E. coli JIE512b (HG970648) | [22] |
| pJIMK3 | pemI gene of pEl1573 in SmaI site of pBCSK+ | This study |
| pJIMK21 | IncL/M rep genes of pEl1573 in pGEM-T Easy | This study |
| pJIMK25 | NotI L/M rep fragment from pJIMK21 in NotI site of pJIMK3 | This study |
| pJIMK39 | pemI and IncL/M rep from pJIMK25 in SmaI site of pBAD18 | This study |
| pJIMK41 | Construct to replace pEl1573 pemK with fosA3 | This study |
| pJIMK43 | Construct to replace pEl1573 MRR with tetA | This study |
| pJIMK45 | pEl1573 with ~28.5 kb including entire MRR replaced by tetA | This study |
| pJIMK46 | pJIMK45 with part of pemK replaced by fosA3 | This study |
| pJIMK50 | Construct to replace pJIE512b blaCMY-2 with fosA3 | This study |
| pJIMK54 | pJIE512b with blaCMY-2 replaced by fosA3 | This study |
| pJIMK55 | Construct to replace pJIE512b pndA with tetA | This study |
| pJIMK56 | pJIMK54 with part of pndA replaced by tetA | This study |
Table 2. Bacterial strains used in this study.
| Bacteria | Characteristics | Source/Reference |
|---|---|---|
| DH5α | E. coli K-12; F‐, 80lacZΔM15 Δ(lacZYA‐rgF)U169 deoR, recA1, endA1, hsdR17(rk‐mK+), phoA, supE44, λ‐thi‐1, gyrA96, relA1 | Invitrogen (USA) |
| UB5201Rf | Rifampicin resistant E. coli K-12, F ‐, pro, met, recA56, gyrA | [4] |
| J53Azir | Azide resistant E. coli K-12, F-, lac+, pro, met | [41] |
| El1573 | Multi‐drug resistant E. cloacae carrying pEl1573 | [39] |
| Kp1239 | Multi‐drug resistant K. pneumoniae carrying pJIBE401 | [4] |
| JIE512b | Multi-drug resistant E. coli carrying pJIE512b | [22] |
| Kp13883Rf | Rifampicin resistant derivative of K. pneumoniae ATCC13883 | [4] |
| Mm1585Rf | Rifampicin resistant derivative of Morganella morganii Mm1585 | [4] |
| Cf4000Rf | Rifampicin resistant derivative of Citrobacter freundii Cf4000 | [4] |
PCR amplification and cloning
Platinum pfx DNA polymerase (Invitrogen, USA) was used to amplify blunt-ended PCR products. All PCR products were purified (PureLink Quick PCR Purification Kit; Invitrogen, USA). PCR and sequencing was used to confirm all constructs.
Construction of specific plasmids
The antitoxin gene pemI with its own promoter and ribosome binding site (RBS) was amplified from pEl1573 as a blunt-ended PCR product and cloned into the unique SmaI site of pBCSK+ (CHLR; Stratagene, USA) to construct pJIMK3. The IncL/M replication genes (repCBA) were amplified from pEl1573 and cloned into pGEM-T Easy (Promega, USA) to construct pJIMK21. The NotI fragment from pJIMK21 containing repCBA was cloned into the unique NotI site of pJIMK3 to construct pJIMK25. pemI-repCBA was amplified from pJIMK25 and the blunt-ended product cloned into the unique SmaI site of pBAD18 (AMPR) to construct pJIMK39.
E. coli DH5α (β-galactosidase-negative, white on CHROMagar) carrying pJIMK39 (AMPR CTXS) was mated with E. coli UB5201Rf (β-galactosidase-positive, pink on CHROMagar) carrying pEl1573 (AMPR CTXR). Six white E. coli DH5α transconjugants picked from CHROMagar containing CTX were all confirmed to contain both pJIMK39 and pEl1573 (AMPR CTXR) by PCR. After incubation (4 h, 37°C, 220 rpm) in LB broth containing AMP plus either glucose or arabinose (0.2% w/v) and subculture on antibiotic-free CHROMagar, 12 colonies from each of the 12 subcultures were screened for blaIMP-4 by PCR.
Construction of conjugative interference plasmids
The fosA3 fosfomycin resistance gene (e.g. GenBank accession no. JF411006) from E. coli 78AJTi [42] and the tetA tetracycline resistance gene (94% identical to tetA(A)) from plasmid N3 (FR850039) were each amplified with their native promoter and RBS. Short regions upstream and downstream of the regions to be replaced were amplified using primers overlapping with tetA or fosA3 specific primers. Fusion products from Gibson assembly PCR [43] of the three amplicons (1:1:1 molar ratio) were cloned into pGEM-T Easy for use as templates to amplify larger amounts. Amplicons (~1.0 μg) were electroporated into UB5201Rf carrying pJIBE401 or J53Azir carrying pJIE512b, both also containing pKM200 encoding lambda Red recombinase [44]. Homologous recombination (at 30°C) was used to replace the target region with the antibiotic resistance marker, with subsequent growth at 37°C to remove pKM200. Colonies were selected on CHROMagar containing appropriate antibiotics and the replacements confirmed by PCR and sequencing.
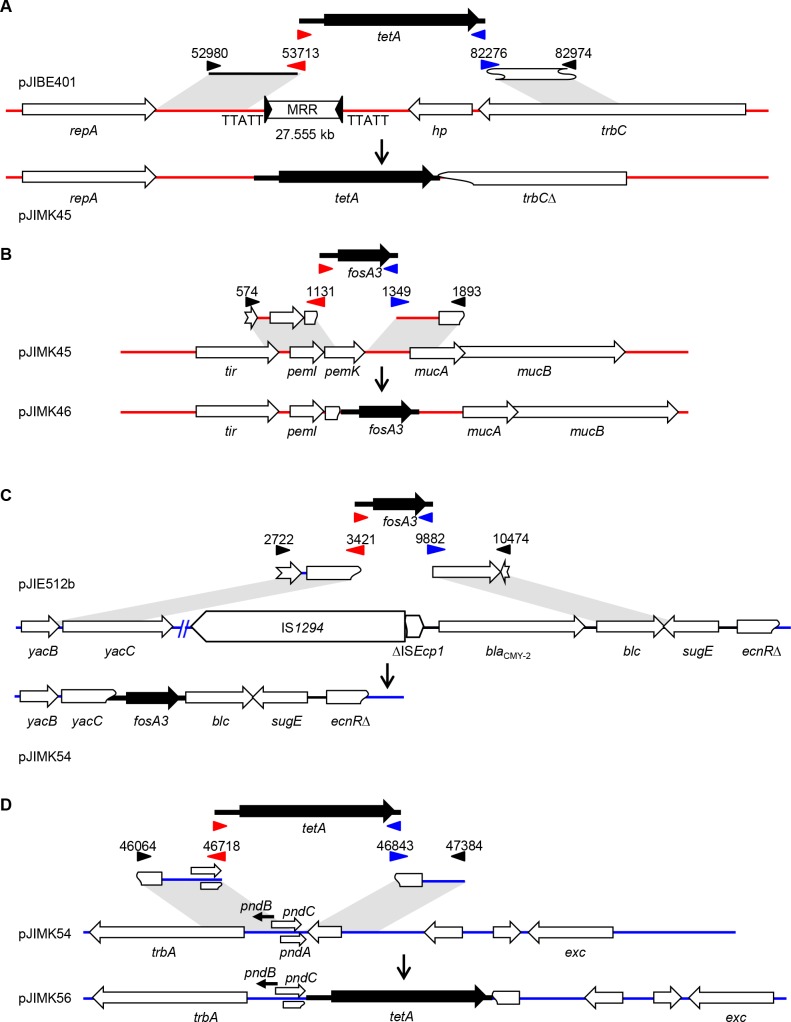
For pJIBE401, a 734 bp region upstream and a 699 bp region (including part of the trbC gene) downstream of the antibiotic multi-resistance region (MRR) were amplified for Gibson PCR with tetA to give pJIMK43. This was used to replace the entire 27.555 kb MRR of pJIBE401 (containing blaIMP-4) plus ~1.0 kb of flanking sequence to create pJIMK45 (Fig 2A). A 558-bp region including 116 bp of pemK and a 545-bp region immediately downstream of pemK were amplified from pJIBE401 for Gibson PCR with fosA3 to give pJIMK41. This was used to replace 217 bp of the 333 bp pemK toxin gene of pJIMK45 to create pJIMK46 (Fig 2B). pJIBE401 (sequenced here) is identical to pEl1573 (JX101693) except for a single C→T change at position 60364 in ISCR1, which is in the antibiotic resistance region that was deleted to construct pJIMK46.
Fig 2. Construction of conjugative interference plasmids.
pJIMK46 (A, B) was constructed from pJIBE401 by replacing 28.5 kb including the MRR with tetA and then part of the pemK toxin gene with fosA3. pJIMK56 (C, D) was constructed from pJIE512b by replacing the blaCMY-2 gene and flanking IS with fosA3 and then part of the pndA toxin gene with tetA. Numbers indicate the positions of the amplified regions in GenBank accession nos. JX101693.1 (pEl1573) or HG970648.1 (pJIE512b). Blue and red arrows indicate overlapping primers, black arrows indicate other primers.
For pJIE512b a 700 bp region (part of the yacBC hypothetical genes) upstream and a 593 bp region (blc and part of sugE) downstream of blaCMY-2 were amplified for Gibson PCR with fosA3 to give pJIMK50. This was used to replace a 6.460 kb region containing hypothetical proteins, IS1294, the truncated ISEcp1 and blaCMY-2 (pJIMK54; Fig 2C). A 655 bp region (218 bp of trbA, all of pndC) upstream and a 542 bp region downstream of pndBCA were amplified from pJIE512b for Gibson PCR with the tetA amplicon to give pJIMK55. This was used to replace the last 7 bp of the pndA toxin gene of pJIMK54 to give pJIMK56 (Fig 2D).
pJIBE401, pJIMK46 and pJIMK56 DNA was purified (HiSpeed plasmid midikit; Qiagen, Germany) and 1 ng of each used for library preparation (Nextera XT DNA sample preparation kit; Illumina, Inc., USA), with each of the three libraries indexed for sequencing (Illumina MiSeq; Australian Genome Research Facility, Melbourne, Australia). Geneious V7 (Biomatters, New Zealand) was used to map raw reads from pJIBE401 and pJIMK46 against pEl1573 (JX101693) and those from pJIMK56 against pJIE512b (HG970648).
Mouse experiments
All research and animal care procedures were approved by the Animal Ethics Committee of the Western Sydney Local Health District (protocol 4205.06.13) in accordance with the ‘Australian Code of Practice for the Care and Use of Animals for Scientific Purposes’. Five week old female BALB/c mice (Animal Resource Centre; Perth, WA, Australia) were housed in groups of three in open-lid M1 polypropylene cages (Able Scientific, Australia) on a 12 h light/dark cycle, with food and water available ad libitum (Westmead Hospital small animal research facility). Mice were acclimatized (d-6 to d0) prior to experiments, followed by run-in (d1-d3) in the experimental room to introduce the new gelatine food [45] (10% w/v Davis gelatine, GELITA NZ Ltd; 10% w/v Splenda artificial sweetener, Johnson-Johnson Pacific Pty Ltd, Australia; 10% v/v flavouring (Flavouring Essence Imitation Strawberry, Queen Fine Foods Pty. Ltd. QLD, Australia). Mice were fasted for 6 h, allowed access to gelatine food and then normal food was continuously available. Bacteria (1 mL culture) carrying a given plasmid were resuspended in PBS to an OD600 of ~0.4–0.5 and fed to mice on specified days in gelatine, with antibiotics in drinking water (20 mg/L) to follow, as previously described [45]. Mice were euthanized by an overdose of CO2 immediately after completion of experiments.
Group 1 received gelatine with no antibiotics. Group 2 received gelatine containing antibiotics. Groups 3, 4, 5 and 6 received bacteria with target plasmid (pEl1573 or pJIE512b, respectively) conferring cefotaxime (CTX) resistance in gelatine plus CTX to select for the plasmid. Only groups 4 and 6 subsequently received bacteria with the matching interference plasmid (TETR FOSR) plus TET to select for it, then CTX in water at the end of the protocol to select for any residual resistant bacteria (Table 3). On the specified days each mouse was briefly transferred into a separate plastic box for weighing and to collect fresh faeces. Faeces (100 μg/mouse) was suspended in 1 mL PBS, dilutions plated on CHROMagar with appropriate antibiotics and the number of E. coli/100 μg faeces after 16 h incubation at 37°C calculated.
Table 3. Protocol for mouse experiments.
| Day(s) | Group 1 | Group 2 | Group 3 | Group 5 | Group 4 | Group 6 |
|---|---|---|---|---|---|---|
| Controls | Antibiotics | Antibiotics+resistance plasmid | Antibiotics+resistance+interference plasmid | |||
| -6-0 | acclimatization—unrestricted normal diet and water | |||||
| 1–3 | run-in of gelatine food protocol (6 h fast, gelatine food 3 h, unrestricted normal diet and water) | |||||
| 4–6 | gelatine | gelatine+CTX | gelatine+CTX+(J53Azir+pEl1573)~2x106 cfu/cage/day | gelatine+CTX+ (UB5201Rf+pJIE512b)~2x106 cfu/cage/day | gelatine+CTX+(J53Azir+pEl1573)~2x106 cfu/cage/day | gelatine+CTX+ (UB5201Rf+pJIE512b)~2x106 cfu/cage/day |
| 7 | unrestricted normal diet and water | |||||
| 8–10 | gelatine | gelatine+TET | gelatine | gelatine | gelatine+TET+(UB5201Rf+pJIMK46)~6x107 cfu/cage/day | gelatine+TET+(J53Azir+pJIMK56)~7x107 cfu/cage/day |
| 11 | unrestricted normal diet and water | |||||
| 12 | move to clean cages | |||||
| 13–22 | unrestricted normal diet and water | |||||
| 23–24 | unrestricted normal diet and water | CTX in water | ||||
| 28 | end of experiment | |||||
Results
Strong association between replicon type and addiction systems
A survey of the literature and available sequences confirmed that addiction systems are common among conjugative plasmids in the Enterobacteriaceae, with predictable associations between addiction systems and replicon types (Table 4). All IncL/M and IncI1 plasmids available in GenBank (November 2016) were examined with tools designed to search for putative addiction systems [46, 47]. A single addiction system (pemIK) was evident in 50/57 IncL/M plasmid sequences, most (n = 42/50) encoding an identical PemI antitoxin, with a single conservative amino acid change (Val to Ala at position 79) in seven and this change plus Ala80Thr in the eighth. Similarly, all 125 available IncI1 plasmid sequences had the pndBCA addiction system with identical pndB antisense RNA (antitoxin) sequences. An additional putative relE-RHH-like addiction system was found in 72 of these 125 IncI1 plasmids, including pJIE512b.
Table 4. Associated replicon types and addiction systems in Enterobacteriaceae plasmids.
| Replicon | Associated addiction systems | Associated resistance genes | Source/Reference |
|---|---|---|---|
| IncF | pemIK, ccdAB, hok-sok, vagCD | blaCTX-M, blaKPC, blaCMY-2-like, blaDHA | [20, 21, 48, 49] |
| IncL/M | pemIK | blaIMP, blaCTX-M, blaTEM | [20, 21, 40] |
| IncI1 | pndBCA, relE-RHHa (in some) | blaCMY-2-like, blaCTX-M, blaTEM | [20–22] |
| IncA/C | relE-vapIa | blaCMY-2-like, blaNDM, blaSHV, blaVEB | |
| IncHI2 | vagCD | blaCTX-M, blaSHV | [21] |
| IncN | stbBC | blaKPC, blaCTX-M, blaSHV | [50, 51] |
| IncX | hicA-hicB-like (in some) | blaCTX-M, blaKPC, blaNDM | [52–55] |
a putative systems identified using RASTA-Bacteria (http://genoweb1.irisa.fr/duals/RASTA-Bacteria/) and TA-finder (http://202.120.12.133/TAfinder/TAfinder.php).
In vitro cure of antibiotic resistance plasmids from Enterobacteriaceae
The conjugative plasmids pEl1573 [40] and pJIBE401 [56] are almost identical (one nucleotide difference in the MRR) representatives of an IncL/M plasmid type that is common in Sydney hospitals [4, 39]. They carry genes encoding resistance to gentamicin (GEN) in addition to blaIMP-4 encoding a metallo-β-lactamase that efficiently hydrolyses extended spectrum β-lactams (e.g. cefotaxime, CTX) and carbapenems.
In order to evaluate plasmid stability, E. coli UB5201Rf carrying only pEl1573 [56] was passaged in serial culture in LB without antibiotic selection for 100 consecutive days. All of 100 colonies retrieved on antibiotic-free growth media at the end of this period were resistant to GEN and CTX on subculture and all were still positive by PCR with primers specific for blaIMP-4 (CTXR) and the IncL/M plasmid replicon (S1 Table), confirming the long-term stability of pEl1573 in the absence of antibiotic selection.
By contrast, provision of the specific antitoxin gene pemI from pEl1573 expressed in trans from the unrepressed Plac promoter in a high-copy chloramphenicol-resistant (CHLR) vector (pJIMK3; Table 1) transformed into the same strain (E. coli UB5201Rf with pEl1573) resulted in significant loss of pEl1573. After six passages over 48 h in LB supplemented with CHL, ~30% of E. coli colonies recovered were GENS CTXS and no longer yielded blaIMP-4 or IncL/M amplicons with specific PCR. The remainder retained the antibiotic-resistant phenotype and genetic markers of pEl1573.
The pEl1573 IncL/M replicon region (rep) was then added to pJIMK3 to generate pJIMK25, in which pemI and IncL/M rep are constitutively expressed from Plac (Table 1), which was transformed into E. coli UB5201Rf with pEl1573. After overnight incubation in LB supplemented with CHL, as purifying selection for pJIMK25, all transformants were GENS CTXS and blaIMP-4 was no longer detected by specific PCR. Complete loss of pEl1573 was confirmed by gel electrophoresis after S1 nuclease treatment of extracted DNA to linearise plasmids [57] (not shown). Complete loss of pEl1573 was also observed after specific expression of IncL/M rep and pemI from an arabinose-inducible promoter in a low-copy vector (pJIMK39) in E. coli UB5201Rf with pEl1573 (Table 2) after 6 h growth in the presence of arabinose (expressing IncL/M rep and pemI). By contrast, pEl1573 and pJIMK39 stably coexisted in the presence of glucose (the promoter-repressed state; data not shown), confirming that the effect was wholly attributable to expression of specific rep and antitoxin.
Construction of conjugative interference plasmids
Having demonstrated plasmid displacement using small high copy number plasmids, specific conjugative ‘interference plasmids’ were constructed. The resistance region and principal toxin gene of pJIBE401 and pJIE512b were replaced with tetA or fosA3, retaining the antitoxin gene and introducing resistance to tetracycline (TETR) and fosfomycin (FOSR) (Fig 2). Sequencing of these specific interference plasmids (pJIMK46, pJIMK56) confirmed that each was otherwise identical to their parent resistance plasmid (pEl1573/pJIBE401 and pJIE512b, respectively).
In order to gauge the likely impact of exclusion systems and the need for purifying selection in favour of interference plasmids, the recovery of interference plasmids after prolonged (overnight) in vitro filter mating was examined. Establishment of both IncI1 and IncL/M interference plasmids was around 8-fold less efficient when incubated with cells in which the incompatible resistance plasmid was already resident (Table 5).
Table 5. Effect of entry exclusion on conjugative transfer efficiency.
| Donor | Recipient | Donor(cfu/ml) | Transconjugant(cfu/ml) | Conjugation frequency(transconjugants/donor) | Difference in frequency(fold) |
|---|---|---|---|---|---|
| UB5201Rf (pJIMK46); RIFR-TETR | J53Azi; AZIR | 3.6x108 | 6.3 x107(AZIR-TETR) | 1.75 x10-1 | 8.02 |
| UB5201Rf (pJIMK46); RIFR-TETR | J53Azi(pEl1573); AZIR-CTXR | 3.6x108 | 7.86x106 (AZIR-TETR) | 2.18 x10-2 | |
| J53Azi (pJIMK56);AZIR-TETR | UB5201Rf; RIFR | 5.6x107 | 1.9x106(RIFR-TETR) | 3.39 x10-2 | 7.9 |
| J53Azi (pJIMK56);AZIR-TETR | UB5201Rf (pJIE512b);RIFR-CTXR | 5.6x107 | 2.4x105(RIFR-TETR) | 4.28 x10-3 |
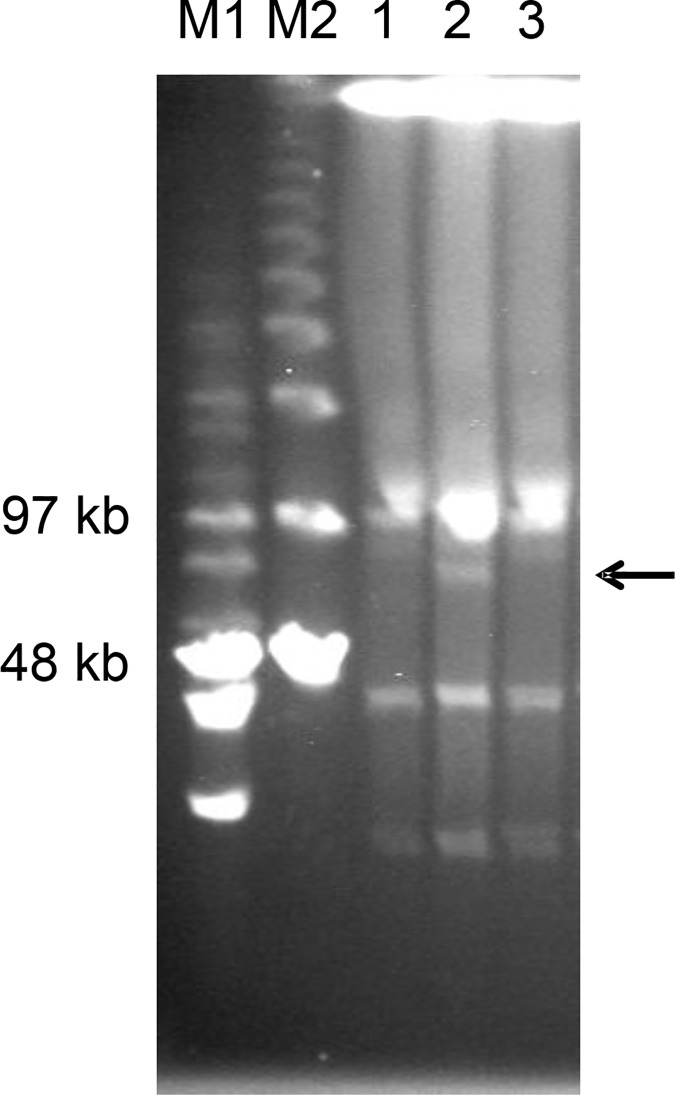
Conjugation of the interference plasmid pJIMK46 (TETR) into rifampicin-resistant (RIFR) E. coli, K. pneumoniae, Citrobacter freundii or Morganella morganii (Table 2) carrying the respective CTXR resistance plasmid (pEl1573 or pJIE512b) appeared to result in loss of the resistance plasmid only after purifying selection on RIF-TET agar, with bystander plasmids preserved (Fig 3). Plasmid curing was confirmed by PCR for blaIMP-4 (pEl1573) or blaCMY-2 (pJIE512b). No CTXR (resistance) or TETR (interference) bacteria could be detected subsequently by selective subculture.
Fig 3. Acquisition and loss of pEl1573 from K. pneumoniae 13883.
Pulsed-field gel electrophoresis of S1-endonuclease treated extracts of Kp13883 before (1) and after (2) acquisition of pEl1573 (horizontal arrow) and after cure (3), showing other ‘bystander’ plasmids. M1, Mid-range and M2, Lambda PFG ladders (New England Biolabs, USA).
In vivo cure of AbR plasmids from mouse gut
Having demonstrated in vitro efficacy and specificity of interference plasmids, BALB/c mice were selected as a suitable model for in vivo study [58]. Groups of three mice per cage were shown to be initially free of CTXR Enterobacteriaceae by faecal culture for three consecutive days. Protocols based on published work [45] were used to introduce resistance and then interference plasmids in bacteria with different chromosomal resistance markers (rifampicin (RIFR) or azide (AZIR; Table 3), to allow tracking of both strains and plasmids by differential subculture. Mouse weights were stable throughout and no feeding disturbance or diarrhoea was observed during the 28 day protocols (not shown).
In a preliminary experiment, mice received pEl1573 (TETS CTXR) in RIFR E. coli and then pJIMK46 (TETR CTXS) in AZIR E. coli, the latter being administered either with or without TET. In those mice that received pJIMK46 in AZIR E. coli, pJIMK46 was found in all culturable E. coli after three days of purifying TET selection while pEl1573 was no longer detected. In those mice that received pJIMK46 without purifying TET selection, the RIFR E. coli originally used to introduce pEl1573 were found to contain both pEl1573 and pJIMK46 in an approximate ~10:1 ratio after three days. This relatively poor penetration of pJIMK46 into the RIFR E. coli population in the absence of TET selection is consistent with exclusion by the resident incompatible pEl1573 and confirms the need for purifying selection in vivo.
A more detailed experiment introducing either pEl1573 (IncL/M; CTXR) or pJIE512b (IncI1; CTXR) and then the respective interference plasmid (pJIMK46/pJIMK56; TETR) was next conducted. Host E. coli strains used to introduce pJIE512b (AZIR) or pJIMK56 (RIFR) were switched for pEl1573/pJIMK46 to ensure that each E. coli strain was used both as initial colonizer and also to introduce interference plasmid, to control for any strain-related effects as experimental confounders.
Different CTXR E. coli populations were retrieved from faeces immediately after introduction of pEl1573 or pJIE512b resistance plasmid, indicating effective colonization of E. coli already resident in the mouse gut in the presence of CTX selection (Tables 6 and 7). In those groups of mice that then received specific interference plasmid with TET selection, TETR E. coli with either RIFR or AZIR chromosomal markers (used to bring in resistance or interference plasmid) or with no such markers (previously resident) were soon detected in approximately equal proportions (Fig 4A and 4B; Tables 6 and 7). After the three days of administration of interference plasmid (TETR) along with relevant antibiotic (TET) in water, CTXR Enterobacteriaceae were no longer culturable. None of 360 colonies of TETR E. coli (AZIR-TETR for curing pEl1573 and RIFR-TETR for curing pJIE512b) subcultured from faeces in each experiment were CTXR and CTXR genes (blaIMP-4 or blaCMY-2) could not be amplified from cultured bacteria or faecal extracts. This indicated that no interference plasmids had acquired CTXR, nor had resistance plasmids acquired TETR, nor did CTXR and TETR traits persist together in any isolate.
Table 6. In vivo cure of pEl1573-colonized mice.
| Day | Mouse no. | Group 3 (resistance plasmid)J53Azir+pEl1573 D4-6 | Group 4 (resistance plasmid followed by interference plasmid)J35Azir+pEl1573 D4-6; UB5201Rf+pJIMK46 D8-10; CTX D23-24 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CTXR | AZIR-CTXR | CTXR | AZIR-CTXR | TETR | RIFR-TETR | AZIR-TETR | CTXS | ||
| D5 | M1 | 1.7x103 | 1.5x103 | 2.3x103 | 1.9x103 | ||||
| M2 | 2.1x104 | 1.9x104 | 3.5x103 | 2.9x103 | |||||
| M3 | 1.3x103 | 1.0x103 | 1.9x102 | 1.5x102 | |||||
| D6 | M1 | 2.7x105 | 2.1x105 | 1.8x105 | 1.5x105 | ||||
| M2 | 3.4x105 | 2.2x105 | 3.6x105 | 3.1x105 | |||||
| M3 | 5.4x105 | 4.8x105 | 4.7x104 | 4.0x104 | |||||
| D7 | M1 | 3.4x105 | 2.8x105 | 3.4x107 | 2.9x107 | ||||
| M2 | 3.7x106 | 3.2x106 | 3.7x105 | 3.5x105 | |||||
| M3 | 4.3x105 | 3.8x105 | 4.3x105 | 4.1x105 | |||||
| D9 | M1 | 5.6x104 | 3.1x 103 | 3.6x104 | |||||
| M2 | 3.2x104 | 6.2x102 | 4.3x104 | ||||||
| M3 | 2.5x104 | 4.7x103 | 5.4x104 | ||||||
| D10 | M1 | nil | 2.5x105 | 5.4x104 | 3.6x105 | ||||
| M2 | 4.0x101 | 2.9x103 | 2.1x103 | 3.8x102 | |||||
| M3 | 2.1x 102 | 1.9x105 | 1.5x104 | 4.8x104 | |||||
| D11 | M1 | 1.8x104 | nil | 3.0x105 | 4.8x104 | 5.3x104 | |||
| M2 | 2.3x103 | 1.0x101 | 1.0x105 | 1.2x104 | 8.9x103 | ||||
| M3 | 1.3x103 | nil | 1.2x105 | 3.1x104 | 3.5x104 | ||||
| D12 | M1 | nil | 1.4x104 | ||||||
| M2 | nil | 3.2x104 | |||||||
| M3 | nil | 1.8x104 | |||||||
| D14 | M1 | 5.1x103 | nil | 1.1x103 | |||||
| M2 | 3.1x103 | nil | 2.0x102 | ||||||
| M3 | 1.8x103 | nil | 4.3x103 | ||||||
| D16 | M1 | nil | 3.0x102 | ||||||
| M2 | nil | 1.3x102 | |||||||
| M3 | nil | 1.1x102 | |||||||
| D18 | M1 | 3.6x103 | nil | 1.3x102 | |||||
| M2 | 8.8x102 | nil | nil | ||||||
| M3 | 6.7x102 | nil | 2.0x101 | ||||||
| D20 | M1 | nil | 5.0x101 | ||||||
| M2 | nil | nil | |||||||
| M3 | nil | nil | |||||||
| D21 | M1 | 7.2x102 | nil | nil | 2.4x103 | ||||
| M2 | 9.3x102 | nil | nil | 5.6x102 | |||||
| M3 | 4.7x102 | nil | nil | 3.1x103 | |||||
| D22 | M1 | nil | nil | 8.3x103 | |||||
| M2 | nil | nil | 7.2x103 | ||||||
| M3 | nil | nil | 4.3x103 | ||||||
| D27 | M1 | 4.4x102 | nil | nil | |||||
| M2 | 3.9x102 | nil | nil | ||||||
| M3 | 5.0x102 | nil | nil | ||||||
E. coli/100 μg faeces with indicated antibiotic resistance/susceptibility phenotype. CTX, cefotaxime; AZI, azide; TET, tetracycline; RIF, rifampicin. Mice were transferred to clean cages D12 and CTX re-administered D23-24 (Group 4 only). Blank, not applicable/not determined; nil, none cultured.
Table 7. In vivo cure of pJIE512b-colonized mice.
| Day | Mouse no. | Group 5 (resistance plasmid)UB5201+pJIE512b D4-6 | Group 6 (resistance plasmid followed by interference plasmid)UB5201+pJIE512b D4-6; J35Azir+pJIMK56 D8-10; CTX D23-24 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CTXR | RIFR-CTXR | CTXR | RIFR-CTXR | TETR | RIFR-TETR | AZIR-TETR | CTXS | ||
| D5 | M1 | 2.5x102 | 2.1x102 | 3.1x103 | 3.0x103 | ||||
| M2 | 1.9x102 | 1.5x102 | 1.1x104 | 9.5x103 | |||||
| M3 | 7.3x103 | 7.0x103 | 1.8x103 | 1.3x103 | |||||
| D6 | M1 | 7.1x104 | 6.4x104 | 5.2x105 | 5.0x105 | ||||
| M2 | 1.6x105 | 1.5x105 | 3.9x105 | 3.2x105 | |||||
| M3 | 2.4x104 | 2.2x104 | 3.5x105 | 3.1x105 | |||||
| D7 | M1 | 1.1x106 | 1.0x106 | 3.1x107 | 3.1x107 | ||||
| M2 | 6.1x105 | 5.7x105 | 1.3x105 | 1.2x105 | |||||
| M3 | 1.2x107 | 1.0x107 | 2.2x105 | 2.0x105 | |||||
| D9 | M1 | 1.1x105 | 5.1x102 | 1.4X103 | |||||
| M2 | 6.5x104 | 3.7x102 | 3.9x104 | ||||||
| M3 | 4.3x104 | 1.3x103 | 5.3x104 | ||||||
| D10 | M1 | 2.0x102 | 8.3x105 | 1.7x103 | 1.8x104 | ||||
| M2 | 4.0x101 | 3.3x105 | 2.3x104 | 1.5x104 | |||||
| M3 | nil | 4.2x105 | 1.9x104 | 3.1x103 | |||||
| D11 | M1 | 1.3x103 | 5.0x101 | 8.4x105 | 1.6x104 | 1.4x105 | |||
| M2 | 3.3x103 | nil | 3.8x105 | 1.9x104 | 3.1x105 | ||||
| M3 | 2.5x103 | nil | 7.2x105 | 3.0x104 | 8.7x104 | ||||
| D12 | M1 | nil | 3.2x103 | ||||||
| M2 | nil | 2.0x104 | |||||||
| M3 | nil | 8.6x103 | |||||||
| D14 | M1 | 1.1x103 | nil | 2.5x103 | |||||
| M2 | 5.1x103 | nil | 1.5x103 | ||||||
| M3 | 3.1x103 | nil | 2.0x103 | ||||||
| D16 | M1 | nil | 1.1x102 | ||||||
| M2 | nil | 1.0x102 | |||||||
| M3 | nil | 4.0x101 | |||||||
| D18 | M1 | 3.6x102 | nil | nil | |||||
| M2 | 7.2x102 | nil | 3.0x101 | ||||||
| M3 | 2.1x103 | nil | 5.0x101 | ||||||
| D20 | M1 | nil | nil | ||||||
| M2 | nil | nil | |||||||
| M3 | nil | 2.0x101 | |||||||
| D21 | M1 | 2.3x102 | nil | nil | 5.3x103 | ||||
| M2 | 1.2x102 | nil | nil | 6.4x103 | |||||
| M3 | 2.0x102 | nil | nil | 2.1x103 | |||||
| D22 | M1 | nil | nil | 5.5x103 | |||||
| M2 | nil | nil | 8.3x102 | ||||||
| M3 | nil | nil | 4.6x103 | ||||||
| D27 | M1 | 1.0x102 | nil | nil | |||||
| M2 | 2.2x102 | nil | nil | ||||||
| M3 | 1.6x102 | nil | nil | ||||||
E. coli/100 μg faeces with indicated antibiotic resistance/susceptibility phenotype. CTX, cefotaxime; AZI, azide; TET, tetracycline; RIF, rifampicin. Mice were transferred to clean cages D12 and CTX re-administered D23-24 (Group 6 only). Blank, not applicable/not determined; nil, none cultured.
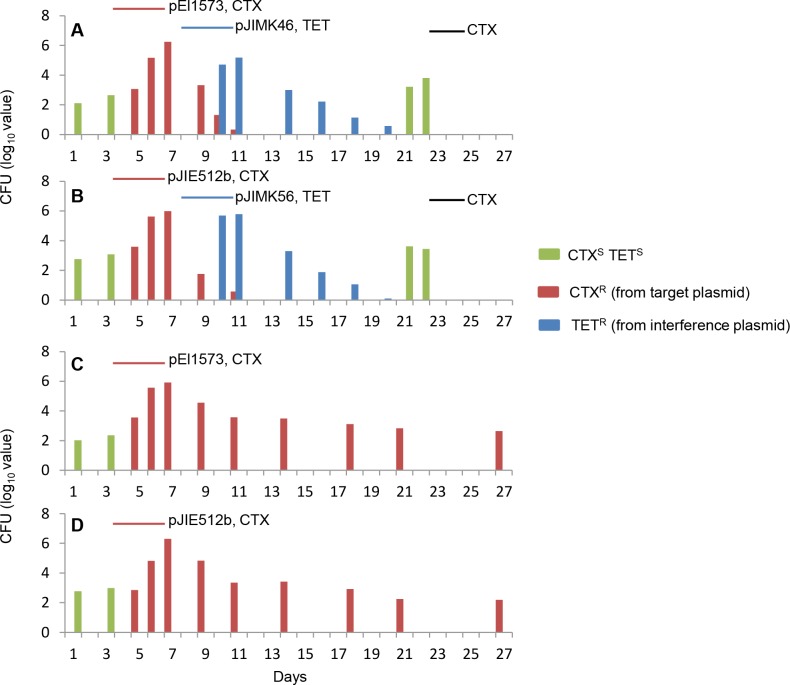
Fig 4. In vivo cure of antibiotic resistance plasmids.
CTXSTETS E. coli (green) were detected in all four groups of mice at the start of the experiments (A-D). All groups of mice were then fed bacteria carrying CTXR target plasmid (pEl1573 or pJIE512b) with CTX, days 4–6 (red lines, A-D) and CTXR E. coli (red) appeared. Two groups of mice (A, B) then received the corresponding TETR interference plasmid (pJIMK46 or pJIMK56) with TET, days 8–10 (blue lines) resulting in a decline in number of CTXR E. coli. TETR E. coli (blue) also appeared and then declined. CTXSTETS E. coli appeared again after curing of target and interference plasmids but were killed by CTX administered on days 23–24 (black lines, A, B). CTXR E. coli persisted to end of protocol in control groups that did not receive interference plasmid (C, D).
After administration of interference plasmid (TETR) to mice in the treatment groups, all mice were transferred into new cages to exclude reinfection from residual faecal contamination of their environment. Ten days after cessation of TET, no TETR Enterobacteriaceae could be cultured from mice who had received TETR interference plasmid (Fig 4A and 4B; Tables 6 and 7). None of the specific resistance (blaCMY-2, blaIMP-4, tetA) or replicon (IncL/M and IncI1 rep) genes from any of the introduced plasmids could be amplified from faecal pellets, confirming the loss of the interference plasmid (which lacks the addiction toxin gene). Only antibiotic-susceptible (originally resident, TETS CTXS) E. coli, AZIR TETS CTXS E. coli and RIFR TETS CTXS E. coli (bacteria used to introduce resistance or interference plasmid, now carrying neither) were retrieved, and these were present in comparable proportions (~104−105 cfu/mg of faeces; Tables 6 and 7).
CTX was finally re-administered (days 23, 24) to select for any residual resistance CTXR plasmids or transferred genes that may not have been detected by culture or direct PCR. blaIMP-4 and blaCMY-2 (CTXR) remained undetectable in stool extracts, and neither CTXR or TETR bacteria could be recovered (Fig 4, Tables 6 and 7). Elimination of CTXS E. coli populations further demonstrated the return of the efficacy of the antibiotic that had been rendered ineffective by the presence of the resistance plasmids.
In control mice that received the resistance plasmid but not the interference plasmid, CTXR bacteria remained (Fig 4C and 4D; Tables 6 and 7) and specific rep and resistance genes remained readily detectable throughout the experiment, confirming the addictive nature of the resistance plasmids.
Discussion
Eradication of resistant bacterial populations by more powerful antibiotics continues the escalation of the antibiotic arms race, leaves the microflora open to invasion by other species, and will not save antibiotics trusted for decades. This study shows that addictive antibiotic resistance plasmids can be specifically and completely eradicated from enteric bacterial populations and these bacterial populations recovered in their antibiotic-susceptible state in vivo. Effective targeting of incompatibility and addiction may provide solutions for a variety of antibiotic resistance plasmids, and high levels of conservation in these systems may even allow specific off-the-shelf solutions to be developed in the future. Two representative antibiotic resistance plasmids with different replicon and entry exclusion systems and with the two most common types of addiction systems were used to demonstrate this. IncL/M and IncI1 plasmids are found in different species of Enterobacteriaceae, often carry genes conferring resistance to important β-lactam and/or to aminoglycoside antibiotics, and are typical examples of plasmids that require specific eradication.
The ability to generalize this approach to other replicons and addiction systems must now be systematically tested: addiction systems are yet to be characterized, some may interact, and not all apparently similar systems are interchangeable. PemI and PemK encoded by IncL/M plasmids such as pEl1573, for example, differ by 4/84 amino acids and 10/133 amino acids respectively from PemI and PemK encoded by IncF plasmids, in which they appear to function in conjunction with the hok-sok addiction system [59]. Further, while the putative relE-RHH-like addiction system identified in pJIE512b clearly does not prevent plasmid loss from mouse gut microflora in vivo and neutralization of pndBCA alone was sufficient to cure this plasmid, it is not known whether this would be true for microflora containing different host species than those in the mouse gut model used here. Evidently this system is not essential for IncI1 plasmid stability in all E. coli populations in which it is found.
The results regarding the inhibitory effect of entry exclusion are at the lowest end of published data [13] but are internally consistent both in vitro and in vivo and in both systems. Entry exclusion of the IncI1 plasmid R64, which is closely related to the target plasmid pJIE512b and derivative pJIMK56, has been reported to inhibit in vitro conjugative transfer by ~700-fold in 90 min surface mating studies [14]. The differences reported here may relate to differences between pJIE512b (GenBank accession no. HG970648) and R64 (AP005147) in both excA and the adjacent traY (13/220 and 91/744 amino acids, respectively), as these differences are in key functional regions of the proteins [14] (S1 Fig). Strains expressing standard PSK/addiction system toxins may also have a relative fitness disadvantage in vivo and in a longer mating protocols such as used here, when compared to toxin-deleted interference plasmids, although this was not specifically tested here.
In the system used here, TET (or FOS) resistance can be used as purifying selection for bacteria which acquire and retain the interference plasmid at the expense of the incompatible CTXR target plasmid. Cells from which the TETR interference plasmid is lost, or that it does not enter at all (e.g. due to entry exclusion), are killed by TET (Fig 5). With the subsequent loss of the non-addictive interference plasmid in the absence of specific selection, all cells are thus free of both the interference plasmid and the original target resistance plasmid.
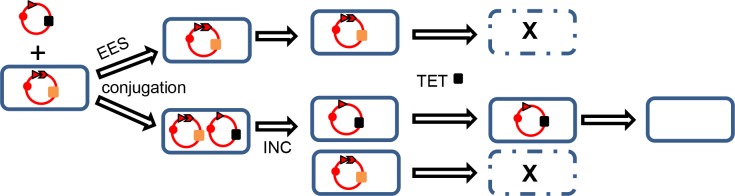
Fig 5. Exclusion and incompatibility.
Replicon (solid circle), antitoxin and toxin genes (arrowhead, arrow) and antibiotic resistance genes (CTXR, orange and TETR, black solid blocks). Interference plasmid not excluded by entry exclusion system (EES) is incompatible (INC) with resident CTXR plasmid and is selected by TET.
This process should therefore be generally efficacious even at low plasmid transfer efficiencies in the presence of brief positive selection for the interference plasmid, but there are potential risks to be considered. Theoretically, homologous recombination might restore toxin or antibiotic resistance genes to an interference plasmid, although a less resistant addictive plasmid or a non-addictive resistance plasmid both seem preferable options to persistence of the original addictive resistance plasmid. Nevertheless, no evidence of recombination was found in any experiments here. This is not unexpected as large co-integrates of interference and resistance plasmids would be subject to multimer resolution mechanisms and to the relative plasmid instability that evidently arises from specific antitoxin excess. Simultaneous antibiotic selection for both (mutually incompatible) plasmids might also favour chromosomal acquisition of resistance genes from the interference plasmid. The associated genetic elements that usually mobilize these genes were deliberately removed and transfer of fosA3 or tetA was not observed.
It is also theoretically possible that strong and simultaneous co-selection for both target and interference plasmids might select for a mutation in the replication region that restores compatibility. However, replicon diversity is naturally limited by strong functional constraints. Even closely evolutionarily related compatible plasmids with cross-complementing primases and helicases have highly specific oriV-Rep DNA binding requirements [60]. Even if target plasmids develop oriV mutations so that they are no longer incompatible and can then co-inhabit a cell with the interference plasmid, these plasmids are still expected to be rendered relatively unstable by specific antitoxin excess. It is also noteworthy that not all plasmid T/A systems are simple addiction mechanisms nor will they necessarily behave identically in different hosts or conditions. Likewise, simple antitoxin excess, evidently effective in these experiments, may also be insufficient in some systems.
Purifying selection using antibiotics is not ideal, both because of the effects of the drug on other cells and because effective antibiotics for this purpose may be increasingly hard to identify in future. TETR bacteria are not uncommon in the human gut but TETR (or FOSR) populations that arise by plasmid acquisition will not only lose their TETR or FOSR plasmids spontaneously but are made immediately vulnerable to other commonly used antibiotics (e.g. GEN, CTX) in the process. A target plasmid that acquires FOSR by recombination with the interference plasmid will likely acquire the immediately adjacent antitoxin (see Fig 2). An important future challenge is to develop interference plasmids with non-antibiotic selection but the inclusion of fosA3 also means that plasmid eradication is compatible with existing use of fosfomycin as a ‘rescue’ therapy of last resort [61]. A final caveat is that not all antibiotic resistance is carried on large plasmids that can be manipulated in this way. Nearly half of all severe sepsis is due to Gram-positive bacteria such as Staphylococci and Streptococci, in which much of the important resistance is chromosomally encoded, as it is in many clinically important Gram-negative bacteria such as Pseudomonas and Acinetobacter.
Specific plasmid interference is not only a useful research tool but may be suitable for clinical use in colonized individuals. The ability to eradicate plasmids detected in the gut flora of patients may prevent later development of antibiotic resistant sepsis, a condition with potentially lethal consequences [5]. Hospitals managing critically ill people at high risk of infection now routinely screen for antibiotic resistance using genetic methods and could similarly provide rapid identification of specific plasmid markers. The simple oral administration route makes in vivo plasmid curing approaches highly feasible, and may allow us to protect important medical advances from the growing threat of antibiotic resistance.
Supporting information
(DOCX)
The internal variable region of TraY (A, aa 430–522) and C-terminal region of Exc (B) are shown. Variable amino acids are shown by black shading, numbers correspond to amino acid positions in proteins.
(DOCX)
Acknowledgments
We thank Jian-Hua Liu and Virve Enne for providing plasmids carrying fosA3 or tetA.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by grant numbers G1001021 and G1084672, National Health and Medical Research Council (NHMRC), Australia.
References
- 1.Peleg AY, Franklin C, Bell JM, Spelman DW. Dissemination of the metallo-β-lactamase gene blaIMP-4 among Gram-negative pathogens in a clinical setting in Australia. Clin Infect Dis. 2005; 41(11):1549–1556. 10.1086/497831 [DOI] [PubMed] [Google Scholar]
- 2.Chu YW, Afzal-Shah M, Houang ET, Palepou MI, Lyon DJ, Woodford N, et al. IMP-4, a novel metallo-β-lactamase from nosocomial Acinetobacter spp. collected in Hong Kong between 1994 and 1998. Antimicrob Agents Chemother. 2001; 45(3):710–714. 10.1128/AAC.45.3.710-714.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994; 264(5157):375–382 [DOI] [PubMed] [Google Scholar]
- 4.Espedido B, Iredell J, Thomas L, Zelynski A. Wide dissemination of a carbapenemase plasmid among Gram-negative bacteria: implications of the variable phenotype. J Clin Microbiol. 2005; 43(9):4918–4919. 10.1128/JCM.43.9.4918-4919.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hal SJ, Wiklendt A, Espedido B, Ginn A, Iredell JR. Immediate appearance of plasmid-mediated resistance to multiple antibiotics upon antibiotic selection: an argument for systematic resistance epidemiology. J Clin Microbiol. 2009; 47(7):2325–2327. 10.1128/JCM.02141-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers BA, Kennedy KJ, Sidjabat HE, Jones M, Collignon P, Paterson DL. Prolonged carriage of resistant E. coli by returned travellers: clonality, risk factors and bacterial characteristics. Eur J Clin Microbiol Infect Dis. 2012; 31(9):2413–2420. 10.1007/s10096-012-1584-z [DOI] [PubMed] [Google Scholar]
- 7.Daneman N, Sarwar S, Fowler RA, Cuthbertson BH, Su DCSG. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis. 2013; 13(4):328–341. 10.1016/S1473-3099(12)70322-5 [DOI] [PubMed] [Google Scholar]
- 8.de Smet AM, Kluytmans JA, Blok HE, Mascini EM, Benus RF, Bernards AT, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis. 2011; 11(5):372–380. 10.1016/S1473-3099(11)70035-4 [DOI] [PubMed] [Google Scholar]
- 9.Cuthbertson BH, Campbell MK, MacLennan G, Duncan EM, Marshall AP, Wells EC, et al. Clinical stakeholders' opinions on the use of selective decontamination of the digestive tract in critically ill patients in intensive care units: an international Delphi study. Crit Care. 2013; 17(6):R266 10.1186/cc13096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan EM, Cuthbertson BH, Prior ME, Marshall AP, Wells EC, Todd LE, et al. The views of health care professionals about selective decontamination of the digestive tract: an international, theoretically informed interview study. J Crit Care. 2014; 29(4):634–640. 10.1016/j.jcrc.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 11.Novick RP. Plasmid incompatibility. Microbiol Rev. 1987; 51(4):381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin S, Nordstrom K. Partition-mediated incompatibility of bacterial plasmids. Cell. 1990; 60(3):351–354. [DOI] [PubMed] [Google Scholar]
- 13.Garcillan-Barcia MP, de la Cruz F. Why is entry exclusion an essential feature of conjugative plasmids? Plasmid. 2008; 60(1):1–18. 10.1016/j.plasmid.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 14.Sakuma T, Tazumi S, Furuya N, Komano T. ExcA proteins of IncI1 plasmid R64 and IncIγ plasmid R621a recognize different segments of their cognate TraY proteins in entry exclusion. Plasmid. 2013; 69(2):138–145. 10.1016/j.plasmid.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 15.Thisted T, Sorensen NS, Wagner EG, Gerdes K. Mechanism of post-segregational killing: Sok antisense RNA interacts with Hok mRNA via its 5'-end single-stranded leader and competes with the 3'-end of Hok mRNA for binding to the mok translational initiation region. EMBO J. 1994; 13(8):1960–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sengupta M, Austin S. Prevalence and significance of plasmid maintenance functions in the virulence plasmids of pathogenic bacteria. Infect Immun. 2011; 79(7):2502–2509. 10.1128/IAI.00127-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi Y, Park JH, Inouye M. Toxin-antitoxin systems in bacteria and archaea. Annu Rev Genet. 2011; 45:61–79. 10.1146/annurev-genet-110410-132412 [DOI] [PubMed] [Google Scholar]
- 18.Unterholzner SJ, Poppenberger B, Rozhon W. Toxin-antitoxin systems: Biology, identification, and application. Mob Genet Elements. 2013; 3(5):e26219 10.4161/mge.26219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes F. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science. 2003; 301(5639):1496–1499. 10.1126/science.1088157 [DOI] [PubMed] [Google Scholar]
- 20.Carattoli A. Plasmids in Gram negatives: molecular typing of resistance plasmids. Int J Med Microbiol. 2011; 301(8):654–658. 10.1016/j.ijmm.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 21.Mnif B, Harhour H, Jdidi J, Mahjoubi F, Genel N, Arlet G, et al. Molecular epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in Tunisia and characterization of their virulence factors and plasmid addiction systems. BMC Microbiol. 2013; 13:147 10.1186/1471-2180-13-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tagg KA, Iredell JR, Partridge SR. Complete sequencing of IncI1 sequence type 2 plasmid pJIE512b indicates mobilization of blaCMY-2 from an IncA/C plasmid. Antimicrob Agents Chemother. 2014; 58(8):4949–4952. 10.1128/AAC.02773-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bravo A, Ortega S, de Torrontegui G, Diaz R. Killing of Escherichia coli cells modulated by components of the stability system ParD of plasmid R1. Mol Gen Genet. 1988; 215(1):146–151 [DOI] [PubMed] [Google Scholar]
- 24.Goeders N, Van Melderen L. Toxin-antitoxin systems as multilevel interaction systems. Toxins (Basel). 2014; 6(1):304–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocker A, Meinhart A. Type II toxin: antitoxin systems. More than small selfish entities? Curr Genet. 2016; 62(2):287–290. 10.1007/s00294-015-0541-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carattoli A. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother. 2009; 53(6):2227–2238. 10.1128/AAC.01707-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baquero F, Coque TM, de la Cruz F. Ecology and evolution as targets: the need for novel eco-evo drugs and strategies to fight antibiotic resistance. Antimicrob Agents Chemother. 2011; 55(8):3649–3660. 10.1128/AAC.00013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hale L, Lazos O, Haines A, Thomas C. An efficient stress-free strategy to displace stable bacterial plasmids. Biotechniques. 2010; 48(3):223–228. 10.2144/000113366 [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Gao Z, Wang H, Feng E, Zhu L, Liu X, et al. Curing both virulent mega-plasmids from Bacillus anthracis wild-type strain A16 simultaneously using plasmid incompatibility. J Microbiol Biotechnol. 2015; 25(10):1614–1620. 10.4014/jmb.1503.03083 [DOI] [PubMed] [Google Scholar]
- 30.Ni B, Du Z, Guo Z, Zhang Y, Yang R. Curing of four different plasmids in Yersinia pestis using plasmid incompatibility. Lett Appl Microbiol. 2008; 47(4):235–240 [DOI] [PubMed] [Google Scholar]
- 31.Amabile-Cuevas CF, Heinemann JA. Shooting the messenger of antibiotic resistance: plasmid elimination as a potential counter-evolutionary tactic. Drug discovery today. 2004; 9(11):465–467. 10.1016/S1359-6446(03)02989-1 [DOI] [PubMed] [Google Scholar]
- 32.Denap JC, Thomas JR, Musk DJ, Hergenrother PJ. Combating drug-resistant bacteria: small molecule mimics of plasmid incompatibility as antiplasmid compounds. J Am Chem Soc. 2004; 126(47):15402–15404. 10.1021/ja044207u [DOI] [PubMed] [Google Scholar]
- 33.Thomas JR, DeNap JC, Wong ML, Hergenrother PJ. The relationship between aminoglycosides' RNA binding proclivity and their antiplasmid effect on an IncB plasmid. Biochemistry. 2005; 44(18):6800–6808. 10.1021/bi0473298 [DOI] [PubMed] [Google Scholar]
- 34.Schaufler K, Wieler LH, Semmler T, Ewers C, Guenther S. ESBL-plasmids carrying toxin-antitoxin systems can be "cured" of wild-type Escherichia coli using a heat technique. Gut pathogens. 2013; 5(1):34 10.1186/1757-4749-5-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Getino M, Sanabria-Rios DJ, Fernandez-Lopez R, Campos-Gomez J, Sanchez-Lopez JM, Fernandez A, et al. Synthetic fatty acids prevent plasmid-mediated horizontal gene transfer. mBio. 2015; 6(5):e01032–01015. 10.1128/mBio.01032-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Getino M, Fernandez-Lopez R, Palencia-Gandara C, Campos-Gomez J, Sanchez-Lopez JM, Martinez M, et al. Tanzawaic acids, a chemically novel set of bacterial conjugation inhibitors. PLoS One. 2016; 11(1):e0148098 10.1371/journal.pone.0148098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zong Z, Partridge SR, Thomas L, Iredell JR. Dominance of blaCTX-M within an Australian extended-spectrum β-lactamase gene pool. Antimicrob Agents Chemother. 2008; 52(11):4198–4202. 10.1128/AAC.00107-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995; 177(14):4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espedido BA, Partridge SR, Iredell JR. blaIMP-4 in different genetic contexts in Enterobacteriaceae isolates from Australia. Antimicrob Agents Chemother. 2008; 52(8):2984–2987. 10.1128/AAC.01634-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Partridge SR, Ginn AN, Paulsen IT, Iredell JR. pEl1573 carrying blaIMP-4, from Sydney, Australia, is closely related to other IncL/M plasmids. Antimicrob Agents Chemother. 2012; 56(11):6029–6032. 10.1128/AAC.01189-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacoby GA, Han P. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol. 1996; 34(4):908–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou J, Huang X, Deng Y, He L, Yang T, Zeng Z, et al. Dissemination of the fosfomycin resistance gene fosA3 with CTX-M β-lactamase genes and rmtB carried on IncFII plasmids among Escherichia coli isolates from pets in China. Antimicrob Agents Chemother. 2012; 56(4):2135–2138. 10.1128/AAC.05104-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009; 6(5):343–345. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- 44.Murphy KC, Campellone KG. Lambda Red-mediated recombinogenic engineering of enterohemorrhagic and enteropathogenic E. coli. BMC Mol Biol. 2003; 4:11 10.1186/1471-2199-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L. Voluntary oral administration of drugs in mice. Protocol Exchange. 2011: [Google Scholar]
- 46.Sevin EW, Barloy-Hubler F. RASTA-Bacteria: a web-based tool for identifying toxin-antitoxin loci in prokaryotes. Genome biology. 2007; 8(8):R155 10.1186/gb-2007-8-8-r155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao Y, Harrison EM, Bi D, Tai C, He X, Ou HY, et al. TADB: a web-based resource for Type 2 toxin-antitoxin loci in bacteria and archaea. Nucleic acids research. 2011; 39(Database issue):D606–611. 10.1093/nar/gkq908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doumith M, Dhanji H, Ellington MJ, Hawkey P, Woodford N. Characterization of plasmids encoding extended-spectrum β-lactamases and their addiction systems circulating among Escherichia coli clinical isolates in the UK. J Antimicrob Chemother. 2012; 67(4):878–885. 10.1093/jac/dkr553 [DOI] [PubMed] [Google Scholar]
- 49.Mnif B, Vimont S, Boyd A, Bourit E, Picard B, Branger C, et al. Molecular characterization of addiction systems of plasmids encoding extended-spectrum β-lactamases in Escherichia coli. J Antimicrob Chemother. 2010; 65(8):1599–1603. 10.1093/jac/dkq181 [DOI] [PubMed] [Google Scholar]
- 50.Perez-Chaparro PJ, Cerdeira LT, Queiroz MG, de Lima CP, Levy CE, Pavez M, et al. Complete nucleotide sequences of two blaKPC-2-bearing IncN plasmids isolated from sequence type 442 Klebsiella pneumoniae clinical strains four years apart. Antimicrob Agents Chemother. 2014; 58(5):2958–2960. 10.1128/AAC.02341-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L, Hu H, Chavda KD, Zhao S, Liu R, Liang H, et al. Complete sequence of a KPC-producing IncN multidrug-resistant plasmid from an epidemic Escherichia coli sequence type 131 strain in China. Antimicrob Agents Chemother. 2014; 58(4):2422–2425. 10.1128/AAC.02587-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kassis-Chikhani N, Frangeul L, Drieux L, Sengelin C, Jarlier V, Brisse S, et al. Complete nucleotide sequence of the first KPC-2- and SHV-12-encoding IncX plasmid, pKpS90, from Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013; 57(1):618–620. 10.1128/AAC.01712-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen L, Chavda KD, Fraimow HS, Mediavilla JR, Melano RG, Jacobs MR, et al. Complete nucleotide sequences of blaKPC-4- and blaKPC-5-harboring IncN and IncX plasmids from Klebsiella pneumoniae strains isolated in New Jersey. Antimicrob Agents Chemother. 2013; 57(1):269–276. 10.1128/AAC.01648-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Partridge SR, Ellem JA, Tetu SG, Zong Z, Paulsen IT, Iredell JR. Complete sequence of pJIE143, a pir-type plasmid carrying ISEcp1-blaCTX-M-15 from an Escherichia coli ST131 isolate. Antimicrob Agents Chemother. 2011; 55(12):5933–5935. 10.1128/AAC.00639-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng J, Qiu Y, Yin Z, Chen W, Yang H, Yang W, et al. Coexistence of a novel KPC-2-encoding MDR plasmid and an NDM-1-encoding pNDM-HN380-like plasmid in a clinical isolate of Citrobacter freundii. J Antimicrob Chemother. 2015; 70(11):2987–2991. 10.1093/jac/dkv232 [DOI] [PubMed] [Google Scholar]
- 56.Espedido BA, Thomas LC, Iredell JR. Metallo-β-lactamase or extended-spectrum βlactamase: a wolf in sheep's clothing. J Clin Microbiol. 2007; 45(6):2034–2036. 10.1128/JCM.02538-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barton BM, Harding GP, Zuccarelli AJ. A general method for detecting and sizing large plasmids. Anal Biochem. 1995; 226(2):235–240. 10.1006/abio.1995.1220 [DOI] [PubMed] [Google Scholar]
- 58.Seedorf H, Griffin NW, Ridaura VK, Reyes A, Cheng J, Rey FE, et al. Bacteria from diverse habitats colonize and compete in the mouse gut. Cell. 2014; 159(2):253–266. 10.1016/j.cell.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pimentel B, Nair R, Bermejo-Rodriguez C, Preston MA, Agu CA, Wang X, et al. Toxin Kid uncouples DNA replication and cell division to enforce retention of plasmid R1 in Escherichia coli cells. Proc Natl Acad Sci U S A. 2014; 111(7):2734–2739. 10.1073/pnas.1308241111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gardner MN, Rawlings DE. Evolution of compatible replicons of the related IncQ-like plasmids, pTC-F14 and pTF-FC2. Microbiology. 2004; 150(Pt 6):1797–1808. 10.1099/mic.0.26951-0 [DOI] [PubMed] [Google Scholar]
- 61.Raz R. Fosfomycin: an old—new antibiotic. Clin Microbiol Infect. 2012; 18(1):4–7. 10.1111/j.1469-0691.2011.03636.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
The internal variable region of TraY (A, aa 430–522) and C-terminal region of Exc (B) are shown. Variable amino acids are shown by black shading, numbers correspond to amino acid positions in proteins.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.