Abstract
Objectives
For this guideline, we investigated the effectiveness of radiotherapy with curative intent in medically inoperable patients with early-stage non-small-cell lung cancer (nsclc).
Methods
The guideline was developed by Cancer Care Ontario’s Program in Evidence-Based Care and by the Lung Cancer Disease Site Group through a systematic review of mainly retrospective studies, expert consensus, and formal internal and external reviews.
Recommendations
- ■ Stereotactic body radiation therapy (sbrt) with curative intent is an option that should be considered for patients with early-stage, node-negative, medically inoperable nsclc.
Qualifying Statements
- ■ Because of the high dose per fraction, the planning process and treatment delivery for sbrt require the use of advanced technology to maintain an appropriate level of safety. Consistent patient positioning and 4-dimensional analysis of tumour and critical structure motion during simulation and treatment delivery are essential.
- ■ Preliminary results for proton-beam therapy have been promising, but the technique requires further clinical study.
- ■ Recommended fractionation schemes for sbrt should result in a biologically effective dose of 100 or greater by the linear quadric model, choosing an α/β value of 10 [bed10(LQ) ≥ 100].
Qualifying Statements
- ■ Because of the increased risk of treatment-related adverse events associated with centrally located tumours, consideration of tumour size and proximity to critical central structures is required when determining the dose and fractionation.
- ■ Examples of dose–fractionation schemes used in the included studies have been provided.
- ■ Based on the current evidence and the opinion of the authors, radiation doses at bed10(LQ) greater than 146 might significantly increase toxicity and should be avoided.
- ■ Determination of the radiation bed by the linear quadratic model has limitations for the extreme hypofractionated schemes used in sbrt.
Keywords: Early-stage disease, inoperable tumours, non-small-cell lung cancer, stereotactic body radiation therapy, stereotactic ablative radiation therapy, clinical practice guidelines
INTRODUCTION
Non-small-cell lung cancer (nsclc) is the most common type of lung cancer1. The standard treatment for patients with early-stage nsclc is surgery; however, some patients are unable to undergo surgery because of medical comorbidities such as abnormal underlying cardiovascular or pulmonary function2. Patients with early-stage nsclc who are medically inoperable were previously offered conventional radiotherapy [rt (60–66 Gy in 1.8–2.0 Gy fractions)] or were observed without specific cancer treatment. The outcomes for such patients were not ideal, with 2-year survival being less than 40% with either conventional radiation or observation, and local control being only 40%–50% with conventional rt3,4.
Stereotactic rt uses specialized equipment to position patients so that high-dose fractions can be delivered precisely to a small target or volume of disease. The technique requires complex treatment planning to ensure the accuracy and precision of treatment delivery that is characterized by a steep dose gradient beyond the target volume. Stereotactic body rt (sbrt) and stereotactic ablative rt are considered synonymous for the purposes of this guideline and will be referred to as sbrt from this point forward.
Because outcomes for patients with early-stage nsclc receiving observation or conventional radiation have not been ideal, Cancer Care Ontario’s Radiation Treatment Program, together with its Lung Cancer Disease Site Group (dsg), developed the present guideline containing recommendations for the use of rt with curative intent in medically inoperable patients with early-stage nsclc.
METHODS
The development of this guideline used the methods of the practice guidelines development cycle5,6. The process included a systematic review with interpretation of the evidence by the authors, who then drafted recommendations based on the evidence and expert consensus; internal review by content and methodology experts; and external review by Ontario clinicians and other stakeholders. The authors had expertise in radiation oncology, medical oncology, and health research methodology.
Further details of the methods and findings of the systematic review that informed these recommendations have been published elsewhere7. Briefly, medline, embase, and the Cochrane Library were searched for studies comparing stereotactic radiation treatment with curative intent, observation, and other types of rt for early-stage medically inoperable nsclc. Comparisons of radiation dose or fractionation schedules for sbrt were included. Preplanned study selection criteria were used to screen the literature. Studies were assessed for quality using the robins-i tool (Risk of Bias in Non-randomized Studies of Interventions, http://www.riskofbias.info).
Internal Review
For the guideline document to be approved, at least 75% of the Lung Cancer dsg have to vote on whether they approve the document, and of those that vote, 75% have to approve the document. The Lung Cancer dsg consists of experts in radiation oncology, medical oncology, and surgical oncology in Ontario. In addition, the Program in Evidence-Based Care’s (pebc’s) Report Approval Panel, a 3-person panel with methodology expertise, had to approve the document.
External Review
Two processes were used to obtain feedback on the approved draft guideline from content experts and target users. In the targeted peer review, 7 individuals with content expertise were identified by the authors and were asked to review and provide feedback on the guideline document. In the professional consultation, health care providers with an interest in lung cancer in the pebc database were contacted and asked to complete a brief online survey about the guideline recommendations. That consultation was intended to facilitate the dissemination of the final guidance report to Ontario practitioners.
RESULTS
The authors held teleconferences to develop and approve the recommendations through informal consensus. Each recommendation took into consideration evidence from the systematic review.
Internal Review
On 18 November 2015, the draft guideline was sent to the Lung Cancer dsg members for approval. Of the 24 members of the Lung Cancer dsg, 21 (88%) voted. Of those 21 voters, 21 (100%) approved the document. Also, 3 Report Approval Panel members, including the pebc Director and 2 methodology experts, reviewed and approved the draft guideline in December 2015.
External Review
After approval of the document at internal review, the authors circulated the draft document to external review participants for review and feedback. Of the 7 experts in radiation oncology contacted, 4 agreed to be targeted peer reviewers and provided feedback. Table i summarizes the survey results.
TABLE I.
Responses to nine items on the targeted peer reviewer questionnaire
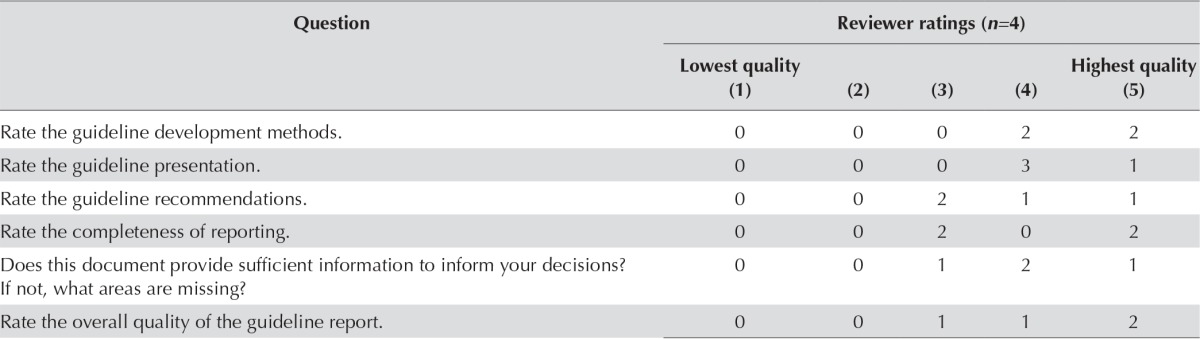
| Question | Reviewer ratings (n=4) | ||||
|---|---|---|---|---|---|
|
| |||||
| Lowest quality (1) | (2) | (3) | (4) | Highest quality (5) | |
| Rate the guideline development methods. | 0 | 0 | 0 | 2 | 2 |
| Rate the guideline presentation. | 0 | 0 | 0 | 3 | 1 |
| Rate the guideline recommendations. | 0 | 0 | 2 | 1 | 1 |
| Rate the completeness of reporting. | 0 | 0 | 2 | 0 | 2 |
| Does this document provide sufficient information to inform your decisions? If not, what areas are missing? |
0 | 0 | 1 | 2 | 1 |
| Rate the overall quality of the guideline report. | 0 | 0 | 1 | 1 | 2 |
|
|
|||||
| Strongly disagree (1) | (2) | Neutral (3) | (4) | Strongly agree (5) | |
|---|---|---|---|---|---|
|
|
|||||
| I would make use of this guideline in my professional decisions. | 0 | 0 | 0 | 2 | 2 |
| I would recommend this guideline for use in practice. | 0 | 0 | 0 | 2 | 2 |
What are the barriers or enablers to the implementation of this guideline report?
| |||||
In the professional consultation, 102 professionals who practice in Ontario and 19 who practice outside Ontario were contacted. Responses were received from 20 (17%) of the professionals, including from 6 who stated that they did not have an interest in the topic or were unavailable to review the guideline at the time. Table ii summarizes the results of the survey responses from the small sample of 14 professionals.
TABLE II.
Responses to four items on the professional consultation survey
| General questions | Overall guideline assessment [n (%)] | ||||
|---|---|---|---|---|---|
|
| |||||
| Lowest quality (1) | (2) | (3) | (4) | Highest quality (5) | |
| Rate the overall quality of the guideline report. | 0 | 0 | 0 | 6 (43) | 8 (57) |
|
|
|||||
| Strongly disagree (1) | (2) | (3) | (4) | Strongly agree (5) | |
|---|---|---|---|---|---|
|
|
|||||
| I would make use of this guideline in my professional decisions. | 0 | 0 | 3 (21) | 6 (43) | 5 (36) |
| I would recommend this guideline for use in practice. | 0 | 0 | 1 (7) | 4 (29) | 9 (64) |
What are the barriers or enablers to the implementation of this guideline report?
| |||||
PRACTICE GUIDELINE
The present report integrates available evidence from observational studies found in the systematic review7 with feedback obtained through the external review process, and has obtained final approval from the Lung Cancer dsg and the Report Approval Panel of the pebc. The target population for the guideline consists of adult patients with potentially curable early-stage (stage i or ii) nsclc (tumours < 5 cm, without nodal involvement or metastases) who are deemed medically inoperable or who refuse surgery. The intended users of the guideline are radiation planning and treatment providers, oncologists, thoracic surgeons, respirologists, diagnostic assessment groups, and other health care providers involved with lung cancer.
Recommendation 1
Stereotactic body rt with curative intent is an option that should be considered for patients with early-stage, node-negative, medically inoperable nsclc.
Qualifying Statements
Because of the high dose per fraction, the planning process and treatment delivery for sbrt require the use of advanced technology to maintain an appropriate level of safety. Consistent patient positioning and 4-dimensional analysis of tumour and critical structure motion during simulation and treatment delivery are essential.
Preliminary results for proton-beam therapy have been promising, but the technique requires further clinical study. More randomized controlled trials are required.
Key Evidence
No randomized trials have compared sbrt with other forms of rt or with observation. One meta-analysis of noncomparative studies8 and eight retrospective cohort studies9–16 compared sbrt with observation or with other forms of rt such as accelerated hypofractionated rt, 3-dimensional conformal rt, conventionally fractionated rt, external-beam rt, and proton-beam or carbon-ion therapy. The evidence was considered to be very low quality because of the potential increase in the risk of bias associated with retrospective designs. However, all studies consistently demonstrated that, compared with observation or alternative rt techniques, sbrt was associated with similar or better survival or local control and with similar or fewer adverse effects (for comparisons with alternative rt techniques). The meta-analysis by Grutters et al.8 found that, compared with sbrt, conventional rt was associated with lower rates of overall survival (os) at 2 years {53% [95% confidence interval (ci): 46% to 60%] vs. 70% [95% ci: 63% to 77%], p < 0.001} and 5 years [20% (95% ci: 15% to 24%) vs. 42% (95% ci: 34% to 50%), p < 0.001] and with lower rates of disease-specific survival at 2 years [67% (95% ci: 59% to 76%) vs. 83% (95% ci: 75% to 92%), p = 0.006] and 5 years [44% (95% ci: 31% to 56%) vs. 63% (95% ci: 50% to 75%), p = 0.045]8.
Interpretation of the Evidence
Although the evidence came from retrospective cohort studies, the consistency of the results led the dsg to believe that the potential benefits in os and local control with sbrt compared with observation and with other rt techniques, especially older conventional rt techniques, outweighed the potential harms associated with sbrt for medically inoperable patients with early-stage nsclc. They therefore considered sbrt to be a recommended treatment option for this patient population.
Recommendation 2
Recommended fractionation schemes for sbrt should result in a biologically effective dose of 100 or greater by the linear quadric model, choosing an α/β value of 10 [bed10(LQ) ≥ 100].
Qualifying Statements
Because of the increased risk of treatment-related adverse events associated with centrally located tumours, consideration of tumour size and proximity to critical central structures is required when determining dose and fractionation.
Examples of dose–fractionation schemes from the studies included in the systematic review can be found in Table iii7. Evidence from the use of those schemes showed consistent tumour control and survival outcomes. Ongoing trials might yield new evidence about optimal stereotactic schedules and recommended doses that are different from those presented in the systematic review.
TABLE III.
Examples of dose–fractionation schemes used in the studies included in the systematic review
| Location | Total dose (Gy) | Fractions (n) | BED10 |
|---|---|---|---|
| Peripheral | |||
| 60 | 3 | 180 | |
| 54 | 3 | 151.2 | |
| 55 | 5 | 115.5 | |
| 48 | 4 | 105.6 | |
| 66 | 3 | 211.2 | |
| 60 | 5 | 132 | |
| Central | |||
| 50 | 5 | 100 | |
| 48 | 4 | 105.6 | |
| 60 | 8 | 105 |
BED10 = biologically effective dose by the linear quadric model, choosing an α/β value of 10.
Based on the current evidence and the opinion of the authors, radiation doses at bed10(LQ) greater than 146 might significantly increase toxicity and should be avoided.
Although the use of radiation doses expressed as beds has been advocated, it is important to understand the limitations of the linear quadratic model in determining radiation beds for the extreme hypofractionated schemes used in sbrt.
Key Evidence
Twelve retrospective observational studies investigated the most appropriate bed cut-off in association with patient outcomes17–28. Again, the studies were considered to be very low quality because of their retrospective design. A meta-regression by Zhang et al.29 found a significant os benefit at 2 years and 3 years with the delivery of a medium bed [83.2–106 (2-year: 76%; 95% ci: 62% to 92%; 3-year: 64%; 95% ci: 57% to 71%)] or a medium-to-high bed [106–146 (2-year: 68%; 95% ci: 61% to 76%; 3-year: 63%; 95% ci: 56% to 71%)] compared with a high bed [>146 (2-year: 56%; 95% ci: 50% to 63%; p < 0.001; 3-year: 50%; 95% ci: 43% to 57%; p < 0.001)] or a low bed at 3 years only [<83.2 (3-year: 52%; 95% ci: 44% to 62%; p < 0.005]. The occurrence of severe adverse events of grades 3–5 was significantly different only between the low and high bed groups. That observation suggests that medium or medium-to-high beds might be the most optimal. However, the cut-off was difficult to determine. Several studies suggested that a bed cut-off of approximately 100 is significantly correlated with patient outcome17,19–22,26; however, other studies, including the meta-regression by Zhang et al., did not show that association18,24,25,27,29.
Interpretation of the Evidence
Although variability in the results with the use of a bed cut-off of approximately 100 was evident, the largest studies suggested that a bed close to 100 was associated with os and local control17,19–22,26. The dsg believed that recommending a minimal bed threshold would maximize the beneficial outcomes associated with sbrt without increasing harm. They chose to use 100 as the bed threshold because most of the larger cohort studies found an association of patient outcomes with bed cut-offs of 100, 105, and 10617,19–22,26. The dsg selected the lowest value because the Zhang meta-analysis found that, compared with lower values, medium values between 83.2 and 106 were associated with significantly better survival29.
Many of the included studies assigned the dose based on the size and location of the tumour. That approach is based on a 2006 study by Timmerman et al.30, which suggested that an increase in damage to critical structures and in the incidence of serious adverse events and toxicity had been found in patients with centrally located tumours when higher dose–fractionation schemes were used. Delivering lower doses, with a minimum bed of 100, to central tumours (compared with peripheral tumours) did not predict inferior os, local control, or increased toxicity31. Those factors should therefore be taken into consideration when deciding on the dose or fractionation schedule.
Although the dsg advocated the use of radiation doses expressed as a bed, it is important to understand the limitations of using the linear quadratic model to determine radiation bed for the extreme hypofractionated schemes used in sbrt. The linear quadratic model has been used as a convenient—and slightly simplified—model to calculate effective dose when treating tumours with conventional fractionated rt. At sbrt’s high-dose fractions, other models of tissue injury have been suggested32–34. Users should therefore exercise caution when using bed models in comparisons of various sbrt schemes.
UPDATES
All pebc documents are maintained and updated as described in the pebc Document Assessment and Review Protocol.
ACKNOWLEDGMENTS
The pebc is supported by the Ontario Ministry of Health and Long-Term Care through Cancer Care Ontario. All work produced by the pebc is editorially independent from its funding source. The authors thank the Lung Cancer dsg members in Ontario for their comments on this project. They also thank Melissa Brouwers, Patrick Cheung, Sheila McNair, Hans Messersmith, Gunita Mitera, Gordon Okawara, Raymond Poon, Kenneth Schneider, Marko Simunovic, Cindy Walker-Dilks, Pardraig Warde, and Eric Winquist for providing feedback on draft versions, and Andrea Bezjak for participating in the early stages of this guideline’s development.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Cancer Care Ontario (cco) Patient Pathway: Is It Lung Cancer? Toronto, ON: CCO; n.d. [Available online at: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=256030; cited 16 October 2015] [Google Scholar]
- 2.McGarry RC, Song G, des Rosiers P, Timmerman R. Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest. 2002;121:1155–8. doi: 10.1378/chest.121.4.1155. [DOI] [PubMed] [Google Scholar]
- 3.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dosoretz DE, Katin MJ, Blitzer PH, et al. Medically inoperable lung carcinoma: the role of radiation therapy. Semin Radiat Oncol. 1996;6:98–104. doi: 10.1016/S1053-4296(96)80006-3. [DOI] [PubMed] [Google Scholar]
- 5.Browman GP, Newman TE, Mohide EA, et al. Progress of clinical oncology guidelines development using the Practice Guidelines Development Cycle: the role of practitioner feedback. J Clin Oncol. 1998;16:1226–31. doi: 10.1200/JCO.1998.16.3.1226. [DOI] [PubMed] [Google Scholar]
- 6.Browman GP, Levine MN, Mohide EA, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13:502–12. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- 7.Falkson CB, Vella ET, Yu E, et al. Radiotherapy with curative intent in patients with early-stage, medically inoperable, non-small-cell lung cancer: a systematic review. Clin Lung Cancer. 2016. [Epub ahead of print]. [DOI] [PubMed]
- 8.Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non–small cell lung cancer: a meta-analysis. Radiother Oncol. 2010;95:32–40. doi: 10.1016/j.radonc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Borst GR, Ishikawa M, Nijkamp J, et al. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother Oncol. 2009;91:307–13. doi: 10.1016/j.radonc.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol. 2013;52:1552–8. doi: 10.3109/0284186X.2013.813635. [DOI] [PubMed] [Google Scholar]
- 11.Koshy M, Malik R, Mahmood U, Husain Z, Sher DJ. Stereotactic body radiotherapy and treatment at a high volume facility is associated with improved survival in patients with inoperable stage i non–small cell lung cancer. Radiother Oncol. 2015;114:148–54. doi: 10.1016/j.radonc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Lanni TB, Jr, Grills IS, Kestin LL, Robertson JM. Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol. 2011;34:494–8. doi: 10.1097/COC.0b013e3181ec63ae. [DOI] [PubMed] [Google Scholar]
- 13.Lucas JT, Jr, Kuremsky JG, Soike M, et al. Comparison of accelerated hypofractionation and stereotactic body radiotherapy for stage 1 and node negative stage 2 non–small cell lung cancer (nsclc) Lung Cancer. 2014;85:59–65. doi: 10.1016/j.lungcan.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non–small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys. 2012;84:1060–70. doi: 10.1016/j.ijrobp.2012.07.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong AN, Yan P, Yuan GH, et al. Advantages of CyberKnife for inoperable stage i peripheral non-small-cell lung cancer compared to three-dimensional conformal radiotherapy. Mol Clin Oncol. 2015;3:442–8. doi: 10.3892/mco.2014.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widder J, Postmus D, Ubbels JF, Wiegman EM, Langendijk JA. Survival and quality of life after stereotactic or 3D-conformal radiotherapy for inoperable early-stage lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:e291–7. doi: 10.1016/j.ijrobp.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 17.Davis JN, Medbery C, 3rd, Sharma S, et al. Stereotactic body radiotherapy for early-stage non–small cell lung cancer: clinical outcomes from a National Patient Registry. J Radiat Oncol. 2015;4:55–63. doi: 10.1007/s13566-014-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Factor OB, Vu CC, Schneider JG, et al. Stereotactic body radiation therapy for stage i non–small cell lung cancer: a small academic hospital experience. Front Oncol. 2014;4:287. doi: 10.3389/fonc.2014.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grills IS, Hope AJ, Guckenberger M, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol. 2012;7:1382–93. doi: 10.1097/JTO.0b013e318260e00d. [DOI] [PubMed] [Google Scholar]
- 20.Guckenberger M, Allgauer M, Appold S, et al. Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol. 2013;8:1050–8. doi: 10.1097/JTO.0b013e318293dc45. [DOI] [PubMed] [Google Scholar]
- 21.Kestin L, Grills I, Guckenberger M, et al. Dose–response relationship with clinical outcome for lung stereotactic body radiotherapy (sbrt) delivered via online image guidance. Radiother Oncol. 2014;110:499–504. doi: 10.1016/j.radonc.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Kohutek ZA, Wu AJ, Zhang Z, et al. fdg-pet maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non–small cell lung cancer. Lung Cancer. 2015;89:115–20. doi: 10.1016/j.lungcan.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koshy M, Malik R, Weichselbaum RR, Sher DJ. Increasing radiation therapy dose is associated with improved survival in patients undergoing stereotactic body radiation therapy for stage i non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;91:344–50. doi: 10.1016/j.ijrobp.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Lee DS, Kim YS, Yoo IR, et al. Long-term clinical experience of high-dose ablative lung radiotherapy: high pre-treatment 18Ffluorodeoxyglucose–positron emission tomography maximal standardized uptake value of the primary tumor adversely affects treatment outcome. Lung Cancer. 2013;80:172–8. doi: 10.1016/j.lungcan.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Mak RH, Hermann G, Lewis JH, et al. Outcomes by tumor histology and KRAS mutation status after lung stereotactic body radiation therapy for early-stage non-small-cell lung cancer. Clin Lung Cancer. 2015;16:24–32. doi: 10.1016/j.cllc.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (hypofxsrt) for stage i non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(suppl 3):S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 27.Ricardi U, Frezza G, Filippi AR, et al. Stereotactic ablative radiotherapy for stage i histologically proven non–small cell lung cancer: an Italian multicenter observational study. Lung Cancer. 2014;84:248–53. doi: 10.1016/j.lungcan.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki O, Mitsuyoshi T, Miyazaki M, et al. Dose–volume–response analysis in stereotactic radiotherapy for early lung cancer. Radiother Oncol. 2014;112:262–6. doi: 10.1016/j.radonc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for stage i non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys. 2011;81:e305–16. doi: 10.1016/j.ijrobp.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 30.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase ii study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 31.Park HS, Harder EM, Mancini BR, Decker RH. Central versus peripheral tumor location: influence on survival, local control, and toxicity following stereotactic body radiotherapy for primary non-small-cell lung cancer. J Thorac Oncol. 2015;10:832–7. doi: 10.1097/JTO.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 32.Chi A, Wen S, Liao Z, et al. What would be the most appropriate alpha/beta ratio in the setting of stereotactic body radiation therapy for early stage non–small cell lung cancer. Biomed Res Int. 2013;2013:391021. doi: 10.1155/2013/391021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong C, Guo WJ, Zha WW, et al. A new index comparable to bed for evaluating the biological efficacy of hypofractionated radiotherapy schemes on early stage non–small cell lung cancer: analysis of data from the literature. Lung Cancer. 2014;84:7–12. doi: 10.1016/j.lungcan.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Song CW, Cho LC, Yuan J, Dusenbery KE, Griffin RJ, Levitt SH. Radiobiology of stereotactic body radiation therapy/stereotactic radiosurgery and the linear-quadratic model. Int J Radiat Oncol Biol Phys. 2013;87:18–19. doi: 10.1016/j.ijrobp.2013.03.013. [DOI] [PubMed] [Google Scholar]



