Abstract
HIV viral proteins within the central nervous system are associated with the development of neurocognitive impairments in HIV-infected individuals. Dopamine transporter (DAT)-mediated dopamine transport is critical for normal dopamine homeostasis. Abnormal dopaminergic transmission has been implicated as a risk determinant of HIV-induced neurocognitive impairments. Our published work has demonstrated that Tat-induced inhibition of DAT is mediated by allosteric binding site(s) on DAT, not the interaction with the dopamine uptake site. The present study investigated whether impaired DAT function induced by Tat exposure in vitro can be documented in HIV-1 transgenic (HIV-1Tg) rats. We assessed kinetic analyses of [3H]dopamine uptake into prefrontal and striatal synaptosomes of HIV-1Tg and Fisher 344 rats. Compared with Fisher 344 rats, the capacity of dopamine transport in the prefrontal cortex (PFC) and striatum of HIV-1Tg rats was increased by 34% and 32%, respectively. Assessment of surface biotinylation indicated that DAT expression in the plasma membrane was reduced in PFC and enhanced in striatum, respectively, of HIV-1Tg rats. While the maximal binding sites (Bmax) of [3H]WIN 35,428 was decreased in striatum of HIV-1Tg rats, an increase in DAT turnover proportion was found, relative to Fisher 344 rats. Together, these findings suggest that neuroadaptive changes in DAT function are evidenced in the HIV-1Tg rats, perhaps compensating for viral protein-induced abnormal dopaminergic transmission. Thus, our study provides novel insights into understanding mechanism underlying neurocognitive impairment evident in neuroAIDS.
Keywords: HIV-1 tat, transgenic rat, DA uptake, dopamine transporter, trafficking, striatum
Introduction
Although the common use of efficacious antiretroviral therapies to control HIV infection and improve the life of HIV patients, HIV-1-associated neurocognitive disorders (HAND) are a highly prevalent and significant health problem (Heaton et al, 2010; Mothobi and Brew, 2012; Simioni et al, 2010). The incidence (~70%) of HAND is dramatically increased due to substance abuse, such as cocaine (Buch et al, 2011; Fiala et al, 1998; Larrat and Zierler, 1993; Norman et al, 2009; Webber et al, 1999). Viral replication and proviral DAT induction within the central nervous system (CNS) in early HIV-1 infection (Nath and Clements, 2011) have been implicated as a risk determinant of HAND (Berger and Arendt, 2000; Purohit et al, 2011). Since most antiretroviral therapy medications cannot cross the blood-brain barrier (Buckner et al, 2006), these medications have no influence on the production of viral proteins in the CNS. Therefore, viral proteins are associated with the persistence of HIV infection-induced neuropathology and subsequent cognitive deficits (Brack-Werner, 1999; Frankel and Young, 1998; Johnston et al, 2001; Power et al, 1998). Prolonged exposure to the viral protein impairs the central dopamine (DA) system (Berger and Arendt, 2000; Koutsilieri et al, 2002; Nath et al, 1987) and the brain pathways controlling motivation (Berridge, 2007; Everitt and Robbins, 2005; Wise and Bozarth, 1987). The HIV regulatory protein, transactivator of transcription (Tat) protein has pivotal effects on the neurotoxicity and cognitive dysfunction evident in neuroAIDS (Rappaport et al, 1999). Tat and cocaine exacerbates the development of cognitive impairments in HIV infected individuals (Gannon et al, 2011). Viral replication in HIV-1 infected macrophages/microglia within dopaminergic brain regions results in Tat induction (Gaskill et al, 2009). The interplay of Tat with cocaine augments synaptic DA levels by inhibiting DA transporter (DAT) activity (Ferris et al, 2010). Importantly, the augmented DA levels by Tat and cocaine further stimulates viral replication in human macrophages within DA-rich brain regions, resulting in viral protein release (Gaskill et al, 2009), and this contributes to the pathophysiology of HAND (Li et al, 2009; Purohit et al, 2011). Additionally, recent studies show that other viral proteins, such as gp120 and Nef also influence DAT activity (Acharjee et al, 2014; Hu et al, 2013). On the other hand, clinical observations have demonstrated that DAT activity is significantly reduced in HIV-infected individuals with cocaine use (Chang et al, 2008; Wang et al, 2004), which is correlated with impaired learning and memory performance (Hsieh et al, 2010; Mozley et al, 2001). Reports from the observations made in postmortem brain tissue from HAND patients found significantly increased DAT expression in the striatum (Gelman et al, 2006) but no changes in DAT levels in the substantia nigra (Silvers et al, 2007). In the early stage of HIV infection, increased levels of DA are found in the cerebrospinal fluid of therapy naïve HIV patients in asymptomatic infection (Scheller et al, 2010), which may contribute to decreased levels of DA in DA-rich brain areas in the advanced stages of HIV infection (Kumar et al, 2009; Sardar et al, 1996). Currently, it is unclear how HIV-1 viral proteins impair the DA system in the CNS of the patients with HAND, thereby producing neurocognitive impairment.
In the last several years, we have studied molecular mechanisms underlying the interplay of HIV-1 Tat with cocaine and DAT. Despite our published data demonstrate that Tat in vitro modulates DAT activity allosterically (Midde et al, 2012; Midde et al, 2013; Zhu et al, 2011; Zhu et al, 2009b), it is unclear whether DAT function is altered in a rodent model that are exposed acutely or chronically to low levels of Tat protein. Since HIV does not infect rodents, several approaches have been used for studying the effects of viral proteins on DA system through: 1) rodent brain tissue in the presence of recombinant Tat (Zhu et al, 2009b), 2) intra-brain region infusion of recombinant Tat (Ferris et al, 2009; Harrod et al, 2008), 3) Tat transgenic mouse model (Kim et al, 2003), and 4) HIV-1 transgenic (HIV-1Tg) rat model that mimics the conditions of HIV-1-infected patients receiving antiretroviral medications in which viral replication is substantially suppressed, but viral proteins continually expressed throughout the animal life (Ray et al, 2003; Reid et al, 2001). Tat transgenic mice exhibit a significant increase in DAT levels (Perry et al, 2010) and deficits in learning and memory performance (Carey et al, 2012). The HIV-1Tg rats carry the gag-pol deleted HIV-1 provirus and continually express seven viral proteins: env, tat, rpr, rev, vif, vpu, and nef (Reid et al, 2001). Although no differences in DAT mRNA levels (Liu et al, 2009) and DAT immunoreactivity (Webb et al, 2010) were found in the brains between HIV-1Tg and control Fisher 344 rats, HIV-1Tg rats exhibited a greater affinity for cocaine inhibiting DAT binding site compared to control Fisher 344 rats (McIntosh et al, 2015). This study was set to characterize DAT activity and its distribution in the plasma membrane of the prefrontal cortex (PFC) and striatum in HIV-1Tg rats.
Materials and Methods
Subjects
Male HIV-1Tg and Fisher 344 (F344) rats at age of 13 weeks were obtained from Harlan Laboratories, Inc. (Indianapolis, IN). Rats were pair housed in a temperature (21 ± 2 °C)- and humidity (50 ± 10%)-controlled vivarium which was maintained at, on a 12-h light/dark cycle with lights on at 0700 h (EST) as reported previously (Midde et al, 2011). F344 rats were used as the controls. Rodent food (Pro-Lab Rat, Mouse Hamster Chow #3000) and water were provided ad libitum. All rats were habituated for one week prior to all experiments. Animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina, Columbia.
Synaptosomal Preparation
Drug-naïve rats were rapidly decapitated and the dissection of PFC and striatum were conducted on an ice-cold plate. The PFC and striatum were chosen because these two regions are critical to HIV infection-mediated neurocognitive and motivation impairments (Chang et al, 2008; Coulehan et al, 2014). In addition, DAT is relatively expressed in the PFC and striatum (Zhu et al, 2005). Synaptosomes were prepared using our published method (Zhu et al, 2004). Bovine serum albumin was used as the standard (Bradford, 1976) to measure protein concentration for all samples.
[3H]DA Uptake Assay
To assess the difference in DAT reuptake between HIV-1Tg and F344 rats, the maximal velocity (Vmax) or Michaelis-Menten constant (Km) of [3H]DA uptake were conducted in the PFC or striatum of these animals as described previously (Zhu et al, 2004). Notably, DA uptake into prefrontal synaptosomes was performed in the presence of desipramine (1 μM), a norepinephrine transporter inhibitor, and paroxetine (5 μM), a serotonin transporter inhibitor as reported previously (Zhu et al, 2004). To determine whether the genetic expression of HIV-1 viral proteins alters the inhibitory effects of substrate and DAT inhibitors on specific [3H]DA uptake into striatal synaptosomes, IC50 values for these substrate and inhibitors inhibiting [3H]DA uptake were examined in the presence of various concentrations of DA, cocaine, WIN 35,428 or GBR12909.
Biotinylation and Western Blot Assays
To determine whether viral proteins alter DAT distribution between the plasma membrane and cytoplasmic compartment, we performed cell surface biotinylation assay followed by DAT Western blotting. Synaptosomes from the PFC and striatum were prepared as described above. Biotinylation and DAT Western blotting assays were performed to determine DAT expression in total levels, plasmalemma, and cytoplasm membrane using a previously published method (Zhu et al, 2005).
[3H]WIN 35,428 Binding Assay
[3H]WIN 35,428 binding sites are pharmacologically responsible for the characters with DA transport carrier and the cocaine binding domain (Reith and Coffey, 1994). To determine whether viral proteins alter DA uptake sites, we performed kinetic analysis of [3H]WIN 35,428 binding in striatum of HIV-1Tg and F344 rats. Synaptosomes were prepared as described above and [3H]WIN 35,428 binding assay was determined using previously described methods (Zhu et al, 2007).
Molecular Modeling
DAT-mediated DA transport involves three typical conformational states of DAT: outward-open state (i.e. the extracellular side of substrate-binding site for the transmitter is open, while the intracellular side is blocked); the outward-occluded state (i.e. both the extracellular and intracellular sides of binding site are blocked such that the binding site is occluded and no longer accessible for substrate); and the inward-open state (i.e. the intracellular side of substrate-binding site is open, while the extracellular side is blocked)(Forrest et al, 2007; Penmatsa et al, 2013; Singh et al, 2007; Wang et al, 2012; Yamashita et al, 2005).
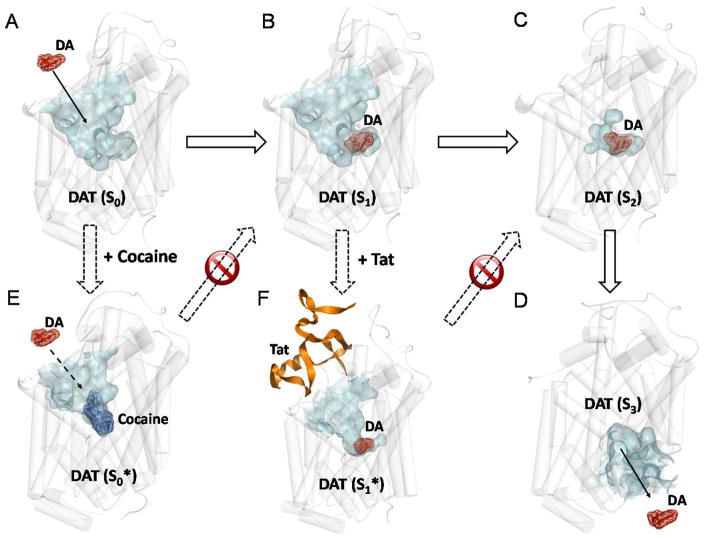
Based on the reported structures of DAT in three typical conformational states (Huang and Zhan, 2007; Yuan et al, 2015), Tat binds most favorably with the outward-open state of DAT in our previous work (Yuan et al, 2015), and cocaine also binds to the outward-open state of DAT (Huang et al, 2009). The structures of DAT and its complexes with Tat and cocaine all came from the previously reported molecular dynamics (MD) simulation studies (Huang et al, 2009; Huang and Zhan, 2007; Yuan et al, 2015). The MD-simulated structures were energy-minimized further by using the AMBER 12 (Case et al, 2012) program package. Then, the PyMol software (Schrödinger, 2010) was used to visualize the structures (depicted in Figure 6).
Figure 6. Schematic representation of the mechanisms concerning how cocaine and Tat block the DA transporting cycle.
All of the structures were generated with the PyMol software from the energy-minimized structures. All of the initial structures used in the energy minimization were obtained from our previous studies (Huang et al, 2009; Midde et al, 2013; Midde et al, 2015). DAT is represented by white semi-transparent cylinder. The channel for substrate entry or leaving in DAT is represented by semi-transparent surface in cyan. Dopamine (DA) and cocaine are shown as mesh surface and colored in red and blue, respectively. Tat is showed as cartoon and colored in orange. A. S0 indicates the apo DAT in the outward-open state. The substrate binding site is opened to extracellular side of the neuro cell for DA uptake. B. S1 indicates the holo DAT in outward-open state. C. S2 indicates the holo DAT in the outward-occluded state. The substrate binding site is occluded and no longer accessible for DA entry. D. S3 indicates the apo DAT in the inward-open state. The substrate binding site is opened to intracellular cytoplasm and dopamine could leave. The change from S0 to S3 represents the process of DAT transporting dopamine from the extracellular side to the intracellular side. E. Cocaine can also bind to the S0 state of DAT with the same binding site of dopamine (S0*). The competitive inhibition effect of cocaine blocks the S0→S1 transition step of DAT transporting. (F) Tat could bind to the S1 state of DAT without occupying the dopamine binding site, which stabilizes the S1 state (S1*). The non-competitive inhibition effect of Tat blocks the S1→S2 transition step of DAT transporting. As a result, although cocaine and Tat adopt slightly different mechanisms in impairing DAT function, they are expected to show similar biological effects on DAT transporting.
Data Statistical Analysis
Data are expressed as mean values ± S.E.M., and n means the number of independent experiments for individual treatment group. IC50 values for inhibiting [3H]DA uptake or [3H]WIN 35,428 were examined from inhibition curves by nonlinear regression analysis using a single site model with variable slope. Kinetic parameters (Km and Vmax for [3H]DA uptake; Kd and Bmax for [3H]WIN 35,428 binding) were examined from saturation curves by nonlinear regression analysis using a single site model with variable slope. These kinetic parameters involving comparisons between groups were analyzed using unpaired Student’s t tests. DAT expression levels were expressed as the ratio of DAT immunoreactivity to control proteins, and analyzed by separate unpaired Student’s t tests. IBM SPSS Statistics version 20 was used for all statistical analyses, and differences at p < 0.05 were determined as significant.
Results
HIV-1Tg rats exhibit an increase in synaptosomal [3H]DA uptake in the PFC and striatum
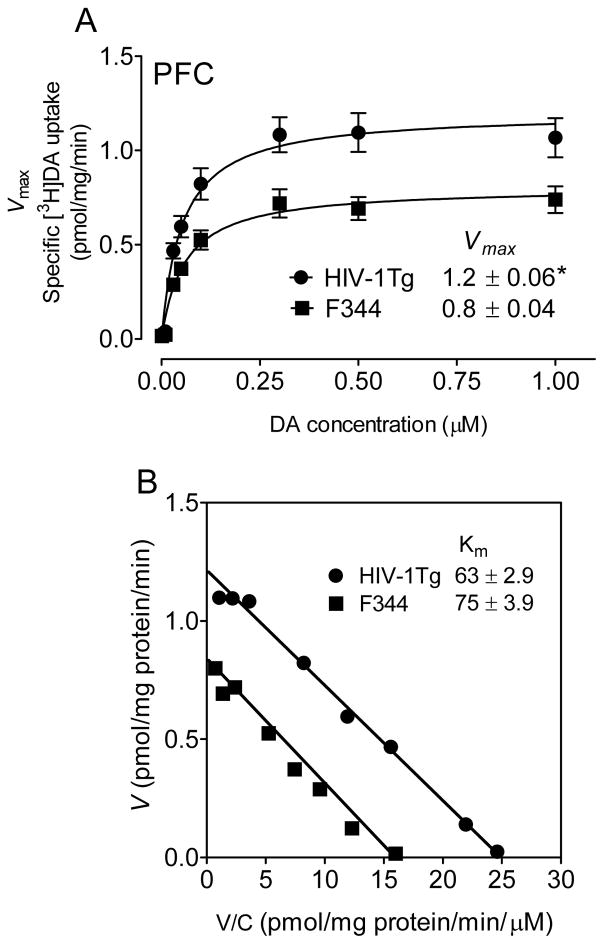
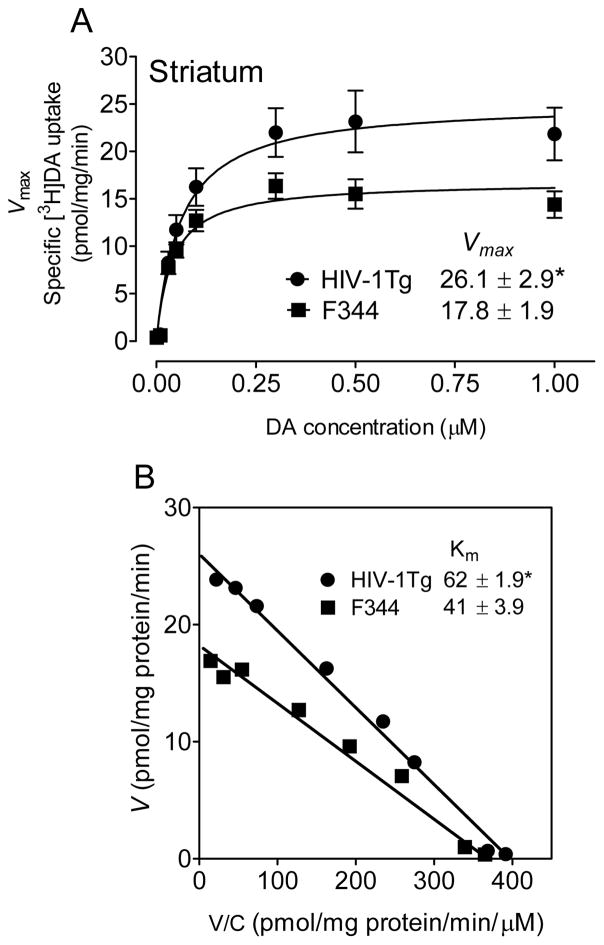
We performed kinetic analyses of synaptosomal [3H]DA uptake to determine the differences between HIV-1Tg and F344 rats. In the PFC, the Vmax values for [3H]DA uptake were significantly increased by 34 ± 2.0 % in HIV-1Tg rats (1.2 ± 0.06 pmol/mg/min) compared with F344 rats [0.8 ± 0.04 pmol/mg/min; t(12) = 2.6, p < 0.05] (Figure 1A). There was no change in the Km between HIV-1Tg rats (63 ± 2.9 nM) and F344 rats (75 ± 3.9 nM, Figure 1B). Similarly, in the striatum, the Vmax values were significantly increased by 32 ± 3.6% in HIV-1Tg rats (26.1 ± 2.90 pmol/mg/min) compared with F344 rats [17.8 ± 1.89 pmol/mg/min; t(12) = 2.4, p < 0.05] (Figure 2A). The Km values were increased in HIV-1Tg rats (62 ± 1.9 nM) relative to F344 rats [41 ± 3.9 nM; t(12) = 2.8, p < 0.05, Figure 2B]. Therefore, HIV-1 viral proteins enhanced DAT reuptake function in both PFC and striatum of HIV-1Tg rats. There were no changes in the IC50 values for DA, cocaine, WIN 35,428 or GBR12909 inhibiting [3H]DA uptake between HIV-1Tg and F344 rats (Table 1).
Figure 1. HIV-1Tg rats exhibit an increase in [3H]DA uptake in the prefrontal synaptosomes.
Kinetic analysis of the synaptosomal [3H]DA uptake was determined in the prefrontal cortex (PFC) of HIV-1Tg and F344 rats. Synaptosomes were preincubated with a range of mixed DA concentrations (1 – 1000 nM, final concentration). In competition, nonspecific uptake (in the presence of 10 μM nomifensine, 1 μM desipramine, 5 nM paroxetine, final concentration) was determined in the presence of subtracted from total uptake to calculate DAT-mediated uptake. A. The Vmax (pmol/mg/min) and Km (nM) values were calculated by fitting the data to the Michaelis-Menten equation and represent the means from five independent experiments ± S.E.M. B. Eadie-Hofstee transformation of the same kinetic data. *, p < 0.01 compared to F344 group.
Figure 2. HIV-1Tg rats exhibit an increase in [3H]DA uptake in the striatal synaptosomes.
Kinetic analysis of the synaptosomal [3H]DA uptake was determined in the striatum of HIV-1Tg and F344 rats. Striatal synaptosomes were preincubated with one of eight mixed concentrations of the [3H]DA (1 – 1000 nM, final concentration). In competition, nonspecific uptake (in the presence of 10 μM nomifensine, final concentration) was calculated from total uptake to calculate DAT-mediated uptake. A. The Vmax (pmol/mg/min) and Km (nM) values were analyzed by fitting the data to the Michaelis-Menten equation and represent the means from five independent experiments ± S.E.M. B. Eadie-Hofstee transformation of the same kinetic data. *, p < 0.01 compared to F344 group.
Table 1.
Effects of substrate and inhibitors on inhibiting [3H]DA uptake and [3H]WIN35,428 binding in the striatal synaptosomes of HIV-1Tg and F344 rats
| [3H]DA uptake | [3H]WIN35,428 | |||
|---|---|---|---|---|
| IC50 | ||||
| HIV-1Tg | F344 | HIV-1Tg | F344 | |
| DA (μM) | 0.17 ± 0.02 | 0.59 ± 0.39 | ||
| Cocaine (μM) | 0.90 ± 0.07 | 0.92 ± 0.13 | 0.17 ± 0.03 | 0.20 ± 0.05 |
| WIN 35,428 (nM) | 87.2 ± 8.76 | 93.3 ± 8.93 | ||
| GBR 12909 (nM) | 3.94 ± 0.36 | 3.62 ± 0.29 | 2.77 ± 0.21 | 2.72 ± 0.28 |
Data are presented as mean ± S.E.M. of 5 independent experiments performed in duplicate.
The DAT expression was altered in HIV-1Tg rats
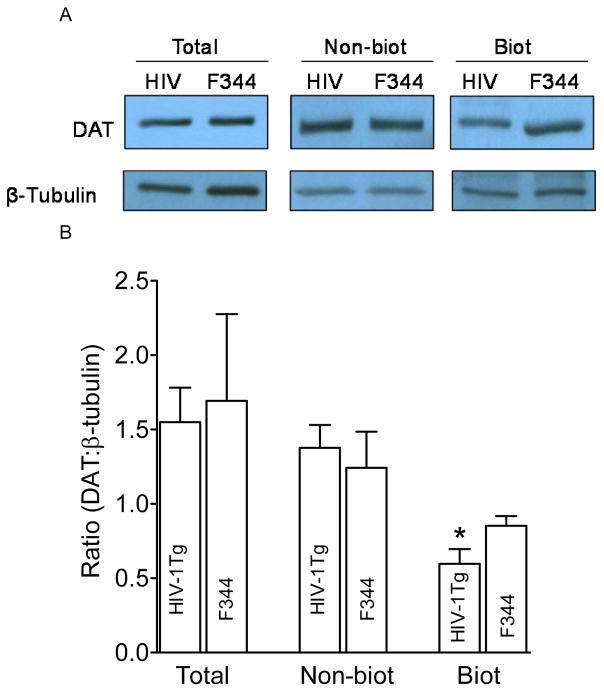
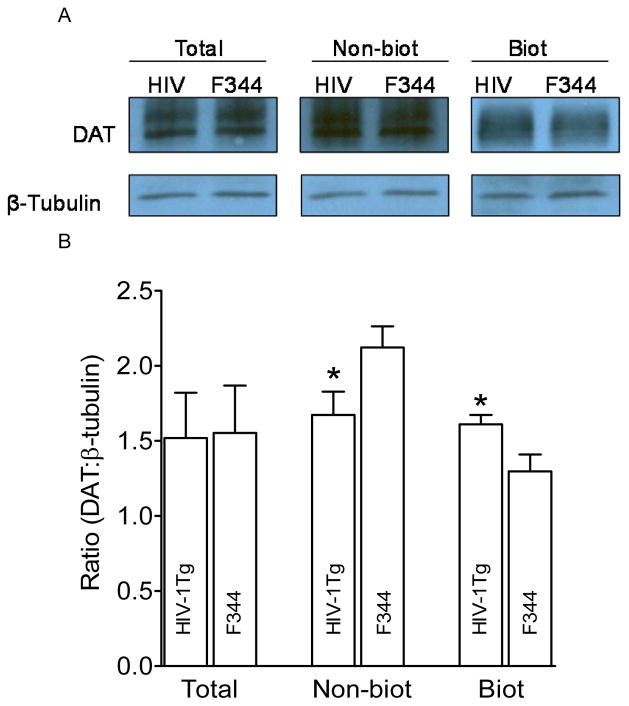
We next assessed cell surface biotinylation and immunoblotting assays to determine whether the increased Vmax in the PFC and striatum of HIV-1Tg rats was result from changes in DAT expression in the plasma membrane. As illustrated in Figure 3, in the PFC, DAT expression in total and non-biotinylated (cytoplasmic pool) fractions were not different between HIV-1Tg and F344 rats, whereas DAT expression in the biotinylated fraction (plasma membrane) from HIV-1Tg rats was lower (37 ± 4.0%) than that in F344 rats [t(6) = 2.2, p < 0.05]. In the striatum (Figure 4), no difference in total DAT expression between HIV-1Tg and F344 rats was found, whereas DAT expression in non-biotinylated fraction from HIV-1Tg rats was reduced by 22 ± 2.5% relative to F344 rats [t(6) = 2.2, p < 0.05]. DAT expression in the biotinylated fraction from HIV-1Tg rats was higher (23 ± 1.5%) than that in F344 rats [t(6) = 2.4, p < 0.05]. These results suggest a region specific correlation between Vmax and DAT cell surface.
Figure 3. HIV-1Tg rats exhibit an increase in cell surface expression of dopamine transporters in the PFC.
A. Representative immunoblots of total synaptosomal fraction (Total), cytoplasmic fraction (non-biotinylated, Non-biot), and plasma membrane fraction (biotinylated, Biot) from PFC of HIV-1Tg (HIV) and F344 rats. β-tubulin was used for monitoring protein loading between HIV-1Tg and F344. B. Mean ± S.E.M. ratio of densitometry values of DAT immunoreactivity to β-tubulin immunoreactivity. * p< 0.05 compared to F344 group (n=4).
Figure 4. HIV-1Tg rats exhibit an increase in cell surface expression of dopamine transporters in the striatum.
A, Representative immunoblots of total synaptosomal fraction (Total), cytoplasmic fraction (non-biotinylated, Non-biot), and plasma membrane fraction (biotinylated, Biot) from striatum of HIV-1Tg (HIV) and F344 rats. β-tubulin was used for monitoring protein loading between HIV-1Tg and F344. B. Mean ± S.E.M. ratio of densitometry values of DAT immunoreactivity to β-tubulin immunoreactivity. * p< 0.05 compared to F344 group (n=4).
HIV-1Tg rats exhibit a decrease in [3H]WIN 35,428 binding in striatal synaptosomes
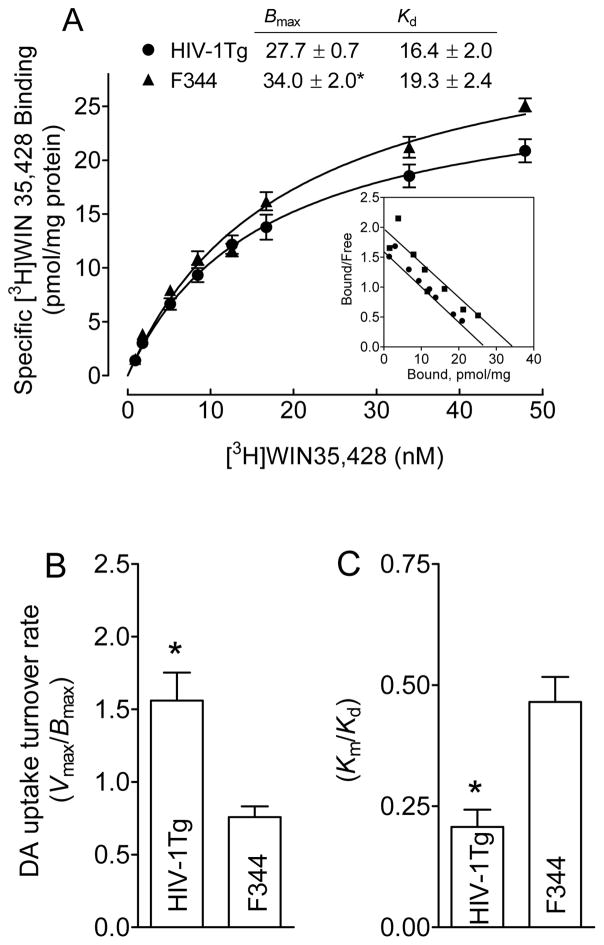
We next determined the Bmax and Kd of [3H]WIN 35,428 binging in striatum from HIV-1Tg and F344 rats. As shown in Figure 5A, the Bmax value of [3H]WIN 35,428 binding was reduced by 25 ± 0.2% (27.3 ± 0.7 pmol/mg/protein) in HIV-1Tg rats compared with F344 rats (34.0 ± 2.0 pmol/mg/protein; t(6) = 2.1, p < 0.05]. There were no changes in the Kd between HIV-1Tg (16.4 ± 2.0 nM) and F344 rats [19.3 ± 2.4 nM; t(6) = 1.8, p = 0.0589]. Thus, taking into account the increase DA uptake (Vmax) in striatum of HIV-1Tg rats (Figure 2A), a significant increase in uptake turnover rate (Vmax/Bmax) in striatum of HIV-1Tg rats (1.5 ± 1.6) was revealed, as compared to in F344 rats [0.78 ± 0.05; t(6) = 3.1, p < 0.05] (Figure 5B). Note: on Oct 10 (in China), I did online check proof, and found the values for Figure 5B and C not match: so yellow highlighted part was changed to: a significant increase in uptake turnover rate (Vmax/Bmax) in striatum of HIV-1Tg rats (1.6 ± 0.19) was revealed, as compared to in F344 rats [0.76 ± 0.07; t(6) = 3.8, p < 0.01] (Figure 5B). Jun also added new sentence for Figure 5C as “In addition, asignificant decrease in the ratio (Km/Kd) in HIV-1Tg rats (0.2 ± 0.04) was found, as compared to F344 rats [0.5 ± 0.05; t(6) = 4.1, p < 0.01] (Fig 5C). There were no changes in the IC50 values for substrate and DAT inhibitors inhibiting [3H]WIN 35,428 binding in striatum of HIV-1Tg and F344 rats (Table 1).
Figure 5. HIV-1Tg rats exhibit a decrease in [3H]WIN 35,428 binding in striatum.
For a single experiment, synaptosomes from a single rat were prepared, and half was used for [3H]DA uptake (Figure 2) and the other half was used for saturation isotherm of [3H]WIN 35,428 binding. A. Bmax (pmol/mg protein) and Kd (nM) values of [3H]WIN 35,428 binding in synaptosomes of HIV-1Tg and F344 rats are presented. Scatchard transformations of same data (Insert) are presented. Data were best fit to a single class of binding site and are presented as means ± S.E.M. from four independent experiments. Nonspecific binding for [3H]WIN 35,428 was determined in the presence of 30 μM cocaine. DA uptake turnover rate values are determined from (B) Vmax of [3H]DA uptake/Bmax of [3H]WIN 35,428 and (C) Km/Kd, respectively. *, p < 0.05 compared to F344 group.
Discussion
This study investigated whether the genetic expression of HIV-1 viral proteins alters DAT reuptake function and expression in the rat PFC and striatum. The major finding is that HIV-1Tg rats exhibit increased DAT reuptake in both PFC and striatum, which is opposite to our previous finding showing decreased DAT reuptake in vitro in rat synaptosomes and cells expressing hDAT in the presence of Tat protein (Midde et al, 2013; Midde et al, 2015; Zhu et al, 2009b). Moreover, we found that the increased Vmax in the PFC and striatum is accompanied by distinctly different alterations in DAT expression in the plasma membrane in a brain region-specific manner. Furthermore, we found that HIV-1Tg rats exhibit decreased Bmax for [3H]WIN 35,428 binding in striatum relative to F344 rats. Considering the increased turnover proportion of DA transport, HIV-1Tg rats may have a neuroadaptive change in DAT function to compensate for viral protein-induced abnormal dopaminergic transmission.
The current findings show that the Vmax was increased by 34% and 32% in PFC and striatum, respectively, with a slight decrease in the Km in striatum but not in the PFC. This findings support the previous studies showing Tat-induced increased DAT-DA uptake in primary neurons of Tat-expressing transgenic mice (Perry et al, 2010) and the elevated DAT levels in striatum of and HIV-infected individuals (Gelman et al, 2006). In contrast, Tat inhibits [3H]DA uptake in rat striatal synaptosomes and cells expressing hDAT in vitro (Midde et al, 2013; Zhu et al, 2009b). Several possibilities may explain the discrepancy between in vitro and in vivo observations. First, Tat in vivo regulates DAT function and expression by disrupting neuronal signaling pathways, such as GSK-3β, thereby elevating DA transport activity and DAT expression in plasma membrane in primary neurons or animals (Perry et al, 2010). However, in vitro Tat-induced decrease in DA transport and DAT plasma membrane expression in rat synaptosomes could be due to the isolation of synaptosomes from the neuronal regulatory pathway. Second, the different types of Tat and its concentration may also cause the different Tat effects observed in vitro and in vivo. For example, a detectable Tat concentration in the frontal cortex of HIV-1 infected individuals is about 140 pmol (Hudson et al, 2000), whereas ~500 nM concentration of recombinant Tat1–86 was used in the synaptosomal DA uptake (Zhu et al, 2009b). Importantly, the native HIV-1 Tat actually detected in the sera (Westendorp et al, 1995) or brain (Hudson et al, 2000) of HIV-1 infected patients has more biological function and is more neurotoxic to neuronal targets, such as DAT. Third, as documented in the recent reports, the decreased DAT function is caused by not only Tat but also gp120 and Nef (Acharjee et al, 2014; Hu et al, 2013), implicating that DAT induction could be differentially modulated by the combined or individual of these HIV-1 viral proteins. Nevertheless, results from the present study and another (Perry et al, 2010) demonstrate that DAT function can be modulated by viral proteins despite increased Vmax and increased DAT cell surface expression, which may be a compensatory response to decreased transporting efficiency of individual DAT molecules.
The efficacy of DA uptake largely depends on DAT expression in the plasma membrane, which is dynamically modulated by a trafficking mechanism (Zhu and Reith, 2008). In general, surface biotinylation assay (Zhu et al, 2005; Zhu et al, 2009a) or sub-fractionation method (Middleton et al, 2007) are extensively used to assess the DAT trafficking. In the present observation, HIV-1Tg rats exhibit a 37% increase in striatal DAT expression in plasma membrane, which is comparable to the magnitude (32%) of the increase in Vmax. However, the magnitude levels of the changes in DAT in plasma membrane were less than (Zhu et al, 2009a) or parallel (Zhu et al, 2005) to the magnitude of the alterations in Vmax. This suggests that HIV-1 viral proteins regulate DAT function in striatum via a trafficking-dependent mechanism. Contrasting with the current results, we have demonstrated that in vitro Tat exposure decreases DAT expression in plasma membrane and increases DAT level in cytoplasmic compartment without changing the total DAT level in rat striatal synaptosomes (Midde et al, 2012). Tat-mediated reduction in the efficiency of DA transport is generally regulated by (1) increasing DAT protein degradation, (2) decreasing DAT turnover efficacy, and/or (3) changing in DAT trafficking on the plasma membrane without changing the DAT protein. Our current results and previous report (Midde et al, 2012) demonstrate that total DAT expression in the striatum was not altered after in vitro Tat exposure and in HIV-1Tg rats, indicating that Tat-induced changes in DAT reuptake is not caused by DAT degradation. Results from [3H]WIN 35,428 binding experiments show that an reduction in the Bmax was observed in striatum of HIV-1Tg rats, which is supported by our previous report showing the decreased specific [3H]WIN 35,428 after in vitro exposure of rat striatal synaptosomes to Tat protein (Midde et al, 2012). DAT turnover proportion reveals the efficacy of DA molecules transported per second per site (Lin et al, 2000). Considering the reduced Bmax along with the increased Vmax in striatum of HIV-1Tg rats, an increase in DA uptake turnover (Vmax/Bmax) was found; however, in vitro exposure of synaptosomes to Tat did not affect DA uptake turnover (Midde et al, 2012). This may help explain the difference in Tat-induced redistribution of DAT (e.g. upregulation or downregulation of DAT plasma membrane expression) between the present observation and our previous report (Midde et al, 2012).
Of interest, our previous report showed that Tat in vitro increased IC50 value of cocaine inhibiting synaptosomal [3H]DA uptake in striatum, indicating that Tat decreases cocaine’s affinity on DAT (Zhu et al, 2011). However, the Tat’s effect was not documented in HIV-1Tg rats. One possible reason is that Tat expression in HIV-1Tg rats is lower than the concentration in vitro study, suggesting that Tat modulates cocaine’s binding sites in a concentration-dependent manner. We hypothesize that Tat protein allosterically regulates DAT activity (Zhu et al, 2011; Zhu et al, 2009b). Indeed, previous study showed that SRI-20041, a novel DAT allosteric modulator, altered cocaine’s affinity in a concentration-dependent manner (Pariser et al, 2008). Therefore, determining Tat concentration-dependent effect on cocaine’s affinity for inhibiting DA uptake will be essential for future study. Notably, viral protein-induced increase of DAT expression is similar to cocaine-induced increase of DAT expression in striatum. For example, chronic administration of cocaine upregulates striatal DAT expression in animals and humans (Schmitt and Reith, 2010), because cocaine can block DA uptake by competitive binding with DA uptake sites in DAT (Huang et al, 2009). Mechanistically, as illustrated in Figure 6, HIV-1 Tat can block DA uptake by allosteric modulation of DA transport (Midde et al, 2013; Midde et al, 2015; Zhu et al, 2009b). Specifically, Tat also binds to DAT in the outward-open state; however, the Tat-DAT binding is not to interfere DA binding to DAT rather than prevent the further conformational change of DAT from the outward-open state to the other states (outward-occluded and inward-open states) such that DA cannot be transported by DAT (Yuan et al, 2015). Therefore, both cocaine and Tat can independently (and synergistically) disrupt the process of DA transport and, thus, could have the similar role in modulation of the DAT function and expression. Hence, Tat protein likely contributes to the increase of DAT expression in striatum of HIV-1Tg rats observed in the current study.
Assessment of surface biotinylation indicates that the expression of a higher amount of DAT in the striatal plasma membrane, but a relative lower DAT density in the PFC in HIV-1Tg rats, suggesting a region-specific regulatory mechanism underlying DAT function and plasma membrane expression. For example, previous study shows that Vmax for DA uptake was elevated in both rat striatum and nucleus accumbens; however, the elevated Vmax was accompanied by increased cell surface DAT in the striatum but not in nucleus accumbens (Samuvel et al, 2008). Regulation of DAT function and trafficking involves in DAT distribution, protein-protein interaction, DAT phosphorylation, and presynaptic receptors (Zhu and Reith, 2008), suggesting that DAT expression in the plasma membrane is not always consistent with changes in DAR reuptake. In general, the process of DA uptake reflects a proportion of the DAT expression in the plasma membrane. It is possible that the regulations of DA transport and DAT expression in the PFC of HIV-1Tg rats are mediated via two independent mechanisms. First, the increased Vmax in the PFC was not supported by elevated DAT in plasma membrane, suggesting a trafficking-independent pathway. Although the current study did not directly determine DAT phosphorylation, our recent report demonstrated that distinct basal phosphorylated levels of cAMP response element binding protein (pCREB) and extracellular regulated kinase 2 (pERK2) were observed among the PFC, nucleus accumbens and ventral tegmental area between HIV-1Tg and F344 rats (Midde et al, 2011). Therefore, we speculate that the different phosphorylation levels of signaling pathways may account for the opposite correlations between Vmax and DAT in the plasma membrane between the PFC and striatum of HIV-1 Tg rats. Furthermore, the basal level of pERK2 in the PFC is higher in HIV-1Tg rats than that in F344 rats (Midde et al, 2011), whereas the inhibition of MEK/ERK activity produces an reduction in DAT expression in plasma membrane (Lin and Uhl, 2003). Second, DA D1 receptor in the PFC has been shown to be highly expressed in HIV-1Tg rats than F344 rats (Liu et al, 2009). Given that activation of D1 receptor is associated with an increase in DA transport (Kimmel et al, 2001), the increased Vmax in the PFC of HIV-1Tg rats could be mediated by viral protein-induced increase in D1 expression. Finally, our data showing increased Vmax along with a lower plasma membrane DAT expression in the PFC suggest a compensatory functional enhancement of efficiency of DAT molecules. Future studies involving determination of the effects of ERK and other signaling proteins, and DA receptors on surface DAT expression in the PFC are necessary to fully understand how viral proteins modulates DAT function and DAT redistribution.
One caveat is that the current study cannot address which viral protein contributes to the alteration of DAT reuptake and DAT plasma membrane expression in HIV-1Tg rats. Although the levels of viral proteins in the PFC and striatum were not measured in this study, previous report has indicated that the mRNA levels of Tat, pg120, nef and vif are detected in these two regions of the HIV-1Tg rats. Particularly, the detected Tat levels are region-specific and age-dependent changes with a higher level in the PFC during 2–3 months and in striatum during 10–11 months (Peng et al, 2010). Therefore, determining DAT activity in HIV-1Tg rats across different ages will be essential future work. Several studies have demonstrated that among the viral proteins, Tat plays a critical role in viral protein-induced neurotoxicity (Rappaport et al, 1999). For example, our previous report shows that HIV-1Tg rats exhibited attenuated nicotine-mediated behavior sensitization and altered pERK1/2, a protein kinase associated with cell death (Kulich and Chu, 2001; Stanciu et al, 2000), in the mesocorticolimbic regions (Midde et al, 2011). These behavioral and neurochemical alterations in HIV-1Tg rats can be documented in rats with intra-ventral tegmental area injection of HIV-1 Tat1–86 (Zhu et al, 2015). Given that gp120 and Nef also inhibit DAT activity (Acharjee et al, 2014; Hu et al, 2013), the current results may represent a combination effect of viral proteins on DAT activity. Nevertheless, the current findings showing viral protein-mediated alterations in DAT activity provide insights into understanding the role of DA neurotransmission in HIV-1-induced cognitive impairment in HIV-infected individuals.
In conclusion, the present findings suggest that HIV-1Tg animals exhibit neuroadaptive changes in DAT reuptake and DAT plasma membrane expression in the PFC and striatum, which, at least in part, support a compensatory mechanism underlying viral protein-induced inhibition of DAT function observed in vitro. We hypothesize that such compensatory response to viral proteins-mediated disruption of DAT reuptake may occur in early HIV-1 infection. Long lasting viral protein-induced dysfunction of DAT activity eventually causes DAT-mediated dysregulation of dopaminergic transmission to accelerate the progression of HAND. Therefore, an intervention for HIV infection-induced dysfunction of DA system has the potential to improve neurocognitive function in patients with the early-stage o HAND. Identifying the binding sites in DAT where Tat interacts with will provide insights into exploring molecular targets for developing potential compound(s) that specifically block Tat binding site(s) in DAT without affecting DA transport. Ideally, the effectiveness of early intervention for HAND may combine such compounds with anti-retroviral therapy, which will be beneficial to the preservation of neurocognitive function in patients with HAND.
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse to Jun Zhu (R01DA035714, R03DA024275 and R03DA026721). This work was previously presented at the 12th International Symposium on NeuroVirology; October 29-November 2, 2013, Washington D.C., USA. We would like to acknowledge Richard Sterling McCain Jr for his excellent technical assistance.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- Bmax
maximal number of [3H]ligand binding sites
- DA
dopamine
- DAT
dopamine transporter
- EDTA
ethylenediamine tetraacetic acid
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- GBR 12909
1-[2-(bis[4-fluorophenyl]methoxy)ethyl]-4-[3-phenylpropyl]piperazine
- HBS-TE
HEPES Buffered Saline with Tween 20 and EDTA
- hDAT
human dopamine transporter
- HIV
human immunodeficiency virus
- HAD
HIV-associated dementia
- Kd
equilibrium dissociation constant
- Km
Michaelis-Menten constant
- Tat
trans-activator of transcription
- Tween 20
polyethylene glycol sorbilan monolaurate
- Vmax
maximal velocity
- WIN 35
428, 2β-Carbomethoxy-3-β-(4-fluorophenyl)tropane
- MD
molecular dynamics
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Acharjee S, Branton WG, Vivithanaporn P, Maingat F, Paul AM, Dickie P, Baker GB, Power C. HIV-1 Nef expression in microglia disrupts dopaminergic and immune functions with associated mania-like behaviors. Brain Behav Immun. 2014;40:74–84. doi: 10.1016/j.bbi.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14:214–21. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buch S, Yao H, Guo M, Mori T, Su TP, Wang J. Cocaine and HIV-1 interplay: molecular mechanisms of action and addiction. J Neuroimmune Pharmacol. 2011;6:503–15. doi: 10.1007/s11481-011-9297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW. Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. J Neuroimmune Pharmacol. 2006;1:160–81. doi: 10.1007/s11481-006-9017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case DA, Darden TA, Cheatham TE, Iii, Simmerling CL, Wang J, Duke RE, Luo R, Walker RC, Zhang W, Merz KM, Roberts B, Hayik S, Roitberg A, Seabra G, Swails J, Goetz AW, Kolossváry I, Wong KF, Paesani F, Vanicek J, Wolf RM, Liu J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, Cai Q, Ye X, Wang J, Hsieh MJ, Cui G, Roe DR, Mathews DH, Seetin MG, Salomon-Ferrer R, Sagui C, Babin V, Luchko T, Gusarov S, Kovalenko A, Kollman PA. AMBER 12. University of California; San Francisco: 2012. [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–78. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulehan K, Byrd D, Arentoft A, Monzones J, Fuentes A, Fraser F, Rosario A, Morgello S, Mindt MR. The role of decision-making ability in HIV/AIDS: impact on prospective memory. J Clin Exp Neuropsychol. 2014;36:730–41. doi: 10.1080/13803395.2014.935705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 Tat-induced alterations in dopamine transmission. Synapse. 2009;63:181–5. doi: 10.1002/syn.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. Hyperdopaminergic tone in HIV-1 protein treated rats and cocaine sensitization. J Neurochem. 2010;115:885–96. doi: 10.1111/j.1471-4159.2010.06968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Gan XH, Zhang L, House SD, Newton T, Graves MC, Shapshak P, Stins M, Kim KS, Witte M, Chang SL. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine’s connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- Forrest LR, Tavoulari S, Zhang YW, Rudnick G, Honig B. Identification of a chloride ion binding site in Na+/Cl -dependent transporters. Proc Natl Acad Sci U S A. 2007;104:12761–6. doi: 10.1073/pnas.0705600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Young JA. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998;67:1–25. doi: 10.1146/annurev.biochem.67.1.1. [DOI] [PubMed] [Google Scholar]
- Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24:275–83. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175:1148–59. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Spencer JA, Holzer CE, 3rd, Soukup VM. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. J Neuroimmune Pharmacol. 2006;1:410–20. doi: 10.1007/s11481-006-9030-6. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Fitting S, Hasselrot U, Booze RM. Intra-accumbal Tat1–72 alters acute and sensitized responses to cocaine. Pharmacol Biochem Behav. 2008;90:723–9. doi: 10.1016/j.pbb.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh PC, Yeh TL, Lee IH, Huang HC, Chen PS, Yang YK, Chiu NT, Lu RB, Liao MH. Correlation between errors on the Wisconsin Card Sorting Test and the availability of striatal dopamine transporters in healthy volunteers. J Psychiatry Neurosci. 2010;35:90–4. doi: 10.1503/jpn.090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Rock RB. CB2 receptor agonists protect human dopaminergic neurons against damage from HIV-1 gp120. PLoS One. 2013;8:e77577. doi: 10.1371/journal.pone.0077577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Gu HH, Zhan CG. Mechanism for cocaine blocking the transport of dopamine: insights from molecular modeling and dynamics simulations. J Phys Chem B. 2009;113:15057–66. doi: 10.1021/jp900963n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhan CG. How dopamine transporter interacts with dopamine: insights from molecular modeling and simulation. Biophys J. 2007;93:3627–39. doi: 10.1529/biophysj.107.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6:145–55. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Johnston JB, Zhang K, Silva C, Shalinsky DR, Conant K, Ni W, Corbett D, Yong VW, Power C. HIV-1 Tat neurotoxicity is prevented by matrix metalloproteinase inhibitors. Ann Neurol. 2001;49:230–41. doi: 10.1002/1531-8249(20010201)49:2<230::aid-ana43>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, Joyce AR, Carroll FI, Kuhar MJ. Dopamine D1 and D2 receptors influence dopamine transporter synthesis and degradation in the rat. J Pharmacol Exp Ther. 2001;298:129–40. [PubMed] [Google Scholar]
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Involvement of dopamine in the progression of AIDS Dementia Complex. J Neural Transm. 2002;109:399–410. doi: 10.1007/s007020200032. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson’s disease. J Neurochem. 2001;77:1058–66. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol. 2009;15:257–74. doi: 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrat EP, Zierler S. Entangled epidemics: cocaine use and HIV disease. J Psychoactive Drugs. 1993;25:207–21. doi: 10.1080/02791072.1993.10472272. [DOI] [PubMed] [Google Scholar]
- Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res. 2009;16:205–20. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- Lin Z, Itokawa M, Uhl GR. Dopamine transporter proline mutations influence dopamine uptake, cocaine analog recognition, and expression. FASEB J. 2000;14:715–28. doi: 10.1096/fasebj.14.5.715. [DOI] [PubMed] [Google Scholar]
- Lin Z, Uhl GR. Human dopamine transporter gene variation: effects of protein coding variants V55A and V382A on expression and uptake activities. Pharmacogenomics J. 2003;3:159–68. doi: 10.1038/sj.tpj.6500169. [DOI] [PubMed] [Google Scholar]
- Liu X, Chang L, Vigorito M, Kass M, Li H, Chang SL. Methamphetamine-induced behavioral sensitization is enhanced in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2009;4:309–16. doi: 10.1007/s11481-009-9160-8. [DOI] [PubMed] [Google Scholar]
- McIntosh S, Sexton T, Pattison LP, Childers SR, Hemby SE. Increased Sensitivity to Cocaine Self-Administration in HIV-1 Transgenic Rats is Associated with Changes in Striatal Dopamine Transporter Binding. J Neuroimmune Pharmacol. 2015;10:493–505. doi: 10.1007/s11481-015-9594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Harrod SB, Zhu J. Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats. Pharmacol Biochem Behav. 2011;98:587–97. doi: 10.1016/j.pbb.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Zhu J. HIV-1 Tat Protein Decreases Dopamine Transporter Cell Surface Expression and Vesicular Monoamine Transporter-2 Function in Rat Striatal Synaptosomes. J Neuroimmune Pharmacol. 2012;7:629–39. doi: 10.1007/s11481-012-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Huang X, Gomez AM, Booze RM, Zhan CG, Zhu J. Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions. J Neuroimmune Pharmacol. 2013;8:975–87. doi: 10.1007/s11481-013-9464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Yuan Y, Quizon PM, Sun WL, Huang X, Zhan CG, Zhu J. Mutations at tyrosine 88, lysine 92 and tyrosine 470 of human dopamine transporter result in an attenuation of HIV-1 Tat-induced inhibition of dopamine transport. J Neuroimmune Pharmacol. 2015;10:122–35. doi: 10.1007/s11481-015-9583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton LS, Apparsundaram S, King-Pospisil KA, Dwoskin LP. Nicotine increases dopamine transporter function in rat striatum through a trafficking-independent mechanism. Eur J Pharmacol. 2007;554:128–36. doi: 10.1016/j.ejphar.2006.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothobi NZ, Brew BJ. Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr Opin Infect Dis. 2012;25:4–9. doi: 10.1097/QCO.0b013e32834ef586. [DOI] [PubMed] [Google Scholar]
- Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–9. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- Nath A, Clements JE. Eradication of HIV from the brain: reasons for pause. AIDS. 2011;25:577–80. doi: 10.1097/QAD.0b013e3283437d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Jankovic J, Pettigrew LC. Movement disorders and AIDS. Neurology. 1987;37:37–41. doi: 10.1212/wnl.37.1.37. [DOI] [PubMed] [Google Scholar]
- Norman LR, Basso M, Kumar A, Malow R. Neuropsychological consequences of HIV and substance abuse: a literature review and implications for treatment and future research. Curr Drug Abuse Rev. 2009;2:143–56. doi: 10.2174/1874473710902020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariser JJ, Partilla JS, Dersch CM, Ananthan S, Rothman RB. Studies of the biogenic amine transporters. 12. Identification of novel partial inhibitors of amphetamine-induced dopamine release. J Pharmacol Exp Ther. 2008;326:286–95. doi: 10.1124/jpet.108.139675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Penmatsa A, Wang KH, Gouaux E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature. 2013;503:85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SW, Barbieri J, Tong N, Polesskaya O, Pudasaini S, Stout A, Lu R, Kiebala M, Maggirwar SB, Gelbard HA. Human immunodeficiency virus-1 Tat activates calpain proteases via the ryanodine receptor to enhance surface dopamine transporter levels and increase transporter-specific uptake and Vmax. J Neurosci. 2010;30:14153–64. doi: 10.1523/JNEUROSCI.1042-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, McArthur JC, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson RT, Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol. 1998;72:9045–53. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol. 2011;44:102–10. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- Rappaport J, Joseph J, Croul S, Alexander G, Del Valle L, Amini S, Khalili K. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. J Leukoc Biol. 1999;65:458–65. doi: 10.1002/jlb.65.4.458. [DOI] [PubMed] [Google Scholar]
- Ray PE, Liu XH, Robinson LR, Reid W, Xu L, Owens JW, Jones OD, Denaro F, Davis HG, Bryant JL. A novel HIV-1 transgenic rat model of childhood HIV-1-associated nephropathy. Kidney Int. 2003;63:2242–53. doi: 10.1046/j.1523-1755.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–6. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith ME, Coffey LL. [3H]WIN 35,428 binding to the dopamine uptake carrier. II. Effect of membrane fractionation procedure and freezing. J Neurosci Methods. 1994;51:31–8. doi: 10.1016/0165-0270(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Manohar S, Kaliyaperumal K, See RE, Ramamoorthy S. Dysregulation of dopamine transporter trafficking and function after abstinence from cocaine self-administration in rats: evidence for differential regulation in caudate putamen and nucleus accumbens. J Pharmacol Exp Ther. 2008;325:293–301. doi: 10.1124/jpet.107.130534. [DOI] [PubMed] [Google Scholar]
- Sardar AM, Czudek C, Reynolds GP. Dopamine deficits in the brain: the neurochemical basis of parkinsonian symptoms in AIDS. Neuroreport. 1996;7:910–2. doi: 10.1097/00001756-199603220-00015. [DOI] [PubMed] [Google Scholar]
- Scheller C, Arendt G, Nolting T, Antke C, Sopper S, Maschke M, Obermann M, Angerer A, Husstedt IW, Meisner F, Neuen-Jacob E, Muller HW, Carey P, Ter Meulen V, Riederer P, Koutsilieri E. Increased dopaminergic neurotransmission in therapy-naive asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. J Neural Transm. 2010;117:699–705. doi: 10.1007/s00702-010-0415-6. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–40. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Schrödinger L. The PyMOL Molecular Graphics System, Version 1.3r1. 2010. [Google Scholar]
- Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Neurotoxicity of HIV-1 Tat protein: involvement of D1 dopamine receptor. Neurotoxicology. 2007;28:1184–90. doi: 10.1016/j.neuro.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–50. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–6. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, DeFranco DB. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275:12200–6. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–8. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wang H, Elferich J, Gouaux E. Structures of LeuT in bicelles define conformation and substrate binding in a membrane-like context. Nat Struct Mol Biol. 2012;19:212–9. doi: 10.1038/nsmb.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb KM, Aksenov MY, Mactutus CF, Booze RM. Evidence for developmental dopaminergic alterations in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2010;16:168–73. doi: 10.3109/13550281003690177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13:257–62. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–23. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Huang X, Midde NM, Quizon PM, Sun WL, Zhu J, Zhan CG. Molecular mechanism of HIV-1 Tat interacting with human dopamine transporter. ACS Chem Neurosci. 2015;6:658–65. doi: 10.1021/acschemneuro.5b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM. Recombinant human immunodeficiency virus-1 transactivator of transcription1–86 allosterically modulates dopamine transporter activity. Synapse. 2011;65:1251–4. doi: 10.1002/syn.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–43. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Dwoskin LP. Nicotinic receptor activation increases [3H]dopamine uptake and cell surface expression of dopamine transporters in rat prefrontal cortex. J Pharmacol Exp Ther. 2009a;328:931–9. doi: 10.1124/jpet.108.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Bardo MT, Bruntz RC, Stairs DJ, Dwoskin LP. Individual differences in response to novelty predict prefrontal cortex dopamine transporter function and cell surface expression. Eur J Neurosci. 2007;26:717–28. doi: 10.1111/j.1460-9568.2007.05690.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–17. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009b;329:1071–83. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Midde NM, Gomez AM, Sun WL, Harrod SB. Intra-ventral tegmental area HIV-1 Tat1–86 attenuates nicotine-mediated locomotor sensitization and alters mesocorticolimbic ERK and CREB signaling in rats. Front Microbiol. 2015;6:540. doi: 10.3389/fmicb.2015.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Reith ME. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets. 2008;7:393–409. doi: 10.2174/187152708786927877. [DOI] [PMC free article] [PubMed] [Google Scholar]