Abstract
Background
Our study evaluated long-term survival outcomes in rectal cancer patients treated with preoperative radiotherapy, and the impact on survival of concomitant and postoperative adjuvant chemotherapy (ctx), among other prognostic factors.
Methods
The study included 196 patients [median age: 58 years (range: 20–86 years); 63.0% men] with locally advanced rectal carcinoma and, in some cases, resectable liver metastasis. Rates of distant metastasis and local recurrence and of 5-year distant metastasis-free survival (dmfs) and overall survival (os) were determined.
Results
The 5-year os rate was 57.0%, with a median duration of 81.5 months (95% confidence interval: 73.7 months to 89.4 months), and the 5-year dmfs rate was 54.1%, with a median duration of 68.4 months (95% confidence interval: 40.4 months to 96.4 months). Prognostic factors for higher os and dmfs rates were downstaging (p = 0.013 and p = 0.005 respectively), radiotherapy dose (50 Gy vs. 56 Gy or 45–46 Gy, both p = 0.002), and concomitant ctx use (p = 0.004 and p = 0.001) and type (5-fluorouracil–leucovorin–folinic acid vs. tegafur–folinic acid, p = 0.034 and p = 0.043). Adjuvant ctx after neoadjuvant long-term concomitant chemoradiotherapy (ccrt) and surgery was associated with better 5-year os rates for postoperative T0–T3 disease (p = 0.003) and disease at all lymph node stages (p = 0.001).
Conclusions
Our findings revealed a favourable survival outcome with long-term fractionated irradiation and concomitant 5-fluorouracil–based ctx, achieving 5-year os and dmfs rates of 57.0% and 54.1% respectively. Preoperative administration of radiotherapy (50 Gy) and postoperative adjuvant ctx were associated with a significant survival benefit. Radiation doses above 50 Gy and the interval between ccrt and surgery had no significant effect on survival.
Keywords: Rectal cancer, preoperative chemoradiotherapy, survival, prognostic factors
INTRODUCTION
Rectal cancer is the 3rd most common malignancy worldwide and a major cause of cancer-related death in the developed world1,2. According to 2007–2008 data from 12 cancer centres in Turkey, the incidence of colorectal cancers is 7.8% in women and 7.5% in men, respectively representing the 3rd and 4th most common types of cancer3.
In view of high rates of local recurrence and poor survival in rectal cancer patients even after curative resection (40%–55% at 5 years), multimodal therapeutic options have gained importance to provide the optimum sequence and combination of radiotherapy (rt), chemotherapy (ctx), and surgery4,5.
Guidelines from the U.S. National Comprehensive Cancer Network recommend preoperative pelvic rt at a dose of 50.4 Gy in 28 fractions, with concurrent 5-fluorouracil (fu)–based ctx for 6 weeks, as a standard concomitant chemoradiotherapy (ccrt) regimen for stages ii and iii rectal cancer6.
The newer-generation chemotherapeutics such as capecitabine, S-1, oxaliplatin, and irinotecan have also been suggested to further enhance the disease control rate and to improve survival in patients with advanced colorectal cancer7–9. However, in a limited number of large-scale clinical trials, those agents have been associated with possible risks of acute and late toxicities7–9.
Since 2007, legislation for health care in Turkey has prohibited the use of uft-fa regimens [combination chemotherapy with the oral fu prodrug tegafur (uft), and folinic acid (fa)], while allowing for the use of intravenous fu and fa for preoperative ccrt in patients with locally advanced rectal cancer.
For about 10 years now in our clinic, preoperative ccrt has been implemented in selected patients with locally advanced rectal cancer. We therefore set out to evaluate long-term outcomes in rectal cancer patients treated with preoperative rt and the effect of concomitant ctx and postoperative adjuvant ctx, together with other prognostic factors, on survival.
METHODS
Our retrospective study included 196 consecutive patients [median age: 58 years (range: 20–86 years); 63.0% men] with locally advanced (T3/4) or any radiologically pelvic node–positive rectal carcinoma with or without resectable liver metastasis who were treated with various doses of preoperative rt in our department between 2004 and 2012. Patients with disseminated multiple metastases and unresectable liver metastasis were excluded from the study. Additional treatment options (based on records in the hospital database) included concomitant fu-based ctx, curative resection, and postoperative adjuvant ctx.
Permission for the use of patient data for publication purposes was obtained from our institutional ethics committee.
Assessments
Abdominopelvic magnetic resonance and computed tomography imaging were performed in all patients; endoscopic ultrasonography or phase-array magnetic resonance imaging was applied only if required for preoperative staging. Any identifiable lymph nodes were recorded as N1. Histopathologic parameters were evaluated in each patient. Operability after neoadjuvant therapy, rates of distant metastasis and local recurrence, 5-year distant metastasis–free survival (dmfs) and overall survival (os) were determined postoperatively. The prognostic factors evaluated were staging, lymphovascular invasion, perineural invasion, rt dose, course of neoadjuvant ccrt, interval from ccrt to surgery, and adjuvant ctx. Survival outcomes and rt doses were also analyzed with respect to rt device.
Standard Treatment Protocol
For patients of advanced age with high comorbidity and for patients with resectable liver metastasis, the treatment protocol consisted of rt (5×500 cGy), followed by surgery (after 7–10 days on average), and postoperative ctx (preferably oxaliplatin-based, depending on performance status and age).
For patients with either or both of advanced T3/4 or lymph node–positive disease who gave consent, concomitant ctx (5-day intravenous bolus fu-fa on weeks 1 and 5 of rt or oral uft-fa on irradiation days throughout treatment) was followed by a 4- to 6-week interval between last day of rt and surgery. Postoperative ctx was then initiated, preferably within 4–6 weeks. The ctx regimen was chosen based on the pathology report (oxaliplatin-based regimens in lymph node–positive cases; and consistent with the type of preoperative ctx, fu-fa or uft in lymph node–negative cases, even for patients with complete tumour response). Adjuvant ctx was not applied in patients who were admitted after the 6th postoperative month or who did not give consent for treatment.
RT
During 2004–2007, conventional two-dimensional (2D) pelvic rt was given using 60Co (Alcyon II: General Electric, Milwaukee, WI, U.S.A.) or the Saturn 41 Linear Accelerator (General Electric, Buc, France) and an anteroposterior or 3- or 4-field technique. Starting in 2007, three-dimensional (3D) conformal pelvic rt with linear accelerators was given [Onco Impression (Siemens Healthineers, Erlangen, Germany) with the XiO planning system (Elekta, Stockholm, Sweden), or Clinac DHX with the Eclipse planning system (Varian Medical Systems, Palo Alto, CA, U.S.A.)]. Pelvic rt was delivered at a dose of 50–50.4 Gy in 25–28 fractions, 45–46 Gy in 23–25 fractions, or 56 Gy (50 Gy plus 6 Gy boost) in 28–31 fractions. In patients treated with 2D systems, boost doses to the tumour site in the rectum were implemented with anteroposterior fields; in 3D systems, boost doses were implemented to the gross tumour volume, with 1 cm margins. In patients with low performance status or resectable liver metastasis, short-term rt (25 Gy in 5 fractions) was given. A few patients received 39 Gy in 13 fractions because of their performance status.
Concomitant CTx with Irradiation
Most patients received ctx either in the form of fu-fa [fu 350–400 mg/m2 daily, and fa 20 mg/m2 as a bolus at weeks 1 and 5 of irradiation; or uft-fa (tegafur 300 mg/m2 daily in 2 divided doses with fa 15 mg tablet, 2 times daily) on irradiation days.
Adjuvant CTx
After long-term neoadjuvant ccrt and curative resection, patients with any postoperative tumour stage and negative lymph node involvement received 4–6 cycles of fu-fa or uft-fa depending on their initial concomitant ctx. Patients with positive lymph node involvement received an oxaliplatin-based adjuvant ctx regimen [either xelox (oxaliplatin 130 mg/m2 on day 1, followed by oral capecitabine 1000–1200 mg/m2 twice daily for days 1–14 every 3 weeks) or folfox4 (oxaliplatin 85 mg/m2 on day 1, followed by leucovorin 200 mg/m2 by intravenous infusion on day 1 and fu 400 mg/m2 by intravenous bolus given over 2–4 minutes, followed by fu 600 mg/m2 by 22-hour continuous intravenous infusion on days 1 and 2 every 2 weeks for a total of 12 cycles)].
Statistical Analysis
The statistical analysis was conducted using the MedCalc software application (version 12.7.7: MedCalc Software, Ostend, Belgium). Chi-square and Fisher exact tests were used to analyze categorical data. Numerical data were analyzed using the Student t-test and Mann–Whitney U-test for normally and non-normally distributed variables respectively. The survival analysis used the Kaplan–Meier method, and the log-rank test was used for comparisons. Data are expressed as mean ± standard error of the mean, medians with ranges, and percentages as appropriate. Statistical significant was accepted at p < 0.05.
RESULTS
Patient Demographics and Histopathologic Tumour Characteristics
Table i shows the characteristics of the patient cohort. Preoperative evaluation showed that tumours were located mostly at the distal rectum (35.0%) and were identified as T3 (75.0%) and N1 (51.0%) tumours.
TABLE I.
Patient demographics and tumour histopathologic characteristics
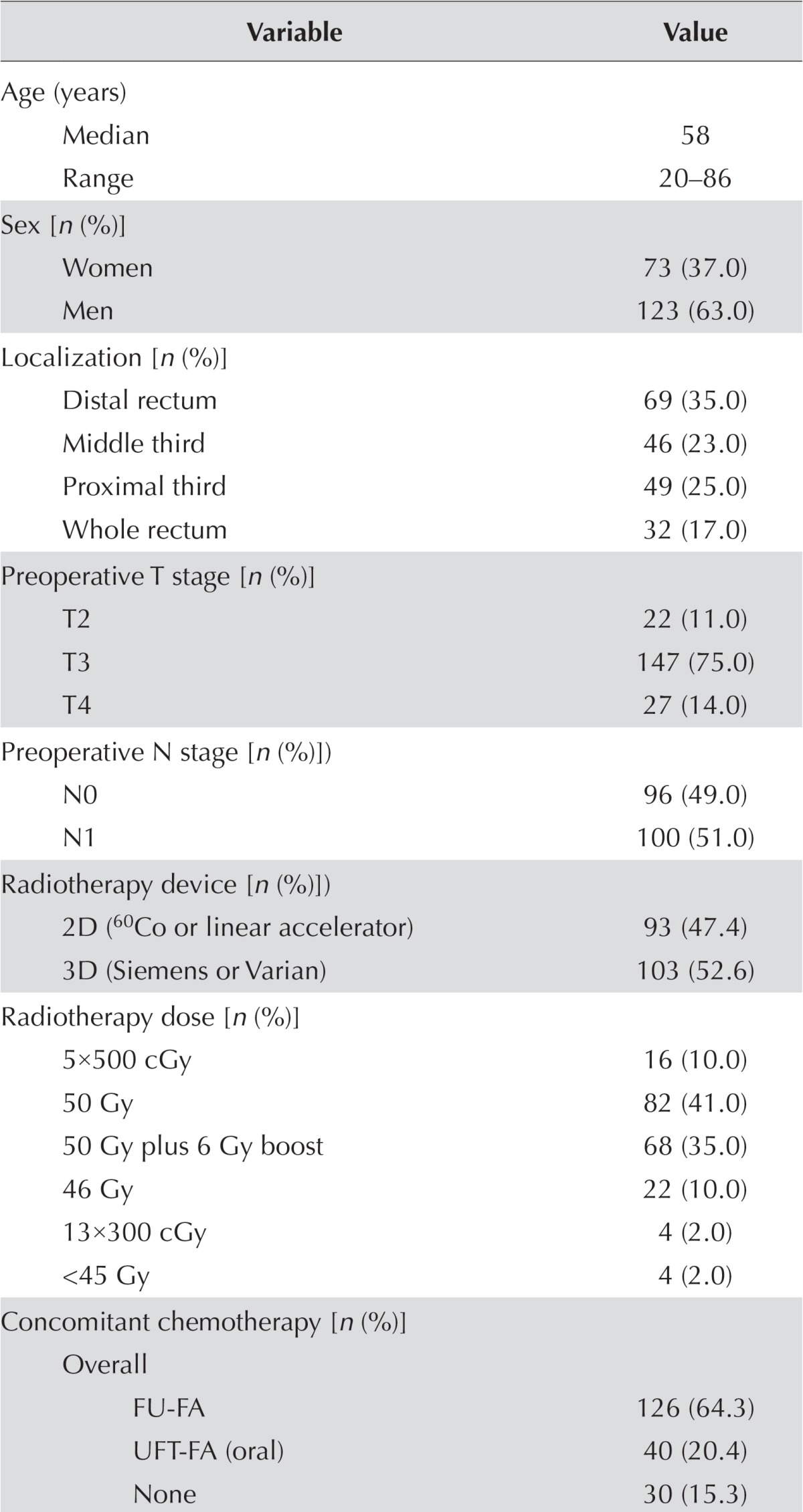
| Variable | Value |
|---|---|
| Age (years) | |
| Median | 58 |
| Range | 20–86 |
| Sex [n (%)] | |
| Women | 73 (37.0) |
| Men | 123 (63.0) |
| Localization [n (%)] | |
| Distal rectum | 69 (35.0) |
| Middle third | 46 (23.0) |
| Proximal third | 49 (25.0) |
| Whole rectum | 32 (17.0) |
| Preoperative T stage [n (%)] | |
| T2 | 22 (11.0) |
| T3 | 147 (75.0) |
| T4 | 27 (14.0) |
| Preoperative N stage [n (%)]) | |
| N0 | 96 (49.0) |
| N1 | 100 (51.0) |
| Radiotherapy device [n (%)]) | |
| 2D (60Co or linear accelerator) | 93 (47.4) |
| 3D (Siemens or Varian) | 103 (52.6) |
| Radiotherapy dose [n (%)] | |
| 5×500 cGy | 16 (10.0) |
| 50 Gy | 82 (41.0) |
| 50 Gy plus 6 Gy boost | 68 (35.0) |
| 46 Gy | 22 (10.0) |
| 13×300 cGy | 4 (2.0) |
| <45 Gy | 4 (2.0) |
| Concomitant chemotherapy [n (%)] | |
| Overall | |
| FU-FA | 126 (64.3) |
| UFT-FA (oral) | 40 (20.4) |
| None | 30 (15.3) |
| Excluding inoperable cases | |
| FU-FA | 117 (59.7) |
| UFT-FA (oral) | 38 (19.4) |
| None | 24 (12.2) |
| Excluding patients treated with hypofractionated radiotherapy | |
| FU-FA | 114 (58.2) |
| UFT-FA (oral) | 38 (19.4) |
| None | 11 (5.6) |
| Operation type [n (%)] | |
| Miles | 66 (36.9) |
| Low anterior resection | 113 (63.1) |
| Postoperative T stage [n (%)] | |
| Complete response | 23 (13.0) |
| T1 focal | 18 (10.0) |
| T2 | 35 (20.0) |
| T3 | 93 (51.0) |
| T4 | 10 (6.0) |
| Postoperative N stage [n (%)] | |
| N0 | 107 (54.5) |
| N1 | 39 (19.9) |
| N2 | 33 (16.8) |
| Adjuvant chemotherapy (n=125) | |
| FU-FA | 70 (57.0) |
| XELOX | 15 (12.4) |
| UFT-FA (oral) | 10 (8.3) |
| FOLFOX | 30 (22.3) |
FU = 5-fluorouracil (350–400 mg/m2 daily); FA = leucovorin–folinic acid (20 mg/m2) as a bolus at 1st and 5th weeks of irradiation; UFT = a fixed combination of the oral FU prodrug tegafur and folinic acid 300 mg/m2 daily in two divided doses; XELOX = capecitabine–oxaliplatin; FOLFOX = oxaliplatin–FU–folinic acid.
Radiotherapy treatment was given using 2D systems in 93 patients (47.4%) and using 3D conformal rt 103 patients (52.6%). An anteroposterior technique was used in 34 patients (17.3%) and a 4-field box technique in 162 patients (82.7%). Pelvic rt was delivered at a dose of 50–50.4 Gy in 41.0% of patients and at 50 Gy plus a 6 Gy boost dose in 35.0% of patients. Concomitant ctx was given before surgery in 85% of the patients, mostly using fu-fa regimens (65.0%). Low anterior resection was the most common type of surgery (58.2%), followed by the Miles procedure (32.6%).
Postoperative evaluation reported T3 (51.0%) and N0 (54.5%) tumours in just more than half the patients. Adjuvant ctx using fu-fa (57.0%) and folfox (22.3%) was given after neoadjuvant ccrt and surgery in 125 of the 179 operated patients (69.8%, Table i).
Differentiation was noted to be intermediate in most patients (n = 120), with smaller numbers having poorly differentiated (n = 30) or well-differentiated (n = 21) tumours. No significant differences in terms of histologic grade were observed (data not shown).
Baseline Characteristics by RT Dose and Device
Apart from a significantly lower percentage of female patients receiving therapy by 2D Co60 than by 2D and 3D linear accelerator (p = 0.02), and a significantly higher percentage of T4 tumours being treated with a 46 Gy dose than with a 50 Gy dose of rt (p = 0.04), no significant differences were evident in patient demographics or baseline tumour T and N stages with respect to rt dose and type of rt device used (Table ii).
TABLE II.
Patient demographics and baseline tumour characteristics, by radiotherapy device and dose
| Radiation therapy variable | Characteristic [n (%)] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Age | Sex | T Stage | N Stage | Total | ||||||
|
|
|
|
|
|
||||||
| <58 Years | ≥58 Years | Women | Men | T2 | T3 | T4 | N0 | N1 | ||
| Device | ||||||||||
| 2D (60Co) | 12 (75.0) | 4 (25.0) | 2 (12.5) | 14 (87.5) | 2 (12.5) | 10 (62.5) | 4 (25.0) | 8 (50.0) | 8 (50.0) | 16 (100.0) |
| 2D (linear accelerator) | 38 (49.4) | 39 (50.6) | 36 (46.7) | 41 (53.2) | 10 (13.0) | 56 (72.7) | 11 (14.3) | 38 (49.4) | 39 (50.6) | 77 (100.0) |
| 3D (Siemens or Varian) | 52 (50.5) | 51 (49.5) | 35 (34.0) | 68 (66.0) | 10 (10.0) | 81 (78.4) | 12 (11.6) | 51 (49.5) | 52 (50.5) | 103 (100.0) |
| p=0.27 a | p= 0.02a | p=0.61a | p=0.99 | |||||||
| Dose | ||||||||||
| 50 Gy | 47 (57.3) | 35 (42.7) | 26 (31.7) | 56 (68.3) | 12 (14.6) | 66 (80.4) | 4 (5.0) | 42 (51.2) | 40 (48.8) | 82 (100.0) |
| 56 Gy | 37 (54.4) | 31 (45.6) | 29 (42.6) | 39 (57.4) | 7 (10.3) | 51 (75.0) | 10 (14.7) | 33 (48.5) | 35 (51.5) | 68 (100.0) |
| 46 Gy | 13 (59.1) | 9 (40.9) | 9 (41.0) | 13 (59.0) | 2 (9) | 14 (63.6) | 6 (27.4) | 11 (50.0) | 11 (50.0) | 22 (100.0) |
| p=0.90 | p=0.35 | p=0.04a | p=0.90 | |||||||
Fisher exact test; others, chi-square test.
Survival Analysis in the Overall Study Population
Overall, the median os duration was 81.5 months (95% confidence interval: 73.7 months to 89.4 months), and the dmfs duration was 68.4 months (95% confidence interval: 40.4 months to 96.4 months). Median os duration for patients who received long-course ccrt (n = 165), excluding those with inoperable tumours and those receiving hypofractionated rt, was 110.8 months overall, 73.8 months for T3 tumours, and 24.2 months for T4 tumours (95% confidence interval: 0.0 months to 54.3 months). For patients whose tumours were located in the distal rectum and who received long-course ccrt (n = 58), median os duration was 110.8 months for T2 and 9.7 months for T4 tumours.
Prognostic Factors for OS Duration
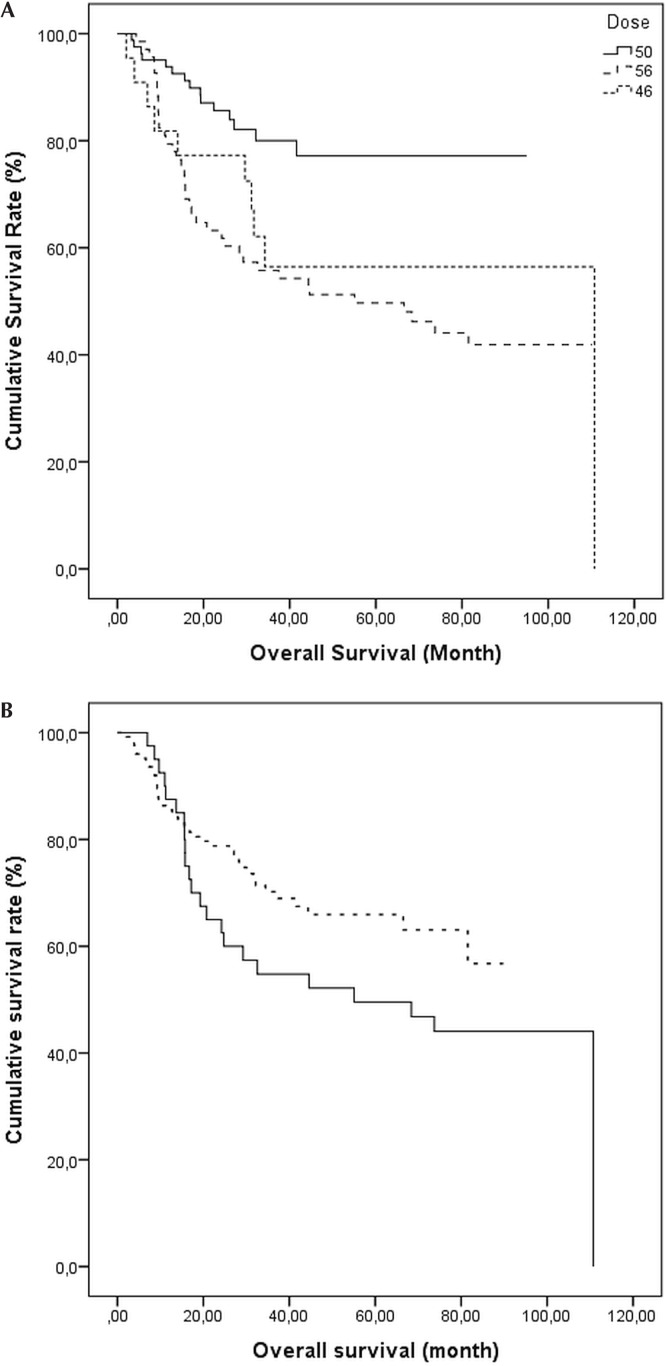
Analysis of survival time by dose of rt, with the exclusion of patients receiving hypofractionated rt (log-rank p = 0.002), revealed a higher os duration for patients receiving 50 Gy compared with 56 Gy or 45–46 Gy [p < 0.001, Figure 1(A)].
FIGURE 1.
Kaplan–Meier survival curves for overall survival with (A) conventionally fractionated irradiation [50 Gy, 50 Gy plus 6 Gy boost, and 46 Gy (excludes hypofractionated radiotherapy)], log-rank p = 0.002; and (B) concomitant chemotherapy (broken line: 5-fluorouracil–leucovorin–folinic acid; solid line: tegafur–folinic acid), log-rank p = 0.04.
A significant difference in survival time was noted depending on the type of concomitant ctx, with a longer os duration being observed in patients receiving fu-fa than in those receiving uft-fa [p = 0.045, Figure 1(B)].
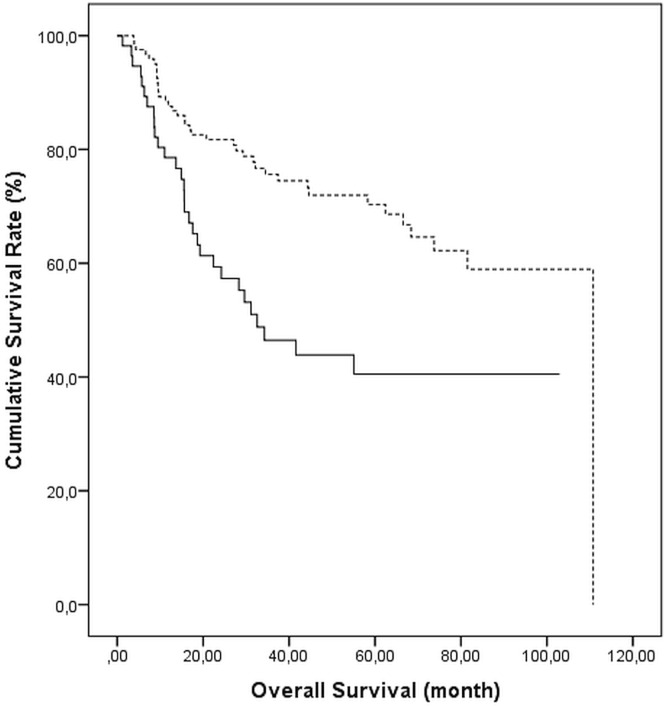
Median os duration was significantly longer in patients receiving than not receiving postoperative adjuvant ctx (110.7 months vs. 31.1 months, p = 0.0005, Figure 2).
FIGURE 2.
Survival probability for patients receiving (broken line) and not receiving (solid line) adjuvant chemotherapy, log-rank p = 0.001.
Prognostic Factors for 5-Year DMFS and OS
In the overall study population, the 5-year os rate was 57.0%, and the dmfs rate was 54.1%. Considering prognostic factors, downstaging was positive in 46.9% of patients, lymphovascular invasion was negative in 55.3%, and perineural invasion was negative in 50.3%.
Presence of downstaging was associated with a significantly higher os rate (71.7% vs. 53.8%, p = 0.013) and dmfs rate (71.6% vs. 51.1%, p = 0.005). Lack of perineural invasion was associated with a significantly higher dmfs rate (76.4% vs. 50.7%, p = 0.029, Table iii).
TABLE III.
Prognostic factors for 5-year rates of distant metastasis–free (DMFS) and overall survival (OS)
| Factor | Patients (n of 196) | OS | DMFS | ||
|---|---|---|---|---|---|
|
|
|
||||
| (%) | p Valuea | (%) | p Valuea | ||
| Downstaging | |||||
| Positive | 84 | 71.7 | 0.013 | 71.6 | 0.0050 |
| Negative | 95 | 53.8 | 51.1 | ||
| Lymphovascular invasion | |||||
| Positive | 110 | 59.8 | 0.853 | 59.2 | 0.711 |
| Negative | 63 | 62.1 | 62 | ||
| Perineural invasion | |||||
| Positive | 71 | 53 | 0.054 | 50.7 | 0.029 |
| Negative | 100 | 78.33 | 76.4 | ||
| Radiotherapy doseb | |||||
| 46 Gy | 22 | 56.4 | 0.002 | 56.3 | 0.002 |
| 50 Gy | 82 | 77.2 | 77.2 | ||
| 56 Gy | 68 | 49.7 | 49.3 | ||
| Preoperative T stage | |||||
| T2 | 22 | 57.9 | 0.040 | 59.1 | 0.010 |
| T3 | 147 | 61.7 | 61.4 | ||
| T4 | 27 | 33.4 | 32.9 | ||
| Postoperative T stage | |||||
| Complete response | 23 | 62.0 | 0.042 | 62.0 | 0.015 |
| T1–T2 | 52 | 72.2 | 72.4 | ||
| T3 | 93 | 61 | 58.5 | ||
| T4 | 11 | 25.5 | 26.9 | ||
| Postoperative nodal stage | |||||
| N0 | 107 | 69.5 | 0.0005 | 69.7 | 0.0005 |
| N1 | 39 | 67.3 | 63.8 | ||
| N2 | 33 | 28.5 | 25.9 | ||
| Concomitant chemotherapy | |||||
| Overall | |||||
| FU-FA | 126 | 65.9 | 0.004 | 67.7 | 0.001 |
| UFT-FA | 40 | 49.6 | 49.6 | ||
| None | 30 | 35.7 | 29.9 | ||
| Exclusions appliedc | |||||
| FU-FA | 114 | 71.1 | 0.034 | 72.8 | 0.043 |
| UFT-FA | 38 | 50.0 | 50.0 | ||
By log-rank test; significant values shown in boldface type.
Based on conventional fractionated radiation therapy.
Excludes patients with inoperative tumours and those who received hypofractionated radiotherapy.
FU = 5-fluorouracil (350–400 mg/m2 daily); FA = leucovorin–folinic acid (20 mg/m2) as a bolus at 1st and 5th weeks of irradiation; UFT = a fixed combination of the oral FU prodrug tegafur and fluoropyrimidine 300 mg/m2 daily in two divided doses.
Compared with T1–3 tumours, T4 tumours were associated with significantly lower os rates (preoperative p = 0.040 and postoperative p = 0.042) and dmfs rates (p = 0.010 and p = 0.015 respectively). The 5-year os and dmfs rates were significantly lower for postoperative N2 tumours (each p = 0.0005) than for N0–1 tumours (Table iii).
Compared with other long-term rt doses, a dose of 50 Gy was associated with a significantly higher os rate (77.2%) and dmfs rate (77.2%, p = 0.002, Table iii).
The 5-year os and dmfs rates were significantly higher in patients who received concomitant ctx than in those who did not (p = 0.004 and p = 0.001 respectively). Survival rates were noted to be better with fu-fa than with uft-fa regimens (os: 71.1% vs. 50.0%, p = 0.034; dmfs: 72.8% vs. 50.0%, p = 0.043; Table iii).
Whether a 2D Co60, 2D linear accelerator, or 3D linear accelerator system was used, 5-year os rates and dmfs rates were similar (os: 54.2%, 50.1%, and 57.5% respectively, p = 0.28; dmfs: 52.7%, 43.2%, and 61.7% respectively, p = 0.28), despite the use of significantly different radiation doses: 45–46 Gy [27.0% (n = 6) for 2D Co60, 18.0% (n = 4) for 2D linear accelerator, and 55.0% (n = 12) for 3D linear accelerator], 50 Gy [6.0% (n = 5), 12.0% (n = 10), and 82.0%
Postoperative Adjuvant CTx and Survival
Use of adjuvant ctx after neoadjuvant long-term ccrt and surgery was associated with better 5-year os rates for all postoperative T stages (apart from T4) and also for all postoperative lymph node stages. The survival benefit obtained with adjuvant ctx was particularly remarkable for patients postoperatively staged T2 (73.6% vs. 56.1% without ctx) and T3 (69.3% vs. 17.9% without ctx, p = 0.0005) and even in patients postoperatively staged as N0 (77.2% vs. 52.3% without ctx, p = 0.0005, Table iv).
TABLE IV.
5-Year rates of overall survival (OS) in patients receiving and not receiving adjuvant chemotherapy, by postoperative staging
| Variable | Adjuvant chemotherapy | |||
|---|---|---|---|---|
|
| ||||
| Yes (n=125) | No (n=54) | |||
|
|
|
|||
| Pts (n) | OS (%) | Pts (n) | OS (%) | |
| Postoperative T stage | ||||
| Complete response | 11 | 90.9 | 12 | 41.0 |
| Focal T1 | 12 | 83.3 | 6 | 80.0 |
| T2 | 22 | 73.6 | 13 | 56.1 |
| T3 | 75 | 69.3 | 18 | 17.9 |
| T4 | 5 | 20.0 | 5 | 30.0 |
| p=0.0005a,b | ||||
| Postoperative lymph node stage | ||||
| N0 | 74 | 77.2 | 33 | 52.3 |
| N1 | 29 | 75.8 | 10 | 15.2 |
| N2 | 22 | 38.7 | 11 | 11.4 |
| p=0.0005a,c | ||||
By Mantel–Cox log-rank test.
Adjusted for postoperative tumour stage.
Adjusted for postoperative nodal stage.
Pts = patients.
Operability After Neoadjuvant Treatment
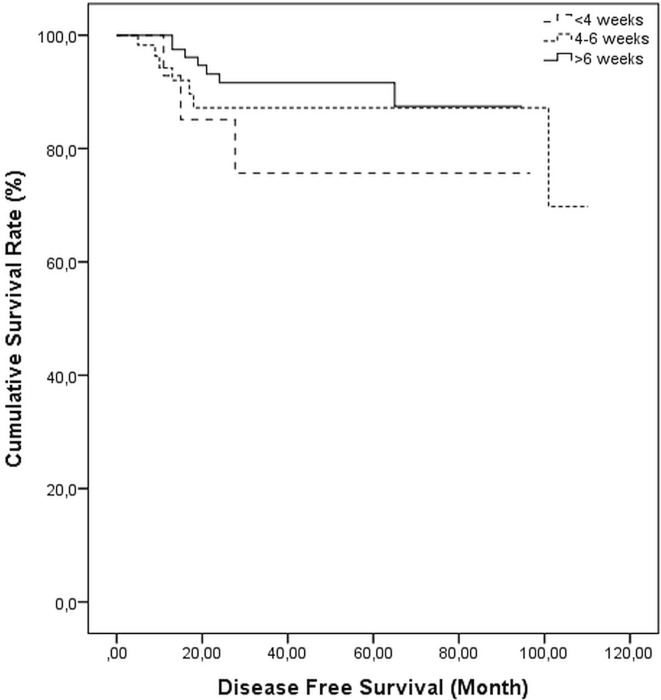
Given that tumours in 17 patients (8.7%) were determined to be inoperable, 179 patients (91.3%) underwent surgery. Of those 179 patients, 99 (55.3%) received 6 weeks of ccrt, ending within 4–6 weeks of surgery in 59 patients (33.0%) and ending 4 weeks before surgery in 21 patients (11.7%). A median 45-day interval (range: 22–339 days) was noted between the last day of ccrt and surgery. No difference in the duration of dmfs was observed with respect to the interval from ccrt to surgery (log-rank p = 0.652, Figure 3).
FIGURE 3.
Kaplan–Meier survival curves for distant metastasis-free survival, by interval in weeks between chemoradiotherapy and surgery, log-rank p = 0.652.
Distant metastasis, mostly to liver (11.6%), was noted in 19.6% of the patients; local recurrence was noted in 3.6% of patients.
DISCUSSION
Our findings reveal that concomitant ctx (85.0%) and postoperative adjuvant ctx (69.8%) were given in most of our rectal cancer patients. Rates for 5-year os, dmfs, distant metastasis, and local recurrence were 57.0%, 54.1%, 19.6%, and 3.6% respectively. Median os duration extended to 110.8 months in operable patients and in patients with a tumour of the distal rectum, particularly for tumours staged T2 or lower. The 5-year os and dmfs rates were significantly higher in patients with rather than without TN downstaging, in patients receiving a rt dose of 50 Gy rather than 56 Gy, and in patients receiving rather than not receiving concomitant ctx. Patients postoperatively staged T0–3 or those of any lymph node stage who received adjuvant ctx after neoadjuvant long-term ccrt and surgery experienced better survival. A radiation dose exceeding 50 Gy and the interval between ccrt and surgery had no significant effect on survival.
An interval longer than 6–8 weeks between preoperative irradiation and curative surgery has been suggested to provide increased tumour downstaging with no detrimental effect10, together with an increased response rate to radiation, and thus a chance to undergo radical surgery11,12. In a meta-analysis of 3584 patients, an interval longer than the conventional 6–8 weeks was reported to be associated with more pathologic complete responses (relative risk: 1.42; an absolute increase to 20% from 14%)13. Notably, with a median 45-day interval between preoperative ccrt and surgery, our findings indicate that prolongation of surgery beyond 6 weeks after ccrt had no significant effect on survival or tumour response. Similarly, in a study conducted with 1593 patients attending 92 Dutch hospitals who underwent preoperative ccrt, the highest chance of pathologic complete response was noted when the interval between ccrt and surgery was about 11 weeks, with no apparent further increase being noted beyond that time14. Prolongation of the interval to 6–8 weeks or more from 4–6 weeks to improve rates of local control or survival has been considered not to be reasonable in tumours considered upfront to be resectable15.
The rate of local tumour downstaging through preoperative short-course rt followed by capecitabine, oxaliplatin, and bevacizumab and delayed surgery was reported to be 47.0% in rectal cancer patients16. Also, delivery of radiation at a dose of 33 Gy in 10 fractions to the pelvis for 2 weeks before curative surgery was reported to be associated with a local tumour downstaging rate of 33.8%12. In the present study, downstaging achieved using 6-week conventionally fractionated irradiation together with fu-based ctx seems to agree with earlier studies aimed at applying short-course irradiation with newer chemotherapeutics, while not supporting the survival benefit associated with prolongation of the interval between ccrt and surgery12,16–18.
Pathologic T and N stage, lymphatic invasion, and extent of tumour regression were reported to significantly predict disease-free survival (dfs) and dmfs in rectal cancer patients19,20, and the presence of positive nodes after surgery was associated with poor dfs21. Similarly, our findings revealed that preoperative T stage, postoperative TN stage, and TN downstaging are among the prognostic factors associated with survival benefit in terms of longer os duration and higher os and dmfs rates.
Perineural invasion has been considered to be highly predictive of long-term outcome, with an effect on adjuvant decision-making in rectal cancer patients22,23. Notably, the rate of perineural invasion in our cohort (35.7%) seems slightly higher than has been seen in other series22,23 and was also associated with a lower rate of dmfs.
That 5-year os and dmfs were achieved in more than half the patients in our cohort with the use of conventionally fractionated ccrt supports the likelihood that neoadjuvant ccrt is associated with improved rates of locoregional control and dfs19,24–27.
Considering the patients in our cohort who received long-term rt, the os duration was longer overall in operable patients with tumours staged T2 than in those staged T4 (110.8 months vs. 24.2 months) and also in patients with tumours located in the distal rectum (110.8 months vs. 9.7 months for tumours in other locations). Nonetheless, T downstaging as a correlate of pathologic response to neo-adjuvant ccrt could be jeopardized, given that a tumour with slight regression might be downstaged from T3 to T2, but a that tumour showing a good response with only microscopic foci of tumour cells in the subserosa might still be staged T322.
Compared with patients not receiving adjuvant ctx, those in our cohort who did receive ctx experienced a better survival rate in all postoperative T-stage groups (0–3) and all lymph node stage groups. Similarly, data from a pooled analysis of five randomized studies revealed an association of postoperative ctx with or without postoperative rt with a 20% absolute survival benefit compared with observation or postoperative rt alone28. Also, even when including patients of all stages receiving all treatment modalities with or without rt or ccrt, rectal cancer patients showed a significant gain in os when treated with single-agent fu as adjuvant ctx (hazard ratio: 0.83), as reported in a Cochrane review29.
Nonetheless, the efficacy of adjuvant ctx after neoadjuvant long-term ccrt and surgery is not well documented and remains controversial9,30–38. Most available data do not support the routine use of adjuvant ctx for patients who have received preoperative ccrt, and there is a controversy about whether patients with a good response to ccrt would benefit from adjuvant ctx30. In past studies, no benefit of adjuvant ctx has been suggested for patients with ypN0 rectal cancer—especially for those with ypT0–2N0 disease31–33. And no clarity has yet been achieved about whether patients with ypN+ or ypT3–4N0 disease characterized by a poor response to preoperative ccrt would benefit from adjuvant ctx9,34,35.
In a meta-analysis of randomized trials involving rectal cancer patients, surgery with or without fluoropyrimidine was compared with surgery plus fluoropyrimidine with or without oxaliplatin. The authors reported that, in two of the four randomized trials analyzed, a significantly higher dfs rate was associated with adjuvant fu plus oxaliplatin than with adjuvant fu only; and after a pooled analysis, the authors concluded that the use of postoperative adjuvant ctx in patients with rectal cancer receiving preoperative rt or ccrt was not based on strong scientific evidence36.
Nonetheless, in a recent phase iii cao/aro/aio-04 trial, a significantly improved dfs rate was reported with the addition of oxaliplatin to postoperative adjuvant ctx in 445 rectal cancer patients with clinical stage cT3–4 or cN1–2 tumours9. Also, findings from the phase ii randomized adore trial, conducted in 321 rectal cancer patients with pathologic TNM stage ii or iii disease, revealed a significant improvement in 3-year dfs with use of the folfox regimen (fluorouracil–leucovorin–oxaliplatin) compared with fluoropyrimidines alone after ccrt and surgery, only in pN+ patients37. Thus, the authors emphasized the potential benefit of adding oxaliplatin to adjuvant ctx in patients with disease less responsive to fluoropyrimidine-based ccrt37.
In another trial in 110 rectal cancer patients who were preoperatively treated with long-course rt plus concurrent fluoropyrimidines, the use of postoperative adjuvant ctx was based on a risk-adapted strategy, with administration of fluoropyrimidines alone in the good-prognosis group (postoperative downstaging to pT0–2N0) and administration of an oxaliplatin-based combination in the postoperative poor-prognosis group (pT3–4 or N+). Results revealed high 5-year dfs rates (79.4% and 66.3% respectively)38.
Based on better 5-year survival rates for patients in our cohort treated with adjuvant ctx after neoadjuvant long-term ccrt and surgery at all postoperative T0–3 stages and even in the N0 stage, our findings emphasize the further benefit and feasibility of adjuvant ctx even in patients with a good response to preoperative ccrt38–41.
The local recurrence rate in our cohort (3.6%) is in line with the idea that any improvement in os will require better control of systemic disease while holding the rate of local recurrence below 5%–10%5. However, given that distant metastasis was noted in 19.6% of our patients, mostly to liver (11.6%), our findings support consideration of more intensive adjuvant treatment, such as ctx for high-risk patients after preoperative ccrt and radical surgery, to achieve better disease control and to improve os42.
Comparison of various schedules of rt and ccrt in terms of extension and dose used in past studies revealed no significant difference in long-course preoperative rt doses of 46 Gy and 50 Gy43, short- or long-course ccrt schedules44, or long-course preoperative rt at 45 Gy plus capecitabine compared with an intensified neoadjuvant ccrt schedule using 50 Gy plus capecitabine and oxaliplatin45. In our series, we preferred to choose short-course treatment based on the performance status of the patients rather than age. We treated 10 patients more than 80 years of age, with 3 of them receiving 5×500 cGy, 2 receiving 13×300 cGy, and 5 receiving conventional doses of 46–56 Gy. Although the present study is not a randomized controlled trial, a good number of our patients (n = 68) received 50 Gy plus a 6 Gy boost. However, in contrast to the documented role of an extra dose of rt in counteracting accelerated tumour repopulation in the case of a long course of conventionally fractionated rt46,47, a dose of 50 Gy seemed to have the best outcome in our cohort.
Despite there being no significant difference in terms of distribution of T and N stages and patient demographics between the groups receiving rt doses of 50 Gy versus 56 Gy and 46 Gy versus 56 Gy, a dose of 50 Gy (compared with 56 Gy) was associated with a significant survival benefit in our cohort. No significant difference was noted between 56 Gy and other rt doses with respect to rates of distant metastasis and downstaging in the postoperative follow-up. It should also be noted that technology differences had no effect on survival in our cohort: similar oncologic outcomes were noted for 2D and 3D treatment systems despite the higher frequency of 56 Gy doses in patients treated using 2D systems. Hence, the low survival rate associated with 56 Gy doses compared with 50 Gy doses in our cohort seems not to be a result of differences in rt device, distant metastasis, or downstaging rate. That finding emphasizes the need for larger-scale randomized trials to address the survival benefit associated with rt doses exceeding 50 Gy in rectal cancer patients.
Given the inconsistent findings from studies considering concurrent administration of oxaliplatin or alternative biologic agents and rt48, approaches for appropriate integration of more effective therapies into combined-modality programs to enhance disease control and treatment response are still under investigation7.
A conventional 6-week course of preoperative rt concomitant with a fu-fa or uft-fa ctx regimen had favourable outcomes in our cohort in terms of downstaging and survival rates, with no benefit to the os rate of using a higher radiation dose. However, prudence must be used when interpreting those results, given that new-generation agents such as capecitabine or oxaliplatin could not be used in our cohort on account of current legislation for health care in Turkey, which has prohibited the use of uft-fa regimens since 2007, while allowing for the use of intravenous fu and fa for preoperative ccrt in patients with locally advanced rectal cancer.
Temporal sequencing of treatment modalities, integration of rt and ctx, and radiation dose fractionation have been indicated as part of the main focus in developing new therapeutic strategies to improve local tumour control and os benefit49. In that regard, our findings indicate longer os with use of neoadjuvant ccrt regardless of a higher dose of radiation or a longer interval from ccrt to surgery, together with the survival benefit obtained by the use of adjuvant ctx after neoadjuvant long-term ccrt and surgery in locally advanced rectal carcinoma.
Certain limitations to the present study should be considered. First, because of the retrospective single-centre design, establishing temporality between cause and effect and also generalizing our findings to the overall patient population seems difficult. Second, although no significant difference in terms of T or N stage and patient characteristics was evident between patients treated with 50 Gy and those treated with 56 Gy, most patients receiving an external radiation boost had 2D treatment planning (60 of 68). However, although the use of 2D and 3D treatment systems showed significant differences with respect to the radiation doses, survival outcomes were similar. Nonetheless, it seems reasonable not to exceed 50 Gy during treatment (such as in 2D systems) given potential limitations about the identification of dose to high-risk organs and the precision of tumour targeting. However, well-designed randomized trials for advanced techniques (intensity-modulated rt, for instance) using boost irradiation are needed to assess the survival benefit of radiation doses exceeding 50 Gy in rectal cancer patients and to exclude the likelihood of overtreatment. Despite those limitations, our findings provide data on long-term outcomes in rectal cancer patients who successfully complete planned treatment schedules and contribute to the literature on this topic.
CONCLUSIONS
Our findings in a single-centre cohort of rectal cancer patients revealed favourable survival outcomes, with 5-year os and dmfs rates of 57.0% and 54.1% respectively, after a 6-week schedule of conventionally fractionated irradiation with concomitant fu-based ctx. Use of a 50 Gy dose of rt preoperatively and administration of postoperative adjuvant ctx were associated with a significant survival benefit, and neither the use of a radiation dose higher than 50 Gy nor the interval between ccrt and surgery had a significant effect on survival. Given the observed survival benefit, our findings seem to suggest that, to achieve better disease control, consideration be given to using adjuvant ctx for patients at any postoperative stage after neoadjuvant long-term ccrt and curative resection. Future larger-scale randomized trials of newer-generation agents and boost irradiation are needed to provide data about tumour response, toxicity, and survival, and thus more convenient administration of ctx and rt.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Winawer SJ. The multidisciplinary management of gastrointestinal cancer. Colorectal cancer screening. Best Pract Res Clin Gastroenterol. 2007;21:1031–48. doi: 10.1016/j.bpg.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Turkish Ministry of Health, Public Health Institute . Turkey Public Health Agency, Cancer Control Directorate, Evaluation Reports: Colorectal Cancer Screening [Turkish] Ankara, Turkey: Cancer Control Directorate; 2008. [Available online at: http://www.kanser.gov.tr/Dosya/Bilgi-Dokumanlari/raporlar/kolorektal.pdf; cited 13 July 2014] [Google Scholar]
- 4.Tsai HL, Wang JY. Predictors of response in locally advanced rectal cancer following concurrent chemoradiotherapy. Genomic Med. 2013;5:18–22. [Google Scholar]
- 5.Rödel C, Liersch T, Becker H, et al. on behalf of the German Rectal Cancer Study Group Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German cao/aro/aio-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–87. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer Ver. 2.2016. Fort Washington, PA: NCCN; 2012. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (free registration required); cited 17 November 2016] [Google Scholar]
- 7.Shin SJ, Kim NK, Keum KC, et al. Phase ii study of preoperative chemoradiotherapy (crt) with irinotecan plus S-1 in locally advanced rectal cancer. Radiother Oncol. 2010;95:303–7. doi: 10.1016/j.radonc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L, Bai C, Shao Y, et al. A phase ii study of neoadjuvant chemoradiotherapy with oxaliplatin and capecitabine for rectal cancer. Cancer Lett. 2011;310:134–9. doi: 10.1016/j.canlet.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Rödel C, Graeven U, Fietkau R, et al. on behalf of the German Rectal Cancer Study Group Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German cao/aro/aio-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–89. doi: 10.1016/S1470-2045(15)00159-X. [DOI] [PubMed] [Google Scholar]
- 10.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396–402. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 11.Peng JY, Di JZ, Wang Y. Delayed surgery for rectal cancer patients receiving neoadjuvant chemoradiotherapy: a promising method in its infancy. Dig Surg. 2012;29:281–6. doi: 10.1159/000341661. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Kim JG, Oh ST, et al. Two-week course of preoperative chemoradiotherapy followed by delayed surgery for rectal cancer: a phase ii multi-institutional clinical trial (krog 11-02) Radiother Oncol. 2014;110:150–4. doi: 10.1016/j.radonc.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studies. Ann Surg. 2016;263:458–64. doi: 10.1097/SLA.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 14.Sloothaak DA, Geijsen DE, van Leersum NJ, et al. on behalf of the Dutch Surgical Colorectal Audit Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg. 2013;100:933–9. doi: 10.1002/bjs.9112. [DOI] [PubMed] [Google Scholar]
- 15.Glimelius B. Optimal time intervals between pre-operative radiotherapy or chemoradiotherapy and surgery in rectal cancer? Front Oncol. 2014;4:50. doi: 10.3389/fonc.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dijk TH, Tamas K, Beukema JC, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage iv rectal cancer. Ann Oncol. 2013;24:1762–9. doi: 10.1093/annonc/mdt124. [DOI] [PubMed] [Google Scholar]
- 17.Yeo SG, Oh JH, Kim DY, et al. Preoperative short-course concurrent chemoradiation therapy followed by delayed surgery for locally advanced rectal cancer: a phase ii multicenter study (krog 10-01) Int J Radiat Oncol Biol Phys. 2013;86:34–9. doi: 10.1016/j.ijrobp.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–33. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 19.Shivnani AT, Small W, Jr, Stryker SJ, et al. Preoperative chemoradiation for rectal cancer: results of multimodality management and analysis of prognostic factors. Am J Surg. 2007;193:389–93. doi: 10.1016/j.amjsurg.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Ruo L, Tickoo S, Klimstra DS, et al. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg. 2002;236:75–81. doi: 10.1097/00000658-200207000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engineer R, Basu T, Chopra S, et al. Factors influencing response to neoadjuvant chemoradiation and outcomes in rectal cancer patients: tertiary Indian cancer hospital experience. J Gastrointest Oncol. 2015;6:155–64. doi: 10.3978/j.issn.2078-6891.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhadda AS, Bessell EM, Scholefield J, Dickinson P, Zaitoun AM. Mandard tumour regression grade, perineural invasion, circumferential resection margin and post-chemoradiation nodal status strongly predict outcome in locally advanced rectal cancer treated with preoperative chemoradiotherapy. Clin Oncol (R Coll Radiol) 2014;26:197–202. doi: 10.1016/j.clon.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Liebig C, Ayala G, Wilks J, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131–7. doi: 10.1200/JCO.2009.22.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fietkau R, Rödel C, Hohenberger W, et al. on behalf of the German Rectal Cancer Study Group Rectal cancer delivery of radiotherapy in adequate time and with adequate dose is influenced by treatment center, treatment schedule, and gender and is prognostic parameter for local control: results of study cao/aro/aio-94. Int J Radiat Oncol Biol Phys. 2007;67:1008–19. doi: 10.1016/j.ijrobp.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. on behalf of the Polish Colorectal Study Group Prediction of mesorectal nodal metastases after chemoradiation for rectal cancer: results of a randomised trial: implication for subsequent local excision. Radiother Oncol. 2005;76:234–40. doi: 10.1016/j.radonc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Weiss J, Moghanaki D, Plastaras JP, Haller DG. Improved patient and regimen selection in locally advanced rectal cancer: who, how, and what next? Clin Colorectal Cancer. 2009;8:194–9. doi: 10.3816/CCC.2009.n.033. [DOI] [PubMed] [Google Scholar]
- 27.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German cao/aro/aio-94 randomized phase iii trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 28.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol. 2004;22:1785–96. doi: 10.1200/JCO.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 29.Petersen SH, Harling H, Kirkeby LT, Wille-Jørgensen P, Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev. 2012;3:CD004078. doi: 10.1002/14651858.CD004078.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poulsen LØ, Qvortrup C, Pfeiffer P, Yilmaz M, Falkmer U, Sorbye H. Review on adjuvant chemotherapy for rectal cancer—why do treatment guidelines differ so much? Acta Oncol. 2015;54:437–46. doi: 10.3109/0284186X.2014.993768. [DOI] [PubMed] [Google Scholar]
- 31.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–6. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiran RP, Kirat HT, Burgess AN, Nisar PJ, Kalady MF, Lavery IC. Is adjuvant chemotherapy really needed after curative surgery for rectal cancer patients who are node-negative after neoadjuvant chemoradiotherapy? Ann Surg Oncol. 2012;19:1206–12. doi: 10.1245/s10434-011-2044-1. [DOI] [PubMed] [Google Scholar]
- 33.Govindarajan A, Reidy D, Weiser MR, et al. Recurrence rates and prognostic factors in ypN0 rectal cancer after neoadjuvant chemoradiation and total mesorectal excision. Ann Surg Oncol. 2011;18:3666–72. doi: 10.1245/s10434-011-1788-y. [DOI] [PubMed] [Google Scholar]
- 34.Bosset JF, Calais G, Mineur L, et al. Fluorouracil based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the eortc 22921 randomised study. Lancet Oncol. 2014;15:184–90. doi: 10.1016/S1470-2045(13)70599-0. [DOI] [PubMed] [Google Scholar]
- 35.You K, Huang R, Gao Y. Adjuvant chemotherapy for rectal cancer. Lancet Oncol. 2014;15:e194. doi: 10.1016/S1470-2045(14)70108-1. [DOI] [PubMed] [Google Scholar]
- 36.Berardi R, Maccaroni E, Onofri A, et al. Locally advanced rectal cancer: the importance of a multidisciplinary approach. World J Gastroenterol. 2014;20:17279–87. doi: 10.3748/wjg.v20.i46.17279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bujko K, Glimelius B, Valentini V, Michalski W, Spalek M. Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: a meta-analysis of randomized trials comparing surgery ± a fluoropyrimidine and surgery + a fluoropyrimidine ± oxaliplatin. Eur J Surg Oncol. 2015;41:713–23. doi: 10.1016/j.ejso.2015.03.233. [DOI] [PubMed] [Google Scholar]
- 38.Hong YS, Nam BH, Kim KP, et al. Oxaliplatin, fluorouracil and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (adore): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014;15:1245–53. doi: 10.1016/S1470-2045(14)70377-8. [DOI] [PubMed] [Google Scholar]
- 39.Sastre J, Serrano JJ, Fernández C, et al. Risk-adapted adjuvant chemotherapy after concomitant fluoropyrimidine-radiotherapy neoadjuvant treatment for patients with resectable CT3–4 or N+ rectal cancer: five-year disease-free survival results of a single-center series. Clin Colorectal Cancer. 2016;15:128–34. doi: 10.1016/j.clcc.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Musio D, De Felice F, Bulzonetti N, et al. Neoadjuvant-intensified treatment for rectal cancer: time to change? World J Gastroenterol. 2013;19:3052–61. doi: 10.3748/wjg.v19.i20.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaffee P, Osipov A, Tan C, Tuli R, Hendifar A. Review of systemic therapies for locally advanced and metastatic rectal cancer. J Gastrointest Oncol. 2015;6:185–200. doi: 10.3978/j.issn.2078-6891.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chao JY, Wang HM, Chiang FF, et al. Preoperative chemoradiotherapy with oxaliplatin and tegafur–uracil in locally advanced rectal cancer: pathologic complete response rate and preliminary results of overall and disease-free survival in a single institute in Taiwan. J Chin Med Assoc. 2014;77:128–32. doi: 10.1016/j.jcma.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Wiltshire KL, Ward IG, Swallow C, et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int J Radiat Oncol Biol Phys. 2006;64:709–16. doi: 10.1016/j.ijrobp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Guckenberger M, Saur G, Wehner D, et al. Comparison of preoperative short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer. Strahlenther Onkol. 2012;188:551–7. doi: 10.1007/s00066-012-0131-2. [DOI] [PubMed] [Google Scholar]
- 45.Rullier A, Gourgou-Bourgade S, Jarlier M, et al. Predictive factors of positive circumferential resection margin after radiochemotherapy for rectal cancer: the French randomised trial accord12/0405 prodige 2. Eur J Cancer. 2013;49:82–9. doi: 10.1016/j.ejca.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 46.Gasinska A, Richter P, Darasz Z, et al. Gender-related differences in repopulation and early tumor response to preoperative radiotherapy in rectal cancer patients. J Gastrointest Surg. 2011;15:1568–76. doi: 10.1007/s11605-011-1589-4. [DOI] [PubMed] [Google Scholar]
- 47.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (rtog) phase iii randomized study to compare hyper-fractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of rtog 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/S0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 48.Gérard JP, Chamorey E, Gourgou-Bourgade S, et al. Clinical complete response (ccr) after neoadjuvant chemoradiotherapy and conservative treatment in rectal cancer. Findings from the accord 12/prodige 2 randomized trial. Radiother Oncol. 2015;115:246–52. doi: 10.1016/j.radonc.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Valentini V, Lambin P, Myerson RJ. Is it time for tailored treatment of rectal cancer? From prescribing by consensus to prescribing by numbers. Radiother Oncol. 2012;102:1–3. doi: 10.1016/j.radonc.2011.12.001. [DOI] [PubMed] [Google Scholar]