Non-myeloablative (NMA) and reduced intensity conditioning (RIC) allow older patients, those with comorbidities, and heavily pre-treated patients to undergo allogeneic hematopoietic cell transplantation (HCT).1,2,3 Consequently, the use of NMA and RIC regimens has significantly increased in the past two decades. However, as relapse remains a significant limitation, conditioning regimens that minimize non-relapse mortality (NRM) associated with MAC while protecting against relapse are needed.
We have previously reported the results in double unit cord blood transplantation (CBT) recipients of a novel RIC regimen using cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2 and thiotepa 10 mg/kg with 400 cGy total body irradiation (Cy50/Flu150/Thio10/TBI400).4,5 Based on favorable outcomes in CBT recipients, we investigated this regimen in recipients of peripheral blood stem cells (PBSC) transplants. Twenty consecutive patients transplanted at Memorial Sloan Kettering Cancer Center between 2008 and 2013 who received Cy50/Flu150/Thio10/TBI400 were included in this retrospective analysis after obtaining approval from the center’s institutional review and privacy board. Patients with a hematologic malignancy who were 18 years of age or greater with an HLA-matched or single-allele-mismatched related or unrelated donor were included. This regimen, along with GVHD prophylaxis and utilization of anti-thymocyte globulin (ATG), was selected for patients at the discretion of the treating provider if the patient was deemed to have high risk disease for which MAC was not feasible either because of comorbidity or based on prior treatment. HLA matching was established by DNA sequence-specific oligonucleotide typing for HLA-A, -B, -C, -DRB1 and -DQB1 loci. Conditioning consisted of cyclophosphamide 50 mg/kg (day -6), fludarabine 30 mg/m2/day for 5 days (-6 to -2), thiotepa 5 mg/kg/day for 2 (days -5 and -4), and TBI 200 cGy/day for 2 (days -2 and -1), with chemotherapy doses calculated on adjusted body weight in recipients over 125% of ideal body weight. GVHD prophylaxis consisted of tacrolimus and methotrexate (n=5), sirolimus and tacrolimus with methotrexate (n=12) or without methotrexate (n=3). A recipient of a 9/10 HLA-match unrelated donor also received ATG. All patients received conventional PBSC grafts; 9 each from HLA-matched related (MRD) or unrelated donors (MUD) and 2 from HLA-unrelated 1 allele mismatched donors (MMUD). Granulocyte colony-stimulating factor (5 μg/kg/day) was given from day 7 until neutrophil recovery. GVHD was diagnosed clinically, confirmed pathologically by biopsy whenever possible, and classified according to standard criteria.6,7 The hematopoietic stem cell transplant comorbidity index score (HCT-CI)8 was assigned retrospectively. Data was updated as of June 2015. Overall (OS) and progression-free survival (PFS) were defined as the time from HCT until death from any cause and disease relapse or death, respectively. NRM was defined as death in a patient without disease relapse. Univariate probabilities and 95% confidence intervals of OS and PFS were estimated using Kaplan-Meier methodology, and survival distributions were compared across patient and treatment characteristics using the logrank test. The cumulative incidence of NRM, disease relapse, and GVHD was estimated based on the cumulative incidence method of competing risks. Disease relapse, death in the absence of disease relapse, and relapse or death in the absence of GVHD were considered competing risks for NRM, disease relapse, and GVHD, respectively. All analyses were performed using R version 3.0.3.
Twenty patients (median age 52 years, range 27–70) underwent transplantation with Cy50/Flu150/Thio10/TBI400 (Table 1). Patients received an average of 2.2 lines of therapy before HCT (range 1–5). Forty percent of the patients were considered to have high or very high-risk disease according to the Disease Risk Index9. Thirty-five percent of patients (n=7) had a HCT-CI of 0, 15% of 1 (n=3), 15% of 2 (n=3) and 35% ≥ 3 (n=7). Comorbidities included: prior solid tumor (n=1), pulmonary disease (n=7), infection requiring antibiotics at time of transplantation (n=1), hepatic dysfunction (n=7), cardiovascular disease (n=1), arrhythmia (n=1) and obesity (n=1), with 8 patients having more than one comorbid condition. Three patients had received prior transplants (one received an autologous transplant (ASCT) 2 years prior to HCT, one received an ASCT 14 years and an HCT 11 years before second HCT, and one had an HCT five years prior to second HCT). Of the patients with a HCT-CI of 0, one received both an ASCT and HCT, 3 were >60 and 3 received >3 lines of prior therapy.
Table 1.
Patient Characteristics.
| Characteristics | Value | ||
|---|---|---|---|
| Median Age | 51.7 (27.3–69.8) | ||
| Male, n (%) | 16 (80) | ||
| Diagnosis/Disease status | |||
| AML | 6 | ||
| Refractory | 2 | ||
| CR1 | 2 | ||
| CRi | 2 | ||
| Plasmacytoid dendritic neoplasm | 1 | ||
| ALL | 2 | ||
| Refractory | 1 | ||
| CR2 | 1 | ||
| CMML | 2 | ||
| Refractory | 2 | ||
| Non-Hodgkin Lymphoma | 9 | ||
| CR1 | 2 | ||
| CR2 | 1 | ||
| CR3 | 1 | ||
| Refractory | 5 | ||
| Disease risk | |||
| Low | 1 (5%) | ||
| Intermediate | 11 (55%) | ||
| High | 6 (30%) | ||
| Very High | 2 (10%) | ||
| Comorbidity Index | |||
| Low (0) | 7 (35%) | ||
| Intermediate (1–2) | 6 (30%) | ||
| High (>=3) | 7 (35%) | ||
| Donor Type | MRD | 9 (45%) | |
| MURD | 9 (45%) | ||
| Mismatch | 2 (10%) |
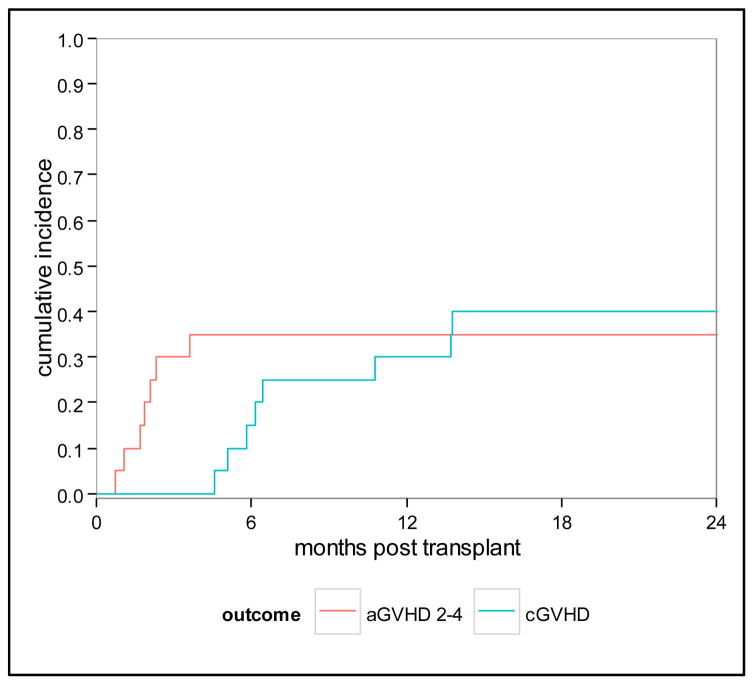
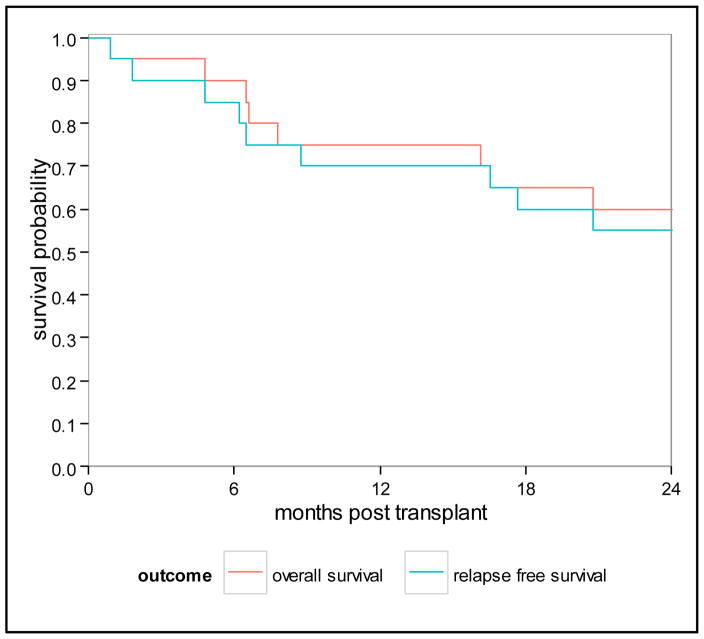
All patients achieved neutrophil engraftment with a median time to neutrophil recovery of 12 days (range 9–19 days). Eighteen patients had platelet recovery, one with delayed platelet recovery (median 12.5 days, range 8–70). Cumulative incidence of acute grade II GVHD (aGVHD) at 100 days was 30% (95% CI 12–51); no patients experienced grade III-IV aGVHD. Patients with aGVHD received grafts from unrelated donors (matched n=6 and mismatch n=1). Cumulative incidence of chronic GVHD (cGVHD) was 40% (95% CI 18–61) at 2 years (Figure 1). Nine patients developed cGVHD, 5 from MRD (including with severe cGVHD), 4 from MUD (matched n=3, mismatched n=1). NRM was 15% (95% CI 4–34) and 25% (95% CI 9–46) at 1 and 2 years, respectively. Five patients died of transplant-related causes, 3 from GVHD (acute in 2, chronic in 1) and 2 from toxicity (1 from liver failure and 1 from diffuse alveolar hemorrhage); no patient died of infection as the primary cause of death. Four patients (2 with NHL, 2 with ALL of whom one also had MDS) relapsed for a cumulative incidence of 20% (95% CI 6–40) at 2 years. Three of these patients died, and one patient with angioimunoblastic T cell lymphoma who was a recipient of a prior transplant and had received 3 lines of therapy is alive at last follow up. As of June 2015, the median follow-up among surviving patients is 41 months (range, 26–82). OS at 1 and 2 years is 75% (95% CI 50–89) and 60% (95% CI 36–78), respectively. PFS at 1 and 2 years is 70% (95% CI 45–85) and 55% (95% CI 31–74), respectively (Figure 2). When analyzing outcomes by HCT-CI, 1-year and 2-year OS were decreased in patients with an HCT-CI ≥ 1 [62% (95% CI 31–82) and 39% (95% CI 14–63), respectively], compared to patients with an HCT CI of 0 [100% OS at both time points, p=0.013)]. Similarly 1-year and 2-year PFS were decreased in patients with an HCT-CI ≥ 1 [54% (95% CI 25–76) and 39% (95% CI 14–63), respectively], compared to patients with an HCT CI of 0 [100% and 86% (95% CI 33–98), p=0.045].
Figure 1.
Cumulative incidence of acute and chronic GVHD
Figure 2.
Overall and Progression Free Survival
In this study we confirm that Cy50/Flu150/Thio10/TBI400 is a promising alternative, radiation-based conditioning regimen of intermediate intensity for patients with a variety of high-risk hematologic malignancies undergoing HCT and ineligible for MAC. Patients with leukemia in this study were either heavily pre-treated or had significant comorbidities precluding the use of MAC. The remainder patients had histologies for which RIC would be considered standard. Although NMA regimens allow older patients and those with greater comorbidities to undergo HCT, relapse rates limit the success of this strategy. Only one study has shown similar outcomes in patients with AML in CR1 receiving RIC vs MAC regimens, although it should be noted that the conditioning in the RIC arm was relatively intense.10 Successful incorporation of radiation to RIC regimens has the potential to decrease relapse rates by increasing conditioning intensity without the toxicities seen in MAC regimens. In the MAC setting, studies have shown comparable outcomes with TBI and non-TBI containing regimens. For Non-Hodgkin lymphoma (NHL) patients specifically, higher intensity regimens and those with radiation, lead to improved outcomes. However, the benefit in disease control is often offset by increased TRM,11 especially after ASCT.12 Herein we present a novel RIC regimen, which intensifies the conditioning over the Cy/Flu/TBI 200 cGy NMA13 by adding 10 mg/kg of thiotepa and increasing the TBI up to 400 cGy with acceptable PFS and OS compared to other RIC regimens.14,15 The Cy50/Flu150/Thio10/TBI400 regimen permits donor engraftment without significant treatment related toxicity. Rates of engraftment were high and no patient died secondary to infectious complications. In this study, only one patient received ATG; however, rates of GVHD were comparable to other studies using conventional grafts, although the regimens for GVHD were variable in this study, limiting any conclusions regarding the optimal GVHD regimen for this conditioning strategy.15,16 Although this study is small, it suggests that ATG can be safely omitted when this regimen is utilized. Non-relapse mortality was 25.0% at 2 years, which is comparable to other studies using RIC.14 Five patients had TRM, all of whom had comorbid conditions prior to treatment (HCT-CI of 1 to 6), and one patient had received a previous autologous and allogeneic transplant. In our study, HCT-CI predicted OS and PFS, despite the number of patients in the study being small. In addition, only one patient with an HCT-CI <3 had a toxicity related death. Limitations of this study include its small size and heterogeneous patient population. However, our data suggest that this reduced intensity regimen incorporating 400 cGy is safe to administer in patients with high-risk disease not eligible for ablative HCT due to age or comorbidities. Prospective studies are required to validate this conditioning strategy for high-risk patients undergoing HCT.
Footnotes
Financial Disclosure: The authors declare no relevant conflicts.
References
- 1.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–54. [PubMed] [Google Scholar]
- 2.Chemnitz JM, von Lilienfeld-Toal M, Holtick U, et al. Intermediate intensity conditioning regimen containing FLAMSA, treosulfan, cyclophosphamide, and ATG for allogeneic stem cell transplantation in elderly patients with relapsed or high-risk acute myeloid leukemia. Annals of hematology. 2012;91:47–55. doi: 10.1007/s00277-011-1253-9. [DOI] [PubMed] [Google Scholar]
- 3.Freytes CO, Zhang MJ, Carreras J, et al. Outcome of lower-intensity allogeneic transplantation in non-Hodgkin lymphoma after autologous transplantation failure. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:1255–64. doi: 10.1016/j.bbmt.2011.12.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponce DM, Sauter C, Devlin S, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19:799–803. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponce DM, Hilden P, Devlin SM, et al. High Disease-Free Survival with Enhanced Protection against Relapse after Double-Unit Cord Blood Transplantation When Compared with T Cell-Depleted Unrelated Donor Transplantation in Patients with Acute Leukemia and Chronic Myelogenous Leukemia. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 7.Filipovich AH. Diagnosis and manifestations of chronic graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:251–7. doi: 10.1016/j.beha.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905–13. doi: 10.1182/blood-2012-03-418202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bornhauser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–44. doi: 10.1016/S1470-2045(12)70349-2. [DOI] [PubMed] [Google Scholar]
- 11.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120:4256–62. doi: 10.1182/blood-2012-06-436725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sureda A, Robinson S, Canals C, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:455–62. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 13.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant A, Nivison-Smith I, Pillai ES, et al. Fludarabine Melphalan reduced-intensity conditioning allotransplanation provides similar disease control in lymphoid and myeloid malignancies: analysis of 344 patients. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2013.142. [DOI] [PubMed] [Google Scholar]
- 16.Blaise D, Tabrizi R, Boher JM, et al. Randomized study of 2 reduced-intensity conditioning strategies for human leukocyte antigen-matched, related allogeneic peripheral blood stem cell transplantation: prospective clinical and socioeconomic evaluation. Cancer. 2013;119:602–11. doi: 10.1002/cncr.27786. [DOI] [PubMed] [Google Scholar]