Abstract
Background
The treatment of children with cancer is associated with significant burden for the entire family. Frequent clinic visits and extended hospital stays can negatively affect quality of life for children and their families.
Methods
Here, we describe the development of a Hospital at Home program (H@H) that delivers therapy to pediatric hematology, oncology, and blood and marrow transplant (bmt) patients in their homes. The services provided include short infusions of chemotherapy, supportive-care interventions, antibiotics, post-chemotherapy hydration, and teaching.
Results
From 2013 to 2015, the H@H program served 136 patients, making 1701 home visits, for patients mainly between the ages of 1 and 4 years. Referrals came from oncology in 82% of cases, from hematology in 11%, and from bmt in 7%. Since inception of the program, no adverse events have been reported. Family surveys suggested less disruption in daily routines and appreciation of specialized care by hematology and oncology nurses. Staff surveys highlighted a perceived benefit of H@H in contributing to early discharge of patients by supporting out-of-hospital monitoring and teaching.
Conclusions
The development of a H@H program dedicated to the pediatric hematology, oncology, or bmt patient appears feasible. Our pilot program offers a potential contribution to improvement in patient quality of life and in cost–benefit for parents and the health care system.
Keywords: Pediatrics, hematology, chemotherapy, program development, home care
INTRODUCTION
The management of cancer, especially in children, often increases burden for families because of the intense stress around the time of diagnosis and the length of treatment, the frequent hospitalizations, and the outpatient clinic appointments required throughout the cancer journey1. Common themes mentioned by families during the cancer treatment experience include exhaustion, disruption of family life, isolation, and confinement to the hospital2. In an attempt to improve the health care experience for children and their families, the Alberta Children’s Hospital developed a quality improvement initiative that aimed to deliver some parts of hematology, oncology, and blood and marrow transplant (bmt) program therapy at home for pediatric patients in Calgary. Here, we describe our experience of piloting the Hospital at Home (H@H) program as an extension of the outpatient clinic, evaluating its feasibility and providing practical aspects of development for similar future initiatives in institutions working in a comparable health care environment.
The H@H Program
The H@H program is an initiative supported by the Childhood Cancer Collaborative, with philanthropic donations through the Alberta Children’s Hospital Foundation. In 2011, a multidisciplinary team was established to help conceptualize the program. The team consisted of a project manager, a unit manager, a clinical nurse specialist, a nurse educator, a nurse practitioner, a pharmacy representative, an oncologist, and a parent representative.
Based on a literature review and results from a family survey, the H@H team identified the types of services that could be offered in the home. Supportive care documents, guidelines, and forms were developed to ensure that procedures were performed safely in the home and were consistent with hospital procedures. A formulary of medications and chemotherapy agents that could be administered in a patient’s home was developed. The formulary was carefully extended over several phases of program expansion, after close monitoring of patient safety and review of internal processes.
Although the H@H program was defined as an extension of the outpatient clinic, further consultation with the existing pediatric home care programs in the Calgary region was undertaken to prevent duplication of services—for example, administration of long-term intravenous (IV) antibiotics. In 2012, two experienced registered nurses were hired to put the pilot project into practice and to further develop supporting documents. Since November 2012, the H@H program has been providing treatment to patients at home, operating during regular outpatient daytime hours and 2 weekends per month.
A communication process was developed to ensure the H@H nurse could reach a physician at any time during the home visit to report urgent concerns. Every medication administered through the H@H program was evaluated in conjunction with the pharmacy to ensure that medications given are low-risk and have 24-hour stability at minimum. To ensure good clinical practice, medications were monitored for temperature during transport. Chemotherapeutic agents were independently double-checked per hospital policies and procedures. Any potential adverse events (defined as staff safety issues, breaches in policy or procedure, or unexpected reactions) were monitored through safety learning reports.
The program had these eligibility criteria:
■ Patient home located within a half-hour drive from the hospital
■ Patient need for an available H@H service
■ Infusion delivery time for chemotherapy or medication less or equal to 1 hour
■ Familial consent to the program
■ Reliable telephone communication available
■ Safe home environment as determined by a safety screen
Once eligible patients had been identified by the primary treatment team, the family was approached by the H@H nurse to introduce the program and to obtain consent to enrol the child.
METHODS
As part of ongoing monitoring of patient satisfaction and quality control processes, we prospectively collected, for each home visit, quantitative data such as disease diagnosis, patient age, number of family members present, distance and nursing drive time from the patient’s home to the hospital, and types of services administered. We also distributed two consecutive surveys to, and interviewed, families who had consented to participate and who had completed at least 3 home visits. The items included in the surveys were developed by the members of the H@H team. Qualitative data collected from the health service online survey tool “Select Survey” included items such as distance from the hospital, method of travel used to reach the hospital, parent and patient experiences in the out patient clinic compared with H@H, perceived benefits with the use of the H@H program, and level of burden within the family with the addition of H@H services. Additionally, one survey was distributed to all staff members of the hematology, oncology, and bmt programs, requesting feedback on the possible effects of H@H.
RESULTS
Population
The Alberta Children’s Hospital is a tertiary centre that cares for patients from southern Alberta, eastern British Columbia, and western Saskatchewan. On average, 100 oncology and bmt patients receive active therapy in the outpatient clinic each year. Of the patients meeting eligibility criteria for H@H, 96% were enrolled into the program. To date, only 5 families referred to the program have declined services. None of the enrolled families prematurely left the program.
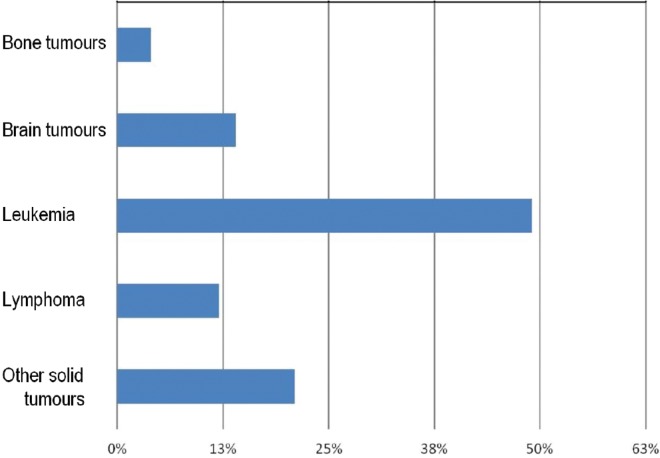
Since inception, the H@H program has served 136 patients and their families during a total of 1701 home visits (last census: December 2015). During the most recent 6-month period, H@H activity represented approximately 14%–20% of the total number of day-treatment visits in the outpatient clinic. During 2013–2015, 82% of referrals involved general oncology patients; 11%, hematology patients; and 7%, bmt patients. Figure 1 highlights the distribution, by diagnosis, of the oncology population served by the program. Children with leukemia predominate (49%), followed by those with solid tumours (21%) and brain tumours (14%). The median number of visits by primary diagnosis was 16 for patients with bone malignancies (range: 9–39), 12 for those with solid tumours (range: 2–131), 9 for those with leukemia (range: 2–72), 8 for those with brain tumours (range: 2–71), and 4 for those with lymphoma (range: 2–14). The age of the patients served by the program ranged from 0 years to 18 years, with most patients being between 1 and 4 years of age.
FIGURE 1.
Distribution of diagnoses for patients enrolled into the Hospital at Home program.
Services
During the 1st year of the program’s operation, the median number of home visits conducted per day was 2 (range: 0–5); in the most recent 2 years, the median number of visits increased to 3 daily (range: 0–6), with an average duration of 90 minutes for an individual visit (range: 60–150), including driving time. With further optimization of time management and extension of services, the projected number of visits should reach 4 daily.
While ensuring that safety procedures were constantly reviewed, the types of interventions offered were diversified and increased in 3 separate phases. Table i summarizes the various treatments and services delivered by the program. During the 1st phase of activity, the main interventions included IV-push chemotherapy and antibiotics, and nasogastric tube care and teaching. In phase 2, chemotherapy agents or medications and fluid bolus administrations (maximum 1 hour) were made available using an ambulatory IV pump.
TABLE I.
Type of services and treatments delivered in the Hospital at Home program
| Chemotherapy | Medications | Supportive care |
|---|---|---|
| Cytosine arabinoside | Ceftriaxone | Nursing physical examination |
| Dactinomycin | Ertapenem | Subcutaneous immunoglobulin |
| Vincristine | Micafungin | Support for factors infusion |
| Vinblastine | Ganciclovir | Nasogastric tube support |
| Vinorelbine | Intravenous push fluid boluses | Instillation and removal of tissue plasminogen activator |
| Methotrexate intravenous push | Home hydration > 24 h (after methotrexate, ifosfamide, cyclophosphamide, or cisplatinum) | Monitoring for low-risk fever and neutropenia |
| Doxorubicin | Ethanol locking | |
| Topotecan | Port access, insertion of indwelling catheter, blood work, injection of granulocyte colony–stimulating factora | |
| Cyclophosphamide (low dose) | ||
| Irinotecan |
These interventions performed during a home visit only in addition to other scheduled treatments.
A home hydration protocol after administration of high-dose methotrexate was also launched to support patients with osteosarcoma. The ability to offer post-chemotherapy continuous IV hydration at home had a significant effect, decreasing hospital days for certain chemotherapy protocols. For patients with osteosarcoma treated according to the aost 0331 protocol and discharged after completion of the methotrexate infusion while continuing their IV hydration with the H@H program, a minimum of 36 nights of hospitalization over 12 rounds of high-dose methotrexate can be spared. The first osteosarcoma patient on home hydration self-reported a decreased need for antiemetics, less weight gain (because of increased mobility at home), better sleep, and ability to attend school and extracurricular activities. Methotrexate clearance followed the expected curve, and no adverse events were reported.
During the 3rd phase of expansion, post-chemotherapy home IV hydration was extended to patients with Ewing sarcoma and rhabdomyosarcoma requiring hydration, and infusions of ifosfamide and post-cyclophosphamide mesna were added. The H@H program also integrated the home monitoring of patients enrolled on the low-risk febrile neutropenia protocol, allowing for early discharge from hospital on oral antibiotics. Subsequent outpatient monitoring included physical examinations in the outpatient clinic, alternating with telephone calls from an outpatient nurse and a H@H visit, until resolution of the neutropenic event. Overall, involvement of the H@H program in the post-discharge monitoring of febrile neutropenia patients contributed to lower the number of outpatient clinic visits to 2 visits from 3 visits per week on average. More recently, the program reached out to hematology patients requiring support with factor infusions and subcutaneous immunoglobulin infusions.
Most home visits were related to chemotherapy administration (58%), followed by provision of supportive care: nursing assessments, nasogastric tube insertions, medication administration (subcutaneous immunoglobulin infusions, instillation of tissue plasminogen activator), monitoring of low-risk febrile neutropenia, infusion of fluid boluses or antibiotics, and teaching appointments.
Ongoing monitoring of adverse events—defined as readmissions, mechanical complications, allergic or anaphylactic reactions, and safety issues involving patients, staff, or breaches in policy—has been embedded in the development of the program. To date, no adverse event has been recorded.
Family and Staff Surveys
Family evaluations of the program were conducted in 2013 and 2014 by survey (Likert and short open questions). In both years, 78% of the families (29 of 37) participated.
Families described an average distance of 45 km from their home to the hospital. With regard to the impact of H@H in their daily life, they reported fewer disruptions in their daily routines (92%), less need for childcare (36%), fewer sick or personal days taken (10%), fewer days off work without pay (28%), and appreciation for care from specialized pediatric oncology nurses (96%). Family feedback also suggested a positive effect of the program on quality of life by decreasing time spent in the hospital and reducing out-of-pocket costs associated with frequent and prolonged hospital visits.
The survey also explored the effect that the program had in reducing the burden felt by families in terms of financial stress, time management, and lifestyle changes. Of the responding families, 88% reported slight to no burden when receiving care at home; 92% described some or great burden when coming to the outpatient clinic. The subjectively reported observation notes by H@H nurses indicated that home visits contributed to a reduction in family anxiety concerning the cancer-specific care of their children. Parents showed greater confidence when demonstrating their skills in the privacy of their own home.
In 2014, staff members of the hematology, oncology, and bmt programs were also surveyed about their experience with the H@H program. Although the response rate was rather low (33%), a closer examination showed that the response rate was more satisfactory for the disciplines working closely with the H@H program (response rate of 100% of pharmacists, 67% of outpatient clinic nurses, 58% of primary nurses, 40% of physicians, and 22% of inpatient nurses). The staff survey indicated support for further expansion of the H@H program. Staff valued the decline in time spent on central line teaching during outpatient clinic hours and the facilitation of earlier discharge from the inpatient unit for newly diagnosed patients.
Because the H@H program is an extension of the outpatient clinic, the cost of supplies, chemotherapy agents, medications, and hospital administration were covered by the operational budget of the outpatient clinic. The additional costs—including 1.46 full time equivalents for nurse salaries, clerical salary, vehicle lease and other associated costs (for example, maintenance, gas, parking), purchase of equipment (the ambulatory pump, for instance)—were funded through the Alberta Children’s Hospital Foundation.
DISCUSSION
Since the mid-1990s, reported experiences of a home care delivery model specifically dedicated to pediatric hematology and oncology patients have been limited in number1,3,4. The published literature discusses the safety and feasibility of this type of program, as well as the types of services that could be offered and the various care providers that could be involved. Those factors vary greatly from institution to institution and also with the unique constraints associated with various health care systems.
In the United Kingdom, a prospective initiative was started to allow parents to administer home IV antibiotics, antiemetics, and low-dose cytosine arabinoside3. Most parents did not find home therapy more stressful than hospital treatment and received enough support at home. The parents also described how receiving home therapy provided a feeling of increased control over their child’s treatment and better knowledge of their child’s disease3. In the United States, a Home Intravenous Chemotherapy by Parents program was established in New York to deliver chemotherapy at the patient’s home with the support of home care nurses4. The chemotherapy agents administered at home included infusions of high-dose methotrexate, high-dose etoposide–ifosfamide, and cyclophosphamide; continuous infusions of doxorubicin–vincristine, irinotecan, carboplatin–etoposide, and cisplatin–etoposide were also administered. The program aimed to decrease disruptions to family life and to children’s education and socialization, and to ease the direct financial burden on parents related to the costs of patient care in the context of the U.S. health care system4. Similarly, another program in New York was able to support a cost-effective home chemotherapy program by starting chemotherapy administrations in the day hospital and then safely completing any remaining overnight chemotherapy infusions or hydration at home by ambulatory pump1.
More recently, a Canadian home-base chemotherapy experience was also reported. In the Greater Toronto Area, pediatric patients with acute lymphoblastic leukemia were enrolled onto a randomized crossover study in which patients acted as their own controls, receiving chemotherapy at home for 6 months and subsequently in hospital for 6 months, and vice versa. The study included administration of two different chemotherapeutic agents (cytosine arabinoside and low-dose methotrexate)5. In that study, the IV chemotherapy was prepared by the community pharmacy, delivered to the patient’s home, and administered by a trained community nurse5. The effect on family and patient quality of life was ambivalent: home treatment allowed for more capability to maintain a usual routine, but parents reported greater emotional distress for the patient. Furthermore, the treatment location was not significantly associated with caregiver burden and cost5. The limited panel of chemotherapy agents administered and the crossover design of the study might have contributed to those findings. Despite the results, the potential benefit of such strategies for certain groups of patients should not dismissed6.
The care model developed for our H@H program combined several aspects of the foregoing models. The initial phase of our pilot project validated the ability of the H@H program to be a safe alternative for the delivery of certain treatments outside the hospital for its patient population. The enrolment rate of 96% and the absence of drop-off reflects the degree of confidence and safety perceived by the families. Although we have not yet formally evaluated the effect of the program on patient quality of life and on cost–benefit balance, the review of our initial surveys showed recurrent themes that warrant future study—for example, enhanced continuity of care, family-centred care in the community, and less time spent in hospital.
To assess the sustainability of such programs in the context of the Canadian health care system, a health economic evaluation should look at the direct costs of the new care program delivery and at the potential effects of the program with respect to optimization of patient flow, decreased length of hospital stay, and buffering of overcapacity workload during seasonal peaks of hospital admissions. The H@H model of care has the potential to significantly affect the cost of treatment for certain pediatric illnesses by lowering the number of inpatient admissions per patient (as illustrated with the implementation of the home IV hydration protocol after chemotherapy). To formally describe the social impact of the program, a social return-on-investment evaluation is currently underway. That evaluation intends to capture unconventional economic metrics—specifically, the monetary value of items such as improved quality of life, improved health, increased self-confidence, and decreased time off work and school7.
FUTURE DIRECTIONS
In light of other published experiences, the H@H program is exploring new directions for reaching out to other patient populations. For instance, the home care program for pediatric oncology patients in Genoa includes physician home visits in their model. In addition to describing the feasibility of their initiative, the authors also showed a significant cost savings for their health care system, even taking into account the cost of staff time10. Adapting such an approach to the Canadian system, the potential involvement of a nurse practitioner in the H@H program could allow for direct physical examinations and chemotherapy administration in the home setting while further decreasing the number of outpatient visits for patients requiring a physical exam before chemotherapy, with an associated positive effect on clinic workload.
Reviewing some adult home care experiences for bmt patients, we are currently investigating expanding our service to pediatric patients who currently undergo prolonged hospitalization for more intensive supportive care management. In Sweden, an adult treatment centre allowed early discharge after an allogenic BMT for 146 patients who had full support at home by a relative or friend8. The study authors noted a significant correlation between a lower incidence of acute graft-versus-host disease and an increase in the number of days spent at home with support from an experienced nurse8. For the same cohort, the patient experience was described using the Sympathy–Acceptance–Understanding Competence questionnaire (completed by 22 patients in hospital and 19 in home care)9. Compared with the hospitalized patients, the patients in home care were, in general, more satisfied with their care. The authors reported no significant negative effects on the overall experience of care and support by the patients during the acute post-transplantation phase, and patients both felt safe and valued the person-centred care experience9. Miano et al.10 also reported their limited experience of a home care program for pediatric bmt patients, offering blood transfusion and supportive care medications. The development of similar supportive-care protocols and monitoring for patients in our pediatric population with acute myeloid leukemia or after bmt will have to be carefully implemented and evaluated. The administration of specialized therapies in the home setting can certainly challenge the existing models of care and shape future health care delivery models for other patients with chronic disease.
We acknowledge that our preliminary findings are probably not generalizable at this stage. Future study to formally evaluate the cost–benefit profile of our program is underway to determine its sustainability within our provincial health care system.
CONCLUSIONS
In our experience, the development and operationalization of a specialized pediatric hematology, oncology, and bmt home care program is feasible and safe. By administering a larger number of cytotoxic agents, providing post-chemotherapy home hydration, delivering additional teaching support in the home, and arranging nursing monitoring of patients with low-risk febrile neutro penia, the H@H pilot program has been able to promote early discharge, to decrease time spent in hospital, and to create a strong sense of safety and confidence in the treating team.
ACKNOWLEDGMENTS
We thank the Alberta Children’s Hospital Foundation for financial support to fund this innovative program.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Lashlee M, O’Hanlon Curry J. Pediatric home chemotherapy: infusing “quality of life”. J Pediatr Oncol Nurs. 2007;24:294–8. doi: 10.1177/1043454207304908. [DOI] [PubMed] [Google Scholar]
- 2.Björk M, Wiebe T, Hallström I. An everyday struggle—Swedish families’ lived experiences during a child’s cancer treatment. J Pediatr Oncol Nurs. 2009;24:423–32. doi: 10.1016/j.pedn.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 3.Hooker L, Kohler J. Safety, efficacy, and acceptability of home intravenous therapy administered by parents of pediatric oncology patients. Med Pediatr Oncol. 1999;32:421–6. doi: 10.1002/(SICI)1096-911X(199906)32:6<421::AID-MPO5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Jayabose S, Escobedo V, Tugal O, et al. Home chemotherapy for children with cancer. Cancer. 1992;69:574–9. doi: 10.1002/1097-0142(19920115)69:2<574::AID-CNCR2820690249>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Stevens B, Croxford R, McKeever P, et al. Hospital and home chemotherapy for children with leukemia: a randomized cross-over study. Pediatr Blood Cancer. 2006;47:285–92. doi: 10.1002/pbc.20598. [DOI] [PubMed] [Google Scholar]
- 6.Breitfeld PP. Belief in home chemotherapy. Pediatr Blood Cancer. 2006;47:237–8. doi: 10.1002/pbc.20679. [DOI] [PubMed] [Google Scholar]
- 7.Banke-Thomas AO, Madaj B, Charles A, van den Broek N. Social return on investment (sroi) methodology to account for value for money of public health interventions: a systematic review. BMC Public Health. 2015;15:582. doi: 10.1186/s12889-015-1935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ringdén O, Remberger M, Holmberg K, et al. Many days at home during neutropenia after allogeneic hematopoietic stem cell transplantation correlates with low incidence of acute graft versus host disease. Biol Blood Marrow Transplant. 2013;19:314–20. doi: 10.1016/j.bbmt.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Bergkvist K, Larsen J, Johansson UB, Mattsson J, Svahn BM. Hospital care or home care after allogeneic hematopoietic stem cell transplantation—patients’ experiences of care and support. Eur J Oncol Nurs. 2013;17:389–95. doi: 10.1016/j.ejon.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Miano M, Manfredini L, Garaventa A, et al. Home care for children following haematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31:607–10. doi: 10.1038/sj.bmt.1703892. [DOI] [PubMed] [Google Scholar]