Abstract
Pancreatic cancer is a highly aggressive and lethal cancer characterized by high invasiveness, local and extensive dissemination at time of diagnosis and resistance to treatment. Few therapies have shown efficacy in the past and even standard of care therapies yield only modest improvements in the mortality of patients with advanced or metastatic disease. Efforts have been undertaken to study the pancreatic tumor microenvironment and have established its complex and immunosuppressive nature which could explain the high resistance to chemotherapy. Novel therapies targeting the tumor microenvironment with an aim to decrease this resistance, improve immune tolerance and increase the efficacy of the current treatment have shown some promising preliminary results in preclinical and clinical trials. We review the current advances in the field of immunotherapy and their effectiveness as a potential treatment strategy in the pancreatic cancer.
Keywords: adoptive cellular immunotherapy, cancer vaccines, immunotherapy, pancreatic cancer
Introduction
Pancreatic cancer is the twelfth most common cancer and is the seventh most common cause of cancer related deaths worldwide with 338,000 new cases and a 5-year prevalence of 4.1 per 100,000 [Ferlay et al. 2015]. In the United States, it is the third leading cause of cancer-related deaths with an estimated incidence of 48,960 cases, an estimated mortality of 40,560 in 2015 and 5-year survival rate of 7.2% [Howlader et al. 2015]. For many years, gemcitabine had been the primary first-line treatment for pancreatic ductal adenocarcinoma (PDAC). The advent of polychemotherapy approaches for locally advanced and metastatic disease using FOLFIRINOX (folinic acid, 5-fluorouracil, irinotecan, oxaliplatin) [Conroy et al. 2011] was able to attain a median survival (MS) of 11.1 months. Von Hoff and colleagues conducted a phase III MPACT trial to study efficacy and safety of nab-paclitaxel and gemcitabine combination versus monotherapy in pancreatic adenocarcinoma. The median overall survival (OS) was 8.5 months in the nab-paclitaxel–gemcitabine group as compared with 6.7 months in the gemcitabine group. The survival rate at 1 year was 35% and 22% and progression-free survival (PFS) was 5.5 and 3.7 months in combination versus monotherapy groups, respectively [Von Hoff et al. 2013]. More recently, Goldstein and colleagues updated results based on long-term follow up and found OS to be 8.7 versus 6.6 months in combination and monotherapy group and found long-term survivors (>3 years) in the combination group (4%) [Goldstein et al. 2015]. There have been many attempts to create other polychemotherapy regimens by combining additional agents with gemcitabine, without significant benefit noted. A randomized phase III trial of pemetrexed in combination with gemcitabine given to 565 locally advanced or metastatic pancreatic cancer demonstrated no significant survival benefits with a MS of 6.5 months and progression-free interval (PFI) of 3.6 months as compared with single-agent gemcitabine [Oettle et al. 2005]. Despite the advances in the treatment for PDAC, patients eventually experience disease progression and cures are rare.
The effect of gemcitabine on the immune system in advanced pancreatic cancer patients has been studied and was not found to be immunosuppressive. Regardless of decreased memory T cell, gemcitabine promotes naïve T-cell activation and may enhance responses to antitumor vaccines and immunotherapy [Plate et al. 2005]. Further effect of gemcitabine proceeded by pemetrexed, an antifolate drug, on innate and adaptive immunity was studied in a phase II study of 15 patients with advanced pancreatic cancer. The study demonstrated MS of 9.5 months and PFI of 4.75 months. Pemetrexed decreased the levels of natural killer (NK) cytolytic units when combined with gemcitabine and initially increased levels of interferon gamma (IFNγ) in NK cells which returned to baseline after the addition of gemcitabine. The combination led to decreased OX40+ activated T cells, helper T cells and memory T cells but levels were increased in subjects with high expression of B7-H3 in tumor tissue. Innate NK cell immunity and FoxP3+ T cells and CD8+ T cells correlated positively with survival and seemed beneficial in patients with pancreatic tumor. Thus, innate and adaptive immunity are important defenses against pancreatic tumors [Davis et al. 2012]. As such, there is a need to further explore novel therapies to improve morbidity and mortality related to PDAC.
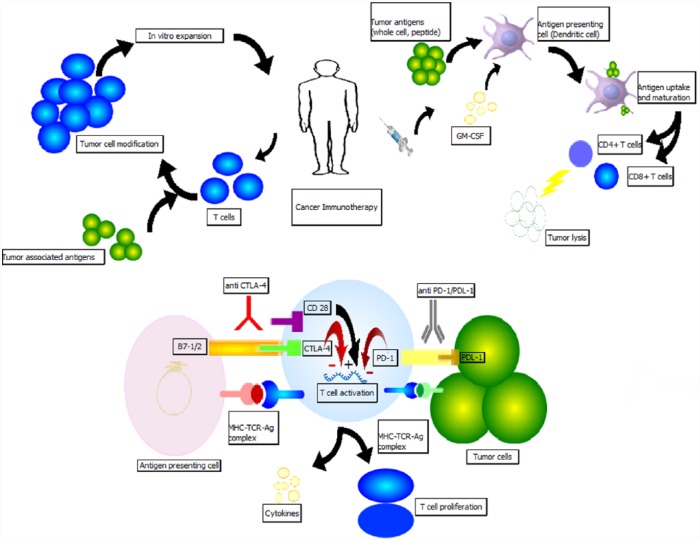
Current progress in immunology-based therapy targets the highly heterogeneous pancreatic tumor immunosuppressive microenvironment. Macrophages, myeloid derived suppressor cells and regulatory T cells (Tregs) are the three major leukocyte subtypes found in early pancreatic intraepithelial (PanIN) stage that are involved in immunosuppressive antitumor activity and more advanced PDAC stage is associated with increased number of Tregs and inactivated effector T cells (Teffs) [Clark et al. 2007]. Various animal and human models have suggested that these immunosuppressive cells also increase in the peripheral blood, stroma and accumulate in PDAC tissue hindering the normal effector T-cell proliferation and response [Liyanage et al. 2002; Zhao et al. 2009; Amedei et al. 2013]. An animal model demonstrated that manipulation of these targets may inhibit or restrict tumor growth [Tan et al. 2009]. These observations advocate for the role of immunosuppression in PDAC. In this article, we review recent advances in immunotherapeutics for pancreatic cancer treatment. See Figure 1 for a diagrammatic depiction of mechanisms.
Figure 1.
Illustrative image describing immunotherapy mechanisms employed in defense against pancreatic cancer.
Immune checkpoint inhibitors
Tumor peptide antigen presentation by major histocompatibility complex (MHC) to the T-cell receptor (TCR) is helpful in eliciting the antitumor immune response. However, this response is modulated by several regulatory molecules expressed on T cells termed ‘immune checkpoints’. These costimulatory or coinhibitory molecules synergize with TCR signaling to promote or inhibit the immune activity under physiologic and pathologic conditions. Co-regulatory molecules serve as immunotherapeutic targets to modify MHC–TCR signaling to optimize the immune response against cancer cells [Pardoll, 2012; Chen and Flies, 2013].
Anti-CTLA-4
CTLA-4 (CD152) is one of the first immune checkpoint receptors to be targeted clinically [Brunet et al. 1987]. It is involved in regulating early stages of T-cell activation. It belongs to the family of CD28 receptors and has an inducible expression on the surface of CD8+ and CD4+ T cells. CTLA-4 binds to ligands B7-1 (CD80) and B7-2 (CD86) on antigen-presenting cells (APCs), and due to its higher avidity, displace the costimulatory receptor CD28. Thus, by blocking the stimulatory effect of CD28 ligand, it attenuates the immune response by inhibiting activation of Teffs, decreasing interleukin (IL)-2 production and activating Tregs to exert suppression [Blank and Enk, 2015]. Several other extrinsic and intrinsic cell mechanisms are also believed to play a role in CTLA-4 function. These include reverse signaling via B7 that can lead to increased production of indoleamine-2,3-dioxygenase (IDO) decreasing T-lymphocyte activation and proliferation and presence of associated phosphatases SHP-2 and PP2A impairing TCR signaling by dephosphorylation leading to overriding of the TCR-induced stop signal required for stable conjugate formation between T cells and APCs, ultimately leading to reduced cytokine production and proliferation [Schneider et al. 2006; Rudd, 2008]. Collectively, all of these factors are believed to limit the body’s immune response to antigens including self-antigens.
The tumor microenvironment in pancreatic cancer has an increased Tregs/Teffs [Ino et al. 2013] and increased expression of negative costimulatory molecules [Chen and Flies, 2013]. Preclinical murine models demonstrated improved tumor control and its shrinkage after CTLA-4 blockade [Leach et al. 1996; Van Elsas et al. 1999; Peggs et al. 2009] which led to the clinical development of the anti-CTLA-4 agent, ipilimumab [Hodi et al. 2010]. Improvement of OS by 4 months (10 versus 6.4 months) led to US Food and Drug Administration (FDA) approval of ipilimumab in metastatic melanoma.
Ipilimumab in pancreatic cancer
Ipilimumab is a fully humanized IgG1 monoclonal antibody (mAb) that antagonizes CTLA-4. A phase II trial [Royal et al. 2010] evaluated the efficacy of ipilimumab for advanced pancreatic cancer in 27 subjects with good performance status. An intravenous dose of 3.0 mg/kg every 3 weeks, four doses per course for a maximum of two courses were given. There were no responders by Response Evaluation Criteria in Solid Tumors (RECIST) criteria but one subject experienced a delayed tumor regression after an initial assessment of progressive disease. Given that RECIST or World Health Organization (WHO) criteria may not provide complete assessment of antitumor response with immunotherapeutics or other such novel agents, a systematic criterion classified as immune-related response criteria was defined to capture and to better interpret any additional atypical responses observed with immune therapy [Wolchok et al. 2009].
A phase Ib trial [ClinicalTrials.gov identifier: NCT00836407] was performed to determine clinical safety, survival rates and T-cell responses to ipilimumab 10 mg/kg alone (arm 1) and in combination with granulocyte macrophage colony-stimulating factor (GM-CSF) vaccine GVAX (arm 2) in patients with previously treated PDAC. Two patients in arm 1 and three patients in arm 2 demonstrated stable disease, and seven patients in arm 2 noted a decline in CA19-9. The combination arm demonstrated an improved median OS (3.6 versus 5.7 months) and 1-year OS (7% versus 27%) [Le et al. 2013]. Additional investigation into optimal use, clinical safety and efficacy of ipilimumab alone or in combination has led to several ongoing clinical trials as documented in Tables 1–5.
Table 1.
Strategy-immune checkpoint inhibitor monotherapy.
| Intervention | Strategy | Date (last updated) | Status | Patient population | Phase | Study design | ClinicalTrials.gov identifier/ Reference |
|---|---|---|---|---|---|---|---|
| Ipilimumab | CTLA-4 inhibitor | 23 September 2015 | Completed | Locally advanced or metastatic pancreatic adenocarcinoma | II | Single arm Ipilimumab | NCT00112580 |
| Pembrolizumab | PD-1 inhibitor | 10 December 2015 | Recruiting | Advanced cancer including pancreatic | Ib/II | Single-agent dose-escalation study | NCT02331251 |
| Pembrolizumab (MK3475) | PD-1 inhibitor | 23 November 2015 | Recruiting | Resectable borderline resectable pancreatic cancer | I/II | Arm 1- pembrolizumab + Neoadjuvant chemoradiation (CRT) Arm 2- Neoadjuvant CRT capecitabine alone |
NCT02305186 |
| Pembrolizumab | PD-1 inhibitor | 2 September 2015 | Recruiting | Advanced solid tumors | I | Single-arm Pembrolizumab | NCT02054806 |
| Pembrolizumab | PD-1 inhibitor | 16 April 2015 | Recruiting | Metastatic cancers including pancreatic adenocarcinoma | I | Pembrolizumab with hypofractionated radiotherapy | NCT02303990 |
| MPDL3280A | PD-L1 inhibitor | 1 December 2015 | Recruiting | Locally advanced or metastatic solid tumors | I | Single-arm i.v. infusion of MPDL3280A | NCT01375842 / [Herbst et al. 2014] |
| MPDL3280A | PD-L1 inhibitor | 11 November 2015 | Recruiting | Solid tumors | II | Single-arm study of MPDL3289A | NCT02458638 |
| MEDI4736 | PD-L1 inhibitor | 11 November 2015 | Recruiting | Advanced solid tumors | I/II | Single-arm dose-expansion study | NCT01693562 |
| Nivolumab + Lirilumab (anti-KIR2DL1/2L3 antibody) | PD-1 Inhibitor, Anti-Killer cell immunoglobulin like receptors | 3 July 2015 | Recruiting | Advanced solid tumors | I | Dose-escalation study | NCT01714739 |
| Nivolumab (BMS-936558) + BMS-986016 (anti-LAG-3) | PD-1 inhibitor, Anti lymphocyte activating gene-3 | 8 December 2015 | Recruiting | Solid tumors | I | Part A-Dose escalation: BMS-986016 Part B-Dose escalation: BMS-986016 + BMS-936558 Part C-Cohort expansion: BMS-986016 + BMS-936558 |
NCT01968109 |
Table 2.
Strategy-dual checkpoint inhibitor combinations.
| Intervention | Strategy | Date (last updated) | Status | Patient population | Phase | Study design | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| Nivolumab + Ipilimumab | PD-1 inhibitor, CTLA-4 inhibitor | 8 December 2015 | Recruiting | Advanced metastatic pancreatic tumor | I/II | Arm N- Nivolumab monotherapy. Arm N-I – Nivolumab + Ipilimumab combination therapy |
NCT01928394 |
| Tremelimumab + MEDI4736 | CTLA-4 inhibitor, PD-L1 inhibitor | 5 December 2015 | Recruiting | Unresectable pancreatic cancer | I | Arm A- MEDI4736 + SBRT Arm B- Tremelimumab + SBRT Arm C-MEDI4736 + Tremelimumab + SBRT |
NCT02311361 |
| Tremelimumab + MEDI4736 | CTLA-4 inhibitor, PD-L1 inhibitor | 7 December 2015 | Recruiting | Advanced solid tumors (Urothelial bladder/ Triple negative Breast cancer/ PDAC) | II | Arm 1- Tremelimumab monotherapy Arm 2- MEDI4736 monotherapy Arm 3- Tremelimumab + MEDI4736 combination |
NCT02527434 |
| Tremelimumab + MEDI4736 + Mogamulizumab (anti- CCR-5) | CTLA-4 inhibitor, PD-L1 inhibitor, Anti chemokine receptor 5 | 4 December 2015 | Recruiting | Advanced solid tumors | I | Arm1- Mogamulizumab + MEDI4736 Arm2- Mogamulizumab + Tremelimumab |
NCT02301130 |
Table 3.
Strategy-immune checkpoint inhibitors and vaccine combination therapy.
| Intervention | Strategy | Date (last updated) | Status | Patient population | Phase | Study design | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| Ipilimumab + GVAX | CTLA-4 inhibitor, Whole cell vaccine | 21 October 2015 | Recruiting | Metastatic Pancreatic cancer | II | Arm1- Ipilimumab + GVAX Arm2- FOLFIRINOX. Endpoint – Efficacy study |
NCT01896869 |
| Nivolumab + GVAX + Cyclophosphamide | PD-1 inhibitor, Whole cell vaccine, Cytotoxic drugs | 1 December 2015 |
Open but not recruiting | Surgically resectable pancreatic cancer | I/II | Arm A- CY/ GVAX Arm B- CY/ GVAX + Nivolumab |
NCT02451982 |
| Nivolumab+ GVAX+ CRS-207 | PD-1 inhibitor, Whole cell vaccine, Listeria vaccine | 23 September 2015 | Recruiting | Metastatic pancreatic cancer | II | Arm 1 – CY/ GVAX/ CRS-207/ Nivolumab Arm 2- CY/ GVAX/ CRS-207 |
NCT02243371 |
Table 4.
Strategy-immune checkpoint inhibitors with chemotherapy.
| Intervention | Strategy | Date (last updated) | Status | Patient population | Phase | Study design | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| Ipilimumab + Gemcitabine | CTLA-4 inhibitor, Cytotoxic drugs | 10 November 2015 | Recruiting | Locally Advanced, unresectable or metastatic pancreatic cancer | Ib | Safety study with induction and maintenance doses of Ipilimumab and Gemcitabine | NCT01473940 |
| Nivolumab + Nab-Paclitaxel + Gemcitabine | PD-1 inhibitor, Cytotoxic drug | 20 October 2015 | Recruiting | Pancreatic cancer | I | Arm A- Nivolumab + nab-paclitaxel Arm B- Nivolumab + nab-paclitaxel + Gemcitabine |
NCT02309177 |
| Pembrolizumab (MK-3475) + mFOLOFOX6 | PD-1 inhibitor | 11 December 2015 | Recruiting | Advanced GI cancer | I/II | Arm- MK-3475 in combination with mFOLFOX6 in a 14 day cycle | NCT02268825 |
| Pidilizumab (CT-011) + Gemcitabine | PD-1 inhibitor | 15 July 2015 | Not recruiting | Resected pancreatic cancer | II | Single arm with CT-011 + Gemcitabine combination therapy | NCT01313416 |
| MPDL3280A + Bevacizumab +/- FOLFOX, Carboplatin, Nab-paclitaxel, Pemetrexed | PD-L1 inhibitor, anti-VEGF antibody, Cytotoxic drugs | 1 December 2015 | Recruiting | Locally advanced or metastatic solid tumors | I | Multiple arms accessing safety and maximum tolerated doses | NCT01633970 |
Table 5.
Strategy-immune checkpoint inhibitors and tyrosine kinase inhibitors combination therapy.
| Intervention | Strategy | Date (last updated) | Status | Patient population | Phase | Study design | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| Ipilimumab + Imatinib mesylate | CTLA-4 inhibitor, Tyrosine kinase inhibitor | 30 November 2015 | Recruiting | Advanced cancer | I | Combination therapy of Ipilimumab with Dose Escalation of Imatinib mesylate | NCT01738139 |
| Pembrolizumab + ACP-196 | PD-1 inhibitor, Bruton tyrosine kinase inhibitor | 30 November 2015 | Active but not recruiting | Advanced or metastatic pancreatic cancer | II | Arm1- ACP-196 alone Arm2- ACP-196 + Pembrolizumab |
NCT02362048 |
| Pembrolizumab + PLX3397 | PD-1 inhibitor, Tyrosine kinase inhibitor | 9 October 2015 | Recruiting | Advanced solid tumors including pancreatic cancer | I | Arm- PLX3397 + pembrolizumab combination therapy | NCT02452424 |
| MEDI4736 + Ibrutinib mesylate | PD-L1 inhibitor, Tyrosine kinase inhibitor | 4 September 2015 | Recruiting | Refractory or relapsed solid tumors | Ib/II | Phase Ib- MEDI4736+ Ibrutinib to determine the recommended phase II dose level Phase II- MEDI4736+ Ibrutinib at recommended phase II dose level |
NCT02403271 |
Tremelimumab in pancreatic cancer
Tremelimumab is a human IgG2 mAb that blocks CTLA-4 [Ribas et al. 2007]. Phase II/III clinical trials in patients with advanced melanoma treated at a dosage of 15 mg/kg every 3 months failed to yield any significant survival benefit [Kirkwood et al. 2010; Ribas et al. 2013]. A phase II single-arm study in patients with malignant mesothelioma yielded a PFS of 6.2 months and a median OS of 10.7 months [Calabrò et al. 2013]. Clinical trials for tremelimumab in pancreatic cancer are underway.
Anti-PD1 and anti-PD-L1
Programmed cell death protein-1 (PD-1)/CD279 is a coinhibitory molecule of the CD28 family. It is expressed predominantly by activated CD4+ and CD8+ T cells, Tregs [Francisco et al. 2010] and is also found on activated B cells [Pedoeem et al. 2014], NK cells, monocytes and certain dendritic cells (DCs). Its binds to the PD-L1 (B7-H1) and PD-L2 (B7-DC) ligands of family B7. PD-L1 is constitutively expressed on several solid tumor cells and tumor-infiltrating lymphocytes (TILs) [Dong et al. 2002] while PD-L2 is expressed on DCs, macrophages, B-cell subsets [Pedoeem et al. 2014] and is upregulated in lymphomas and hematologic malignancies [Rosenwald et al. 2003]. PD-1 engagement with its ligands controls peripheral T-cell tolerance. PD-L1 interaction with PD-1 and B7-1 (CD80) results in negative signals leading to decreased T-cell proliferation, cytokine release and cytolytic activity of PD-1+ T cells. Chronic antigen exposure such as cancer and viral infection, can lead to persistent PD-1 expression inducing a state of exhaustion among antigen-specific T cells and helping the tumor cells escape from host immune attack. Blockade of these targets reverses the tumor protective effects and may provide an effective approach for tumor immunotherapy [Iwai et al. 2002; Okudaira et al. 2009]. Increased expression [Razzaque et al. 2013] of PD-L1 and PD-L2 significantly correlates with worse prognosis, higher grade and is inversely correlated with TILs in pancreatic cancer [Nomi et al. 2007; Geng et al. 2008]. Furthermore, its role in pancreatic adenocarcinoma is defined, given that knocking down of B7-H1 (PD-L1) inhibits PDAC proliferation [Song et al. 2014].
Nivolumab and pembrolizumab, examples of anti-PD1 therapeutic agents, have demonstrated positive results with in various studies involving malignant melanoma [Mahoney et al. 2015], renal cell carcinoma [Weinstock and Mcdermott, 2015] along with preliminary efficacy in a wide array of other tumors. Pembrolizumab has been approved by US FDA for malignant melanoma previously treated with ipilimumab [Robert et al. 2014]. The first PD-L1 antibody to show an objective response in a phase I study including patients with solid tumors was BMS-956559 [Brahmer et al. 2012], but no responses in patients with pancreatic and colorectal cancer were observed. Various PD-1 and PD-L1 inhibitors are presently in clinical trials for pancreatic malignancies.
A broad array of clinical trials in pancreatic cancer alone or advanced solid tumors including pancreatic cancer have been completed or ongoing using immune checkpoint monotherapy (Table 1), dual checkpoint combination therapy (Table 2), immune checkpoint inhibitor and vaccine combinations (Table 3), immune checkpoint inhibitors with cytotoxic chemotherapy (Table 4), immune checkpoint inhibitors with kinase inhibitors (Table 5) and immune checkpoint inhibitor combinations with other agents (Table 6).
Table 6.
Strategy-other therapies in combination with immune checkpoint inhibitors.
| Intervention | Strategy | Date | Status | Patient population | Phase | Study design | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| Nivolumab + FPA008 (CSF1R inhibitor) | PD-1 inhibitor, Colony stimulating factor-1 receptor inhibitor | 2 December 2015 | Recruiting | Advanced pancreatic cancer | I | Arm 1- Phase 1a monotherapy Arm 2- Phase1a combination therapy to determine safety data Arm 3- Phase 1b combination therapy to determine clinical activity and safety profile |
NCT02526017 |
| Nivolumab + Ulocuplumab (anti- CXCR4) | PD-1 inhibitor, Anti alpha chemokine receptor 4 | 8 December 2015 | Recruiting | Metastatic solid tumors | I/II | Combination arm in pancreatic cancer | NCT02472977 |
| Pembrolizumab + AM0010 | PD-1 inhibitor, PEGylated recombinant human IL-10 | 24 March 2015 | Recruiting | Advanced solid tumor including pancreatic cancer | I | Combination therapy | NCT02009449 |
| MPDL3280A + RO6895882(IL-2v with anti-CEA) | PD-L1 inhibitor, Variant of Interleukin-2 against Carcinoembryonic antigen | 1 December 2015 | Recruiting | Advanced solid tumors | I | Part 1- Dose Escalation RO6895882(IL-2v with anti-CEA) + MPDL3280A Part 2- Dose expansion |
NCT02350673 |
| Pembrolizumab+ Modified vaccinia virus Ankara virus | PD-1 inhibitor, Oncolytic virus | 18 December 2015 | Not recruiting | Solid tumors with failed prior therapy including PDAC | I | Combination therapy | NCT02432963 |
Therapeutic vaccine immunotherapy
The principle of using vaccine immunotherapy in cancer treatment is similar to passive immunity against an infectious process. These are biologically designed to mount an immune response against a tumor by administering tumor-specific antigens. They include whole-cell vaccines, DC vaccines, DNA vaccines and peptide vaccines.
Whole-cell vaccines
Whole-cell recombinant vaccines utilize the tumor cells which have a wide range of antigens and express epitopes for CD8+ and CD4+ T cells. Allogeneic cell lines may be used instead of autologous cell lines in pancreatic cancer when it is difficult to harvest these tumor cells due to limited number of surgical candidates, long culture times and risk of contamination [Koido et al. 2005].
Algenpantucel-L
Algenpantucel-L is a hyperacute vaccine that has allogenic irradiated pancreatic cancer cells expressing the enzyme alpha-1,3-galactosyl transferase (αGT) which is required for the synthesis of alpha-galactosyl (αGal) epitopes (Table 7). Humans lack the presence of αGal epitopes but have anti-αGal antibodies [Galili et al. 1985] and these are continuously produced in response to enterobacteria with no immune tolerance to it [Galili et al. 1988]. The hyperacute technology exploits this naturally acquired immunity against αGal labeled tumor cells leading to enhanced antitumor response [Mandell et al. 2009]. Effective tumor responses in animal models [Gorelik et al. 1995; Rossi et al. 2005] led to subsequent human clinical trials. Hardacre and colleagues [Hardacre et al. 2012], presented results of an open-label, single-arm phase II study of algenpantucel-L with gemcitabine and 5-fluorouracil for 70 resected pancreatic cancer patients. One-year disease-free survival (DFS) of 63% and OS of 86% was achieved as compared with historical controls (~45% and 65%). Current ongoing phase III clinical trials are the Immunotherapy for Pancreatic Resectable Cancer Survival Study (IMPRESS) and the Pancreatic Immunotherapy with algenpantucel-L for Locally Advanced non-Resectable (PILLAR) trial.
Table 7.
Algenpantucel-L in clinical trials.
| Intervention | Strategy | Date (last updated) | Status | Patient population | Phase | Study design | ClinicalTrials.gov identifier (acronym) |
|---|---|---|---|---|---|---|---|
| Algenpantucel-L + Gemcitabine +/– 5-FU | Hyperacute (HAPa) vaccine, Cytotoxic drugs | 5 August 2015 | Ongoing but not recruiting | Surgically resected pancreatic cancer | III | Randomized double arm. Arm 1 – HAPa Vaccine + Cytotoxic drugs. Arm 2 – Cytotoxic drugs |
NCT01072981 (IMPRESS) |
| Algenpantucel-L + FOLFIRINOX + Gemcitabine + 5-FU chemoradiation + Capecitabine + Nab-paclitaxel | HAPa, Cytotoxic drugs, Chemoradiation | 28 October 2015 | Recruiting | Borderline resectable or locally advanced unresectable pancreatic cancer | III | Arm 1A – FLOFIRINOX + HAPa Arm 1B – Gemcitabine/Nab-Paclitaxel + HAPa Vaccine Arm 2A – FOLFIRINOX alone Arm 2B – Gemcitabine/Nab-paclitaxel |
NCT01836432 (PILLAR) |
| Algenpantucel-L + mFOLFIRINOX + Gemcitabine + SBRT | HAPa, Cytotoxic drugs, Radiation | 23 September 2015 | Recruiting | Borderline resectable pancreatic cancer | II | Single-arm combination therapy | NCT02405585 |
GM-CSF vaccine
GVAX is an allogenic irradiated whole-cell tumor vaccine transfected with GM-CSF gene and delivering tumor-associated antigens (TAAs) at the same time (Table 8). GM-CSF is a potent cytokine released by macrophages, T cells, mast cells, NK cells that function to stimulate stem cells to produce granulocytes and monocytes, promoting cytolytic activity against tumor cells [Thomas et al. 2004] and also causing influx of bone-marrow derived DCs that process and present TAAs delivered by the vaccine [Huang et al. 1994]. A phase I [Jaffee et al. 2001] clinical trial of GVAX in resectable pancreatic cancer given before and after chemoradiation therapy, assessed 14 patients and showed a mean DFS of 13 months. Three patients who demonstrated delayed-type hypersensitivity (DTH) were disease free from 25 to 30 months. A dose of 50 × 107 vaccine cells was identified as safe with limited side effects and associated with induction of antitumor immune response. These favorable results resulted in phase II trials of GVAX in combination with cyclophosphamide (CY) [Laheru et al. 2008] in metastatic pancreatic cancer patients who failed gemcitabine therapy. Despite no significant statistical difference of OS and 1-year survival between two cohorts, this study suggested enhanced antitumor activity in combination cohort where CY is given prior to immunotherapy based on a higher rate of induction of mesothelin-specific T-cell responses that could be associated with longer PFS and OS. Another phase II study of 60 patients with resected PDAC received GVAX integrated with boosters and 5-FU-based chemoradiation demonstrated a median DFS of 17.3 months with MS of 24.8 months. It also demonstrated a correlation between induction of mesothelin-specific T-cell responses with improved OS proposing a prognostic role [Lutz et al. 2011]. Additional markers that may be associated with prolonged survival in patients treated with GVAX and/or ipilimumab are induction of thyroglobulin antibodies due to thyroid autoimmunity [De Remigis et al. 2015].
Table 8.
Clinical trials that used combination of whole cell vaccine GVAX and chemotherapy.
| Intervention | Strategy | Date (last updated) | Status | Patient population | Phase | Results | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| GVAX + Adjuvant standard of care chemoradiation. | Whole-cell vaccine, Cytotoxic drugs | 17 July 2015 | Completed | 60 patients with resected stage I or II pancreatic adenocarcinoma | II | Overall survival – 24.8 months. Disease-free survival – 17.3 months |
NCT00084383 |
| GVAX + Cyclophosphamide + Cetuximab | Whole-cell vaccine, Cytotoxic drugs | 18 June 2015 | Completed | 60 patients with metastatic or locally advanced pancreatic cancer | II | Total serious adverse events – 12/60 (20%) | NCT00305760 |
| GVAX + Cyclophosphamide. | Whole-cell vaccine, Cytotoxic drugs | 17 November 2105 | Ongoing not recruiting | Stage I or II pancreatic cancer | Pilot | Combination versus GVAX monotherapy. | NCT00727441 |
| GVAX + Cyclophosphamide + SBRT + FOLFIRINOX | Whole-cell vaccine, Cytotoxic drugs, Radiation | 27 July 2015 | Ongoing not recruiting | Pancreatic cancer | Pilot | Arm 1 - SBRT + FOLFIRINOX. Arm 2 - Combination therapy |
NCT01595321 |
Peptide vaccines
Peptide-based vaccines constitute tumor antigenic fragments (epitopes) which elicit a tumor-specific response by stimulating T cells. These vaccines are safe, simple and economically beneficial. However, their minimal immunogenic potential, impaired antigen presentation [Yanagimoto et al. 2005] and defective immune response in patients with advanced cancer have limited their usefulness [Gaudernack, 2006].
Ras vaccine
K-Ras is mutated in almost 90% of pancreatic cancers [Hruban et al. 1993] and is presented as a foreign antigen by MHC class I and II to CD4+ and CD8+ T cells [Abrams et al. 1997; Gjertsen et al. 1997]. Therefore, K-Ras mutated gene product can serve as a focus for therapeutic peptide vaccine inducing anti-Ras immunity in patients. Initial studies testing Ras vaccine in humans [Gjertsen et al. 1995] demonstrated the safety and induced transient T-cell tumor response against mutations in tumor cell, albeit with no clinical responses. Failure to manifest clinically significant immunity was attributed to the inability to elicit the imperative immune response to these antigens. Eventually in an attempt to enhance the immune response, a phase I/II trial combining synthetic mutant Ras peptides with GM-CSF as an adjuvant was given to 48 patients (10 surgically resected and 38 with advance disease) of pancreatic adenocarcinoma. Peptide-specific immunity was induced in 58% of patients and the responders showed an improved survival over nonresponders (MS 148 days versus 61 days, respectively) and 9 of 10 resected PDAC patients showed stability with an OS of 25.6 months (range 10–39 months) and 11 of 34 unresectable patients had a stable disease lasting an average of 10.2 months (range 3–28 months) [Gjertsen et al. 2001].
Adjuvant use of Ras vaccine in a phase II clinical trial [Toubaji et al. 2008] in patients with surgically resected (five pancreatic and seven colorectal cancer patients) with no evidence of disease at enrollment demonstrated a positive immune response with an increase of IFNγ m-RNA copy numbers in 5/11 patients. Pancreatic cancer patients had a mean DFS of 35.2+ months and a mean OS of 44.4+ months providing evidence for adjuvant early use of mutant Ras peptide immunotherapy. Subsequently [Wedén et al. 2011], a study using a long synthetic mutant Ras peptide vaccine described a 10-year follow up of 23 patients from previous phase I/II clinical trials [Carbone et al. 2005; Toubaji et al. 2008] to assess their long-term immunological T-cell reactivity and survival. A total of 17 of 20 (85%) were immune responders with a MS of 28 months. The 5-year survival was 22% and 28% for all and immune responders respectively and a striking 10-year survival of 20% (4/20 evaluable) versus 0% in comparable nonvaccinated cohort, was observed. Recently, another study evaluated 24 patients with resected pancreatic cancer with K-Ras mutation that were vaccinated with peptide vaccine on day 7 of a 10-day course of GM-CSF. A total 25% of patients (9/24) were immune responders, three of them displayed nonspecific DTH and one patient had a K-Ras mutation-specific DTH. Median recurrence-free survival time was 8.6 months and median OS was 20.3 months [Abou-Alfa et al. 2011]. These studies demonstrate promising results and suggest larger controlled studies depicting the role of K-Ras vaccine as an adjuvant immunotherapy for PDAC are needed.
Another strategy applied to activate immune system against the mutated Ras peptide is by using nonpathogenic yeast as a vehicle to carry antigens and present them to DCs. DCs treated with yeast and cocultured with T cells had a lower expression of FoxP3 cells and increase in the ratio of CD4+CD25+ activated T cells to Tregs and production of Th1-related cytokines and IL-6 [Cereda et al. 2011]. GI-4000 is a product series of four tarmogens; each tarmogen is a different heat inactivated yeast Saccharomyces cerevisiae expressing combination of common Ras mutations found in human cancers. Clinical trials studying the K-Ras peptide vaccine alone or as combination therapy in pancreatic cancer are shown in Table 9.
Table 9.
Ras vaccine in clinical trials.
| Intervention | Strategy | Date | Status | Patient population | Phase | Study design | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| Ras vaccine, Detox-B adjuvant |
Peptide cancer vaccine, Immunostimulant | 27 April 2015 | Completed | Pancreatic, Colon or Lung cancer | I | Adjuvant treatment for Stage I–IV resected pancreatic cancer | NCT00019006 |
| Ras vaccine, P53 peptide vaccine, Aldesleukin, Sargramostim, Therapeutic autologous and TILs | Peptide cancer vaccine, DC vaccine, IL-2, Leukocyte growth factor | 19 June 2013 | Completed | Advanced solid tumors | II | Vaccine therapy alone or in combination with cellular immunotherapy | NCT00019084 |
| Ras vaccine, Sargramostim, Aldesleukin, Detox PC | Peptide vaccine, Leukocyte growth factor, IL-2, Immunostimulant | 19 July 2013 | Completed | Metastatic solid tumors | II | Vaccine therapy alone or in combination | NCT00019331 |
| Ras vaccine, QS21 | Peptide vaccine, Natural saponin stimulant | 10 July 2013 | Completed | Advanced pancreatic or colorectal cancer | I | Safety study | NCT00006387 |
| GI-4000, Gemcitabine | Globeimmune heat killed yeast cell transfected with mutated Ras peptide, Chemotherapy | 2 April 2015 | Completed | Nonmetastatic, post-resection PDAC | II | Efficacy of vaccine compared with placebo | NCT00300950 |
Telomerase peptide vaccine
Telomerase is a ribonucleoprotein enzyme found in almost 90% cancer cells [Vasef et al. 1999] including pancreatic cancer cells [Suehara et al. 1997]. The induction of human telomerase reverse transcriptase (hTERT), a telomerase catalytic subunit parallels the increased expression of the enzyme [Counter et al. 1998]. Acknowledging its role in tumorigenesis, telomerase tumor antigen served as a target for the development of peptide-based vaccine, GV1001. It is a 16-aa hTERT peptide that binds to MHC activating hTERT-specific T-cell responses [Su et al. 2005]. GV1001 was studied in a phase I/II trial by Bernhardt and colleagues in 48 patients with unresectable pancreatic cancer. The GV1001 vaccine was given in three dose levels (100, 300, 1000 nmol) along with GM-CSF for a period of 10 weeks with monthly boosters. Immune response (positive DTH test or the presence of GV1001-specific T cells in peripheral blood after vaccination) was observed in 24 of 38 evaluable patients with the highest immune response (75%) in the intermediate dosage group. The MS for the low-, intermediate- and high-dose groups were 4.0, 8.6 and 5.1 months, respectively. As such, prolonged immunologic response correlated with prolonged survival in the study [Bernhardt et al. 2006].
The two phase III trials testing PrimoVax (GV1001 and GVAX) [Buanes et al. 2009] and TeloVac (GV1001) [Middleton et al. 2013] administered with subsequent and concurrent chemotherapy were unable to simulate similar affirmative results and showed no significant survival benefits in patients with metastatic pancreatic cancer. A currently ongoing clinical trial [ClinicalTrials.gov identifier: NCT01342224] is testing GV1001 with GM-CSF in patients with locally advanced pancreatic cancer given in combination with gemcitabine chemotherapy and tadalafil (PDE5 inhibitor) followed by concurrent radiation therapy.
Mucin-1 vaccine
Mucin 1 (MUC-1) is type I transmembrane protein constituting a large extracellular domain with extensive O-linked glycosylation and a small cytoplasmic domain in close contact with the nucleus and plays a role in normal cell signal transduction as well as oncogenic signaling to increase invasion, angiogenesis and metastasis [Behrens et al. 2010]. MUC-1 expression is increased in tumor cells including pancreatic tumors [Burdick et al. 1997; Qu et al. 2004] with a loss of polarity and hypoglycosylation. The overexpression and aberrant glycosylation makes MUC-1 highly immunogenic and, thus, a target for cancer immunotherapy [Besmer et al. 2011]. MUC-1 vaccine has been tested in different forms such as peptide vaccine, peptide transfected DC, DCs transfected with c-DNA22 tandem repeats units of MUC-1, as well as adoptive transfer immunotherapy. The phase I clinical trials by Goydos and colleagues [Goydos et al. 1996], Ramanathan and colleagues [Ramanathan et al. 2005] and most recently Yamamoto [Yamamoto et al. 2005] used peptide-based vaccines. Pecher and colleagues [Pecher et al. 2002] tested three different types of formulation of mucin vaccine namely: (1) MUC-1 peptide admixed with murine GM-CSF as an adjuvant; (2) MUC-1 peptide admixed with adjuvant SB-AS2; and (3) MUC-1 peptide-pulsed DCs. Only MUC-1 peptide loaded DC vaccine was able to elicit tumor rejection and response correlated with induction of MUC-1-specific T-cell response in wild and transgenic mice. This was further tested by Lepisto and colleagues [Lepisto et al. 2008].
These studies (Table 10) that define the effect of MUC-1 immunotherapy on pancreatic cancer reveal the safety of the vaccine and has led to increased survival in few vaccinated subjects. MUC-4, MUC-16 are the other potential targets that play a role in PDAC development and also lead to resistance of chemotherapeutic drugs [Bafna et al. 2009; Skrypek et al. 2013] Overcoming this resistance by combining chemotherapeutics with MUC based therapy may improve the clinical outcomes of patients with pancreatic cancer.
Table 10.
MUC-1 vaccine in clinical trials.
| Intervention | Adjuvant | Strategy | Phase | Patient population | Year | Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| MUC-1 105 amino acid | BCG | Peptide-based | I | Pancreatic adenocarcinoma | 1996 | Vaccine well tolerated with strong T-cell immune response but no clinical response | Goydos et al. [1996] |
| Autologous DC transfected with 22 tandem repeat units of MUC-1 | Gene transfected DC Vaccine | I/II | Advanced pancreatic adenocarcinoma | 2002 | Demonstrated feasibility and safety of vaccine | Pecher et al. [2002] | |
| MUC-1 100 aa peptide | SB-AS2 | Peptide-based vaccine | I | Resected and locally advanced pancreatic cancer | 2005 | Vaccine specific DTH response in 3/10 patients | Ramanathan et al. [2005] |
| MUC-1 100 aa peptide | Extracellular tandem repeats and incomplete Freund | Peptide-based vaccine | I | Advanced pancreatic and biliary cancer | 2005 | PDAC patients with high frequency of IFNγ secreting CD8+ T cells | Yamamoto et al. [2005] |
| MUC-1 peptide loaded DC | DC vaccine | I/II | Resected pancreatic and biliary tumors | 2008 | 4/12 alive at 4 years without recurrence. Median survival is 26 months | Lepisto et al. [2008] |
Anti-VEGFR vaccine
Vascular endothelial growth factor receptor 2 (VEGFR2) is upregulated and essential in tumor angiogenesis. A phase I trial combining VEGFR2-169 with gemcitabine was conducted in advanced pancreatic cancer patients. A total of 15/18 patients developed immunological response (cytotoxic T lymphocyte and Treg response). A total of 15/18 (83%) developed immunological reactions at the injection sites. Specific cytotoxic T lymphocytes (CTLs) reacting to the VEGFR2-169 peptide were induced in 11 (61%) of the 18 patients. The disease control rate was 67%, and median OS time was 8.7 months [Miyazawa et al. 2010]. A phase I study with pancreatic cancer patients testing gemcitabine with antiangiogenic peptide vaccine (VEGFR2-169) NCT00622622 and a phase I/II study [ClinicalTrials.gov identifier: NCT00655785] with VEGFR2-169 and VEGF1-1084 has been completed.
Survivin-based vaccine
Survivin is one of the cancer-specific proteins overexpressed in tumor cells and human fetal tissues, but hardly detectable in normal human tissues. It promotes angiogenesis, inhibits apoptosis and enhances proliferation [Ryan et al. 2009]. Expression of survivin is increased on pancreatic adenocarcinoma tissue as well as in serum [Dong et al. 2015] and is associated with poor prognosis and resistance to chemotherapy and radiation [Kami et al. 2004]. A 77-year-old patient suffering from pancreatic cancer with liver metastasis refractory to gemcitabine therapy received a survivin-based vaccine (consisting of 100 μg of a modified HLA-A2 restricted surviving (96–104) epitope in Montanide(R)) and achieved a complete remission of liver metastasis at 8 months but with recurrence of disease after being weaned from the vaccine [Wobser et al. 2006]. This effect was further tested in a study [Ishizaki et al. 2011] on the Pan02 mouse pancreatic adenocarcinoma cell line that combined gemcitabine and modified vaccinia Ankara (MVA) expressing murine survivin. This study resulted in significant tumor regression and prolonged survival. A total of 50% of the mice in the MVA-survivin + gemcitabine group were alive at 50 days, whereas all other animals in MVA-survivin and MVA-control + gemcitabine groups expired by 38 days post-tumor induction. These results observed with combined immunotherapy were primarily dependent on generation of CD8+ T cells. Gemcitabine and survivin act in synergy creating an antitumor environment by generating effector cells against self-tumor antigen and apoptosis of tumor cells that helps to reduce tolerance against survivin and prolong the survival in combination therapy. The data from the study suggests combination therapy may find its potential for clinical benefit in pancreatic cancer.
Antigastrin vaccine
Gastrin-17 is a growth factor for pancreatic and other gastrointestinal malignancies. Antigastrin-17 vaccine (G17DT) has a gastrin immunogen with diphtheria toxoid (DT) carrier protein. The first phase II study testing G17DT recruited 30 patients with advanced pancreatic cancer, out of which 20 patients produced an antibody response. The MS for immune responders was 217 days and 121 days for nonresponders (p = 0.0023) [Brett et al. 2002]. A randomized clinical trial of 154 patients with advanced pancreatic cancer was well tolerated (2/79 (2.5%) G17DT patients versus 0/74 (0%) placebo patients developed sterile abscess at the injection site). The MS in the G17DT group was 138 days versus 78 days for the control group [Gilliam et al. 2004]. Subsequently, a randomized, multicenter, double-blind, placebo-controlled study of 154 patients with advanced pancreatic cancer gave MS of 151 days as compared with 82 days with placebo [Gilliam et al. 2012]. Another multicenter study evaluated the role of G17DT + gemcitabine versus placebo + gemcitabine in 394 patients with locally advanced, recurrent or metastatic adenocarcinoma of pancreas. The MS was 5.8 months in G17DT + gemcitabine group which was lower than the placebo + gemcitabine (6.6 months) and same PFS of 3.9 months [Shapiro et al. 2005]. A prospective randomized phase III study testing G17DT for the treatment of advanced pancreatic cancer [ClinicalTrials.gov identifier: NCT02118077] was recently completed and results are awaited.
Heat shock protein–peptide complex
Heat shock proteins act as peptide carriers for stabilization and delivery. Overexpression of HSP90 is observed in pancreatic carcinoma and HSP90 β is reported to inhibit apoptosis [Ogata et al. 2000]. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma to determine tolerance and immune as well as clinical responses. A total of 3/10 patients were alive at 2.6, 2.7 and 5 years follow up [Maki et al. 2007]. A phase I clinical trial of immunotherapy with autologous tumor-derived gp96 heat shock protein-peptide complex in patients with resected pancreatic adenocarcinoma [ClinicalTrials.gov identifier: NCT00003025] has been completed and results are awaited.
Dendritic cell vaccine
DCs are the most potent APCs which function to process and present antigens with MHC to activate cytolytic and regulatory T-cell response [Koski et al. 2008]. These characteristics are being employed in development of DC vaccines to mount an antitumor response (Table 11). DC vaccines are designed to present TAAs like peptides, mRNA or cDNA or by fused autologous DC with tumor cells and means by which they are presented including: (a) nontargeted vaccine which consists of tumor-specific peptides or nucleic acids captured in vivo; (b) vaccines with ex vivo generated DCs loaded with antigens; (c) vaccines with tumor antigens coupled to anti-DC-antibodies to mount in vivo response [Palucka and Banchereau, 2013]. Intratumoral response with tumor RNA-pulsed DC was studied in C57BL/6 murine pancreatic tumor model inducing a specific T lymphocyte antitumor effect [Schmidt et al. 2003].
Table 11.
Studies using dendritic cell (DC) vaccines.
| Intervention | Strategy | Patient population | Phase | Year | Results | Reference |
|---|---|---|---|---|---|---|
| CEA loaded DC | mRNA infested DC vaccine | 3 patients with resected pancreatic adenocarcinoma following neoadjuvant chemoradiotherapy. | Pilot | 2002 | Well tolerated, no evidence of adverse events All patients were alive without evidence of disease at more than 30 months from the original diagnosis |
Morse et al. [2002] |
| Mutant p53 and K-Ras loaded DC | Peptide based DC vaccine | 39 patients with pancreatic, lung, colon, breast, ovarian, gastrointestinal and other cancers | I | 2005 | 10/38 evaluable patients had detectable CTL response, 16 patients with positive IFNγ after vaccination Five experienced stable disease. Median survival time of 393 versus 98 days for a positive versus negative CTL response |
Carbone et al. [2005] |
| Adenovirus encoding Interleukin 12 (AFIL-12) gene transfected DC | Engineered DC vaccine | 17 patients with metastatic pancreatic (3), colorectal (5), liver (9) | Pilot | 2005 | Stable disease in two patients and partial response in one. Progression in eight patients Marked increase of CD8+ T lymphocytes in 3/11 tumor biopsies analyzed |
Mazzolini et al. [2005] |
| MUC-1 pulsed DC | Peptide based DC vaccine | 20 patients with unresectable or recurrent pancreatic cancer | Pilot | 2008 | Complete response in one patient with lung metastasis. 5 patients had stable disease. Median survival was 9.8 months | Kondo et al. [2008] |
Further clinical trials to determine the effectiveness of CEA RNA-pulsed DC vaccine alone [ClinicalTrials.gov identifier: NCT00004604] and in combination with chemotherapy gemcitabine [ClinicalTrials.gov identifier: NCT00547144] and chemokine modulatory regimen (CKM) [ClinicalTrials.gov identifier: NCT02151448] are in process.
Recombinant bacterial vaccine
Bacterial agents such as Listeria monocytogenes (LM) and Salmonella enterica typhimurium act as delivery systems for TAAs because of their ability to survive intracellularly [Paterson et al. 2010]. Listeria is a Gram-negative intracellular facultative bacterium which is actively phagocytosed by macrophages or other APCs. Once inside the phagosome it secretes Listeriolysin O (LLO) and phospholipase C which helps it to modify the phagosomes to larger compartment called spacious Listeria-associated phagosomes (SLAPS) [Birmingham et al. 2008] promoting its replication and release from the phagolysosome into the cytoplasm. LM-based immunotherapy utilizes live attenuated LM bacteria to act as a vaccine vector and deliver the TAAs to the APCs and hence eliciting an antitumor immune response.
Preclinical studies have shown the antitumor effect of the vaccine. A study in FVB/N mouse fusing LLO with HER-2/neu antigen delivered by LM showed the increased immunogenicity of self-antigens and provided tumor regression [Singh et al. 2005]. CRS-207 is a live attenuated vaccine of LM bacterium has two gene deletions that encode for virulence and is modified to express mesothelin. A phase I clinical trial [Le et al. 2012] tested ANZ-100 (live attenuated LM strain) and CRS-207 in advanced mesothelin expressing cancers with liver metastasis. CRS-207 induced both LLO and mesothelin-specific T-cell responses and chemokine induction were present at all doses while ANZ-100 induction of chemokines was dose dependent. While the study was statistically unable to show survival benefit, it revealed that 37% of the population survived for more than 15 months with 3 PDAC long-term survivors with minimal adverse events. A more recently completed randomized phase II study assessed safety and survival of CRS-207 with GVAX and low-dose CY for 90 metastatic pancreatic cancer patients [Le et al. 2015]. GVAX/CY inhibits Tregs and had shown earlier preclinical synergy with the vaccine that helps to induce innate and adaptive immunity. OS was 9.7 months with an improvement of 5.6% (2.2 months) as compared with first-line therapy gemcitabine plus nab-paclitaxel. The longer OS was associated with enhanced mesothelin-specific CD8 T-cell response. An ongoing phase II clinical trial is testing the safety and efficacy of GVAX (with CY) and CRS-207 compared with CRS-207 alone in adults with previously treated metastatic pancreatic adenocarcinoma [ClinicalTrials.gov identifier: NCT02004262].
Adoptive T-cell immunotherapy
Adoptive T-cell immunotherapy (ACT) employs the technique of genetically modifying the T cells and infusing them into tumor tissue to increase the immune response against the tumor. Autologous TILs are engineered to equip them with activity against TAAs using modified TCRs or Chimeric Antigen Receptors (CARs). CARs are recombinant receptors with an intracellular signaling portion constituting T-cell receptor–CD3-ζ complex and an extracellular single chain variable fragment of mAb [Gross et al. 1989; Khan et al. 2014]. The first generation CARs have FcRγ or CD3ζ chains and second-generation CARs incorporates additional cytoplasmic costimulatory domains such as CD28 and CD137 and third-generation CARs comprise three signaling domains. This helps T cells to target tumor cells in a HLA-independent manner to a wide range of surface TAAs [Kershaw et al. 1996].
A preclinical study on murine model expressing anti-CEA CAR T cells were able to reduce the size of pancreatic tumors below the limit of detection and long-term tumor eradication in 67% of mice [Chmielewski et al. 2012]. Intratumoral injection of T cells expressing CAR against Her2/neu or CD24 completely eliminated the tumor and more than 50% of animals appeared to be disease-free more than 2 months later [Maliar et al. 2012]. Similarly other studies with T cells expressing MUC-1 CAR T cells plus IL-4 receptor α [Wilkie et al. 2010] and mesothelin CAR T cells [Carpenito et al. 2009, Zhao et al. 2010] resulted in tumor regression in large, pre-established tumors in mice.
MORAb-009 is a chimeric IgG1 antibody targeting tumor-associated mesothelin overexpressed on pancreatic, ovarian, lung and colorectal carcinoma. A preclinical evaluation of MORAb-900 found the increased effectiveness that led to reduction in tumor growth of mesothelin positive tumors when combined with chemotherapy [Hassan et al. 2007]. This was tested further in a phase I clinical study [Hassan et al. 2010] in 24 mesothelin positive patients including 7 pancreatic cancer patients which showed it was safe and well tolerated at a dose of 200 mg/m2. A total of 11 of previously treated patients in this group had stable disease. Recently reported results of randomized phase II clinical trial [ClinicalTrials.gov identifier: NCT00570713] of MORAb-009 in combination with gemcitabine or placebo in 155 advanced pancreatic cancer subjects failed to suggest any significant benefit of MORAb-900 therapy. The OS of MORAb arm was found to be 6.5 months (4.5–8.10) versus 6.9 months (5.4–8.8) in the placebo arm and PFS of 3.4 months (1.9–4.7) and 3.5 (2.8–4.9), respectively. A total of 19.2% of the patients had progressive disease in the MORAb-009 arm as compared with 23.4% patients in the placebo arm.
Despite the results of the above-mentioned trials, majority of patients has failed to respond well to ACT. Tumor-induced immunosuppression, i.e. upregulation of inhibitory receptors and ligands of activated T cells have shown to contribute to failure of T-cell therapy [Abate-Daga et al. 2013]. To overcome these inhibitory signals, Kobold and colleagues developed a new fusion PD-1 – CD28 receptor hypothesizing that CD28 costimulation may overcome peripheral tolerance [Kobold et al. 2015]. Treatment of mice with Panc02 pancreatic carcinoma cell line expressing ovalbumin (Panc-OVA) with PD-1–CD28 fusion receptor led to complete regression of tumor and had a memory response in 9 out of 11 mice. It warrants further development of the strategy to enhance efficacy of ACT in cancer. Another approach tested preclinically to improve the activity of antitumor CAR T cells is through increased constitutive expression of CD40L. T cells demonstrated increased proliferation and secretion of proinflammatory Th1 cytokines and increased CD19-specific CAR/CD40L T cells cytotoxicity against tumor cells. It also augmented immunogenicity of CD40+ tumor cells by upregulated expression of various costimulatory, adhesion molecules thereby counteracting the tumor cells ability for immune evasion. These novel approaches should be tested in additional studies with the goal of ascertaining their usefulness in a clinical setting. Completed and ongoing studies with adoptive T-cell therapy in pancreatic cancer alone or in solid tumor cancer groups including pancreatic cancer are depicted in Table 12.
Table 12.
Adoptive T-cell therapy in clinical trials.
| Intervention | Strategy | Date (last updated) | Status | Patient population | Phase | Study design | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| Autologous T-cells with chimeric anti-mesothelin SS1 receptor | Mesothelin RNA CAR T-cell therapy | 8 January 2015 | Completed | Metastatic PDAC | I | I.v. administration of intervention to study safety and feasibility | NCT01897415 |
| CART-meso +/– Cyclophosphamide | Mesothelin CAR T-cell therapy. Cytotoxic drugs |
30 November 2015 | Recruiting | Mesothelin expressing cancers including metastatic PDAC | I | Dose escalation design transducing CART-meso cells with or without CY | NCT02159716 |
| Anti-mesothelin CAR, Cyclophosphamide, Fludarabine, Aldesleukin |
CAR T cell therapy. Cytotoxic drugs. IL-2 cytokine |
3 November 2015 | Recruiting | Metastatic pancreatic cancer | I/II | Single arm with infusion of lymphodepleting cytotoxic drugs followed by CART-meso + Aldesleukin | NCT01583686 |
| Anti-NY ESO-1 mTCR PBL, Cyclophosphamide, Fludarabine, Aldesleukin | Anti-NY-ESO-1 murine TCR peripheral blood lymphocyte. Cytotoxic drugs. IL-2 cytokine |
7 November 2015 | Recruiting | Metastatic cancer expressing NY-ESO-1 antigen | II | Single arm with infusion of lymphodepleting cytotoxic drugs followed by anti-NY-ESO-1 mTCR PBL and high-dose aldesleukin | NCT01967823 |
| Anti-MAGE-A3-DP4 TCR PBL, Cyclophosphamide, Fludarabine, Aldesleukin | Anti-melanoma antigen-3 reactive T-cell therapy. Cytotoxic drugs. IL-2 cytokine |
22 September 2015 | Recruiting | Metastatic cancer expressing the MAGE-A3-DP4 antigen | I/II | Single arm with infusion of lymphodepleting cytotoxic drugs followed by anti-MAGE-A3-DP4 TCR PBL plus aldesleukin | NCT02111850 |
| Anti VEGFR2 TCR, Cyclophosphamide, Fludarabine, Aldesleukin |
Anti-VEGFR2 reactive T-cell therapy. Cytotoxic drugs. IL-2 cytokine |
30 September 2015 | Ongoing but not recruiting | Metastatic cancer with evaluable disease | I/II | Single arm with infusion of lymphodepleting cytotoxic drugs followed by anti-VEGFR2 TCR plus aldesleukin | NCT01218867 |
| Anti-CEA-CAR T | Carcinogenic embryonic antigen CAR T cell therapy | 21 June 2015 | Recruiting | CEA positive cancer | I | Efficacy study | NCT02349724 |
| Anti-CD133-CAR vector-transduced T cells | CAR T cell therapy | 2 September 2015 | Recruiting | Relapsed or chemotherapy refractory malignancies | I | Dose-escalation study | NCT02541370 |
| CART-meso-19 T cells, Cyclophosphamide | CAR T cell therapy, Cytotoxic drugs. |
20 July 2015 | Recruiting | Metastatic pancreatic cancer. | I | Cyclophosphamide followed by single dose of combined CART-meso and CART-19 cells. | NCT02465983 |
| Young TIL Aldesleukin Cyclophosphamide Fludarabine |
Autologous transfer of tumor infiltrating lymphocytes, Cytotoxic drugs |
26 November 2015 | Recruiting | Metastatic cancer including pancreatic cancer | II | Lymphodepleting drugs followed by i.v. young TILs PBL and aldesleukin | NCT01174121 |
Immune modulating agents
CD40 agonist antibodies
CD40 is a cell surface receptor that belongs to the family of tumor necrosis factor receptor and is expressed on various APCs including DCs, monocytes, macrophages, mast cells etc. and its ligand CD40L (CD154) is a type II transmembrane protein predominantly expressed on activated T cells. CD40–CD40L interactions provide proliferative signals to promote differentiation of CD4+ and CD8+ T cells and hence may play a role in upregulation of immunity [Grewal and Flavell, 1998; Van Kooten and Banchereau, 2000]. As described earlier, pancreatic cancer leads to immune suppression within the tumor environment by inhibiting costimulatory molecules and also via upregulation of inhibitory molecules on inactivated T cells. Activation of CD40 has shown to restrict tumor growth in vitro and in vivo in many preclinical studies [Funakoshi et al. 1994; Eliopoulos et al. 2000; Tong et al. 2001]. Clinical activity of CD40 molecule was studied by giving CP-870,893, a fully human and selective CD40 agonist mAb in 29 patients with advanced solid tumors. A partial response was observed in four patients with metastatic melanoma and dose-related upregulation of costimulatory molecules on B cells in peripheral blood was also noted [Vonderheide et al. 2007].
Pancreatic cancer tissues have a higher expression of CD40 as compared to adjacent normal tissues. In a study by He and colleagues [He et al. 2012] 69% patients had a positive expression of CD40 and it correlated with higher TNM staging and lymph node metastasis. Also there were increased serum sCD40L levels compared with the healthy subjects. Recombinant CD40L leading to activation of CD40 significantly inhibited the proliferation of pancreatic cancer cell lines and induction of apoptosis. In a phase I study patients with advanced solid tumors were infused weekly doses of CP-870,893 which were well tolerated but there was little clinical activity [Rüter et al. 2010]. A combination of CD40 agonist antibody was tested in combination with gemcitabine in genetically engineered mouse model of PDAC. There was a depletion of CD40+ macrophages in stroma which infiltrated the tumor and became tumoricidal suggesting the CD40+ T-cell-independent mechanism of tumor regression [Beatty et al. 2011]. This was subsequently tested in clinical study. Beatty and colleagues investigated maximum tolerated dose, safety and antitumor activity of CP-870,893 with gemcitabine combination in 22 patients with advanced PDAC. Using RECIST criteria, four patients had a partial response, 11 with stable disease with PET demonstrating more than 25% decrease in FDG uptake within primary pancreatic lesions. One patient with partial response also had a complete regression of 7.6 cm liver mass and 47% regression of a second liver metastasis. Increase in B-cell expansion of costimulatory molecules was seen along with markedly increased inflammatory cytokines. PFS of 5.6 months and OS of 7.4 months was promising. Improved OS correlated with a decrease in FDG uptake in hepatic lesions suggesting the early role of FDG-PET/CT imaging [Beatty et al. 2013]. Table 13 summarizes completed and ongoing studies with CD40 agonist antibodies.
Table 13.
Studies using CD40 agonist antibody.
| Intervention | Strategy | Patient population | Phase | Year | Results | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|
| CP-870,893 + Gemcitabine. | Monoclonal antibody and cytotoxic drugs | Pancreatic neoplasms | I | 2013 | Overall survival 8.4 months Progression-free survival 5.3 months | NCT00711191 |
| CP-870,893 + Gemcitabine | Monoclonal antibody and cytotoxic drugs | PDAC | I | 2014 | Pending | NCT01456585 |
| CP-870,893 | Monoclonal antibody | Advanced solid tumors | I | 2014 | Pending | NCT02225002 |
(C-C motif) receptor 2 inhibitor
CCR2 is a chemokine receptor which mediates chemotaxis of immune cells and is expressed on monocytes and macrophages. Chronic tumor infiltrating cells might play a role in tumor progression, metastasis and resistance to chemotherapy. Targeting tumor-associated macrophages (TAMs) by inhibiting CCR2 would decrease the immune cells in tumor environment and increases efficacy of chemotherapy [Mitchem et al. 2013]. PF-04136309 is a novel CCR2 inhibitor with preclinical antitumor activity in PDAC through depleting TAMs and inflammatory monocytes. A phase Ib study of PF-04136309 given with FOLFIRINOX in 41 patients was conducted. A total of 12 of 23 evaluable patients (52.2%) had partial response by RECIST and 11 (47.8%) had stable disease. Curative resection was achieved in four out of five patients with borderline resectable and two with locally advanced disease [Wang-Gillam et al. 2015]. The data thus far are promising and need to be further validated.
Conclusion
Immunotherapy is a promising emerging treatment in cancer care. Manipulation of the immune targets along with traditional chemotherapy displays a synergy in antitumor response suggesting a probable role for immunotherapy as an adjuvant or in combination with radiochemotherapy. Furthermore, combining different immunotherapeutic strategies has shown some favorable outcomes with significant clinical benefits. Additional larger clinical trials testing the complex of immune checkpoint inhibitors with use of one or more types of vaccines and newer personalized therapies are warranted. Pancreatic cancer is a systemic disease which remains highly lethal even when resected. Therefore, early administration of effective combination therapy including immunotherapy may prove beneficial and needs further evaluation.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Komal Thind, Department of Internal Medicine, Cleveland Clinic Akron General, Akron, OH, USA.
Leslie J. Padrnos, Division of Hematology/Oncology, Mayo Clinic Arizona, Scottsdale, AZ, USA
Ramesh K. Ramanathan, Division of Hematology/Oncology, Mayo Clinic Arizona, Scottsdale, AZ, USA
Mitesh J. Borad, Division of Hematology/Oncology, Mayo Clinic Arizona, 5777 E. Mayo Boulevard, Phoenix, AZ 85054, USA.
References
- Abate-Daga D., Hanada K., Davis J., Yang J., Rosenberg S., Morgan R. (2013) Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood 122: 1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Alfa G., Chapman P., Feilchenfeldt J., Brennan M., Capanu M., Gansukh B., et al. (2011) Targeting mutated K-Ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol 34: 321–325. [DOI] [PubMed] [Google Scholar]
- Abrams S., Khleif S., Bergmann-Leitner E., Kantor J., Chung Y., Hamilton J., et al. (1997) Generation of stable CD4+and CD8+T cell lines from patients immunized with Ras oncogene-derived peptides reflecting codon 12 mutations. Cell Immunol 182: 137–151. [DOI] [PubMed] [Google Scholar]
- Amedei A., Niccolai E., Benagiano M., Della Bella C., Cianchi F., Bechi P., et al. (2013) Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol Immunother 62: 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafna S., Kaur S., Momi N., Batra S. (2009) Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer 101: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty G., Chiorean E., Fishman M., Saboury B., Teitelbaum U., Sun W., et al. (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331: 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty G., Torigian D., Chiorean E., Saboury B., Brothers A., Alavi A., et al. (2013) A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res 19: 6286–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M., Grandgenett P., Bailey J., Singh P., Yi C., Yu F., et al. (2010) The reactive tumor microenvironment: MUC1 signaling directly reprograms transcription of CTGF. Oncogene 29: 5667–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt S., Gjertsen M., Trachsel S., Møller M., Eriksen J., Meo M., et al. (2006) Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer 95: 1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer D., Curry J., Roy L., Tinder T., Sahraei M., Schettini J., et al. (2011) Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res 71: 4432–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham C., Canadien V., Kaniuk N., Steinberg B., Higgins D., Brumell J. (2008) Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature 451: 350–354. [DOI] [PubMed] [Google Scholar]
- Blank C., Enk A. (2015) Therapeutic use of anti-CTLA-4 antibodies. Int Immunol 27: 3–10. [DOI] [PubMed] [Google Scholar]
- Brahmer J., Tykodi S., Chow L., Hwu W., Topalian S., Hwu P., et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett B., Smith S., Bouvier C., Michaeli D., Hochhauser D., Davidson B., et al. (2002) Phase II study of anti-gastrin-17 antibodies, raised to G17DT, in advanced pancreatic cancer. J Clin Oncol 20: 4225–4231. [DOI] [PubMed] [Google Scholar]
- Brunet J., Denizot F., Luciani M., Roux-Dosseto M., Suzan M., Mattei M., et al. (1987) A new member of the immunoglobulin superfamily—CTLA-4. Nature 328: 267–270. [DOI] [PubMed] [Google Scholar]
- Buanes T., Maurel J., Liauw W., Hebbar M., Nemunaitis J. (2009) A randomized phase III study of gemcitabine (G) versus GV1001 in sequential combination with G in patients with unresectable and metastatic pancreatic cancer (PC). ASCO Meeting Abstracts 27: 4601. [Google Scholar]
- Burdick M., Harris A., Reid C., Iwamura T., Hollingsworth M. (1997) Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J Biol Chem 272: 24198–24202. [DOI] [PubMed] [Google Scholar]
- Calabrò L., Morra A., Fonsatti E., Cutaia O., Amato G., Giannarelli D., et al. (2013) Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase II trial. Lancet Oncol 14: 1104–1111. [DOI] [PubMed] [Google Scholar]
- Carbone D., Ciernik I., Kelley M., Smith M., Nadaf S., Kavanaugh D., et al. (2005) Immunization with mutant p53- and K-Ras–derived peptides in cancer patients: immune response and clinical outcome. J Clin Oncol 23: 5099–5107. [DOI] [PubMed] [Google Scholar]
- Carpenito C., Milone M., Hassan R., Simonet J., Lakhal M., Suhoski M., et al. (2009) Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A 106: 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda V., Vergati M., Huen N., Di Bari M., Jochems C., Intrivici C., et al. (2011) Maturation of human dendritic cells with Saccharomyces cerevisiae (yeast) reduces the number and function of regulatory T cells and enhances the ratio of antigen-specific effectors to regulatory T cells. Vaccine 29: 4992–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Flies D. (2013) Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13: 227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski M., Hahn O., Rappl G., Nowak M., Schmidt–Wolf I., Hombach A., et al. (2012) T cells that target carcinoembryonic antigen eradicate orthotopic pancreatic carcinomas without inducing autoimmune colitis in mice. Gastroenterology 143: 1095–1107. e1092. doi: 10.1053/j.gastro.2012.06.037. [DOI] [PubMed] [Google Scholar]
- Clark C., Hingorani S., Mick R., Combs C., Tuveson D., Vonderheide R. (2007) Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 67: 9518–9527. [DOI] [PubMed] [Google Scholar]
- Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., et al. (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- Counter C., Meyerson M., Eaton E., Ellisen L., Caddle S., Haber D., et al. (1998) Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene 16: 1217–1222. [DOI] [PubMed] [Google Scholar]
- Davis M., Conlon K., Bohac G., Barcenas J., Leslie W., Watkins L., et al. (2012) Effect of pemetrexed on innate immune killer cells and adaptive immune T cells in subjects with adenocarcinoma of the pancreas. J Immunother 35: 629–640. [DOI] [PubMed] [Google Scholar]
- De Remigis A., De Gruijl T., Uram J., Tzou S., Iwama S., Talor M., et al. (2015) Development of thyroglobulin antibodies after GVAX immunotherapy is associated with prolonged survival. Int J Cancer 136: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Qian D., Wang Y., Meng L., Chen D., Ji X., et al. (2015) Survivin expression and serum levels in pancreatic cancer. World J Surg Oncol 13: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Strome S., Salomao D., Tamura H., Hirano F., Flies D., et al. (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8: 793–800. [DOI] [PubMed] [Google Scholar]
- Eliopoulos A., Davies C., Knox P., Gallagher N., Afford S., Adams D., et al. (2000) CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol Cell Biol 20: 5503–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- Francisco L., Sage P., Sharpe A. (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236: 219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi S., Longo D., Beckwith M., Conley D., Tsarfaty G., Tsarfaty I., et al. (1994) Inhibition of human B-cell lymphoma growth by CD40 stimulation. Blood 83: 2787–2794. [PubMed] [Google Scholar]
- Galili U., Macher B., Buehler J., Shohet S. (1985) Human natural anti-alpha-galactosyl IgG. II. The specific recognition of alpha (1—3)-linked galactose residues. J Exp Med 162: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Mandrell R., Hamadeh R., Shohet S., Griffiss J. (1988) Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun 56: 1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudernack G. (2006) Prospects for vaccine therapy for pancreatic cancer. Best Pract Res Clin Gastroenterol 20: 299–314. [DOI] [PubMed] [Google Scholar]
- Geng L., Huang D., Liu J., Qian Y., Deng J., Li D., et al. (2008) B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol 134: 1021–1027. [DOI] [PubMed] [Google Scholar]
- Gilliam A., Broome P., Topuzov E., Avgust G., Pulay I., Humphreys J., et al. (2012) An international multicenter randomized controlled trial of G17DT in patients with pancreatic cancer. Pancreas 41: 374–379. [DOI] [PubMed] [Google Scholar]
- Gilliam A., Topuzov E., Garin A., Pulay I., Broome P., Watson S., et al. (2004) Randomised, double blind, placebo-controlled, multi-centre, group-sequential trial of G17DT for patients with advanced pancreatic cancer unsuitable or unwilling to take chemotherapy. ASCO Meeting Abstracts 22: 2511. [Google Scholar]
- Gjertsen M., Bakka A., Breivik J., Saeterdal I., Solheim B., Søreide O., et al. (1995) Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet 346: 1399–1400. [DOI] [PubMed] [Google Scholar]
- Gjertsen M., Bjørheim J., Saeterdal I., Myklebust J., Gaudernack G. (1997) Cytotoxic CD4+ and CD8+ T lymphocytes, generated by mutant p21-ras (12Val) peptide vaccination of a patient, recognize 12Val-dependent nested epitopes present within the vaccine peptide and kill autologous tumour cells carrying this mutation. Int J Cancer 72: 784–790. [DOI] [PubMed] [Google Scholar]
- Gjertsen M., Buanes T., Rosseland A., Bakka A., Gladhaug I., Søreide O., et al. (2001) Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer 92: 441–450. [DOI] [PubMed] [Google Scholar]
- Goldstein D., El-Maraghi R., Hammel P., Heinemann V., Kunzmann V., Sastre J., et al. (2015) nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst 107: dju413. doi: 10.1093/jnci/dju413. [DOI] [PubMed] [Google Scholar]
- Gorelik E., Duty L., Anaraki F., Galili U. (1995) Alterations of cell surface carbohydrates and inhibition of metastatic property of murine melanomas by α1,3 galactosyltransferase gene transfection. Cancer Res 55: 4168–4173. [PubMed] [Google Scholar]
- Goydos J., Elder E., Whiteside T., Finn O., Lotze M. (1996) A phase I trial of a synthetic mucin peptide vaccine: induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res 63: 298–304. [DOI] [PubMed] [Google Scholar]
- Grewal I., Flavell R. (1998) CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 16: 111–135. [DOI] [PubMed] [Google Scholar]
- Gross G., Waks T., Eshhar Z. (1989) Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 86: 10024–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardacre J., Mulcahy M., Small W., Talamonti M., Obel J., Rocha Lima C., et al. (2012) Addition of algenpantucel-L immunotherapy to standard of care (SOC) adjuvant therapy for pancreatic cancer. ASCO Meeting Abstracts 30: 4049. [DOI] [PubMed] [Google Scholar]
- Hassan R., Cohen S., Phillips M., Pastan I., Sharon E., Kelly R., et al. (2010) Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAB-009 in patients with mesothelin expressing cancers. Clin Cancer Res 16: 6132–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan R., Ebel W., Routhier E., Patel R., Kline J., Zhang J., et al. (2007) Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun 7: 20. [PMC free article] [PubMed] [Google Scholar]
- He S., Zhao H., Fei M., Wu Y., Wang L., Zhu X., et al. (2012) Expression of the co-signaling molecules CD40-CD40l and their growth inhibitory effect on pancreatic cancer in vitro. Oncol Rep 28: 262–268. [DOI] [PubMed] [Google Scholar]
- Herbst R., Soria J., Kowanetz M., Fine G., Hamid O., Gordon M., et al. (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F., O’day S., Mcdermott D., Weber R., Sosman J., Haanen J., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N., Noone A., Krapcho M., Garshell J., Miller D., Altekruse S., et al. (2015) Seer Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute. [Google Scholar]
- Hruban R., Van Mansfeld A., Offerhaus G., Van Weering D., Allison D., Goodman S., et al. (1993) K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol 143: 545–554. [PMC free article] [PubMed] [Google Scholar]
- Huang A., Golumbek P., Ahmadzadeh M., Jaffee E., Pardoll D., Levitsky H. (1994) Role of bone marrow-derived cells in presenting MHD class I-restricted tumor antigens. Science 264: 961–965. [DOI] [PubMed] [Google Scholar]
- Ino Y., Yamazaki-Itoh R., Shimada K., Iwasaki M., Kosuge T., Kanai Y., et al. (2013) Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 108: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki H., Manuel E., Song G., Srivastava T., Sun S., Diamond D., et al. (2011) Modified vaccinia Ankara expressing survivin combined with gemcitabine generates specific antitumor effects in a murine pancreatic carcinoma model. Cancer Immunol Immunother 60: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 99: 12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee E., Hruban R., Biedrzycki B., Laheru D., Schepers K., Sauter P., et al. (2001) Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 19: 145–156. [DOI] [PubMed] [Google Scholar]
- Kami K., Doi R., Koizumi M., Toyoda E., Mori T., Ito D., et al. (2004) Survivin expression is a prognostic marker in pancreatic cancer patients. Surgery 136: 443–448. [DOI] [PubMed] [Google Scholar]
- Kershaw M., Darcy P., Trapani J., Smyth M. (1996) The use of chimeric human Fc(epsilon) receptor I to redirect cytotoxic T lymphocytes to tumors. J Leukoc Biol 60: 721–728. [DOI] [PubMed] [Google Scholar]
- Khan M., Halfdanarson T., Borad M. (2014) Immunotherapeutic and oncolytic viral therapeutic strategies in pancreatic cancer. Future Oncol 10: 1255–1275. [DOI] [PubMed] [Google Scholar]
- Kirkwood J., Lorigan P., Hersey P., Hauschild A., Robert C., Mcdermott D., et al. (2010) Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res 16: 1042–1048. [DOI] [PubMed] [Google Scholar]
- Kobold S., Grassmann S., Chaloupka M., Lampert C., Wenk S., Kraus F., et al. (2015) Impact of a new fusion receptor on PD-1–mediated immunosuppression in adoptive T cell therapy. J Natl Cancer Inst 107: djv146. doi: 10.1093/jnci/djv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koido S., Hara E., Homma S., Torii A., Toyama Y., Kawahara H., et al. (2005) Dendritic cells fused with allogeneic colorectal cancer cell line present multiple colorectal cancer-specific antigens and induce antitumor immunity against autologous tumor cells. Clin Cancer Res 11: 7891–7900. [DOI] [PubMed] [Google Scholar]
- Kondo H., Hazama S., Kawaoka T., Yoshino S., Yoshida S., Tokuno K., et al. (2008) Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res 28: 379–387. [PubMed] [Google Scholar]
- Koski G., Cohen P., Roses R., Xu S., Czerniecki B. (2008) Reengineering dendritic cell-based anti-cancer vaccines. Immunol Rev 222: 256–276. [DOI] [PubMed] [Google Scholar]
- Laheru D., Lutz E., Burke J., Biedrzycki B., Solt S., Onners B., et al. (2008) Allogeneic GM-CSF secreting tumor immunotherapy (Gvax®) alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility and immune activation. Clin Cancer Res 14: 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D., Brockstedt D., Nir-Paz R., Hampl J., Mathur S., Nemunaitis J., et al. (2012) A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase 1 studies of safety and immune induction. Clin Cancer Res 18: 858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D., Lutz E., Uram J., Sugar E., Onners B., Solt S., et al. (2013) Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 36: 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D., Wang-Gillam A., Picozzi V., Greten T., Crocenzi T., Springett G., et al. (2015) Safety and survival with GVAX pancreas prime and Listeria Monocytogenes–expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 33: 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D., Krummel M., Allison J. (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271: 1734–1736. [DOI] [PubMed] [Google Scholar]
- Lepisto A., Moser A., Zeh H., Lee K., Bartlett D., Mckolanis J., et al. (2008) A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther 6: 955–964. [PMC free article] [PubMed] [Google Scholar]
- Liyanage U., Moore T., Joo H., Tanaka Y., Herrmann V., Doherty G., et al. (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169: 2756–2761. [DOI] [PubMed] [Google Scholar]
- Lutz E., Yeo C., Lillemoe K., Biedrzycki B., Kobrin B., Herman J., et al. (2011) A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma: a phase II trial of safety, efficacy, and immune activation. Ann Surg 253: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney K., Freeman G., McDermott D. (2015) The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther 37: 764–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki R., Livingston P., Lewis J., Janetzki S., Klimstra D., Desantis D., et al. (2007) A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci 52: 1964–1972. [DOI] [PubMed] [Google Scholar]
- Maliar A., Servais C., Waks T., Chmielewski M., Lavy R., Altevogt P., et al. (2012) Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 143: 1375–1384. e1-5. doi: 10.1053/j.gastro.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Mandell R., Flick R., Staplin W., Kaniewski L., Carzoli A., Manuszak R., et al. (2009) The αGal HyperAcute® Technology: enhancing immunogenicity of antiviral vaccines by exploiting the natural αGal-mediated zoonotic blockade. Zoonoses Public Health 56: 391–406. [DOI] [PubMed] [Google Scholar]
- Mazzolini G., Alfaro C., Sangro B., Feijoó E., Ruiz J., Benito A., et al. (2005) Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol 23: 999–1010. [DOI] [PubMed] [Google Scholar]
- Middleton G., Valle J., Wadsley J., Propper D., Coxon F., Ross P., et al. (2013) A phase III randomized trial of chemoimmunotherapy comprising gemcitabine and capecitabine with or without telomerase vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer. ASCO Meeting Abstracts 31: LBA4004. [DOI] [PubMed] [Google Scholar]
- Mitchem J., Brennan D., Knolhoff B., Belt B., Zhu Y., Sanford D., et al. (2013) Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression and improves chemotherapeutic responses. Cancer Res 73: 1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M., Ohsawa R., Tsunoda T., Hirono S., Kawai M., Tani M., et al. (2010) Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci 101: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M., Nair S., Boczkowski D., Tyler D., Hurwitz H., Proia A., et al. (2002) The feasibility and safety of immunotherapy with dendritic cells loaded with CEA mRNA following neoadjuvant chemoradiotherapy and resection of pancreatic cancer. Int J Gastrointest Cancer 32: 1–6. [DOI] [PubMed] [Google Scholar]
- Nomi T., Sho M., Akahori T., Hamada K., Kubo A., Kanehiro H., et al. (2007) Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 13: 2151–2157. [DOI] [PubMed] [Google Scholar]
- Oettle H., Richards D., Ramanathan R., Van Laethem J., Peeters M., Fuchs M., et al. (2005) A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol 16: 1639–1645. [DOI] [PubMed] [Google Scholar]
- Ogata M., Naito Z., Tanaka S., Moriyama Y., Asano G. (2000) Overexpression and localization of heat shock proteins mRNA in pancreatic carcinoma. J Nippon Med Sch 67: 177–185. [DOI] [PubMed] [Google Scholar]
- Okudaira K., Hokari R., Tsuzuki Y., Okada Y., Komoto S., Watanabe C., et al. (2009) Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. International Journal of Oncology 35: 741–749. [DOI] [PubMed] [Google Scholar]
- Palucka K., Banchereau J. (2013) Dendritic cell-based cancer therapeutic vaccines. Immunity 39: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson Y., Guirnalda P., Wood L. (2010) Listeria and Salmonella bacterial vectors of tumor-associated antigens for cancer immunotherapy. Semin Immunol 22: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecher G., Häring A., Kaiser L., Thiel E. (2002) Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial. Cancer Immunol Immunother 51: 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedoeem A., Azoulay-Alfaguter I., Strazza M., Silverman G., Mor A. (2014) Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol 153: 145–152. [DOI] [PubMed] [Google Scholar]
- Peggs K., Quezada S., Chambers C., Korman A., Allison J. (2009) Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti–CTLA-4 antibodies. J Exp Med 206: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate J., Plate A., Shott S., Bograd S., Harris J. (2005) Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother 54: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C., Li Y., Song Y., Rizvi S., Raja C., Zhang D., et al. (2004) MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by (213)Bi-C595 radioimmunoconjugate. Br J Cancer 91: 2086–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R., Lee K., Mckolanis J., Hitbold E., Schraut W., Moser A., et al. (2005) Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother 54: 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque S., Ashraf N., Chavez J., Malafa M., Coppola D., Springett G., et al. (2013) Expression of programmed death ligand 1 (PD-L1) in malignant and nonmalignant pancreatic tissue. ASCO Meeting Abstracts 31: 215. [Google Scholar]
- Ribas A., Hanson D., Noe D., Millham R., Guyot D., Bernstein S., et al. (2007) Tremelimumab (CP-675,206), a cytotoxic T lymphocyte–associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist 12: 873–883. [DOI] [PubMed] [Google Scholar]
- Ribas A., Kefford R., Marshall M., Punt C., Haanen J., Marmol M., et al. (2013) Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 31: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Ribas A., Wolchok J., Hodi F., Hamid O., Kefford R., et al. (2014) Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase I trial. Lancet 384: 1109–1117. [DOI] [PubMed] [Google Scholar]
- Rosenwald A., Wright G., Leroy K., Yu X., Gaulard P., Gascoyne R., et al. (2003) Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 198: 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G., Mautino M., Unfer R., Seregina T., Vahanian N., Link C. (2005) Effective treatment of preexisting melanoma with whole cell vaccines expressing α(1,3)-galactosyl epitopes. Cancer Res 65: 10555–10561. [DOI] [PubMed] [Google Scholar]
- Royal R., Levy C., Turner K., Mathur A., Hughes M., Kammula U., et al. (2010) Phase II trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 33: 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. (2008) The reverse stop-signal model for CTLA4 function. Nat Rev Immunol 8: 153–160. [DOI] [PubMed] [Google Scholar]
- Rüter J., Antonia S., Burris H., Huhn R., Vonderheide R. (2010) Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther 10: 983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan B., O’Donovan N., Duffy M. (2009) Survivin: a new target for anti-cancer therapy. Cancer Treat Rev 35: 553–562. [DOI] [PubMed] [Google Scholar]
- Schmidt T., Ziske C., Märten A., Endres S., Tiemann K., Schmitz V., et al. (2003) Intratumoral immunization with tumor RNA-pulsed dendritic cells confers antitumor immunity in a C57BL/6 pancreatic murine tumor model. Cancer Res 63: 8962–8967. [PubMed] [Google Scholar]
- Schneider H., Downey J., Smith A., Zinselmeyer B., Rush C., Brewer J., et al. (2006) Reversal of the TCR stop signal by CTLA-4. Science 313: 1972–1975. [DOI] [PubMed] [Google Scholar]
- Shapiro J., Marshall J., Karasek P., Figer A., Oettle H., Couture F., et al. (2005) G17DT+gemcitabine [Gem] versus placebo+Gem in untreated subjects with locally advanced, recurrent, or metastatic adenocarcinoma of the pancreas: results of a randomized, double-blind, multinational, multicenter study. ASCO Meeting Abstracts 23: LBA4012. [Google Scholar]
- Singh R., Dominiecki M., Jaffee E., Paterson Y. (2005) Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol 175: 3663–3673. [DOI] [PubMed] [Google Scholar]
- Skrypek N., Duchêne B., Hebbar M., Leteurtre E., Van Seuningen I., Jonckheere N. (2013) The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene 32: 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Liu J., Lu Y., Jin H., Huang D. (2014) Overexpression of B7-H1 correlates with malignant cell proliferation in pancreatic cancer. Oncol Rep 31: 1191–1198. [DOI] [PubMed] [Google Scholar]
- Su Z., Dannull J., Yang B., Dahm P., Coleman D., Yancey D., et al. (2005) Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol 174: 3798–3807. [DOI] [PubMed] [Google Scholar]
- Suehara N., Mizumoto K., Muta T., Tominaga Y., Shimura H., Kitajima S., et al. (1997) Telomerase elevation in pancreatic ductal carcinoma compared to nonmalignant pathological states. Clin Cancer Res 3: 993–998. [PubMed] [Google Scholar]
- Tan M., Goedegebuure P., Belt B., Flaherty B., Sankpal N., Gillanders W., et al. (2009) Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol 182: 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A., Santarsiero L., Lutz E., Armstrong T., Chen Y., Huang L., et al. (2004) Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med 200: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A., Papayoti M., Netto G., Armstrong D., Ordonez G., Lawson J., et al. (2001) Growth-inhibitory effects of CD40 ligand (CD154) and its endogenous expression in human breast cancer. Clin Cancer Res 7: 691–703. [PubMed] [Google Scholar]
- Toubaji A., Achtar M., Provenzano M., Herrin V., Behrens R., Hamilton M., et al. (2008) Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers. Cancer Immunol Immunother 57: 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elsas A., Hurwitz A., Allison J. (1999) Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 190: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kooten C., Banchereau J. (2000) CD40-CD40 ligand. J Leukoc Biol 67: 2–17. [DOI] [PubMed] [Google Scholar]
- Vasef M., Ross J., Cohen M. (1999) Telomerase activity in human solid tumors. diagnostic utility and clinical applications. Am J Clin Pathol 112(Suppl. 1): S68–S75. [PubMed] [Google Scholar]
- Von Hoff D., Ervin T., Arena F., Chiorean E., Infante J., Moore M., et al. (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide R., Flaherty K., Khalil M., Stumacher M., Bajor D., Hutnick N., et al. (2007) Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol 25: 876–883. [DOI] [PubMed] [Google Scholar]
- Wang-Gillam A., Nywening T., Sanford D., Lockhart A., Suresh R., Tan B., et al. (2015) Phase IB study of FOLFIRINOX plus PF-04136309 in patients with borderline resectable and locally advanced pancreatic adenocarcinoma (PC). ASCO Meeting Abstracts 33: 338. [Google Scholar]
- Wedén S., Klemp M., Gladhaug I., Møller M., Eriksen J., Gaudernack G., et al. (2011) Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer 128: 1120–1128. [DOI] [PubMed] [Google Scholar]
- Weinstock M., Mcdermott D. (2015) Targeting PD-1/PD-L1 in the treatment of metastatic renal cell carcinoma. Ther Adv Urol 7: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie S., Burbridge S., Chiapero-Stanke L., Pereira A., Cleary S., Van Der Stegen S., et al. (2010) Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem 285: 25538–25544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobser M., Keikavoussi P., Kunzmann V., Weininger M., Andersen M., Becker J. (2006) Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother 55: 1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok J., Hoos A., O’day S., Weber J., Hamid O., Lebbé C., et al. (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15: 7412–7420. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Ueno T., Kawaoka T., Hazama S., Fukui M., Suehiro Y., et al. (2005) MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res 25: 3575–3579. [PubMed] [Google Scholar]
- Yanagimoto H., Takai S., Satoi S., Toyokawa H., Takahashi K., Terakawa N., et al. (2005) Impaired function of circulating dendritic cells in patients with pancreatic cancer. Clin Immunol 114: 52–60. [DOI] [PubMed] [Google Scholar]
- Zhao F., Obermann S., Von Wasielewski R., Haile L., Manns M., Korangy F., et al. (2009) Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology 128: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Moon E., Carpenito C., Paulos C., Liu X., Brennan A., et al. (2010) Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res 70: 9053–9061. [DOI] [PMC free article] [PubMed] [Google Scholar]