Abstract
Antispasmodic drugs are cheap, effective and generally safe. They may improve outcomes in colonoscopy, however their use has not been consistent or widespread. This manuscript reviews the three most commonly used antispasmodics in colonoscopy, namely, hyoscine butylbromide (and related ammonium compounds), glucagon and peppermint oil. The pharmacology, action and safety of the agents, as well as the evidence for them improving colonoscopic outcomes will be discussed. In addition to polyp detection, other colonoscopic outcome endpoints of interest include cecal and ileal intubation, and patient comfort. The drugs studied were all found to be effective gastrointestinal antispasmodics with good safety profiles. There is insufficient evidence to conclude whether antispasmodics improve cecal intubation rate, predominantly because the baseline rates are already high. Antispasmodics probably have efficacy in reducing cecal intubation time especially in those with marked colonic spasm. Antispasmodics do not offer significant benefit in polyp detection or improving patient comfort during colonoscopy. Future studies should focus on inexperienced colonoscopists as well as those with marked colonic spasm, in whom the greatest benefit seems to lie.
Keywords: adenoma detection, antispasmodic, buscopan, colonoscopy, glucagon, hyoscine, peppermint oil, polyp detection
Introduction
Colonoscopy is the gold standard for the diagnosis and treatment of colon disorders; it has numerous roles including, but not limited to, the diagnosis and therapy of early colonic neoplasia, diagnosis of inflammatory bowel disease and therapy for its complications, and for the evaluation of undifferentiated symptoms of potentially colonic origin. Over 3.3 million outpatient colonoscopies are performed annually in the United States [Rex et al. 2015]. Since the advent of colonoscopy, antispasmodics have been used adjunctively in the belief that the resultant reduction in colonic spasm would improve outcomes. Recently in the United Kingdom, routine antispasmodic use has been recommended during colonoscopy as part of a bowel cancer screening program in the belief that it improves adenoma detection [Rajasekhar et al. 2015]. Despite this, controversy has persisted as to their utility not only in aiding colonoscope insertion but also in improving outcomes such as polyp detection and patient comfort. This article aims to review the use of the three most commonly used antispasmodic drugs in colonoscopy; namely, hyoscine butylbromide (HBB) (Buscopan, Boehringer Ingelheim, Ingelheim, Germany) and related ammonium compounds, glucagon and peppermint oil. An overview of the pharmacology, action and safety of the agents themselves (Table 1) is followed by a review of the evidence for improving colonoscopic outcomes with their use.
Table 1.
Overview of antispasmodic drugs.
| Mechanism(s) of action | Suggested dosing in colonoscopy | Safety considerations | |
|---|---|---|---|
| HBB | Anticholinergic effect | 20 mg intravenous injection, as premedication | Avoid in severe cardiac disease |
| Glucagon | Production of cAMP; neuronal effect; catecholamine release from adrenal medulla | 1 mg intravenous injection, as premedication | Use with caution in people with diabetes; contraindicated in pheochromocytoma |
| Peppermint oil | Calcium channel antagonism | 20 ml 1.6% L-menthol solution via direct intraluminal application, with further doses as required | None |
cAMP, cyclic adenosine-3′,5′-monophosphate; HBB, hyoscine butylbromide.
Methods
A search on the PubMed database was conducted using the following keywords and phrases: hyoscine, hyoscyamine, buscopan, glucagon, peppermint, menthol, L-menthol, antispasmodic, colonoscopy and endoscopy. Furthermore, we reviewed the reference list of the primary and review articles to identify articles not retrieved by electronic searches.
Overview of antispasmodic agents
Hyoscine butylbromide (and related ammonium compounds)
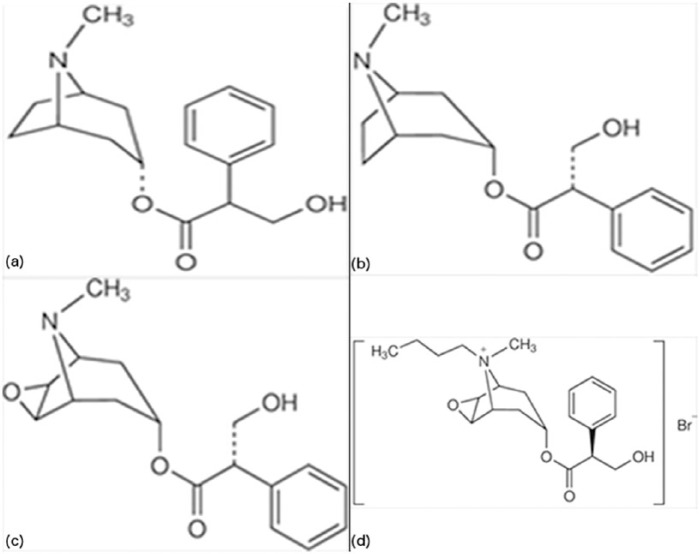
Hyoscine (also called scopolamine), HBB, and hyoscyamine are a group of closely related atropine-like compounds whose chemical structure and relationships are illustrated (Figure 1). All of these agents exert similar antimuscarinic anticholinergic effects.
Figure 1.
Comparison of the chemical structures of the closely related anticholinergic agents atropine (a), tertiary ammonium compounds hyoscyamine (b) and hyoscine (c), and quaternary ammonium compound hyoscine butylbromide (d).
Hyoscine is an alkaloid derived from the leaves of plants of the Datura and Duboisia genera [Tytgat, 2007]. HBB is manufactured from hyoscine by chemical addition of a butyl group, to obtain a quaternary ammonium structure [Evangelista, 2004]. This change in chemical structure causes it to be poorly absorbed from the gastrointestinal tract, and renders it unable to pass through the blood–brain barrier [Rang et al. 2015]. These changes are responsible for the significantly lower systemic side effects seen with HBB when compared with hyoscine [Evangelista, 2004]. Hyoscyamine is a related compound that is used infrequently, but is the focus of some articles included in this review. It is a direct precursor in the plant biosynthesis of hyoscine. Its actions are comparable to hyoscine, aside from having more potent central nervous system anticholinergic effects [Gyermek, 1997].
Pharmacokinetics and pharmacodynamics of HBB
After intravenous administration, HBB undergoes rapid distribution to abdominal and pelvic smooth muscle, and to a lesser extent elsewhere. It does not traverse the blood–brain barrier. The drug is metabolized primarily in the liver, through hydrolytic cleavage of the ester bond. The total clearance is 1.2 liter/min. Two-thirds of intravenously injected drug is renally excreted, and the rest is excreted in the feces [Gyermek, 1997; Tytgat, 2007, 2008].
As an atropine-like drug, HBB exerts gastrointestinal effects by blockade of the action of acetylcholine at muscarinic receptors in the gastrointestinal tract [Evangelista, 2004]. Parenterally administered HBB causes reduction in smooth muscle tone at multiple sites in the gastrointestinal tract, with lower doses required for muscarinic antagonism in the upper than lower gastrointestinal tract [Tytgat, 2007, 2008].
Side effects and contraindications
HBB has an excellent safety profile. Side effects are generally mild and relate to anticholinergic properties. The most common of these observed after intravenous administration are tachycardia, dry mouth and paralysis of visual accommodation [Herxheimer et al. 1966]. Less common anticholinergic side effects include nausea, urinary retention and dizziness. Rash and other immunological reactions, including anaphylaxis, have been rarely described [Treweeke et al. 1987; González-Mendiola et al. 2004]. In colonoscopy, the pooled incidence for any of these side effects is less than 1% [Mui et al. 2004; Corte et al. 2012; Rondonotti et al. 2013]. The side effects are typically short lived, reflecting the short half life and duration of action of HBB.
The presence of cardiac disease is a commonly cited contraindication to the use of HBB. The increase in heart rate induced by HBB [Gitanjali et al. 1998; Pao et al. 2014] theoretically has potential to exacerbate cardiac conditions such as atrial tachyarrhythmias, congestive cardiac failure, coronary artery disease and mitral stenosis. However, since the duration of action of HBB is short, this is unlikely to be of concern in the majority of these patients. We suggest HBB be avoided during colonoscopy of those patients with at least moderately severe forms of these conditions, given other antispasmodics are readily available. Other contraindications listed by the manufacturer and included in studies involving HBB [Corte et al. 2012] include known hypersensitivity to the drug, myasthenia gravis, glaucoma, megacolon, mechanical stenosis of the gastrointestinal tract and prostatic hypertrophy with urinary retention. In the absence of any of the specific conditions listed, HBB is not contraindicated in the elderly.
In colonoscopy, seven randomized controlled trials comparing HBB and placebo with a cumulative number of 1600 patients, primarily examining differences in polyp detection or cecal intubation rates, examined differences in side effects between the treatment arms [Saunders et al. 1996; Marshall et al. 1999; Mui et al. 2004; Chaptini et al. 2008; Byun et al. 2009; Corte et al. 2012; Rondonotti et al. 2013]. None found any difference in significant adverse events. A common finding in a number of the studies was tachycardia in those treated with HBB [Marshall et al. 1999; Mui et al. 2004; Byun et al. 2009; Corte et al. 2012; Rondonotti et al. 2013], although this was never clinically significant and never resulted in hemodynamic compromise.
Dosing and administration
We suggest using a single intravenous dose of 20 mg of HBB as a premedication, which has been used in the vast majority of trials in colonoscopy without significant adverse effects; higher doses have not provided any additional benefit [Mui et al. 2004].
Glucagon
Glucagon is a naturally occurring hormone produced by the α cells of the islets of Langerhans in the pancreas. Genetically modified Escherichia coli bacteria are utilized to produce the compound used clinically. Glucagon relaxes gastrointestinal smooth muscle independently of its metabolic effects of increasing serum glucose via hepatic glycogenolysis and gluconeogenesis [Damm Jorgensen et al. 1983].
Pharmacokinetics and pharmacodynamics
Glucagon must be administered in a parenteral fashion, usually via the intravenous route where it produces a prompt therapeutic effect within 5 min. The mean volume of distribution is small, at 0.25 liter/kg. Glucagon has a short serum half life of 8–18 min, and is rapidly metabolized in the liver, kidney and plasma into its constituent amino acids [Muhlhauser et al. 1985]. The short duration of action may be an advantage over other spasmolytic drugs in those at risk of side effects of anticholinergic agents.
Intravenous glucagon causes smooth muscle relaxation throughout the gastrointestinal tract by an incompletely elucidated mechanism. In vivo and in vitro studies suggest that it may be via activation of the production of cyclic adenosine-3′,5′-monophosphate, through a neuronal effect, by catecholamine release from the adrenal medulla, or a combination of these [Fasth et al. 1971; Gagnon et al. 1980].
Side effects and contraindications
Glucagon is a safe substance, with no severe adverse effects being given in the literature describing its use in endoscopy. Hyperglycemia and in response, delayed hypoglycemia occurring 2 h after administration, are physiological effects that are not of great significance in patients without diabetes [Hashimoto et al. 2002]. However, in people with diabetes, glucagon should hence be used with some caution, although is not contraindicated in these patients. Nausea and vomiting have been reported to occur in around 2% of patients and in a dose-dependent fashion, especially with doses above 1 mg [Cutler et al. 1995; Al-Haddad et al. 2006; Kedia, 2011]. Glucagon is contraindicated in the presence of pheochromocytoma as it has rarely been described to induce pheochromocytoma crisis [Hosseinnezhad et al. 2011].
Dosing and administration
The recommended dosing for glucagon in colonoscopy is 1 mg, via intravenous injection; this has been used in all trials in colonoscopy. We recommend administration as a premedication at least 1 min prior to procedure commencement, to allow sufficient time for onset of action [Tamai et al. 2013].
Peppermint oil
Peppermint oil is an extract derived from the leaves and flowering tops of the Mentha piperita (peppermint) plant [Amato et al. 2014]. The major constituent and active ingredient of peppermint oil is L-menthol [Amato et al. 2014]. Peppermint oil is a substance that has a long history of use for gastrointestinal spasmolysis, with the first documentation of its use for this indication in 1982 [Leicester et al. 1982]. Peppermint oil has also been extensively used for symptomatic relief in irritable bowel syndrome, with evidence for moderate efficacy in a selected subset of these patients [Khanna et al. 2014; Cash et al. 2016].
Pharmacokinetics and pharmacodynamics
When taken orally, menthol is rapidly absorbed in the proximal gut [Grigoleit et al. 2005]. Therapeutic efficacy is reliant on direct uptake by the gastrointestinal mucosa, therefore enteric-coated preparations are required to reach more distally in the gastrointestinal tract [Shavakhi et al. 2012]. For endoscopic applications, the limitation can also be circumvented by direct intraluminal application. Once absorbed, menthol is metabolized in the liver by the cytochrome P450 system, producing inactive metabolites (glucuronides) that are excreted in the bile and urine [Grigoleit et al. 2005]. The likely mechanism of action of menthol is calcium channel antagonism [Grigoleit et al. 2005]. It has been shown in animal and human studies to reduce contractility of gastrointestinal smooth muscle, in all parts of the gastrointestinal tract [Taylor et al. 1984; Hills et al. 1991; Amato et al. 2014].
Side effects and contraindications
Peppermint oil is a safe substance. No significant adverse events are reported in the literature describing its therapeutic use for any indication. Minor and self-limited gastrointestinal side effects such as bloating and abdominal cramps have infrequently been reported [Rogers et al. 1988]. Nonenteric-coated formulations (rarely used in endoscopy) can exacerbate reflux symptoms, due to relaxation of the lower esophageal sphincter [Grigoleit et al. 2005]. With direct intraluminal application bypassing the upper gastrointestinal tract, where most side effects occur, the incidence of even minor side effects is around 1% and no greater than placebo [Inoue et al. 2014; Cash et al. 2016]. There are no contraindications to its use [Rogers et al. 1988].
Dosing and administration
In colonoscopy, the most logical and preferred method of administration is direct intraluminal application. Inoue and colleagues describe the method for preparation of a 1.6% L-menthol solution, of which 20 ml is sprayed via the working channel of the colonoscope directly on to the cecum; further application of the same amount of solution can be performed during withdrawal, wherever significant peristalsis is encountered [Inoue et al. 2014]. Where intraluminal application is not feasible or desired, peppermint oil can be administered orally as a single capsule containing 0.2 ml of peppermint oil, 4 h prior to the procedure. A novel triple microsphere coated formulation of peppermint oil that is insoluble at gastric pH and released only from the small intestine onwards has recently been described; it has the advantage of ease of administration, while avoiding reflux and other upper gastrointestinal side effects associated with traditional oral formulations, but is not yet widely available [Cash et al. 2016].
Outcomes in colonoscopy
Cecal intubation rate
Cecal intubation rates are an indicator of technical expertise and are an important quality marker of colonoscopy [Rex et al. 2015]. Cecal intubation can be defined as passage of the tip of the colonoscope to a point proximal to the ileocecal valve so that the entire cecal pole, including the medial wall of the cecum, is visible [Rex et al. 2015]. Higher cecal intubation rates are associated with higher adenoma detection rates [Lee et al. 2012] and greater protection against right-sided colon cancer [Baxter et al. 2011].
Several studies have examined the hypothesis that antispasmodics ease colonoscope insertion through reduced colonic spasm, by evaluating cecal intubation rates. Five randomized controlled trials have published data regarding this hypothesis with the use of HBB, and none showed any improvement in cecal intubation rate [Saunders et al. 1996; Marshall et al. 1999; Mui et al. 2004; Yoong et al. 2004; Chaptini et al. 2008] (Table 2). However, none of the trials examined cecal intubation rate as the primary outcome, and they were not powered adequately to detect a difference between the two groups. In addition, the cecal intubation rates were 100% in two of the trials in which colonoscopy was performed by very experienced endoscopists [Saunders et al. 1996; Chaptini et al. 2008], making it impossible to detect a benefit from HBB.
Table 2.
Randomized, placebo-controlled trials examining the effect of HBB or hyoscyamine on cecal intubation rate.
| Intervention | n: treatment/placebo | Cecal intubation rate (%): treatment/placebo (p value) |
|---|---|---|
| 40 mg intravenous HBB [Mui et al. 2004] | 60/60 | 91.7/93.3 (0.5) |
| 0.5 mg intravenous hyoscyamine [Marshall et al. 1999] | 57/59 | 94.6/93.2 (0.86) |
| 0.25 mg sublingual hyoscyamine [Chaptini et al. 2008] | 50/50 | 100/100 |
| 20 mg intravenous HBB [Saunders et al. 1996] | 29/27 | 100/100 |
| 20 mg intravenous HBB [Yoong et al. 2004] | 61/56 | 97/88 (0.06) |
HBB, hyoscine butylbromide.
Three trials [Cutler et al. 1995; Yoshikawa et al. 2006; Tamai et al. 2013] examined the effect of glucagon on cecal intubation rate, and none showed any evidence of benefit. In the two studies [Cutler et al. 1995; Tamai et al. 2013] comparing glucagon with placebo, an extremely high cecal intubation rate in both arms (>99%) rendered it impossible to detect a significant difference with the numbers involved. The third [Yoshikawa et al. 2006] found no significant difference in cecal intubation rate between glucagon and HBB (96% versus 98% respectively). Cecal intubation rate was not a primary outcome for any of these studies. No studies have examined the efficacy of peppermint oil on improving cecal intubation rate.
Cecal intubation rate is an important quality measure, but it is difficult to assess outcomes using this metric, as baseline levels are extremely high, particularly in academic centers [Cutler et al. 1995; Saunders et al. 1996; Chaptini et al. 2008; Tamai et al. 2013]. At present there is a lack of quality evidence to make any conclusion about the effect of antispasmodics on improving cecal intubation rates. Further research should focus on inexperienced colonoscopists with lower cecal intubation rates, as well as in the subset of patients with significant colonic spasm. If a role is found for antispasmodics in aiding cecal intubation rates, it may be in trainees or inexperienced colonoscopists.
Ileal intubation
The role of antispasmodics in facilitating ileal intubation during colonoscopy has not been widely studied, and only one randomized controlled trial of antispasmodics examined ileal intubation as an outcome [Misra et al. 2007]. In this study, HBB administered once the ileocecal valve was visualized resulted in greater ease of ileal intubation (as measured by the endoscopist on a visual analog scale) and a greater length of ileum visualized compared with placebo. The ileal intubation rate itself was not an outcome assessed as it was presumed it would be high in both groups amongst experienced operators (94% in this study).
Cecal intubation time
Cecal intubation time is a surrogate marker of ease of colonoscope insertion. Reduction in insertion time allows more time to be spent on withdrawal, which has been shown to correlate with increased adenoma detection rates [Barclay et al. 2006; Lee et al. 2013]. Ease of colonoscope insertion and overall colonoscopy is felt to imply superior colonoscope control [Corte et al. 2010]. Therefore, reduction in cecal intubation time is a valuable outcome.
It was previously hypothesized that reduction in colonic spasm would result in greater ease of colonoscope insertion, by removing hindrance to scope advancement [Saunders et al. 1996]. However, for HBB, the evidence for this is equivocal. The majority of large randomized controlled trials have shown no beneficial effect for premedication with HBB or hyoscyamine in reducing cecal intubation time [Shaheen et al. 1999; Mui et al. 2004; Yoong et al. 2004; Chaptini et al. 2008]. A caveat to these findings is that in three of these studies [Shaheen et al. 1999; Mui et al. 2004; Yoong et al. 2004], there was no reduction in colonic spasm found in the treatment group. One of these three actually showed a significant increase in the cecal intubation time in those given HBB compared with placebo (12.20 versus 9.74 min respectively; p = 0.04) [Mui et al. 2004]. The fact that this was found despite the two groups having no difference in spasm score (as assessed by the endoscopist), on either insertion or withdrawal, suggests that the difference in cecal intubation time in the two groups was due to factors other than colonic spasm.
Of the randomized controlled trials demonstrating a benefit for intravenous HBB or hyoscyamine in reducing insertion time, the largest [Marshall et al. 1999] involved 116 patients and differed from other studies in that the vast majority of procedures were conducted by trainees. It is conceivable that it would be easier to detect a positive effect of an antispasmodic given less proficiency with colonoscopic technique amongst trainees. Two other studies involving a total of 189 patients showed a benefit for HBB in reducing cecal intubation time [Saunders et al. 1996; Kim et al. 2010]. Of note, all three of these studies showing a positive effect for HBB or hyoscyamine and also showed a significant reduction in colonic spasm in the treatment group. The overall conclusion therefore is that HBB seems to aid in reducing colonoscope insertion time when it leads to reduction in colonic spasm.
Four randomized placebo-controlled trials have assessed the efficacy of glucagon in reducing cecal intubation time [Norfleet, 1978; Jamal et al. 1992; Cutler et al. 1995; Tamai et al. 2013], and produced conflicting results. Of these, the most rigorous and well designed study [Tamai et al. 2013] found that cecal intubation time was statistically (and clinically) significantly quicker in those given intravenous glucagon 1 min before the procedure (294 versus 370 s respectively; p < 0.05). Jamal et al. [1992] found a similar significant benefit for glucagon in a trial published in abstract form only (9.73 versus 14.12 min; p < 0.05). The two trials that showed no benefit for glucagon in cecal intubation time have limitations. The first [Norfleet, 1978] is an old study in which cecal intubation rate was so lengthy (around 60 min), that it calls into question its relevance to modern colonoscopic practice. The other study [Cutler et al. 1995] in a cohort of 90 patients concluded that cecal intubation time was no different in patients given intravenous glucagon or placebo at the start of the procedure. A limitation of this study may be that glucagon was not given sufficient time to act, unlike in the abovementioned study [Tamai et al. 2013] where it was administered 1 min before the start of the procedure. This would also explain why Cutler and colleagues found no difference in spasm scores between the glucagon and placebo groups, despite the fact that glucagon is known to be an effective gastrointestinal antispasmodic [Hradsky et al. 1974; Gerner et al. 1983]. Glucagon may be effective in reducing cecal intubation time.
Only one trial has examined the effect of peppermint oil on cecal intubation time [Shavakhi et al. 2012]. This randomized controlled trial of 65 patients found that cecal intubation time was significantly shorter in patients orally administered enteric-coated peppermint oil 4 h pre procedure compared with placebo capsule. This also corresponded with a significant reduction in colonic spasm in the treatment group.
Colonic spasm is probably not a continuous variable, and no validated scoring system for colonic spasm exists. This may explain some of the variability of the results, but despite this, the weight of evidence suggests that antispasmodics might be useful in reducing cecal intubation time. As would be expected, the benefit of hastening insertion is greatest in those with pronounced spasm; therefore even if the endoscopist is not inclined to administer antispasmodics routinely, the data support the rational practice of administering antispasmodics to negotiate areas of persistent spasm and smooth muscle hypertrophy (most frequently in the sigmoid colon).
Patient comfort
Patient comfort and experience is one of the chief quality concerns in colonoscopy, and there is a vast literature on optimizing sedation for the purpose of enhanced patient comfort and ease of procedure [Sipe et al. 2002; Hansen et al. 2004; Vannatta et al. 2006; McQuaid et al. 2008]. Furthermore, patient discomfort correlates with reluctance to return for subsequent procedures [Marshall et al. 1999]. This potentially has relevance for dysplasia detection and treatment through missed opportunity for diagnosis, surveillance or endoscopic therapy. Antispasmodics are beneficial in the treatment of abdominal cramping pain related to gastrointestinal smooth muscle spasm [Tytgat, 2007; Tamai et al. 2013]. Therefore, it has been hypothesized that these drugs may reduce abdominal pain during colonoscopy, which may at least partly be due to the same mechanism.
There are eight randomized controlled trials [Saunders et al. 1995; Dumot et al. 1998; Marshall et al. 1999; Shaheen et al. 1999; Mui et al. 2004; Chaptini et al. 2008; Kim et al. 2010; Rondonotti et al. 2013] evaluating the effect of HBB compared with placebo on abdominal pain during colonoscopy (Table 3). There was no benefit demonstrated in all but one of these trials. It should be noted that these trials were all conducted in the era when air insufflation was used, and may not be applicable to current colonoscopy practice, where carbon dioxide insufflation is the standard of care. The majority used a visual analog pain score completed by the patient at the end of the procedure or at time of discharge. The level of sedation in these studies varied from conscious sedation with benzodiazepines plus opioids to no sedation at all.In one study colonoscopy was performed under patient-controlled sedation [Mui et al. 2004]. This study demonstrated that the amount of sedative drug administered by the patient was significantly higher in those who received HBB, even though there was no difference in pain scores. Only one study showed any benefit in patient comfort in those treated with hyoscyamine compared with placebo [Marshall et al. 1999]. A potential explanation for the aberrant results found in this study could be the greater central nervous system effects of hyoscyamine compared with HBB (causing more sedation or memory loss).
Table 3.
Randomized, placebo-controlled trials assessing the benefit of HBB or hyoscyamine for patient comfort in colonoscopy and flexible sigmoidoscopy.
| Intervention | n: treatment/placebo | Outcome assessed | Results: treatment/placebo (p value) |
|---|---|---|---|
| 0.25 intravenous hyoscyamine/0.25 mg oral hyoscyamine/placebo [Shaheen et al. 1999] | 54/56/55 | Patient’s discomfort on visual analog scale (0–10) | NR (NS) |
| 0.25 sublingual hyoscyamine [Chaptini et al. 2008] | 50/50 | Patient’s discomfort on visual analog scale (0–100) | 9.46/12.78 (0.304) |
| 0.5 mg intravenous hyoscyamine [Marshall et al. 1999] | 57/59 | Patient comfort on scale (1–4) | NR (0.0001) |
| 20 mg intravenous HBB [Rondonotti et al. 2013] | 202/200 | Patient’s perceived bloating on visual analog scale 0–100 | 12.9/13.9 (0.48) |
| Difference in measured abdominal circumference before and after colonoscopy (cm) | 2.5/2.2 (0.22) | ||
| 40 mg intravenous HBB [Mui et al. 2004] | 60/60 | Number of patient-controlled sedation doses administered | 7.25/5.87 (0.04) |
| Patient’s degree of pain on visual analog score (1–10) | 6.35/5.82 (0.35) | ||
| 20 mg intravenous HBB [Kim et al. 2010] | 70/63 | Patient’s rating of procedure as tolerable or pleasant (%) | 78.6/74.6 (0.891) |
| 0.25 mg sublingual hyoscyamine [Dumot et al. 1998] | 75/74 | Patient’s discomfort on visual analog scale (0–100) | 32.4/37.7 (0.18) |
| 10 mg intravenous HBB [Saunders et al. 1995] | 40/43 | Patient’s degree of pain on visual analog scale (0–100) | 34.5/39.9 (NS) |
HBB, hyoscine butylbromide; NR, no quantitative result provided; NS, not significant.
There has been only one well designed placebo controlled trial evaluating whether glucagon improved patient comfort during colonoscopy. Tamai and colleagues found that glucagon significantly improved patient comfort, as measured by both visual analog score as well as intraprocedural salivary amylase activity [Tamai et al. 2013], which has been shown to be an objective marker of stress levels during upper endoscopy [Uesato et al. 2010]. Norfleet conducted a placebo-controlled trial evaluating the same subject and found no benefit for glucagon in patient comfort [Norfleet, 1978]. Apart from the significant limitations of this study already discussed earlier, the ability to draw conclusions from this study is further hindered by its use of an imprecise outcome measure for assessing patient discomfort. Another study compared patient comfort in two groups given glucagon and HBB respectively; it found no significant difference between the groups [Yoshikawa et al. 2006]. There is insufficient evidence to suggest HBB or glucagon routinely be used to improve patient comfort in colonoscopy.
Only one trial [Shavakhi et al. 2012] has analyzed the effect of peppermint oil on patient comfort in colonoscopy. This randomized controlled trial of 65 patients found orally administered enteric-coated peppermint oil to reduce subjective pain score and patients were more willing to have a repeat colonoscopy. These findings must be interpreted with caution, given the small sample size.
Antispasmodics have not consistently been shown to offer significant benefit in reducing patient pain during colonoscopy. This is most likely because colonic spasm is only one factor in the etiology of pain during and following colonoscopy. Pain is more likely to be induced during mesenteric stretch that cannot be abated by simple antispasmodics. In the current era of carbon dioxide insufflation colonoscopy, the utility of antispasmodic medication in reducing postprocedure pain may be further reduced.
Polyp detection
Adenoma detection rate, the percentage of screening colonoscopies where an adenoma is identified, is the most investigated, reproducible and frequently utilized marker of colonoscopic quality. The adenoma detection rate has been shown to be inversely associated with the risk of developing interval colorectal cancer [Corley et al. 2014]. Polyp detection rate is an imperfect surrogate of the adenoma detection rate [Leung, 2013]. Missed polyps are estimated to account for half of all interval cancers. A few reviews have proposed numerous mechanisms for missed polyps, one of which is decreased mucosal view [Robertson et al. 2014; Rex, 2000; Chen et al. 2007]. These and other reviews have proposed antispasmodics to improve mucosal view by reducing colonic spasm, and in support of this hypothesis, a modest but significant increase in percentage surface visualization of the colon following HBB administration has been demonstrated in a study involving computed tomography colonography [East et al. 2015].
The efficacy of HBB in improving polyp detection was examined in eight randomized controlled trials [Saunders et al. 1996; Mui et al. 2004; Byun et al. 2009; Kim et al. 2010; Lee et al. 2010; Corte et al. 2012; De Brouwer et al. 2012; Rondonotti et al. 2013], four meta-analyses [Ashraf et al. 2014; Cui et al. 2014; Rondonotti et al. 2014; Madhoun et al. 2015], and a large cohort study [Lee et al. 2014]. Of the randomized controlled trials, six were large studies with polyp detection as the primary outcome (Table 4) [Byun et al. 2009; Kim et al. 2010; Lee et al. 2010; Corte et al. 2012; De Brouwer et al. 2012; Rondonotti et al. 2013]. None showed any overall benefit for polyp or adenoma detection rate. One showed a significant increase in the mean polyps per patient detected in those treated with HBB [Corte et al. 2012]. Another study suggested that in a subgroup of patients with significant colonic spasm (as assessed by the endoscopist), there was an improvement in polyp detection rate in those given HBB [Lee et al. 2010].
Table 4.
Randomized, placebo-controlled trials evaluating the benefit of intravenous HBB for polyp detection in colonoscopy.
| Intervention | n: HBB/placebo | PDR: HBB/placebo (p value) | ADR: HBB/placebo (p value) | PPP: HBB/placebo (p value) | APP: HBB/placebo (p value) |
|---|---|---|---|---|---|
| 20 mg intravenous HBB; CI [Byun et al. 2009] | 103/102 | 45.6%/39.2% (0.35) | 35%/29.4% (0.40) | NR | NR |
| 20 mg intravenous HBB; CI [Lee et al. 2010] | 58/58 | 34.4%/25.9% (NS) | NR | 0.9/0.6 (NS) | NR |
| 20 mg intravenous HBB; CI [Corte et al. 2012] | 303/298 | 43.6/36.6% (0.08) | 27.1%/21.8% (0.13) | 0.91/0.70 (0.04) | 0.55/0.42 (0.06) |
| 20 mg intravenous HBB; CI [De Brouwer et al. 2012] | 340/334 | 55.9%/60.2% (0.26) | 29.7%/31.4% (0.63) | 1.13/1.21 (0.56) | NR |
| 20 mg intravenous HBB; CI [Rondonotti et al. 2013] | 202/200 | 38.6%/37.0% (0.81) | 31.7%/28% (0.48) | 0.69/0.67 (0.66) | 0.52/0.48 (0.66) |
| 20 mg intramuscular HBB; PM [Kim et al. 2010] | 70/63 | 15.7%/28.6% (0.07) | 10%/15.9% (0.31) | NR | NR |
ADR, adenoma detection rate; APP, adenomas per patient; CI, at cecal intubation; HBB, hyoscine butylbromide; NR, not reported; NS, not significant; PDR, polyp detection rate; PM, premedication; PPP, polyps per patient.
None of the four meta-analyses demonstrated any benefit in polyp detection in those treated with HBB. A cohort study involving over 30,000 screening colonoscopies performed as part of a national bowel cancer screening program demonstrated that those administered HBB compared with no antispasmodic had a significantly increased adenoma detection rate (50.1% versus 44.5%; p < 0.001) [Lee et al. 2014]. However, being an observational study in which the use of HBB was left to the discretion of the endoscopist, significant potential clearly exists for confounding factors to explain the findings.
Based on these data, the routine use of HBB for all patients to facilitate increased polyp detection cannot be recommended; however further studies focusing on the subset of patients with marked spasm, such as those with sigmoid diverticulosis, are needed, as it may prove to be beneficial in this subgroup.
There are scarce reliable data assessing whether administration of glucagon improves polyp detection in colonoscopy. Only two placebo-controlled studies of glucagon have reported on polyp detection rates [Cutler et al. 1995; Tamai et al. 2013] (none described adenoma detection rates); neither showed any benefit for glucagon. These results are limited by polyp detection not being a primary outcome for either study and they were likely underpowered to detect a benefit for glucagon. Sufficient quality evidence is unavailable to make a conclusion as to whether glucagon improves polyp detection in colonoscopy.
Similarly, evidence for peppermint oil in this area is scarce. One randomized controlled trial involving 226 patients has examined the effectiveness of peppermint oil on the adenoma detection rate in colonoscopy [Inoue et al. 2014]. It found that the adenoma detection rate was significantly higher in those administered intraluminal menthol compared with placebo (60.2% versus 42.6%; p = 0.0083). Lack of blinding (the endoscopist was not blinded as to the intervention administered) may have introduced bias in this case. Again, more large high-quality randomized studies are required before its routine use can be recommended.
Conclusion
Antispasmodic agents have a long history of use in colonoscopy for a variety of indications. The pharmacology of these agents is predictable and there have not been any significant safety signals to date. Evidence for their routine use is lacking for polyp detection, but there is reasonable evidence to suggest that antispasmodics aid in shortening cecal intubation time. Overall, the literature also suggests a benefit for a subgroup of patients with pronounced colonic spasm, and use in such patients is reasonable. Future studies should focus on this group of patients, as well as on inexperienced colonoscopists, for whom more benefit can be expected. A robust and validated scoring system for colon spasm is needed before further trials to examine the utility of antispasmodics are conducted.
Acknowledgments
None of the authors have any sources of financial or material support to disclose.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Santosh Sanagapalli, Gastroenterology & Liver Services, Concord Repatriation General Hospital, Hospital Rd, Concord, NSW 2139, Australia.
Kriti Agnihotri, Gastroenterology & Liver Services, Concord Repatriation General Hospital, Sydney, NSW, Australia.
Rupert Leong, Gastroenterology & Liver Services, Concord Repatriation General Hospital, Sydney, NSW, Australia.
Crispin John Corte, Gastroenterology & Liver Services, Concord Repatriation General Hospital, Sydney, NSW, Australia.
References
- Al-Haddad M., Ward E., Scolapio J., Ferguson D., Raimondo M. (2006) Glucagon for the relief of esophageal food impaction does it really work? Dig Dis Sci 51: 1930–1933. [DOI] [PubMed] [Google Scholar]
- Amato A., Liotta R., Mule F. (2014) Effects of menthol on circular smooth muscle of human colon: analysis of the mechanism of action. Eur J Pharmacol 740: 295–301. [DOI] [PubMed] [Google Scholar]
- Ashraf I., Ashraf S., Siddique S., Nguyen D., Choudhary A., Bechtold M. (2014) Hyoscine for polyp detection during colonoscopy: a meta-analysis and systematic review. World J Gastrointest Endosc 6: 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay R., Vicari J., Doughty A., Johanson J., Greenlaw R. (2006) Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 355: 2533–2541. [DOI] [PubMed] [Google Scholar]
- Baxter N., Sutradhar R., Forbes S., Paszat L., Saskin R., Rabeneck L. (2011) Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 140: 65–72. [DOI] [PubMed] [Google Scholar]
- Byun T., Han D., Ahn S., Cho H., Kim T., Eun C., et al. (2009) Role of intravenous hyoscine N-butyl-bromide at the time of colonic withdrawal for polyp detection rates: a randomized double-blinded placebo-controlled trial. Gastrointest Endosc 69: abstract AB229. [Google Scholar]
- Cash B., Epstein M., Shah S. (2016) A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci 61: 560–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaptini L., Janec E., Seltzer G., Peikin S., Elfant A. (2008) Sublingual hyoscyamine spray as premedication for colonoscopy: a randomized double-blinded placebo-controlled trial. Am J Sur 196: 51–55. [DOI] [PubMed] [Google Scholar]
- Chen S., Rex D. (2007) Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol 102: 856–861. [DOI] [PubMed] [Google Scholar]
- Corley D., Jensen C., Marks A., Zhao W., Lee J., Doubeni C., et al. (2014) Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 370: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corte C., Dahlenburg L., Selby W., Griffin S., Byrne C., Chua T., et al. (2012) Hyoscine butylbromide administered at the cecum increases polyp detection: a randomized double-blind placebo-controlled trial. Endoscopy 44: 917–922. [DOI] [PubMed] [Google Scholar]
- Corte C., Kim A., Kaffes A. (2010) M1417: objective measures of colonoscopic difficulty: correlation with polyp detection and operator perception. Gastrointest Endosc 71: abstract AB215–AB216. [Google Scholar]
- Cui P., Yao J., Han H., Zhao Y., Yang J. (2014) Does hyoscine butylbromide really improve polyp detection during colonoscopy? A meta-analysis of randomized controlled trials. World J Gastroenterology 20: 7034–7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler C., Rex D., Hawes R., Lehman G. (1995) Does routine intravenous glucagon administration facilitate colonoscopy? A randomized trial. Gastrointest Endosc 42: 346–350. [DOI] [PubMed] [Google Scholar]
- Damm Jørgensen K., Weis J., Diamant B. (1983) Dissociation of the spasmolytic and metabolic effects of glucagon. Eur J Pharmacol 90: 315–323. [DOI] [PubMed] [Google Scholar]
- De Brouwer E., Arbouw M., Van Der Zwet W., Van Herwaarden M., Ledeboer M., Jansman F., et al. (2012) Hyoscine N-butylbromide does not improve polyp detection during colonoscopy: a double-blind, randomized, placebo-controlled, clinical trial. Gastrointest Endosc 75: 835–840. [DOI] [PubMed] [Google Scholar]
- Dumot J., Verzola E., Nicol S., Easley K., Vargo J., Van Stolk R. (1998) Sublingual hyoscyamine for patient comfort during screening sigmoidoscopy: a randomized, double-blind, placebo-controlled clinical trial. Gastrointest Endosc 48: 283–286. [DOI] [PubMed] [Google Scholar]
- East J., Saunders B., Burling D., Tam E., Boone D., Halligan S., et al. (2015) Mechanisms of hyoscine butylbromide to improve adenoma detection: a case-control study of surface visualization at simulated colonoscope withdrawal. Endosc Int Open 3: E636–E641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista S. (2004) Quaternary ammonium derivatives as spasmolytics for irritable bowel syndrome. Curr Pharm Des 10: 3561–3568. [DOI] [PubMed] [Google Scholar]
- Fasth S., Hulten L. (1971) The effect of glucagon on intestinal motility and blood flow. Acta Physiol Scand 83: 169–173. [DOI] [PubMed] [Google Scholar]
- Gagnon G., Regoli D., Rioux F. (1980) Studies on the mechanism of action of glucagon in strips of rabbit renal artery. Br J Pharmacol 69: 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner T., Myren J., Larsen S. (1983) Premedication in upper gastrointestinal endoscopy. A comparison of glucagon and atropine given in combination with diazepam and pethidine. Scand J Gastroenterol 18: 925–928. [DOI] [PubMed] [Google Scholar]
- Gitanjali B., Rauniar G., Shashindran C. (1998) Effect of hyoscine butylbromide and atropine on heart rate during nocturnal sleep. Indian J Exp Biol 36: 1216–1220. [PubMed] [Google Scholar]
- González-Mendiola R., Sánchez Fernández C., Prieto Montaño P., Cuevas M., Ceña Delgado M., Sánchez Cano M. (2004) Acute urticaria induced by hyoscine butylbromide. Allergy 59: 787–788. [DOI] [PubMed] [Google Scholar]
- Grigoleit H., Grigoleit P. (2005) Gastrointestinal clinical pharmacology of peppermint oil. Phytomedicine 12: 607–611. [DOI] [PubMed] [Google Scholar]
- Gyermek L. (1997) The tropane alkaloids. In: Gyermek L. (ed), Pharmacology of Antimuscarinic Agents. Boca Raton, FL: CRC Press. [Google Scholar]
- Hansen J., Ulmer B., Rex D. (2004) Technical performance of colonoscopy in patients sedated with nurse-administered propofol. Am J Gastroenterol 99: 52–56. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Adachi K., Ishimura N., Hirakawa K., Katsube T., Kurotani A., et al. (2002) Safety and efficacy of glucagon as a premedication for upper gastrointestinal endoscopy: a comparative study with butyl scopolamine bromide. Aliment Pharmacol Ther 16: 111–118. [DOI] [PubMed] [Google Scholar]
- Herxheimer A., Lisette H. (1966) Human pharmacology of hyoscine butylbromide. The Lancet 288: 418–421. [Google Scholar]
- Hills J., Aaronson P. (1991) The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology 101: 55–65. [DOI] [PubMed] [Google Scholar]
- Hosseinnezhad A., Black R., Aeddula N., Adhikari D., Trivedi N. (2011) Glucagon-induced pheochromocytoma crisis. Endocr Pract 17: e51–e54. [DOI] [PubMed] [Google Scholar]
- Hradsky M., Stockbrugger R., Dotevall G., Ostberg H. (1974) The use of glucagon during upper gastrointestinal endoscopy. Gastrointest Endosc 20: 162. [DOI] [PubMed] [Google Scholar]
- Inoue K., Dohi O., Gen Y., Jo M., Mazaki T., Tokita K., et al. (2014) L-menthol improves adenoma detection rate during colonoscopy: a randomized trial. Endoscopy 46: 196–202. [DOI] [PubMed] [Google Scholar]
- Jamal M., Palmer R., Harrington D., Simon K., McCarthy D. (1992) Glucagon facilitates colonoscopy: a double-blind study. Am J Gastroenterol 87: abstract 1323. [Google Scholar]
- Kedia N. (2011) Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes 4: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R., Macdonald J., Levesque B. (2014) Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol 48: 505–512. [DOI] [PubMed] [Google Scholar]
- Kim E., Lee S., Kim D., Lee C., Lee T., Chung I., et al. (2010) A clinical usefulness of premedication with hyoscine N-butyl bromide in colonoscopy. Korean J Gastrointest Endosc 41: 10–15. [Google Scholar]
- Lee T., Blanks R., Rees C., Wright K., Nickerson C., Moss S., et al. (2013) Longer mean colonoscopy withdrawal time is associated with increased adenoma detection: evidence from the bowel cancer screening programme in England. Endoscopy 45: 20–26. [DOI] [PubMed] [Google Scholar]
- Lee J., Cheon J., Park J., Moon C., Kim E., Kim T., et al. (2010) Effects of hyosine N-butyl bromide on the detection of polyps during colonoscopy. Hepato-gastroenterology 57: 90–94. [PubMed] [Google Scholar]
- Lee T., Rees C., Blanks R., Moss S., Nickerson C., Wright K., et al. (2014) Colonoscopic factors associated with adenoma detection in a national colorectal cancer screening program. Endoscopy 46: 203–211. [DOI] [PubMed] [Google Scholar]
- Lee T., Rutter M., Blanks R., Moss S., Goddard A., Chilton A., et al. (2012) Colonoscopy quality measures: experience from the NHS bowel cancer screening programme. Gut 61: 1050–1057. [DOI] [PubMed] [Google Scholar]
- Leicester R., Hunt R. (1982) Peppermint oil to reduce colonic spasm during endoscopy. Lancet 2: 989. [DOI] [PubMed] [Google Scholar]
- Leung F. (2013) PDR or ADR as a quality indicator for colonoscopy. Am J Gastroenterol 108: 1000–1002. [DOI] [PubMed] [Google Scholar]
- Madhoun M., Ali T., Tierney W., Maple J. (2015) Effect of hyoscine N-butylbromide on adenoma detection rate: meta-analysis of randomized clinical trials. Dig Endosc 27: 354–360. [DOI] [PubMed] [Google Scholar]
- Marshall J., Patel M., Mahajan R., Early D., King P., Banerjee B. (1999) Benefit of intravenous antispasmodic (hyoscyamine sulfate) as premedication for colonoscopy. Gastrointest Endosc 49: 720–726. [DOI] [PubMed] [Google Scholar]
- McQuaid K., Laine L. (2008) A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc 67: 910–923. [DOI] [PubMed] [Google Scholar]
- Misra S., Dwivedi M. (2007) Role of intravenously administered hyoscine butyl bromide in retrograde terminal ileoscopy: a randomized, double-blinded, placebo-controlled trial. World J Gastroenterol 3: 1820–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlhauser I., Koch J., Berger M. (1985) Pharmacokinetics and bioavailability of injected glucagon: differences between intramuscular, subcutaneous, and intravenous administration. Diabetes Care 8: 39–42. [DOI] [PubMed] [Google Scholar]
- Mui L., Ng E., Chan K., Ng C., Yeung A., Chan S., et al. (2004) Randomized, double-blinded, placebo-controlled trial of intravenously administered hyoscine N-butyl bromide in patients undergoing colonoscopy with patient-controlled sedation. Gastrointest Endosc 59: 22–27. [DOI] [PubMed] [Google Scholar]
- Norfleet R. (1978) Premedication for colonoscopy: randomized, double-blind study of glucagon versus placebo. Gastrointest Endosc 24: 164–165. [DOI] [PubMed] [Google Scholar]
- Pao Y., Chung K., Chen J., Lee K., Hu W., Juang S., et al. (2014) The hemodynamic effect of an intravenous antispasmodic on propofol requirements during colonoscopy: a randomized clinical trial. Acta Anaesthesiol Taiwanica 52: 13–16. [DOI] [PubMed] [Google Scholar]
- Rajasekhar P., Rees C., Bramble M., Wilson D., Rutter M., Saunders B., et al. (2015) A multicenter pragmatic study of an evidence-based intervention to improve adenoma detection: the Quality Improvement in Colonoscopy (QIC) study. Endoscopy 47: 217–224. [DOI] [PubMed] [Google Scholar]
- Rang H., Ritter J., Flower R., Henderson G. (2015) Cholinergic transmission. In: Rang H., Ritter J., Flower R., Henderson G. (eds), Rang & Dale’s Pharmacology, 8th ed. Edinburgh: Elsevier Health Sciences. [Google Scholar]
- Rex D. (2000) Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc 51: 33–36. [DOI] [PubMed] [Google Scholar]
- Rex D., Schoenfeld P., Cohen J., Pike I., Adler D., Fennerty M., et al. (2015) Quality indicators for colonoscopy. Gastrointest Endosc 81: 31–53. [DOI] [PubMed] [Google Scholar]
- Robertson D., Lieberman D., Winawer S., Ahnen D., Baron J., Schatzkin A., et al. (2014) Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut 63: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Tay H., Misiewicz J., Bell G. (1988) Peppermint oil. The Lancet 332: 98–99. [Google Scholar]
- Rondonotti E., Radaelli F., Paggi S., Amato A., Imperiali G., Terruzzi V., et al. (2013) Hyoscine N-butylbromide for adenoma detection during colonoscopy: a randomized, double-blind, placebo-controlled study. Dig Liver Dis 45: 663–668. [DOI] [PubMed] [Google Scholar]
- Rondonotti E., Zolk O., Amato A., Paggi S., Baccarin A., Spinzi G., et al. (2014) The impact of hyoscine-n-butylbromide on adenoma detection during colonoscopy: meta-analysis of randomized, controlled studies. Gastrointest Endosc 80: 1103–1200. [DOI] [PubMed] [Google Scholar]
- Saunders B., Elsby B., Boswell A., Atkin W., Williams C. (1995) Intravenous antispasmodic and patient-controlled analgesia are of benefit for screening flexible sigmoidoscopy. Gastrointest Endosc 42: 123–127. [DOI] [PubMed] [Google Scholar]
- Saunders B., Williams C. (1996) Premedication with intravenous antispasmodic speeds colonoscope insertion. Gastrointest Endosc 43: 209–211. [DOI] [PubMed] [Google Scholar]
- Shaheen N., Robertson D., Crosby M., Furs S., May D., Harlan W., et al. (1999) Hyoscyamine as a pharmacological adjunct in colonoscopy: a randomized, double blinded, placebo-controlled trial. Am J Gastroenterol 94: 2905–2908. [DOI] [PubMed] [Google Scholar]
- Shavakhi A., Ardestani S., Taki M., Goli M., Keshteli A. (2012) Premedication with Peppermint oil capsules in colonoscopy: a double blind placebo-controlled randomized trial study. Acta Gastroenterol Belg 75: 349–353. [PubMed] [Google Scholar]
- Sipe B., Rex D., Latinovich D., Overley C., Kinser K., Bratcher L., et al. (2002) Propofol versus midazolam/meperidine for outpatient colonoscopy: administration by nurses supervised by endoscopists. Gastrointest Endosc 55: 815–825. [DOI] [PubMed] [Google Scholar]
- Tamai N., Matsuda K., Sumiyama K., Yoshida Y., Tajiri H. (2013) Glucagon facilitates colonoscopy and reduces patient discomfort: a randomized double-blind controlled trial with salivary amylase stress analysis. Eur J Gastroenterol Hepatol 25: 575–579. [DOI] [PubMed] [Google Scholar]
- Taylor B., Luscombe D., Duthie H. (1984) Inhibitory effect of peppermint and menthol on human isolated coli. Gut 25: 68–69. [Google Scholar]
- Treweeke P., Barrett N. (1987) Allergic reaction to buscopan. Br J Radiol 60: 417–418. [DOI] [PubMed] [Google Scholar]
- Tytgat G. (2007) Hyoscine butylbromide: a review of its use in the treatment of abdominal cramping and pain. Drugs 67: 1343–1357. [DOI] [PubMed] [Google Scholar]
- Tytgat G. (2008) Hyoscine butylbromide: a review on its parenteral use in acute abdominal spasm and as an aid in abdominal diagnostic and therapeutic procedures. Curr Med Res Opin 24: 3159–3173. [DOI] [PubMed] [Google Scholar]
- Uesato M., Nabeya Y., Akai T., Inoue M., Watanabe Y., Kawahira H., et al. (2010) Salivary amylase activity is useful for assessing perioperative stress in response to pain in patients undergoing endoscopic submucosal dissection of gastric tumors under deep sedation. Gastric Cancer 13: 84–89. [DOI] [PubMed] [Google Scholar]
- Vannatta M., Rex D. (2006) Propofol alone titrated to deep sedation versus propofol in combination with opioids and/or benzodiazepines and titrated to moderate sedation for colonoscopy. Am J Gastroenterol 101: 2209–2217. [DOI] [PubMed] [Google Scholar]
- Yoong K., Perkin D., Portal J., Strickland I., Heymann T. (2004) Intravenous hyoscine as a premedication for colonoscopy: a randomized double-blind controlled trial. Endoscopy 36: 720–722. [DOI] [PubMed] [Google Scholar]
- Yoshikawa I., Yamasaki M., Taguchi M., Kanda K., Tashiro M., Kume K., et al. (2006) Comparison of glucagon and scopolamine butylbromide as premedication for colonoscopy in unsedated patients. Dis Colon Rectum 49: 1393–1398. [DOI] [PubMed] [Google Scholar]