Abstract
BACKGROUND
Human chorionic gonadotropin (hCG) is used to monitor pregnancy status. Yet the pattern of hCG excretion in the first week following implantation has not been adequately described.
OBJECTIVE
To describe hCG's average profile and its variability during the 7 days following estimated implantation in a population of naturally-conceived pregnancies.
METHODS
We measured daily hCG concentrations in first-morning urine for 142 clinical pregnancies from women with no known fertility problems. Mixed-effects regression models were used to estimate the hCG trajectory and its variability in relation to pregnancy outcomes.
RESULTS
HCG rose three-fold between the day of detection and the next day (95% CI = 2.7–3.4). The relative rate of rise decreased thereafter, reaching 1.6-fold (95% CI = 1.5–1.8) between days 6 and 7. HCG levels followed a log-quadratic trajectory, and the patterns of rise were unrelated to number of fetuses, risk of miscarriage, or sex of the baby. Later implantations (after 10 luteal days) produced slower rates of increase.
CONCLUSIONS
While mean hCG follows a log-quadratic trajectory during the first week of detectability, there is high variability across pregnancies. Later implantation may reflect characteristics of the uterus or conceptus that slow hCG production.
Keywords: pregnancy, placenta, miscarriage, twins, fetal sex
INTRODUCTION
The growth and development of the human embryo are unobservable during the crucial early stages after implantation. The best information on early growth comes indirectly, through the measurement of hCG (human chorionic gonadotropin) produced by the trophoblastic cells of the conceptus. HCG is a robust hormone, measurable in maternal serum and urine. Absolute hCG values and rates of rise in secretion and excretion have been reported to be associated with the embryo's genetic quality, site of implantation (intrauterine or ectopic), and developmental pace (Barnhart et al. 2004; Check et al. 1992; Ho et al. 1997; Kadar et al. 1981; Marshall et al. 1968; Seeber et al. 2006; Urbancsek et al. 2002). Consequently, there is an interest in hCG as a biomarker of embryonic health and a tool to evaluate the effects of endogenous and exogenous challenges on very early gestation (Lohstroh et al. 2007). First, however, it is necessary to describe the normal longitudinal daily excretion patterns of hCG, and its natural variation among individuals.
HCG is an oligosaccharide glycoprotein hormone critical for pregnancy establishment and survival. It exerts a variety of intracrine, autocrine, paracrine, and endocrine actions that play vital roles in the communication between conceptus and mother during early gestation (Yang et al. 2003). HCG's decisive early roles include the “rescue” of the corpus luteum (Zeleznik and Pohl 2006) and formation of the placental syncytium (Yang et al. 2003). Maintenance of the corpus luteum and its progesterone production (Baird et al. 2003; Liu et al. 1995) are crucial in supporting pregnancy until the placenta can produce adequate levels of progesterone (Csapo and Pulkkinen 1978).
Though the mRNA for the hCG subunits has been detected as early as the 6-8-cell stage (Bonduelle et al. 1988), the intact protein first becomes detectable in the mother's blood and urine between 6 and 14 days after fertilization both in vitro and in vivo (Hay and Lopata 1988; Lohstroh et al. 2006; Wilcox et al. 1999). Most research on longitudinal urinary excretion patterns of hCG has been conducted later in pregnancy (6 or more weeks after the last menstrual period (LMP)) (Braunstein et al. 1976; Gerhard and Runnebaum 1984; Gol et al. 2005; Williams et al. 1995). Profiles for earlier gestational intervals have not been adequately characterized. This is particularly true for the first week post-implantation, which coincides with the onset of the placentation process (Malassine and Cronier 2002).
Many past studies of very early hCG excretion have been based on clinical populations and involve women undergoing assisted fertility treatments (AFT), which limits their generalizability. Reproductive profiles of clinical patients might be inherently different from those of women in the general population, and could be affected by the exogenous drugs (or related stress) that accompany treatment (Batzer et al. 1981; Cwikel et al. 2004; Lam et al. 1999). Additionally, many previous studies are based on cross-sectional designs, with each woman contributing only 1 to 3 data points (Lambers et al. 2006; Seeber et al. 2006). Such cross-sectional designs are inadequate for exploring the among-women variations in hCG excretion.
Our objective is to provide a description of daily urinary excretion of hCG during the first week after the estimated onset of implantation, using a sample of naturally conceived pregnancies in women with no known fertility problems. We have previously focused on pregnancies resulting in early miscarriage (before 6 weeks LMP)(Baird et al. 2003; Wilcox et al. 1999). Here we evaluate daily concentrations in first-morning urinary hCG from clinical pregnancies (i.e. those that survived at least 6 weeks LMP), and investigate the extent to which fetal number, fetal viability, or fetal sex might affect the pattern of hCG rise.
METHODS
Study population
Analyses were based on data collected in the context of the North Carolina Early Pregnancy Study (NCEPS), which had as its original objective to determine the incidence of early, sub-clinical, miscarriage (before 6 weeks LMP). The field portion of the study was carried out between 1982 and 1986. Two hundred and twenty-one women planning to conceive were enrolled at the onset of their attempt. Eligibility for participation required that women had no known chronic health or fertility problems, and were not under any hormone treatment. Participants' ages ranged from 21 to 42 years (mean=30); most were white and college-educated (Wilcox et al. 1988). All participants provided informed consent. This research was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences.
Study design
Participants kept daily dairies in which they recorded menstrual bleeding and symptoms of pregnancy. Women collected first morning urine samples daily in 30 ml wide-mouth polypropylene jars with screw tops. The collection of samples continued 8 weeks into the pregnancy or for up to 6 months if no clinical pregnancy was identified. Samples were stored in the participants' home freezers (without preservatives), with weekly pickup and transport to a central storage unit where they were kept at −20°C. Urine specimens were then packed in dry ice and shipped by overnight freight to Columbia University for hCG assay (Wilcox et al. 1988; Wilcox et al. 1985).
Hormonal assays and reproductive parameters
HCG assays were conducted from 1983 to 1987, within 6 to 24 months after the end of each woman's participation. HCG assays were carried out in triplicate, using an immunoradiometric assay (Sepharose-IRMA B101-R525) (Wilcox et al. 1988). Laboratory personnel were blind to the outcomes of the clinical pregnancy. The specific primary target of the IRMA was intact hCG, with cross-reactivity to βhCG (~70%) (McChesney et al. 2005). The detection limit was 0.01 ng/mL (Armstrong et al. 1984). Converting to bioassay units of purified reference preparations, a nanogram in the IRMA is approximately equivalent to 0.013 IU per ng. Within-assay variability in pooled samples was 21%; with this removed the variability among assays was 15% (Wilcox et al. 1988). All specimens for a given woman were measured in the same assay batch, except that additional dilutions were carried out if necessary for high concentrations of hCG. The stability of intact hCG was assessed by subjecting purified analytes in urine to 40 cycles of freezing and thawing and to storage at 4 °C for 4 weeks. No loss of immunodetection for intact hCG analytes was evident during the freeze–thaw process or at any time during that storage period (McChesney et al. 2005).
Specimens were also analyzed for steroid metabolites in duplicate, using radioimmunoassay to determine urinary concentrations of estrone-3-glucuronide (E1G) and pregnanediol-3-glucuronide (PdG) (Samarajeewa et al. 1979; Wright et al. 1978).
Concentrations of intact hCG are similar in urine and serum (McChesney et al. 2005; Norman et al. 1987; Wehmann and Nisula 1981). Urine collection is non invasive, which is an advantage for studies requiring frequent specimens collection and non-clinical settings. Creatinine concentrations were measured for a subset of urine samples. Variations in urine concentration of creatinine were trivial in relation to the steep daily rise of hCG (McChesney et al. 2005). Accordingly, no adjustments for creatinine were performed in the present analysis.
Definitions and reproductive end-points
Hormonal patterns were used to estimate ovulation and the onset of implantation. The day of ovulation was inferred from the rapid decline of the ratio of E1G to PdG (Baird et al. 1995; Baird et al. 1991), which marks the luteinization of the ovarian follicle. This estimator of ovulation has been validated by Ecochard and colleagues, who used ultrasound of the rupturing follicle to demonstrate that the urinary steroid hormone ratio is as good a marker of ovulation as the LH surge, and better than the LH peak (Ecochard et al. 2001).
Conception was inferred when the urinary concentration of hCG exceeded 0.025 ng/mL for 3 consecutive days (Wilcox et al. 1988). For each conception, the onset of implantation was approximated as the first day of sustained production of urinary hCG above 0.015 ng/mL (Wilcox et al. 1999). While hCG is reliably detected in maternal urine only six or more days after fertilization (Lohstroh et al. 2006; Wilcox et al. 1999), the conceptus probably begins secreting hCG earlier (Bonduelle et al. 1988; Hay and Lopata 1988). The specific phase of the implantation process in which hCG reaches maternal circulation and is excreted in urine is unknown (Chang et al. 1998).
Sample size
Of the 199 conceptions chemically detected in our study, 151 survived at least 6 weeks after LMP (“clinical pregnancy”). The initiation of implantation was identified for 142 of the 151 pregnancies. In one of the 142 pregnancies, a urine sample was missing near the onset of hCG rise. We inferred that this was the likely day of implantation, based on the fact that there was no detectable hCG on the previous day, and by the subsequent day hCG was already more than two-fold higher than any confirmed day of first detection in our sample. Critical hCG information was missing for the 9 remaining pregnancies, which are excluded from further analysis. Of the 142 pregnancies in which implantation could be identified, 121 ended in singleton births, 6 in twin births, 13 in miscarriages, 1 in an ectopic pregnancy and 1 in a molar pregnancy (all identified by participants' self-report).
Statistical Analyses
Hormonal values were log-transformed for analysis in order to normalize distributions and reduce the influence of outliers. Urinary hCG excretion trajectories were evaluated using mixed model analyses (Proc Mixed in SAS 9.1; SAS Institute, Cary, NC) with a random-effect term to account for correlations among observations within women. Comparisons of hCG trajectories among pregnancies according to their outcomes, sex of the fetus, and time to implantation were performed by including the characteristic in question as a predictor in the mixed model. The fit of competing models was assessed by comparing the models' restricted log-likelihoods using chi-squared tests.
RESULTS
hCG trajectory during the first week following detection
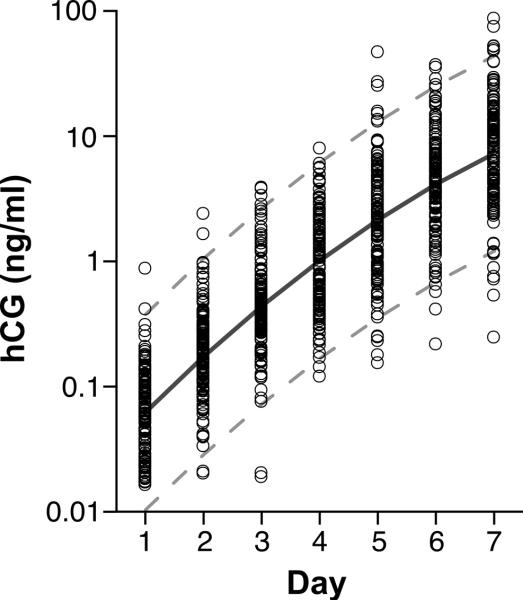
During the first week following detection, average maternal urinary concentration of hCG increased rapidly and continuously (Table I, Table II & Figure 1). The overall pattern of hCG excretion (Figure 1) was well described by the following regression equation:
in which time is day in relation to hCG detection (day of hCG detection = 0), β0 =−2.837 (SE=0.065), β1 =1.070 (SE=0.031), β2 =−0.046 (SE=0.005); p-values <0.0001 for all terms. The rate of increase in hCG excretion was steepest between the first day of hCG detection and the following day. The rate of increase progressively slowed in subsequent days (Table II).
Table I.
Daily geometric mean hCG concentrations in first morning urine during the first week following detection calculated for 142 clinical pregnancies.
| Days1 | n | Geometric mean (ng/ml) | 95% CI |
|---|---|---|---|
| 1 | 141 | 0.05 | 0.05–0.06 |
| 2 | 142 | 0.17 | 0.15–0.20 |
| 3 | 140 | 0.40 | 0.35–0.47 |
| 4 | 137 | 0.91 | 0.78–1.07 |
| 5 | 137 | 1.94 | 1.63–2.31 |
| 6 | 136 | 3.99 | 3.40–4.69 |
| 7 | 133 | 6.76 | 5.66–8.07 |
Day 1 = day of detection (hCG > 0.015 ng/mL).
Table II.
Daily rates of increase in urinary excretion of hCG during the first week following detection for 142 clinical pregnancies.
| Days1 | n | Rates of increase2 | 95% CI | 10 percentile | Median | 90 percentile |
|---|---|---|---|---|---|---|
| 1–2 | 141 | 3.0 | 2.7–3.4 | 1.3 | 2.9 | 7.1 |
| 2–3 | 140 | 2.3 | 2.0–2.5 | 1.1 | 2.2 | 5.3 |
| 3–4 | 137 | 2.3 | 2.1–2.5 | 1.0 | 2.0 | 6.2 |
| 4–5 | 137 | 2.1 | 1.8–2.4 | 0.9 | 2.0 | 5.9 |
| 5–6 | 136 | 2.0 | 1.7–2.3 | 0.9 | 2.0 | 5.3 |
| 6–7 | 133 | 1.6 | 1.4–1.8 | 0.8 | 1.7 | 3.6 |
Day 1 = day of detection (hCG > 0.015 ng/mL).
The rate of increase was calculated by exponentiating the means of daily differences [lnhCG day2- lnhCGday1]. A value of 3.0 reflects a 3-fold increase and a value of < 1 reflects a decline in hCG levels from one day to the next.
Figure 1.
Predicted hCG excretion pattern and observed values during the first week of detection for 142 clinical pregnancies. Circles represent individual data points, the central solid line represents the hCG trajectory predicted by the regression equation and the broken lines represent the 95% probability band for the model. Day 1 = day of detection (hCG > 0.015 ng/mL).
Variability among pregnancies
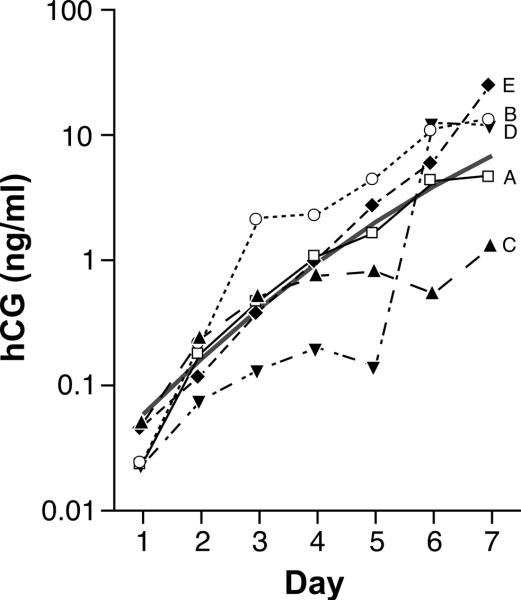
Individual hCG profiles varied markedly. We illustrate this variability in 5 selected individual profiles of surviving pregnancies (Figure 2). Some individual profiles follow a pattern similar to the mean model (e.g. Figure 2.A) while others diverge from it in various ways (2.B–D). There are pregnancies, for example, in which the rate of increase remains relatively constant (Figure 2.E). In other pregnancies, hCG rates of increase slow down for a few days (Figure 2.C, D & B) and then increase abruptly and, in some cases, decrease again afterwards (Figure 2.D and 2.B). Abrupt increases appeared to be more common towards the beginning of the week and decelerations more common towards the end.
Figure 2.
Selected set of five individual hCG urinary excretion trajectories (labeled A to E) to illustrate between-pregnancy variability. The central solid line represents the hCG trajectory predicted by the regression equation for 142 clinical pregnancies. Icons and associated lines represent the 5 individual trajectories. Day 1 = day of detection (hCG > 0.015 ng/mL).
HCG excretion and pregnancy survivability
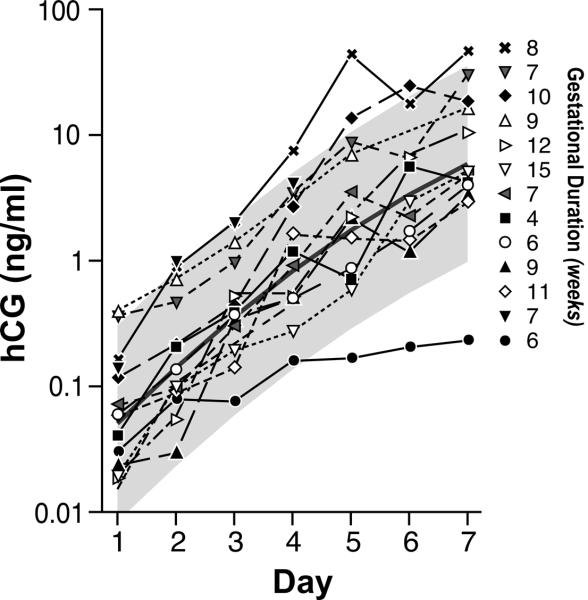
The trajectory of hCG rise in the first week was not different for spontaneous abortions and live births (p=0.80). Pregnancies ending in spontaneous abortion presented as much variability in their hCG trajectories as did surviving singleton pregnancies (Levene's test for homogeneity, p=0.61) (Figure 3). Furthermore, among these clinical pregnancies the mean time to implantation was identical (9.15 days after estimated ovulation) between surviving pregnancies and those resulting in miscarriage.
Figure 3.
Urinary hCG excretion profiles for 13 clinical miscarriages during the first week following implantation. Numbers represent the length of gestation (from ovulation to bleeding). The smooth central solid line represents the hCG trajectory predicted by the regression equation for 121 surviving singletons pregnancies and the gray area represents the 95% confidence interval. Day 1 = day of detection (hCG > 0.015 ng/mL).
HCG excretion in twin pregnancies
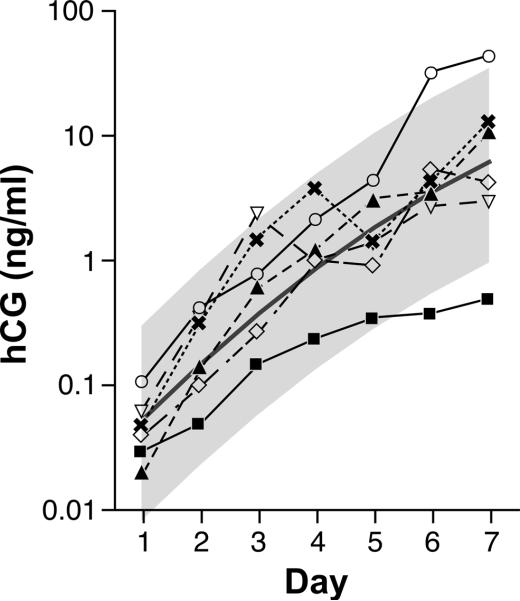
Our sample includes 6 sets of twins, all monozygotic. Zygosity was assessed several years after birth using a validated set of questions answered by the mother. A visual inspection of hCG trajectories for the twin pregnancies shows considerable variation, with an average trajectory very similar to that for surviving singletons (Figure 4).
Figure 4.
Urinary hCG excretion profiles for six sets of monozygotic twins during the first week of detection. The central solid line represents the hCG trajectory predicted by the regression equation for 121 surviving singleton pregnancies and the gray area represents the 95% confidence interval. Icons and associated lines represent the 6 individual trajectories. Day 1 = day of detection (hCG > 0.015 ng/mL).
HCG excretion and sex of the fetus
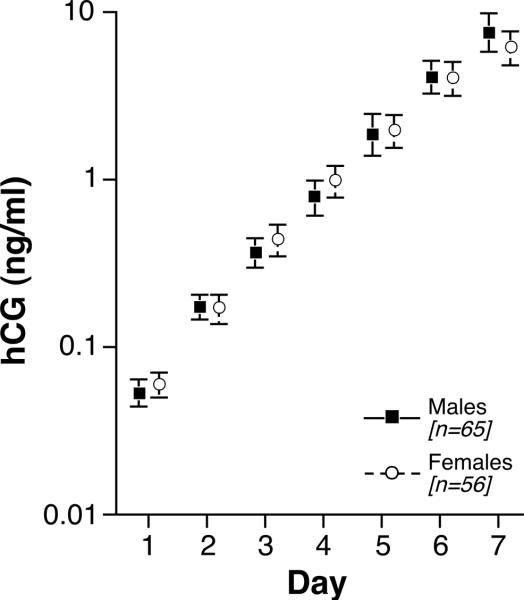
Sex of the fetus (65 boys and 56 girls) was not a significant predictor of urinary hCG levels (p=0.09) among pregnancies resulting in surviving singletons (Figure 5).
Figure 5.
Daily hCG geometric means (GM) for surviving male and female fetuses during the first week of hCG detection. Icons represent daily GMs and bars are drawn to plus/minus one standard error. Day 1 = day of detection (hCG > 0.015 ng/mL).
Time to implantation and hCG excretion trajectories
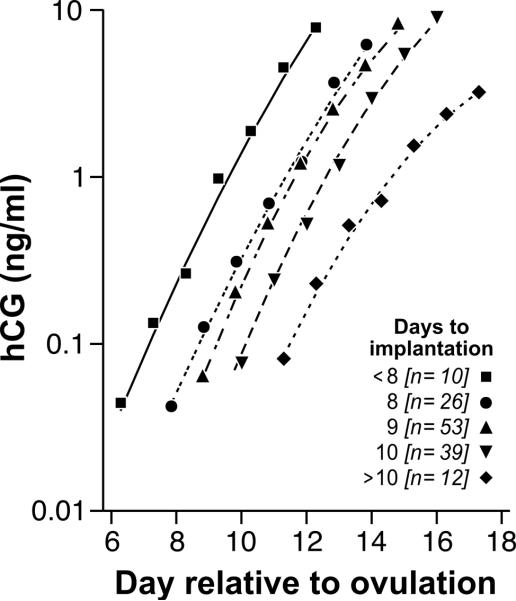
As we previously reported for these data (Wilcox et al.1999), the time between estimated day of ovulation and estimated day of implantation ranged from 6 to 12 days (mean=9.1, SD=1.12, median=9). The time it takes for a conceptus to implant may reflect quality of the conceptus or the uterus. In our sample of clinical pregnancies, time to implantation emerged as a significant predictor of the pattern of hCG rise (p<.0001). As shown in figure 6, conceptuses that implanted early (7 days after ovulation or earlier) tended to have lower hCG levels on the day of implantation but higher rates of increase during the first week, compared with conceptuses that implanted late (11 days or later). By the end of the first week following hCG detection, late implanters showed lower mean levels of hCG.
Figure 6.
Daily hCG trajectories by time elapsed between ovulation and first hCG detection (“time to implantation”) for 142 clinical pregnancies during the first week of detection. Lines represent the trajectories predicted by the regression model for each day for each time to implantation category. The icons associated with each line represent the observed daily geometric means for each category. Day 0 = estimated day of ovulation.
DISCUSSION
We evaluated maternal hCG urinary excretion patterns during the first week following detection in 142 naturally-conceived clinical pregnancies. Daily mean urinary hCG concentrations rose rapidly, with daily increases ranging from an initial 3-fold rise to a 1.6-fold rise by the end of the week. In contrast with the smooth increase in the overall mean, individual hCG trajectories were highly variable. Sharp increases were sometimes interspersed with relative plateaus (Fig. 2). Some of this may be measurement error, although the use of triplicate assays reduces such error. The observed variation may also reflect day-to-day changes in the trophoblastic invasion process, as hCG-producing cells are brought in proximity to the maternal blood supply. Alternatively, there could be variable sequestering of hCG in maternal tissues prior to excretion. Our sample was ethnically and socioeconomically homogeneous; a more diverse sample could possibly show even higher heterogeneity in early hCG profiles.
Comparing our findings with those of previous studies is complicated by differences in design, sensitivity of the hCG assay used, and data analysis methods. A study based on 25 pregnancies achieved via artificial insemination reports higher average hCG values for the first five days following detection than the values we observed for the same period (Ho et al. 1997). The differences in results can be at least partially explained by the higher sensitivity of our assay which allowed earlier pregnancy detections. Two prior studies that examined longitudinal hCG rise patterns at the very earliest time of pregnancy for naturally-conceived pregnancies were based on very small samples (Lenton and Woodward 1988; Lohstroh et al. 2006). Lohstroh et al. (2006) reported hCG data from 13 naturally conceived clinical pregnancies for days 10 to14 from the FSH peak. The daily absolute increases they observed are similar to ours. Lenton et al., (1982, 1988) report data from 18 spontaneously conceived pregnancies. They observed a deceleration in the day-to-day relative rate of increase during the first 12 days following implantation that is consistent with the pattern we describe. A recent review of 9 studies by Chung and colleagues (2006) provides evidence suggesting that the deceleration in the relative rate of increase of hCG levels continues after the first week post-implantation.
The time at which the relative rate of hCG rise starts to slow down as pregnancy advances has long been a topic of debate (Fritz and Guo 1987). Studies discussing this issue focus on different intervals within the first trimester and data from the earliest stages of pregnancy are rarely well represented. Some of these previous studies show an early deceleration pattern (Check et al. 1992; Daya 1987; Fritz and Guo 1987; Lenton et al. 1982; Pittaway and Wentz 1985), while others report a constant exponential rise (Barnhart et al. 2004; Kadar et al. 1981; Zegers-Hochschild et al. 1994).
Early hCG levels are regarded as a predictor of pregnancy outcome (Check et al. 1992; France et al. 1996; Hauzman et al. 2004; Ho et al. 1997; Lohstroh et al. 2007; Yaron et al. 2002). While this is clearly true for very early losses (before 6 weeks) (Baird et al. 2003; Lohstroh et al. 2006; Wilcox et al. 1999), early hCG levels are a poor predictor of clinical miscarriages. In our data, conditional on survival to six weeks, the urinary hCG trajectory during the first week had no association with miscarriage. This observation is consistent with results reported by Lohstroh et al. who found no association between hCG levels on day of detection and pregnancy outcome either in 62 fertile women undergoing artificial insemination (Lohstroh et al. 2005) or in 10 naturally-conceived pregnancies (Lohstroh et al. 2006). There is some evidence however, that differences in hCG slopes may emerge during the second week, but findings are based on relatively small samples and IVF patients (Lohstroh et al. 2006; Lohstroh et al. 2007; Porat et al. 2007). Also, variations in hCG bioactivity and hyperglycosylated hCG levels, which we did not measure, may prove to be useful in assessing pregnancy viability during the peri-implantation period (Ho et al. 1997; Kovalevskaya et al. 2007; Lohstroh et al. 2006).
The number of developing embryos has been proposed to affect the trajectory of hCG (Chung et al. 2006; Hauzman et al. 2001; Urbancsek et al. 2002; Zegers-Hochschild et al. 1994). Some studies focused on the earliest gestational stages have, however, failed to observe an effect (Check et al. 1992; Kelly et al. 1991). During the week following the estimated day of implantation we found no differences in either absolute hCG values or rates of rise between the twins and singleton pregnancies.
By the second trimester women bearing female fetuses have higher hCG levels on average than those carrying males (Brody and Carlstroem 1965; Santolaya-Forgas et al. 1997; Spencer 2000). Yaron and colleagues report sex differences in hCG levels by the third week post-fertilization (Yaron et al. 2002; Yaron et al. 2001), but others have failed to observe differences that early in gestation (Braunstein et al. 1980; Gol et al. 2005; Kletzky et al. 1985). We found no sex-related differences in hCG levels among surviving pregnancies during the first week of detection, suggesting that sex differences arise later. Delay in implantation is a strong predictor of early pregnancy loss (before 6 weeks) (Wilcox et al. 1999). Here we report that, among pregnancies surviving at least 6 weeks, those that implanted after luteal day 10 had a slower hCG rise. The finding of slower hCG rise might seem to support the hypothesis that time to implantation is related to embryo quality (Bolton et al. 1989; Rogers 1995; Woodward and Lenton 1992). Both late implantation and a slower hCG rise may reflect a slower growing conceptus (Liu et al. 1995; Wilcox et al. 1999). However, conditional on survival to 6 weeks, neither late implantation nor a slower rate of hCG rise during the first week were associated with survival in our data.
In summary, maternal urinary hCG levels are quite variable during the first week following implantation. During this gestational stage, hCG patterns rise very rapidly but the relative rate of rise decelerates as the week advances. Among the factors evaluated the only significant predictor of hCG trajectories during the first week was time to implantation.
Acknowledgments
We thank Drs. Shyamal Peddada and Grace Kissling for statistical advice, Dr. Robert McConnaughey for assistance with data management and Sue Edelstein and Brian Mills for assistance in composing the final figures. Joy Pierce managed the field study and collection of urine. Drs. Olga Basso, Freya Kamel and Nicole Berry provided comments on an earlier draft of the manuscript. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
REFERENCES
- Armstrong EG, Ehrlich PH, Birken S, Schlatterer JP, Siris E, Hembree WC, Canfield RE. Use of a highly sensitive and specific immunoradiometric assay for detection of human chorionic gonadotropin in urine of normal, nonpregnant, and pregnant individuals. J Clin Endocrinol Metab. 1984;59:867–874. doi: 10.1210/jcem-59-5-867. [DOI] [PubMed] [Google Scholar]
- Baird DD, McConnaughey DR, Weinberg CR, Musey PI, Collins DC, Kesner JS, Knecht EA, Wilcox AJ. Application of a method for estimating day of ovulation using urinary estrogen and progesterone metabolites. Epidemiology. 1995;6:547–550. doi: 10.1097/00001648-199509000-00015. [DOI] [PubMed] [Google Scholar]
- Baird DD, Weinberg CR, McConnaughey DR, Wilcox AJ. Rescue of the corpus luteum in human pregnancy. Biol Reprod. 2003;68:448–456. doi: 10.1095/biolreprod.102.008425. [DOI] [PubMed] [Google Scholar]
- Baird DD, Weinberg CR, Wilcox AJ, McConnaughey DR, Musey PI. Using the ratio of urinary oestrogen and progesterone metabolites to estimate day of ovulation. Stat Med. 1991;10:255–266. doi: 10.1002/sim.4780100209. [DOI] [PubMed] [Google Scholar]
- Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol. 2004;104:50–55. doi: 10.1097/01.AOG.0000128174.48843.12. [DOI] [PubMed] [Google Scholar]
- Batzer FR, Schlaff S, Goldfarb AF, Corson SL. Serial beta-subunit human chorionic gonadotropin doubling time as a prognosticator of pregnancy outcome in an infertile population. Fertil Steril. 1981;35:307–312. doi: 10.1016/s0015-0282(16)45376-8. [DOI] [PubMed] [Google Scholar]
- Bolton VN, Hawes SM, Taylor CT, Parsons JH. Development of spare human preimplantation embryos in vitro: an analysis of the correlations among gross morphology, cleavage rates, and development to the blastocyst. J In Vitro Fert Embryo Transf. 1989;6:30–35. doi: 10.1007/BF01134578. [DOI] [PubMed] [Google Scholar]
- Bonduelle ML, Dodd R, Liebaers I, Van Steirteghem A, Williamson R, Akhurst R. Chorionic gonadotrophin-beta mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Hum Reprod. 1988;3:909–914. doi: 10.1093/oxfordjournals.humrep.a136808. [DOI] [PubMed] [Google Scholar]
- Braunstein GD, Rasor J, Danzer H, Adler D, Wade ME. Serum human chorionic gonadotropin levels throughout normal pregnancy. Am J Obstet Gynecol. 1976;126:678–681. doi: 10.1016/0002-9378(76)90518-4. [DOI] [PubMed] [Google Scholar]
- Braunstein GD, Rasor JL, Engvall E, Wade ME. Interrelationships of human chorionic gonadotropin, human placental lactogen, and pregnancy-specific beta 1-glycoprotein throughout normal human gestation. Am J Obstet Gynecol. 1980;138:1205–1213. doi: 10.1016/s0002-9378(16)32793-4. [DOI] [PubMed] [Google Scholar]
- Brody S, Carlstroem G. Human Chorionic Gonadotropin Pattern in Serum and Its Relation to the Sex of the Fetus. J Clin Endocrinol Metab. 1965;25:792–797. doi: 10.1210/jcem-25-6-792. [DOI] [PubMed] [Google Scholar]
- Chang PL, Canfield RE, Ditkoff EC, O'Connor JF, Sauer MV. Measuring human chorionic gonadotropin in the absence of implantation with use of highly sensitive urinary assays for intact beta-core and free beta epitopes. Fertil Steril. 1998;69:412–414. doi: 10.1016/s0015-0282(97)00572-4. [DOI] [PubMed] [Google Scholar]
- Check JH, Weiss RM, Lurie D. Analysis of serum human chorionic gonadotrophin levels in normal singleton, multiple and abnormal pregnancies. Hum Reprod. 1992;7:1176–1180. doi: 10.1093/oxfordjournals.humrep.a137817. [DOI] [PubMed] [Google Scholar]
- Chung K, Sammel MD, Coutifaris C, Chalian R, Lin K, Castelbaum AJ, Freedman MF, Barnhart KT. Defining the rise of serum HCG in viable pregnancies achieved through use of IVF. Hum Reprod. 2006;21:823–828. doi: 10.1093/humrep/dei389. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Pulkkinen M. Indispensability of the human corpus luteum in the maintenance of early pregnancy. Luteectomy evidence. Obstet Gynecol Surv. 1978;33:69–81. doi: 10.1097/00006254-197802000-00001. [DOI] [PubMed] [Google Scholar]
- Cwikel J, Gidron Y, Sheiner E. Psychological interactions with infertility among women. Eur J Obstet Gynecol Reprod Biol. 2004;117:126–131. doi: 10.1016/j.ejogrb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Daya S. Human chorionic gonadotropin increase in normal early pregnancy. Am J Obstet Gynecol. 1987;156:286–290. doi: 10.1016/0002-9378(87)90269-9. [DOI] [PubMed] [Google Scholar]
- Ecochard R, Boehringer H, Rabilloud M, Marret H. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. Bjog. 2001;108:822–829. doi: 10.1111/j.1471-0528.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- France JT, Keelan J, Song L, Liddell H, Zanderigo A, Knox B. Serum concentrations of human chorionic gonadotrophin and immunoreactive inhibin in early pregnancy and recurrent miscarriage: A longitudinal study. Australian & New Zealand Journal Of Obstetrics & Gynaecology. 1996;36:325–330. doi: 10.1111/j.1479-828x.1996.tb02722.x. [DOI] [PubMed] [Google Scholar]
- Fritz MA, Guo SM. Doubling time of human chorionic gonadotropin (hCG) in early normal pregnancy: relationship to hCG concentration and gestational age. Fertil Steril. 1987;47:584–589. [PubMed] [Google Scholar]
- Gerhard I, Runnebaum B. Hormone load tests in the first half of pregnancy--a diagnostic and therapeutic approach. Biol Res Pregnancy Perinatol. 1984;5:157–173. [PubMed] [Google Scholar]
- Gol M, Guclu S, Demir A, Erata Y, Demir N. Effect of fetal gender on maternal serum human chorionic gonadotropin levels throughout pregnancy. Arch Gynecol Obstet. 2005;273:90–92. doi: 10.1007/s00404-005-0036-8. [DOI] [PubMed] [Google Scholar]
- Hauzman E, Fedorcsak P, Halmos A, Vass Z, Devenyi N, Papp Z, Urbancsek J. Role of serum hCG measurements in predicting pregnancy outcome and multiple gestation after in vitro fertilization. Early Pregnancy. 2001;5:26–27. [PubMed] [Google Scholar]
- Hauzman E, Fedorcsak P, Klinga K, Papp Z, Rabe T, Strowitzki T, Urbancsek J. Use of serum inhibin A and human chorionic gonadotropin measurements to predict the outcome of in vitro fertilization pregnancies. Fertil Steril. 2004;81:66–72. doi: 10.1016/j.fertnstert.2003.05.007. [DOI] [PubMed] [Google Scholar]
- Hay DL, Lopata A. Chorionic gonadotropin secretion by human embryos in vitro. J Clin Endocrinol Metab. 1988;67:1322–1324. doi: 10.1210/jcem-67-6-1322. [DOI] [PubMed] [Google Scholar]
- Ho HH, O'Connor JF, Nakajima ST, Tieu J, Overstreet JW, Lasley BL. Characterization of human chorionic gonadotropin in normal and abnormal pregnancies. Early Pregnancy. 1997;3:213–224. [PubMed] [Google Scholar]
- Kadar N, Caldwell BV, Romero R. A method of screening for ectopic pregnancy and its indications. Obstet Gynecol. 1981;58:162–166. [PubMed] [Google Scholar]
- Kelly MP, Molo MW, Maclin VM, Binor Z, Rawlins RG, Radwanska E. Human chorionic gonadotropin rise in normal and vanishing twin pregnancies. Fertil Steril. 1991;56:221–224. doi: 10.1016/s0015-0282(16)54475-6. [DOI] [PubMed] [Google Scholar]
- Kletzky OA, Rossman F, Bertolli SI, Platt LD, Mishell DR., Jr. Dynamics of human chorionic gonadotropin, prolactin, and growth hormone in serum and amniotic fluid throughout normal human pregnancy. Am J Obstet Gynecol. 1985;151:878–884. doi: 10.1016/0002-9378(85)90665-9. [DOI] [PubMed] [Google Scholar]
- Kovalevskaya G, Kakuma T, Schlatterer J, O'Connor JF. Hyperglycosylated HCG expression in pregnancy: cellular origin and clinical applications. Mol Cell Endocrinol. 2007;260–262:237–243. doi: 10.1016/j.mce.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Lam YH, Yeung WS, Tang MH, Ng EH, So WW, Ho PC. Maternal serum alpha-fetoprotein and human chorionic gonadotrophin in pregnancies conceived after intracytoplasmic sperm injection and conventional in-vitro fertilization. Hum Reprod. 1999;14:2120–2123. doi: 10.1093/humrep/14.8.2120. [DOI] [PubMed] [Google Scholar]
- Lambers MJ, van Weering HG, van't Grunewold MS, Lambalk CB, Homburg R, Schats R, Hompes PG. Optimizing hCG cut-off values: a single determination on day 14 or 15 is sufficient for a reliable prediction of pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. 2006;127:94–98. doi: 10.1016/j.ejogrb.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Lenton EA, Neal LM, Sulaiman R. Plasma concentrations of human chorionic gonadotropin from the time of implantation until the second week of pregnancy. Fertil Steril. 1982;37:773–778. doi: 10.1016/s0015-0282(16)46337-5. [DOI] [PubMed] [Google Scholar]
- Lenton EA, Woodward AJ. The endocrinology of conception cycles and implantation in women. J Reprod Fertil Suppl. 1988;36:1–15. [PubMed] [Google Scholar]
- Liu HC, Pyrgiotis E, Davis O, Rosenwaks Z. Active corpus luteum function at pre-, peri- and postimplantation is essential for a viable pregnancy. Early Pregnancy. 1995;1:281–287. [PubMed] [Google Scholar]
- Lohstroh P, Dong H, Chen J, Gee N, Xu X, Lasley B. Daily immunoactive and bioactive human chorionic gonadotropin profiles in periimplantation urine samples. Biol Reprod. 2006;75:24–33. doi: 10.1095/biolreprod.105.048363. [DOI] [PubMed] [Google Scholar]
- Lohstroh PN, Overstreet JW, Stewart DR, Nakajima ST, Cragun JR, Boyers SP, Lasley BL. Secretion and excretion of human chorionic gonadotropin during early pregnancy. Fertil Steril. 2005;83:1000–1011. doi: 10.1016/j.fertnstert.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Lohstroh PN, Overstreet JW, Stewart DR, Nakajima ST, Cragun JR, Boyers SP, Lasley BL. Hourly human chorionic gonadotropin secretion profiles during the peri-implantation period of successful pregnancies. Fertil Steril. 2007;87:1413–1418. doi: 10.1016/j.fertnstert.2006.11.053. [DOI] [PubMed] [Google Scholar]
- Malassine A, Cronier L. Hormones and human trophoblast differentiation: a review. Endocrine. 2002;19:3–11. doi: 10.1385/ENDO:19:1:3. [DOI] [PubMed] [Google Scholar]
- Marshall JR, Hammond CB, Ross GT, Jacobson A, Rayford P, Odell WD. Plasma and urinary chorionic gonadotropin during early human pregnancy. Obstet Gynecol. 1968;32:760–764. [PubMed] [Google Scholar]
- McChesney R, Wilcox AJ, O'Connor JF, Weinberg CR, Baird DD, Schlatterer JP, McConnaughey DR, Birken S, Canfield RE. Intact HCG, free HCG beta subunit and HCG beta core fragment: longitudinal patterns in urine during early pregnancy. Human Reproduction. 2005;20:928–935. doi: 10.1093/humrep/deh702. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Menabawey M, Lowings C, Buck RH, Chard T. Relationship between blood and urine concentrations of intact human chorionic gonadotropin and its free subunits in early pregnancy. Obstet Gynecol. 1987;69:590–593. [PubMed] [Google Scholar]
- Pittaway DE, Wentz AC. Evaluation of early pregnancy by serial chorionic gonadotropin determinations: a comparison of methods by receiver operating characteristic curve analysis. Fertil Steril. 1985;43:529–533. doi: 10.1016/s0015-0282(16)48492-x. [DOI] [PubMed] [Google Scholar]
- Porat S, Savchev S, Bdolah Y, Hurwitz A, Haimov-Kochman R. Early serum beta-human chorionic gonadotropin in pregnancies after in vitro fertilization: contribution of treatment variables and prediction of long-term pregnancy outcome. Fertil Steril. 2007;88:82–89. doi: 10.1016/j.fertnstert.2006.11.116. [DOI] [PubMed] [Google Scholar]
- Rogers PA. Current studies on human implantation: a brief overview. Reprod Fertil Dev. 1995;7:1395–1399. doi: 10.1071/rd9951395. [DOI] [PubMed] [Google Scholar]
- Samarajeewa P, Cooley G, Kellie AE. The radioimmunoassay of pregnanediol-3 alpha-glucuronide. J Steroid Biochem. 1979;11:1165–1171. doi: 10.1016/0022-4731(79)90169-9. [DOI] [PubMed] [Google Scholar]
- Santolaya-Forgas J, Meyer WJ, Burton BK, Scommegna A. Altered newborn gender distribution in patients with low mid-trimester maternal serum human chorionic gonadotropin (MShCG) J Matern Fetal Med. 1997;6:111–114. doi: 10.1002/(SICI)1520-6661(199703/04)6:2<111::AID-MFM10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Seeber BE, Sammel MD, Guo W, Zhou L, Hummel A, Barnhart KT. Application of redefined human chorionic gonadotropin curves for the diagnosis of women at risk for ectopic pregnancy. Fertil Steril. 2006;86:454–459. doi: 10.1016/j.fertnstert.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Spencer K. The influence of fetal sex in screening for Down syndrome in the second trimester using AFP and free beta-hCG. Prenat Diagn. 2000;20:648–651. doi: 10.1002/1097-0223(200008)20:8<648::aid-pd869>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Urbancsek J, Hauzman E, Fedorcsak P, Halmos A, Devenyi N, Papp Z. Serum human chorionic gonadotropin measurements may predict pregnancy outcome and multiple gestation after in vitro fertilization. Fertil Steril. 2002;78:540–542. doi: 10.1016/s0015-0282(02)03278-8. [DOI] [PubMed] [Google Scholar]
- Wehmann RE, Nisula BC. Metabolic and renal clearance rates of purified human chorionic gonadotropin. J Clin Invest. 1981;68:184–194. doi: 10.1172/JCI110234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Wehmann RE, Armstrong EG, Canfield RE, Nisula BC. Measuring early pregnancy loss: laboratory and field methods. Fertil Steril. 1985;44:366–374. [PubMed] [Google Scholar]
- Williams MA, Hickok DE, Zingheim RW, Zebelman AM, Mittendorf R, Luthy DA. A Longitudinal-Study Of Maternal Serum Human Chorionic-Gonadotropin Levels During Pregnancy. Gynecologic And Obstetric Investigation. 1995;40:158–161. doi: 10.1159/000292327. [DOI] [PubMed] [Google Scholar]
- Woodward AJ, Lenton EA. Differential responses to a simulated implantation signal at various stages of the luteal phase in women. J Clin Endocrinol Metab. 1992;74:999–1004. doi: 10.1210/jcem.74.5.1569178. [DOI] [PubMed] [Google Scholar]
- Wright K, Collins DC, Musey PI, Preedy JR. Direct radioimmunoassay of specific urinary estrogen glucosiduronates in normal men and nonpregnant women. Steroids. 1978;31:407–426. doi: 10.1016/0039-128x(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Yang M, Lei ZM, Rao Ch V. The central role of human chorionic gonadotropin in the formation of human placental syncytium. Endocrinology. 2003;144:1108–1120. doi: 10.1210/en.2002-220922. [DOI] [PubMed] [Google Scholar]
- Yaron Y, Ochshorn Y, Heifetz S, Lehavi O, Sapir Y, Orr-Urtreger A. First trimester maternal serum free human chorionic gonadotropin as a predictor of adverse pregnancy outcome. Fetal Diagn Ther. 2002;17:352–356. doi: 10.1159/000065384. [DOI] [PubMed] [Google Scholar]
- Yaron Y, Wolman I, Kupferminc MJ, Ochshorn Y, Many A, Orr-Urtreger A. Effect of fetal gender on first trimester markers and on Down syndrome screening. Prenat Diagn. 2001;21:1027–1030. doi: 10.1002/pd.178. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Altieri E, Fabres C, Fernandez E, Mackenna A, Orihuela P. Predictive value of human chorionic gonadotrophin in the outcome of early pregnancy after in-vitro fertilization and spontaneous conception. Hum Reprod. 1994;9:1550–1555. doi: 10.1093/oxfordjournals.humrep.a138747. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Pohl RC. Control of Follicular Development, Corpus Luteum Function, the Maternal Recognition of Pregnancy, and the Neuroendocrine Regulation of the Menstrual Cycle in Higher Primates. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. Third edition Elsevier, Academic Press; San Diego, CA, USA: 2006. pp. 2470–2475. [Google Scholar]