Abstract
Laminins are a major constituent of the basement membranes of the kidney collecting system. Integrins, transmembrane receptors formed by non-covalently bound α and β subunits, serve as laminin receptors, but their role in development and homeostasis of the kidney collecting system are poorly defined. Integrin α3β1, one of the major laminin receptors, plays a minor role in kidney collecting system development, while the role of α6 containing integrins (α6β1 and α6β4), the other major laminin receptors, is unknown. Patients with mutations in α6 containing integrins not only develop epidermolysis bullosa, but also have abnormalities in the kidney collecting system. In this study, we show that selectively deleting the α6 or β4 integrin subunits at the initiation of ureteric bud development in mice does not affect morphogenesis. However, the collecting system becomes dilated and dysmorphic as the mice age. The collecting system in both null genotypes were also highly susceptible to unilateral ureteric obstruction injury with evidence of excessive tubule dilatation and epithelial cell apoptosis. Mechanistically, integrin α6-null collecting duct cells are unable to withstand high mechanical force when adhered to laminin. Thus, we conclude that α6 integrins are important for maintaining the integrity of the kidney collecting system by enhancing tight adhesion of the epithelial cells to the basement membrane. These data give a mechanistic explanation for the association between kidney collecting system abnormalities in patients and epidermolysis bullosa.
Introduction
The multi-branched kidney collecting system develops from the ureteric bud (UB) which undergoes iterative branching morphogenesis following its interactions with the metanephric mesenchyme (MM). This process requires growth factor-mediated cell signaling and integrin-dependent cell-extracellular matrix (ECM) interactions.
Integrins are transmembrane receptors formed by non-covalently bound α and β subunits and they mediate multiple cellular processes including adhesion, migration, proliferation and tubule formation [1–3]. In mammals, 18 α and 8 β subunits combine in a restricted manner to form specific dimers with different ligand binding properties [4]. Of these heterodimers, α3β1, α6β1 and α6β4 act as primary receptors for laminins (LM) which are large heterotrimeric glycoproteins composed of one α, one β, and one γ chain [5, 6]. LMs belong to a multigenic family with five α, four β and three γ chain genes that can assemble into at least 15 different heterotrimers [7]. Preferred ligands for integrin α3β1 are LMs-111 and 332 and the α5-containing LMs, such as LM-511 and 521 [8, 9]. Integrin α6β1 was identified as a receptor for LMs-111, 511 and 521, while integrin α6β4 preferentially binds to LM-332, however it also interacts with the LMs-511 and 521 [9–11].
The contribution of α6 containing integrins to skin development is well described. Mutations of either α6 or β4 result in junctional epidermolysis bullosa with skin blistering in humans [12]. Consistent with this finding, integrin α6- and β4-null mice die at birth with epidermolysis bullosa, despite no skin morphogenesis defects [13–15]. Dysplasia of the kidney collecting system is also found in epidermolysis patients caused by mutations in the subunits of either integrin α6β4 or of LM-332[16], however due to their perinatal fatality, no causal link has been shown in mice. There is good circumstantial evidence that these integrins are important for normal development and maintenance of the kidney collecting system as integrin α6 and β4 subunits are highly expressed in the UB and antibodies directed against either subunit decreased UB branching morphogenesis in ex vivo organ culture models[17]. Also, selectively deleting the other major LM receptor, integrin α3β1, in renal collecting ducts at an early embryonic stage resulted in only a mild renal phenotype [18–20].
In this study, we selectively deleted the α6 or β4 integrin subunits in the developing UB and found that although morphogenesis was normal, the kidney collecting system was pathologically dilated in aged mice. Both null genotypes were also highly susceptible to tubular dilatation and tubular cell apoptosis following ureteric obstruction. Mechanistically integrin α6-null collecting duct (CD) cells cannot withstand high forces when adhered to LM-332. Thus, we conclude that α6 integrin/LM-332 interactions play a key role in maintaining the integrity of the kidney collecting system by mediating tight adhesion of the epithelial cells to the basement membrane. These data provide a mechanistic explanation for the susceptibility of epidermolysis bullosa patients to kidney injury.
Results
Integrin α6 and β4 subunits are not required for normal ureteric bud morphogenesis, but are necessary for maintaining normal kidney collecting duct integrity
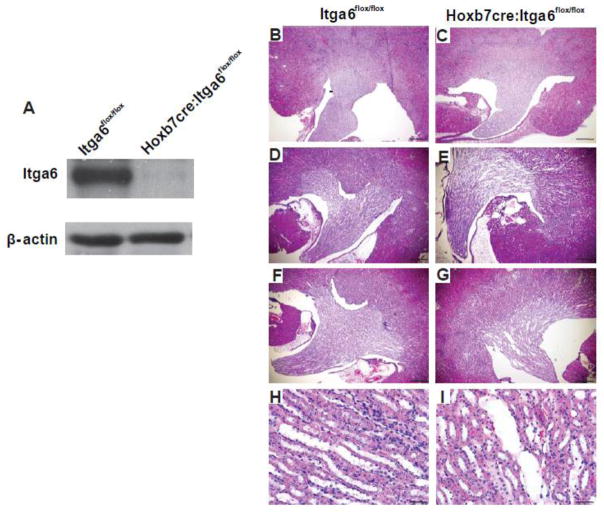
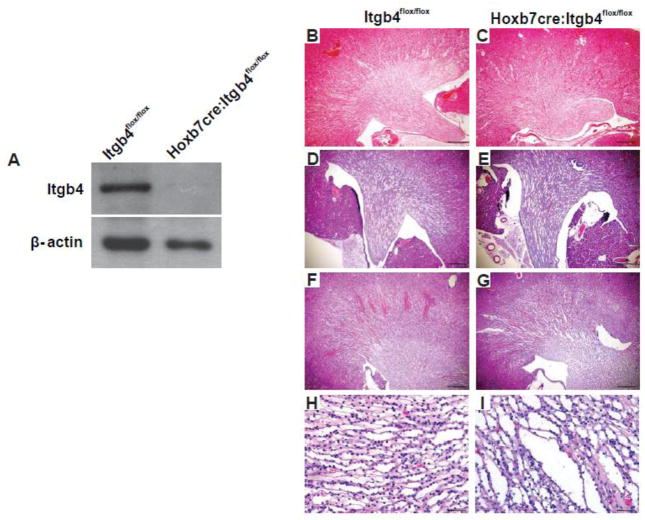
We deleted either the α6 or β4 integrin subunit at the initiation of UB development (E 10.5) by crossing integrin Itga6flox/flox or integrin Itgb4flox/flox mice with the Hoxb7cre mouse. Despite successful deletion of integrin α6 (Fig. 1A), the kidneys of Hoxb7cre:Itga6flox/flox mice developed normally with no defects observed in 3 months old mice (Fig. 1B–C). Interestingly, at 6 months of age these mice had dilated collecting ducts (Fig. 1 D–E) that was worse in 10 month old mice (Fig. 1F–I). Despite these kidney defects, these mice lived a normal life span. We also achieved successful deletion of the integrin β4 in the collecting system of Hoxb7cre:Itgb4flox/flox mice (Fig. 2A) and, similar to the integrin Hoxb7cre:Itga6flox/flox mice, no kidney abnormalities were detected in 3 months old mice (Fig. 2B–C). The Hoxb7cre:Itgb4flox/flox kidneys demonstrated some dilatation of the collecting ducts at both 6 and 12 months, however it was less pronounced than that observed in Hoxb7cre:Itga6flox/flox mice (Fig. 2. D–I). These results suggest that α6 containing integrins are not required for morphogenesis of the developing kidney collecting system but are necessary for maintenance of its integrity over time.
Figure 1. The integrin α6 subunit is not required for normal UB morphogenesis but is necessary to maintain normal kidney collecting duct morphology.
(A) Expression of integrin α6 in papillary lysates from a 3-day old Itga6flox/flox and Hoxb7cre:Itga6flox/flox mice was analyzed by Western blotting analysis. α6 is indicated by the arrowhead. (B–I) Morphology of H&E stained kidney sections from Itga6flox/flox and Hoxb7cre:Itga6flox/flox mice at 2 months (magnification 40×, scale bar =500um) (B, C), 6 months (magnification 40×, scale bar =500um), (D, E) and 10 months of age (F, G) (magnification 40×, scale bar =500um) and (H, I) (magnification 200×, scale bar =100um).
Figure 2. The integrin β4 subunit is not required for normal UB morphogenesis but is necessary to maintain normal kidney collecting duct morphology.
A) Expression of integrin β4 in papillary lysates from a 3-day old Itgb4flox/flox and Hoxb7cre:Itgb4flox/flox mice analyzed by Western blotting analysis. (B–I) Morphology of H&E stained kidney sections from Itgb4flox/flox and Hoxb7cre:Itgb4flox/flox mice at 2 months (magnification 40×, scale bar =500um) (B, C), 6 months (magnification 40×, scale bar =500um), (D, E) and 12 months of age (F, G) (magnification 40×, scale bar =500um) and (H, I) (magnification 200×, scale bar =100um).
The integrin α6 and β4 subunits protect the kidney collecting system from injury
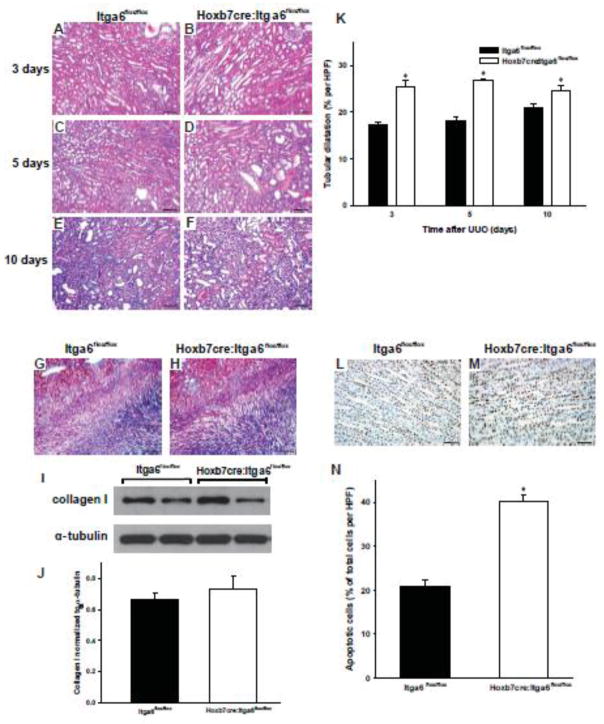
Based on the finding that the collecting system of the Hoxb7cre:Itga6flox/flox and Hoxb7cre:Itgb4flox/flox mice developed dysplastic papillae over time, we tested the hypothesis that α6-containing integrins play a critical role in maintaining kidney structure following injury by subjecting them to unilateral ureteric obstruction (UUO). Three days after UUO the Hoxb7cre:Itga6flox/flox kidneys displayed increased tubular dilation, flattened collecting duct epithelium and proteinacious casts relative to kidneys from Itga6flox/flox mice (Fig. 3 A–B). On day 5 after UUO, we also observed an increase in cellularity at the corticomedullary junction of the kidneys of the Hoxb7cre:Itga6flox/flox mice (Fig. 3 C–D). By day 10 the increased cellularity in the Hoxb7cre:Itga6flox/flox kidneys was easily seen (Fig. 3 E–F). Despite this phenotype, kidneys of injured Hoxb7cre:Itga6flox/flox mice did not develop worse fibrosis than Itga6flox/flox mice as assessed by trichrome staining (Fig 3G–H) and immunoblotting for collagen I in isolated kidneys (Fig. 3I–J). The degree of tubular injury was scored (see Concise Methods) at days 3, 5 and 10 after UUO, and the Hoxb7cre:Itga6flox/flox mice always had a significantly worse injury score than the Itga6flox/flox mice. The respective scores for the Hoxb7cre:Itga6flox/flox and Itga6flox/flox mice were 3.25 ± 0.5 (SE) versus 2.00 ± 0.15 (SE) at 3 days; 3.75 ± 0.00 (SE) versus 2.25 + 0.25 (SE) at 5 days and 4.00 ± 0.00 (SE) versus 3.00 + 0.25 (SE) at 10 days. Furthermore, the kidneys of Hoxb7cre:Itga6flox/flox mice developed a significant increase in tubular dilatation compared to Itga6flox/flox mice (Fig. 3 K). here was also a significant increase in TUNEL staining in the Hoxb7cre:Itga6flox/flox collecting duct epithelium 2 days after UUO (Fig. 3 L–N) and this difference persisted at 3 and 5 days (data not shown). Taken together, α6-containing integrins protect the kidney collecting system from injury in the setting of UUO model by decreasing collecting duct dilatation and apoptosis, but not the fibrotic response.
Figure 3. Hoxb7cre:Itga6flox/flox mice have increased injury after UUO.
(A–F) Morphology of H&E stained kidney sections from Itga6flox/flox (A, C, E) and Hoxb7cre:Itga6flox/flox (B, D, F) 2 month old mice subjected to UUO for 3 (A, B), 5 (C, D) or 10 (E, F) days (magnification 200×, scale bar =100um). (G, H) Fibrillar collagen was detected 7 days after UUO by Trichrome staining. (I, J) Levels of collagen I in kidney lysates of Itga6flox/flox and Hoxb7cre:Itga6flox/flox mice at 7 days after UUO was assessed by Western analysis and quantified by densitometry, normalized to α-tubulin and reported as mean measurements ±SEM. (K) Quantitative analysis of tubular dilatation in renal sections of Itga6flox/flox (black bars) and Hoxb7cre:Itga6flox/flox (white bars) mice subjected to UUO for 3, 5 or 10 days. Values are means with SEM from 6 mice; *p<0.01between Itga6flox/flox and Hoxb7cre:Itga6flox/flox mice. (L, M) Kidney papilla sections from UUO-treated Itga6flox/flox and Hoxb7cre:Itga6flox/flox mice for 2 days were stained for TUNEL (apoptosis) (scale bar= 100um). (N) Quantitative analysis of apoptosis in renal papilla sections of UUO-treated Itga6flox/flox and Hoxb7cre:Itga6flox/flox mice. Apoptosis was quantified and expressed as percent of apoptotic cells per microscopic field (9 fields of 9 kidneys from either genotype were analyzed. *p<0.01 between Itga6flox/flox and Hoxb7cre:Itga6flox/flox mice.
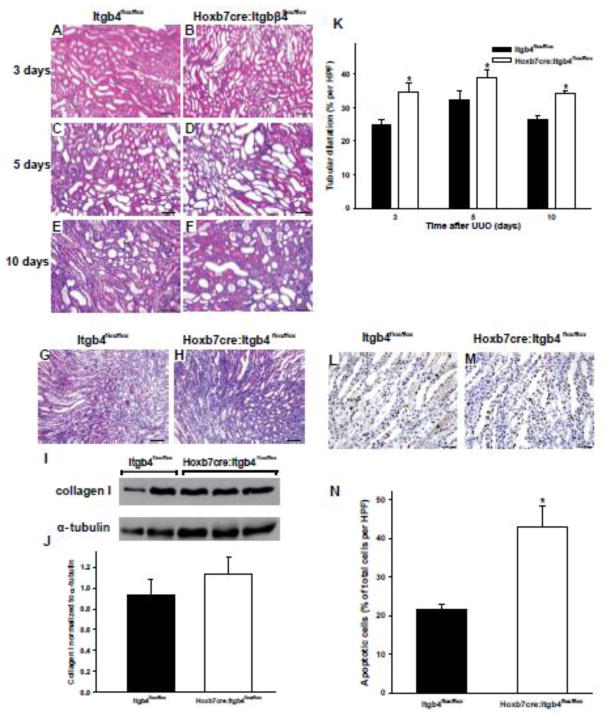
We next investigated the relative contribution of integrin α6β4 in mediating this protection in the setting of UUO injury model by performing the same experiments in the Itgb4flox/flox and Hoxb7cre:Itgb4flox/flox mice. Similar to the Hoxb7cre:Itga6flox/flox mice, the Hoxb7cre:Itgb4flox/flox mice developed more severe injury than the Itgb4flox/flox mice at 3, 5 and 10 days after injury (Fig 4A–F), which was characterized by increased tubular dilation, flattened collecting duct epithelium, proteinacious casts and excessive cellularity but no increase in fibrosis. When the injury was scored it was significantly worse in the Hoxb7cre:Itgb4flox/flox when compared with the Itgb4flox/flox mice at 3, 5 and 10 days respectively. (3.5 ± 0.5 (SE) versus 2.25 ± 0.15 (SE) at 3 days; 4 ± 0.00 (SE) versus 2.5 + 0.25 (SE) at 5 days and 4.00 ± 0.00 (SE) versus 3.00 + 0.25 (SE) at 10 days). There were no significant differences in fibrosis between the two genotypes as assessed by trichrome staining and immunoblotting for collagen I (Fig. 4G–J). The tubular dilatation was significantly increased in the Hoxb7cre:Itgb4flox/flox relative to the Itgb4flox/flox mice in all three days (Fig. 4K). There was also a significantly increased amount of apoptosis in the collecting ducts of the injured Hoxb7cre:Itgb4flox/flox when compared to Itgb4flox/flox mice at 2, 3 and 5 days (Fig. 4 L–N). Thus the differences in severity of injury in the α6 and β4 null mice relative to their wild type controls were very similar, suggesting the protection from injury after UUO was primarily mediated by integrin α6β4.
Figure 4. Hoxb7cre:Itgb4flox/flox mice have increased injury after UUO.
(A–F) Morphology of H&E stained kidney sections from Itgb4flox/flox (A, C, E) and Hoxb7cre:Itgb4flox/flox (B, D, F) 2 month old mice subjected to UUO for 3 (A, B), 5 (C, D) or 10 (E, F) days (magnification 200×, scale bar =100um). (G, H) Fibrillar collagen was detected 7 days after UUO by Trichrome staining. (I, J) Levels of collagen I in kidney lysates of Itgb4flox/flox and Hoxb7cre:Itgb4flox/flox mice at 7 days after UUO was assessed by Western analysis and quantified by densitometry, normalized to α-tubulin and reported as mean measurements ±SEM. (K) Quantitative analysis of tubular dilatation in renal sections of Itgb4flox/flox (black bars) and Hoxb7: Itgβ4flox/flox (white bars) mice subjected to UUO for 3, 5 or 10 days. Values are means with SEM from 6 mice;a significant *p<0.01 between Itgb4flox/flox and Hoxb7cre:Itgb4flox/flox mice. (L, M) Kidney sections from UUO-treated Itgb4flox/flox and Hoxb7cre:Itgb4flox/flox mice for 2 days were stained for TUNEL (apoptosis)( scale bar =100um). (N) Quantitative analysis of apoptosis in renal sections of UUO-treated Itgb4flox/flox and Hoxb7cre:Itgb4flox/flox mice. Apoptosis was quantified and expressed as percent of apoptotic cells per microscopic field (9 fields of 9 kidneys from either genotype were analyzed *p<0.01 between Itgb4flox/flox and Hoxb7cre:Itgb4flox/flox mice.
Integrin α6-null collecting duct cells adhere and signal normally on LM-511 but not on LM-332
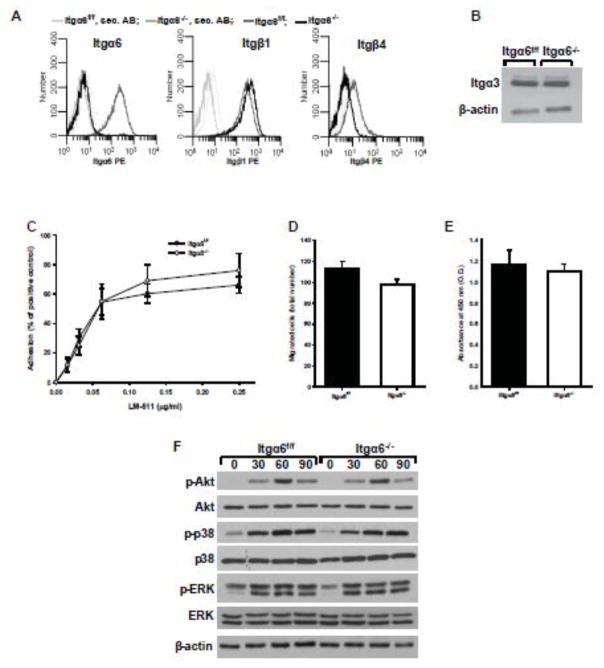
To study the mechanisms whereby integrin α6-LM interactions regulate the homeostasis of the kidney collecting system during aging and after UUO, we isolated collecting duct cells from Itga6flox/flox mice (Itga6f/f cells) and deleted Itgα6 in vitro using Cre adenovirus (Itga6−/− cells). The α6 integrin subunit was efficiently deleted resulting in no surface expression on CD cells (Fig. 5A). This deletion also decreased the surface expression of β4 but did not significantly alter β1 surface expression or total expression of the α3 (Fig. 5A–B) or αv integrin subunits (data not shown). We next assessed the functional consequences of deleting integrin α6 on CD cell functions on LM-511, the principal LM in the collecting duct. Surprisingly, CD cells lacking the integrin α6 subunit did not show significant changes in cell adhesion, migration and proliferation (Fig. 5 C–E). Furthermore, when Itgα6−/− CD cells were plated on purified LM-511, there were no differences in adhesion-dependent Akt, p38-MAPK or ERK activation (Fig. 5F). Thus, in cells expressing the LM-binding integrin α3β1, loss of the integrin α6 does not play a role in mediating interactions between CD cells and LM-511.
Figure 5. Itgα6−/− CD cells adhere, migrate, proliferate and signal normally on LM-511.
(A) Surface expression of integrin α6, β1 and β4 subunits were determined on Itgα6f/f and Itgα6−/− CD cells by flow cytometry using R-phycoerythrin (PE) conjugated secondary antibodies. (B) Lysates from Itgα6f/f and Itgα6−/− CD cells (20 μg total protein/lane) were immunoblotted for integrin α3 subunits or β-actin (loading control). Adhesion (C), migration (D) and proliferation (E) on LM-511 were evaluated as described in the Methods. For migration and proliferation, 1 μg/ml LM-511 was used. Shown are mean measurements ±SEM of 4–6 independent experiments. (F) Itgα6f/f and Itgα6−/− CD cells were plated in serum-free medium on LM-511 (1 μg/ml). Cells were lysed at 30, 60 and 90 min after plating and lysates (20 μg total protein/lane) were analyzed by Western blot for levels of phosphorylated and total Akt, p38, and ERK1/2 or β-actin (loading control).
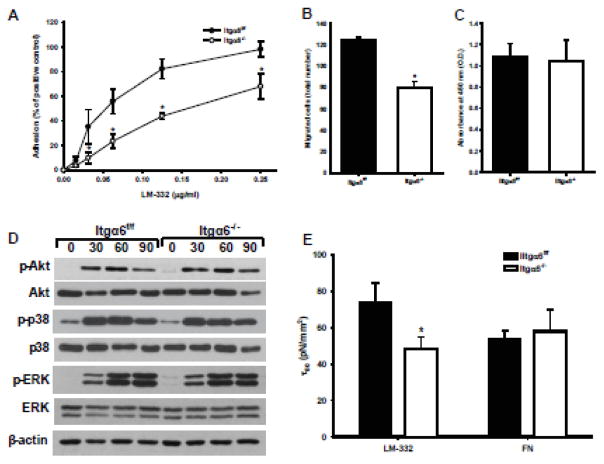
Although LM-511 is the principal LM in the collecting duct, we showed that LM-332 is also expressed in the developing collecting system, where it is required for normal CD development [18]. As both integrins α6β1 and α6β4 are receptors for LM-332, we defined the functions of Itga6−/− CD cells on LM-332. Unlike on LM-511, these cells had significant adhesion and migration defects on LM-332 (Fig. 6A, B) with no overall changes in their ability to proliferate on this substrate (Fig. 6C). When we determined whether there were adhesion-dependent signaling defects of the Itga6-\- CD cells on LM-332, none were found (Fig. 6D). Finally, based on the severe dilatation of the tubules seen in the UUO model in the Hoxb7cre:Itga6flox/flox mice, we assessed the adhesion strength of Itga6−/− CD cells on LM-332 by performing the spinning disc assay. The adhesive strength of these cells was significantly less compared to Itga6f/f CD cells on LM-332, but not on fibronectin, a ligand for α5β1 and αv integrins (Fig. 6E). These results suggest that integrin Itga6−/− CD cells have an adhesion and migration defect on LM-332 and they cannot withstand high forces when adhered to LM-332.
Figure 6. Itgα6−/− CD cells show adhesion, migration and adhesion strength defects on LM-332.
Adhesion (A), migration (B), proliferation (C), and replating assay (D) on LM-322 (1 μg/ml) were evaluated as described in the Methods and in Fig. 5. Shown are mean measurements ±SEM of 4–6 independent experiments; *p<0.01between Itgα6−/− and Itgα6f/f CD cells. (E) The shear stress for 50% detachment (τ50), which represents the mean cell adhesion strength, was determined for Itgα6−/− and Itgα6f/f CD cells on LM-332 and fibronectin (which is not a ligand of a6 containing integrin) coated cell culture plates. Shown are the averaged τ50 values of 3 independent experiments; *p<0.01between Itgα6−/− and Itgα6f/f CD cells.
Discussion
Normal UB development requires integrin-dependent cell-ECM interactions, however the role of specific integrins in this process is poorly defined. Deleting the integrin β1 subunit (which deletes 12 integrins) at the initiation of the developing UB resulted in a severe UB branching phenotype that ultimately caused lethal kidney failure [21]. Surprisingly, deleting the α3 subunit in the UB only results in a mild UB development disorder [18–20] and integrin α1 and α2 null mice have normal kidney collecting systems [3, 4]. The addition of blocking α6 and β4 antibodies to in vitro kidney and UB cultures inhibited branching morphogenesis suggesting these LM-binding receptors are important for collecting system development [17, 22]. In this study we directly investigated the role of the α6 integrins in UB development by selectively deleting the α6 or β4 subunit at the initiation of UB development. We show that normal UB morphogenesis does not require expression of either of these integrin subunits, however as the mice age the collecting system becomes dilated and dysmorphic, suggesting a role for the α6 integrins in maintenance of the normal architecture of the kidney collecting system. Consistent with this finding the collecting systems of both the α6- and β4-null mice were highly susceptible to UUO injury, exhibiting excessive tubule dilatation and apoptosis of the kidney tubules. Mechanistically integrin α6-null CD cells have an adhesion and migration defect on LM-332 and they cannot withstand high forces when adhered to this LM. Thus, α6 integrins are important for maintaining the integrity of the kidney collecting system by providing sufficient adhesive strength to epithelial cells on BMs. In the absence of α6 integrins, there is less tight adhesion of CD cells to the BM leading to tubular dilatation over time and increased susceptibility to injury. These data suggest a mechanistic explanation for the higher incidence of renal abnormalities in patients with epidermolysis bullosa [16, 23–25].
Our result that deleting the α6 or β4 integrin subunit from the developing UB did not alter branching morphogenesis was consistent with the observation that newborn constitutive integrin α6-null mice had no renal phenotype [13]. These data contrast with the minor UB branching abnormalities seen in the integrin α3 and LM γ2, or α5 null mice[18–20], which suggests that integrin α3β1 is the principal LM receptor required for normal UB development in vivo. As the renal phenotype of the integrin α3-null mouse is so mild and the α6, α1 and α2 null mice are normal there does not appear to be a major αβ1 integrin that regulates UB morphogenesis. This contrasts with the glomerulus, where deleting the α3 or β1 integrin subunit causes very similar phenotypes [26–29].
The kidney collecting system in both the Hoxb7cre:Itga6flox/flox and Hoxb7cre:Itgb4flox/flox mice become dilated and dysmorphic over time. This phenotype is worse in the α6-null mice, suggesting that both integrin α6β1 and α6β4 play a role in this process. Both mice were equally susceptible to injury after UUO where they exhibited excessive tubular dilatation and epithelial cell apoptosis. These data are consistent with the primary role of α6β4 as an integrin that mediates tight adhesion to a basement membrane. Integrin α6β4 is a major component of both the type I and II hemidesmosome structures [30, 31]. Type I are found in stratified and pseudostratified epithelia such as the skin, while type II are found in simple epithelia such as the uroepithelial layers [32–34]. There are reports of junctional epidermolysis bullosa patients with either α6 or β4 mutations that have a dilated renal pelvis and hydronephrosis[16, 23–25], features similar to those seen in the aged mice. Interestingly, the urinary tract signs in patients do not always correlate with the skin manifestations. Thus our mouse data provides mechanistic insights as to why patients with epidermolysis bullosa caused by α6β4 integrin mutations have kidney collecting system defects. Furthermore, it suggests these patients are potentially susceptible to exacerbated injury in conditions with excessive intraluminal tubular pressure such as obstruction or after the passage of renal stones.
Deleting the integrin α6 subunit from CD cells resulted in a mild adhesion and migration defect as well as decreased adhesion strength on LM-332. Surprisingly, α6 integrins do not play a role in CD cell interactions with LM-511, nor do they regulate any form of ligand-dependent cell signaling. These results are consistent with findings that LM-332 supports stable adhesion of many cell types by interacting with both integrin α6β4 and α3β1, while the latter is the principal integrin that promotes CD cell adhesion to LM-511[9–11]. Thus in CD cells that express α3β1 at high levels, α6 integrins play a minor role in supporting adhesion to LM-332 and do not alter adhesion to LM-511. These data suggest that the principal role of integrin α6β4, like in the skin, is probably to mediate tight adhesion and adhesive strength of CD cells to the tubular basement membrane.
Our results showing that integrin α6β4 is required for normal maintenance of the kidney collecting system adds to its described functions. Other than the severe blistering of the skin despite normal morphogenesis, all the phenotypes are subtle and occur postnatally in an organ specific manner. The only other branching organ where integrin α6β4 was shown to be important is the mammary gland. Deleting β4 resulted in small glands that had increased apoptosis in the surrounding mesenchyme due to decreased PTHrP expression and signaling[35], however these results contrast with another study where mammary glands were shown to develop normally in integrin α6-null mice[36]. Similar to our results, deleting the β4 integrin subunit in Schwann cells in peripheral nerves resulted in abnormal myelin folding and slower nerve recovery after injury because integrin α6β4 is required to anchor Schwann cells to the basal lamina[37, 38]. Other defects in the nervous system associated with the loss of α6, but not β4, have also been described. Deletion of the α6 subunit in the brain using the nestin-Cre mouse, did not result in major abnormalities in the laminar organization of the cerebral cortex and only mild defects in the cerebellar foliation. Compensation by other LM-binding proteins was advanced as the reason for this surprisingly subtle phenotype [39]. Similarly, when the α6 subunit was specifically deleted from Schwann cells using the mP0TOT-Cre mice, there were no abnormalities in axonal sorting and only minor ensheathment and myelin anomalies. The LM receptor, integrin α7β1, partially compensated for α6β1 in Schwann cells [40]. Integrin α6 was also shown to play a role in the development of the olfactory bulb where it is required for neural migration[41]. Thus, with the exception of the skin where it is absolutely required for the tight adhesion of the epidermis to the basement membrane, it appears that other organs can compensate for the lack of integrin α6.
In conclusion our data suggests that α6 integrins provide adhesion strength for CD cell attachment to BMs and that deleting α6 containing makes the kidney collecting system susceptible to age related degeneration and injury due to increased intraluminal pressure of the tubules. These data provide a molecular explanation for the increased incidence of urogenital abnormalities in patients with epidermolysis bullosa due to integrin α6β4 mutations and suggests physicians should protect the kidney collecting system of these patients from trauma and injury.
Concise Methods
Reagents and antibodies
Human LMs 332 and 511 were produced as previously described[42, 43]. Collagen I was purchased from BD Biosciences (San Jose, CA, USA); fibronectin and vitronectin were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). The following antibodies were used in Western immunoblot analyses: integrin α3 (AB1920, Millipore, Temecula, CA, USA and AF2787, R&D Systems); integrin β1 (AB1952, Millipore); integrin β1 (MAB1997, Millipore); integrin β4 (AF4054, R&D Systems); integrin α6 (3750), phospho-AktThr308 (9275), phospho-AktSer473 (9271), total Akt (9272), phospho-p38Thr180/Tyr182 (9211) and total p38 (9212), phospho-ERK1/2Thr202/Tyr204 (9101), total ERK1/2 (9102), (all from Cell Signaling Technologies) and collagen I (MD Biosciences). Antibody to β-actin (A4700, Sigma-Aldrich) and alpha-tubulin (3873, Cell Signaling Technologies) were used to evaluate protein loading. Anti-mouse β1 (550530), α1 (555001), α2 (553819), α5 (553350), α6 (555734) and αv (550024) integrin antibodies were purchased from BD Biosciences. R-phycoerythrin-conjugated secondary antibodies were bought from Invitrogen (Carlsbad, CA, USA).
Generation of Hoxb7cre:Itgα6flox/flox and HoxB7cre:Itgβ4flox/flox mice
Integrin α6flox/flox (Itgα6flox/flox) mice [13, 39], 1996) and integrin β4flox/flox (Itgβ4flox/flox) mice[15] were crossed with the HoxB7cre mice (generous gift of Dr. A. McMahon)[44]. Age-matched littermates homozygous for the integrin Itgα6flox/flox or Itgβ4flox/flox gene but lacking cre were used as controls. The expression of integrin α6 and β4 in the developing mouse collecting ducts was determined using Western immunoblot analysis.
Unilateral ureter obstruction
All procedures using animals were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Vanderbilt University. Unilateral ureteric obstruction was performed on mice aged 8 weeks. Mice were anesthetized with ketamine and administered pre-operative buprenorphine and isotonic saline. The right ureter was ligated with 4.0 silk tie sutures. Mice were euthanized 2, 3, 5, 7, 10 and 14 days’ post-surgery and obstructed kidneys were collected for histology and western blot analysis. Tubular dilatation was quantified using the ImageJ NIH software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2014). At least 10 different animals were analyzed per each group.
Protein extraction and immunoblotting
Protein from the renal papilla of individual 3-day old pups or the whole kidney after UUO was isolated and lysed using a Polytron homogenizer in T-PER reagent (Thermo Scientific, Waltham, MA, USA) with protease inhibitors (P8340 Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitors cocktails 1 and 2 (P5726 and P0044, Sigma-Aldrich). The homogenate was centrifuged at 17,000×g for 20 min at 4°C. The supernatant was collected and stored at 80°C. Cell lysates were prepared using M-PER reagent (Thermo Scientific, Waltham, MA, USA). Lysates were centrifuged at 17,000×g for 15 min at 4°C. Protein concentration in the supernatants was measured using BCA reagent (Thermo Scientific). Protein extracts were subjected to Western immunoblot analysis and developed using the Western Lightning Chemiluminescence Plus detection system (Perkin Elmer-Cetus, Wellesley, MA) according to the manufacturer’s protocol. Densitometry was performed using the ImageJ program. Normalization of each protein of interest was performed relative to α-tubulin or β-actin value.
Analyses of kidney tissue morphology, apoptosis and fibrosis
Kidneys were cut in half, fixed on 10% formalin and embedded in paraffin. Severity of tubular injury was assessed on hematoxylin and eosin (H&E) sections. The percentage of tubules with cell necrosis, loss of brush border, cast formation, and tubular dilation as follows: 0, none; 1, 10%; 2, 11 to 25%; 3, 26 to 45%; 4, 46 to 75%; and 5, >76%. At least 10 fields (×200) were reviewed for each slide in a blinded manner. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed as described by the manufacturer’s instructions (Promega Corporation, Madison WI). TUNEL-positive (apoptotic) cells were counted from 10 randomly selected high-power fields using five kidney samples per genotype. All slides were analyzed in a blinded fashion. Trichrome staining was performed according to the kit’s instructions (Sigma-Aldrich).
Generation of Itgα6f/f and Itgα6 / CD cells
CD cells were isolated from 5- to 6-wk-old Itgα6flox/flox mice as previously described[45] and immortalized with pSV40 plasmid. Loci for the α 6 integrin subunit in CD cells were deleted with adenovirus expressing cre recombinase. CD cells were grown in DMEM/F12 medium containing 10% fetal bovine serum and 1% penicillin/streptomycin.
Flow cytometry
Flow cytometry analysis was performed as previously described[21]. CD cells were incubated with anti-mouse β1, β4, α1, α2, α6 and αv integrin antibodies followed by FITC-conjugated secondary antibodies.
Cell adhesion
Cell adhesion assays were performed in 96-well plates as previously described [46]. Cells (1×105) were seeded in serum-free medium onto plates containing different concentrations of ECM for 60 min. Non-adherent cells were removed and the remaining cells were fixed, stained with crystal violet, and solubilized, and the optical densities of the cell lysates were read at 570 nm (OD570). Adhesion was calculated as percent of positive control (adhesion to serum).
Cell migration
Cell migration was assayed as previously described [46]. Transwells with 8-μm pores were coated with different ECM components, and 1×105 cells were added to the upper well in serum-free medium. Cells that migrated through the filter after 8 h were counted.
Cell proliferation
Cell proliferation was assessed by measuring incorporation of 5-bromodeoxyuridine (BrdU) in an enzyme-linked immunosorbent assay–based 5-Bromo-2′-deoxy-uridine Labeling and Detection Kit III (Roche Applied Science, Indianapolis, IN) as previously described [47]. BrdU incorporation was quantified by a change of absorbance (optical density) at 405 nm.
Cell replating assay
Cell replating assays were performed on CD cells that were trypsinized, washed, suspended in serum-free DMEM, plated on LM-332 or LM-511 (1 μg/ml) and harvested at 0, 30 and 60 min later. Cells were washed in PBS and lysed using M-PER reagent with protease and phosphatases inhibitor cocktails (Sigma). Protein extracts (20–40 μg) were subjected to Western immunoblot analysis.
Spinning disk adhesion assay
Mean cell adhesion strength was measured using a spinning disk system as previously described[48, 49]. Briefly, coverslips with adherent cells were mounted on the spinning platform and spun. After spinning, remaining cells were fixed in 3.7% formaldehyde, permeabilized in 0.1% Triton X-100, stained with ethidium homodimer-1, and counted. Cell counts were normalized to the number of cell counts at the center of the disk, where the applied force is zero. The shear stress for 50% detachment (τ50) was used as the mean cell adhesion strength.
Statistical analyses
The mean and SEM of each treatment group were calculated for all experiments. At least 4 independent experiments (some in triplicates each) were performed. Student’s t test was used to compare two groups. All statistical tests were two-sided and statistical analysis was done with the use of SigmaStat software (Systat Software Inc., San Jose, CA). Statistical significance was defined as p less than or equal to 0.05.
Highlights.
This manuscript highlights the fact that although integrins α6β1 and α6β4 are highly expressed in the ureteric bud if the kidney, they are not required for its normal development. However, these integrins are vital in protecting the kidney collecting system against injury and deterioration with the ageing process. It also highlights the possibility that patients with mutations in the α6 or β4 integrins that develop bullous skin diseases are more susceptible to injury of the kidney collecting system.
Acknowledgments
The authors would like to thank Cathy Alford for performing the cell sorting. This research was funded by VA Merit Reviews grant numbers 1I01BX002025 to A.P., 1I01BX002196 to R.Z.; by the National Institutes of Health grant numbers R01-DK083187 to R.Z., R01-DK075594 to R.Z., R01-DK069221 to R.Z., R01-DK095761 to A.P.
Footnotes
The authors declare no competing or financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathew S, Chen X, Pozzi A, Zent R. Integrins in renal development. Pediatric nephrology. 2012;27(6):891–900. doi: 10.1007/s00467-011-1890-1. [DOI] [PubMed] [Google Scholar]
- 2.Pozzi A, Zent R. Extracellular matrix receptors in branched organs. Curr Opin Cell Biol. 2011;23(5):547–53. doi: 10.1016/j.ceb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pozzi A, Zent R. Integrins in kidney disease. Journal of the American Society of Nephrology : JASN. 2013;24(7):1034–9. doi: 10.1681/ASN.2013010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pozzi A, Zent R. Integrins: sensors of extracellular matrix and modulators of cell function. Nephron Exp Nephrol. 2003;94(3):e77–84. doi: 10.1159/000072025. [DOI] [PubMed] [Google Scholar]
- 5.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 6.Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15(12):1277–94. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24(5):326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara K, Tsuruta D, Ishii M, Jones JC, Kobayashi H. Laminin-332 and -511 in skin. Exp Dermatol. 2008;17(6):473–80. doi: 10.1111/j.1600-0625.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- 9.Delwel GO, de Melker AA, Hogervorst F, Jaspars LH, Fles DL, Kuikman I, Lindblom A, Paulsson M, Timpl R, Sonnenberg A. Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5(2):203–15. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikkawa Y, Yu H, Genersch E, Sanzen N, Sekiguchi K, Fassler R, Campbell KP, Talts JF, Ekblom P. Laminin isoforms differentially regulate adhesion, spreading, proliferation, and ERK activation of beta1 integrin-null cells. Exp Cell Res. 2004;300(1):94–108. doi: 10.1016/j.yexcr.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by alpha 3 beta 1, alpha 6 beta 1 and alpha 6 beta 4 integrins. J Cell Sci. 2000;113(Pt 5):869–876. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- 12.Bruckner-Tuderman L, Has C. Disorders of the cutaneous basement membrane zone--the paradigm of epidermolysis bullosa. Matrix Biol. 2014;33:29–34. doi: 10.1016/j.matbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13(3):370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 14.Niculescu C, Ganguli-Indra G, Pfister V, Dupe V, Messaddeq N, De Arcangelis A, Georges-Labouesse E. Conditional ablation of integrin alpha-6 in mouse epidermis leads to skin fragility and inflammation. Eur J Cell Biol. 2011;90(2–3):270–7. doi: 10.1016/j.ejcb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 15.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet. 1996;13(3):366–9. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 16.Schumann H, Kiritsi D, Pigors M, Hausser I, Kohlhase J, Peters J, Ott H, Hyla-Klekot L, Gacka E, Sieron AL, Valari M, Bruckner-Tuderman L, Has C. Phenotypic spectrum of epidermolysis bullosa associated with alpha6beta4 integrin mutations. Br J Dermatol. 2013;169(1):115–24. doi: 10.1111/bjd.12317. [DOI] [PubMed] [Google Scholar]
- 17.Zent R, Bush KT, Pohl ML, Quaranta V, Koshikawa N, Wang Z, Kreidberg JA, Sakurai H, Stuart RO, Nigam SK. Involvement of Laminin Binding Integrins and Laminin-5 in Branching Morphogenesis of the Ureteric Bud during Kidney Development. Dev Biol. 2001;238(2):289–302. doi: 10.1006/dbio.2001.0391. [DOI] [PubMed] [Google Scholar]
- 18.Yazlovitskaya EM, Tseng HY, Viquez O, Tu T, Mernaugh G, McKee KK, Riggins K, Quaranta V, Pathak A, Carter BD, Yurchenco P, Sonnenberg A, Bottcher RT, Pozzi A, Zent R. Integrin alpha3beta1 regulates kidney collecting duct development via TRAF6-dependent K63-linked polyubiquitination of Akt. Mol Biol Cell. 2015;26(10):1857–74. doi: 10.1091/mbc.E14-07-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Chattopadhyay N, Qin S, Szekeres C, Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner JH, Kreidberg JA. Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis. Development. 2009;136(5):843–53. doi: 10.1242/dev.027805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Mernaugh G, Yang DH, Gewin L, Srichai MB, Harris RC, Iturregui JM, Nelson RD, Kohan DE, Abrahamson D, Fassler R, Yurchenco P, Pozzi A, Zent R. beta1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development. 2009;136(19):3357–66. doi: 10.1242/dev.036269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk M, Salmivirta K, Durbeej M, Larsson E, Ekblom M, Vestweber D, Ekblom P. Integrin alpha 6B beta 1 is involved in kidney tubulogenesis in vitro. J Cell Sci. 1996;109 ( Pt 12):2801–2810. doi: 10.1242/jcs.109.12.2801. [DOI] [PubMed] [Google Scholar]
- 23.Fine JD, Johnson LB, Weiner M, Stein A, Cash S, DeLeoz J, Devries DT, Suchindran C. Genitourinary complications of inherited epidermolysis bullosa: experience of the national epidermylosis bullosa registry and review of the literature. J Urol. 2004;172(5 Pt 1):2040–4. doi: 10.1097/01.ju.0000143200.86683.2c. [DOI] [PubMed] [Google Scholar]
- 24.Fine JD, Johnson LB, Weiner M, Stein A, Cash S, DeLeoz J, Devries DT, Suchindran C. Inherited epidermolysis bullosa and the risk of death from renal disease: experience of the National Epidermolysis Bullosa Registry. Am J Kidney Dis. 2004;44(4):651–60. [PubMed] [Google Scholar]
- 25.Cuesta-Estelles G, Escobedo-Rumoroso JM, Garces-Lopez L, Perez-Garcia A. Epidermolysis bullosa and chronic renal failure. Nephrol Dial Transplant. 1998;13(8):2133–4. doi: 10.1093/ndt/13.8.2133. [DOI] [PubMed] [Google Scholar]
- 26.Sachs N, Claessen N, Aten J, Kreft M, Teske GJ, Koeman A, Zuurbier CJ, Janssen H, Sonnenberg A. Blood pressure influences end-stage renal disease of Cd151 knockout mice. The Journal of clinical investigation. 2012;122(1):348–58. doi: 10.1172/JCI58878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175(1):33–9. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008;316(2):288–301. doi: 10.1016/j.ydbio.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, Gattone VH, Jr, Kalluri R. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313(2):584–93. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LMH, Falcioni R, Kennel SJ, Aplin JD, Baker J, Loizidou M, Garrod D. Integrin alpha6/beta4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991;113:907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16(7):376–83. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Sterk LM, Geuijen CA, Oomen LC, Calafat J, Janssen H, Sonnenberg A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6beta4 and may regulate the spatial organization of hemidesmosomes. J Cell Biol. 2000;149(4):969–82. doi: 10.1083/jcb.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uematsu J, Nishizawa Y, Sonnenberg A, Owaribe K. Demonstration of type II hemidesmosomes in a mammary gland epithelial cell line, BMGE-H. J Biochem. 1994;115(3):469–76. doi: 10.1093/oxfordjournals.jbchem.a124361. [DOI] [PubMed] [Google Scholar]
- 34.Fontao L, Stutzmann J, Gendry P, Launay JF. Regulation of the type II hemidesmosomal plaque assembly in intestinal epithelial cells. Exp Cell Res. 1999;250(2):298–312. doi: 10.1006/excr.1999.4549. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Sun H, Feltri ML, Mercurio AM. Integrin beta4 regulation of PTHrP underlies its contribution to mammary gland development. Dev Biol. 2015;407(2):313–20. doi: 10.1016/j.ydbio.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klinowska TC, Alexander CM, Georges-Labouesse E, Van der Neut R, Kreidberg JA, Jones CJ, Sonnenberg A, Streuli CH. Epithelial development and differentiation in the mammary gland is not dependent on alpha 3 or alpha 6 integrin subunits. Dev Biol. 2001;233(2):449–67. doi: 10.1006/dbio.2001.0204. [DOI] [PubMed] [Google Scholar]
- 37.Van der Zee CE, Kreft M, Beckers G, Kuipers A, Sonnenberg A. Conditional deletion of the Itgb4 integrin gene in Schwann cells leads to delayed peripheral nerve regeneration. J Neurosci. 2008;28(44):11292–303. doi: 10.1523/JNEUROSCI.3068-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nodari A, Previtali SC, Dati G, Occhi S, Court FA, Colombelli C, Zambroni D, Dina G, Del Carro U, Campbell KP, Quattrini A, Wrabetz L, Feltri ML. Alpha6beta4 integrin and dystroglycan cooperate to stabilize the myelin sheath. J Neurosci. 2008;28(26):6714–9. doi: 10.1523/JNEUROSCI.0326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchetti G, De Arcangelis A, Pfister V, Georges-Labouesse E. alpha6 integrin subunit regulates cerebellar development. Cell Adh Migr. 2013;7(3):325–32. doi: 10.4161/cam.25140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellegatta M, De Arcangelis A, D’Urso A, Nodari A, Zambroni D, Ghidinelli M, Matafora V, Williamson C, Georges-Labouesse E, Kreidberg J, Mayer U, McKee KK, Yurchenco PD, Quattrini A, Wrabetz L, Feltri ML. alpha6beta1 and alpha7beta1 integrins are required in Schwann cells to sort axons. J Neurosci. 2013;33(46):17995–8007. doi: 10.1523/JNEUROSCI.3179-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitley M, Treloar H, De Arcangelis A, Georges Labouesse E, Greer CA. The alpha6 integrin subunit in the developing mouse olfactory bulb. J Neurocytol. 2005;34(1–2):81–96. doi: 10.1007/s11068-005-5049-5. [DOI] [PubMed] [Google Scholar]
- 42.Tripathi M, Nandana S, Yamashita H, Ganesan R, Kirchhofer D, Quaranta V. Laminin-332 is a substrate for hepsin, a protease associated with prostate cancer progression. J Biol Chem. 2008;283(45):30576–84. doi: 10.1074/jbc.M802312200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKee KK, Capizzi S, Yurchenco PD. Scaffold-forming and Adhesive Contributions of Synthetic Laminin-binding Proteins to Basement Membrane Assembly. The Journal of biological chemistry. 2009;284(13):8984–94. doi: 10.1074/jbc.M809719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132(12):2809–23. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- 45.Husted RF, Hayashi M, Stokes JB. Characteristics of papillary collecting duct cells in primary culture. Am J Physiol. 1988;255(6 Pt 2):F1160–9. doi: 10.1152/ajprenal.1988.255.6.F1160. [DOI] [PubMed] [Google Scholar]
- 46.Chen D, Roberts R, Pohl M, Nigam S, Kreidberg J, Wang Z, Heino J, Ivaska J, Coffa S, Harris RC, Pozzi A, Zent R. Differential expression of collagen- and laminin-binding integrins mediates ureteric bud and inner medullary collecting duct cell tubulogenesis. Am J Physiol Renal Physiol. 2004;287(4):F602–11. doi: 10.1152/ajprenal.00015.2004. [DOI] [PubMed] [Google Scholar]
- 47.Linkous AG, Yazlovitskaya EM, Hallahan DE. Cytosolic phospholipase A2 and lysophospholipids in tumor angiogenesis. J Natl Cancer Inst. 2010;102(18):1398–412. doi: 10.1093/jnci/djq290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia AJ, Ducheyne P, Boettiger D. Quantification of cell adhesion using a spinning disc device and application to surface-reactive materials. Biomaterials. 1997;18(16):1091–8. doi: 10.1016/s0142-9612(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 49.Garcia AJ, Huber F, Boettiger D. Force required to break alpha5beta1 integrin-fibronectin bonds in intact adherent cells is sensitive to integrin activation state. J Biol Chem. 1998;273(18):10988–93. doi: 10.1074/jbc.273.18.10988. [DOI] [PubMed] [Google Scholar]