Abstract
In this work, we developed a simple method to load drugs into commercially available contact lenses utilizing fluorous chemistry. We demonstrated this method using model compounds including fluorous-tagged fluorescein and antibiotic ciprofloxacin. We showed that fluorous interactions facilitated the loading of model molecules into fluorocarbon-containing contact lenses, and that the release profiles exhibited sustained release. Contact lenses loaded with fluorous-tagged ciprofloxacin exhibited antimicrobial activity against Pseudomonas aeruginosa in vitro, while no cytotoxicity towards human corneal epithelial cells was observed. To mimic the tear turnover, we designed a porcine eye infection model under flow conditions. Significantly, the modified lenses also exhibited antimicrobial efficacy against Pseudomonas aeruginosa in the ex vivo infection model. Overall, utilizing fluorous chemistry, we can construct a drug delivery system that exhibits high drug loading capacity, sustained drug release, and robust biological activity.
Keywords: Fluorous chemistry, contact lenses, drug delivery, antimicrobial activity
1. Introduction
Currently more than 90% of ophthalmic drugs are delivered in the form of eye drop solutions.[1] However, only ∼ 1-7% of the administered dose is actively absorbed.[2] Therefore, high drug dosage and frequent administration are necessary in order to have a therapeutic effect, which results in undesired toxicity and low patient compliance. In the search for alternative approaches, contact lenses as ocular drug delivery systems have attracted tremendous attention due to high ocular drug availability, less frequent administration, and low drug toxicity, which could potentially provide a more convenient treatment regime and better patient compliance.[3-8]
The approaches to incorporate drugs into contact lenses have evolved in recent years. Initially, simple immersion of contact lenses in drug solution was tested and found to be insufficient due to its low loading efficiency and fast burst release within the first few minutes, which does not offer significant advantages compared to eye drops.[9-17] In some cases, lens transparency was largely decreased due to drug precipitation.[14] In attempts to overcome these issues, several strategies have emerged, such as molecular imprinting, covalent attachment of drug molecules to contact lenses, plasma treatment of the contact lenses, and delivery of drugs using nanomaterials. Molecular imprinting, a method that creates binding sites within contact lenses with high affinity for specific drugs, greatly improved loading and reproducibility.[18-28] A “layer-by-layer” design of contact lenses,[29, 30] in which a layer of drugs was sandwiched between two polymeric layers, may offer extended drug release. However, this method is difficult to be incorporated in to the contact lens manufacturing process, and some drugs may lose their biological activity during the process. Plasma treatment, a technology that has been long used to improve the wettability of contact lenses, has shown to desirably slow down the initial burst release of drugs from lenses.[31, 32] However, the plasma conditions have to be carefully controlled as insufficient and aggressive treatments either do not have any effect or have the potential to result in the loss of the drugs' bioactivity. Recently contact lenses have been combined with nano-/micro- scale drug delivery systems such as liposomes,[33] polymeric materials,[15, 27, 30, 34] and micelles[35]. These nano-/micro- scale drug delivery systems offer high drug loading capacity and suitability for nearly any kind of drug by rational design of the system. One recent approach described a polymeric “nanowafer” disk that was fabricated using lithography and loaded with drug into nano-/micro- sized holes, which greatly increased drug loading.[36] Each method has its own advantages in one or two areas, but unfortunately, none to date has provided simple manufacture process, high drug loading efficacy, satisfactory drug release kinetics, and high optical quality after drug incorporation. Furthermore, contact lens wear is associated with high risk of complications such as potentially vision threatening infection and inflammation.[37-42] With the widespread and rapid growth of contact lens wear not only for optical correction but also for purposes such as cosmesis and smart lenses with biosensors[43-51], it is essential to make contact lenses safer to wear by equipping them with antimicrobial and anti-inflammatory abilities. To this end, a recent design for safer contact lenses with covalently attached antimicrobial peptide has been tested in laboratory animals and humans.[52] Overall, novel designs of contact lens drug delivery systems are needed to address all the above issues. In this work, we developed a simple yet effective method based on fluorous chemistry to incorporate drugs into contact lenses that has great potential to provide the desirable characteristics of an ideal contact lens drug delivery system.
Fluorous chemistry is based on the unique fluorous interactions with which fluorous-tagged carbon chains are attracted to each other much more than other media.[53-55] These interactions are neither hydrophilic nor hydrophobic. Fluorous interactions have been widely used for anti-fouling coatings.[56-58] Furthermore, perfluorocarbons are used to enhance oxygen delivery in contact lenses, ultrasound imaging and therapy.[59-64] We have used fluorous interactions for incorporating active groups such as alkynes or carboxylic acids onto various substrate surfaces including contact lens surfaces pre-modified with fluorous chains.[65] The functional molecules such as antimicrobial peptides are then covalently attached to the surface via click chemistry. However, this approach has its limitations. In particular, this approach is a 2-step procedure, and during the covalent attachment step, fluorous molecules are susceptible to dissociation from the surface. To keep the molecules on the surface requires a very strong fluorous interaction, thus limiting its application to contact lenses with a high content of fluorocarbon chains, which is not commonly found in commercial contact lenses. Furthermore, the toxic copper catalysts used for covalent modification of contact lenses may be difficult to remove.
In this work, we overcome the above limitations by attaching a short fluorous tag to the drug molecule. We show that the fluorous-tagged drugs can be easily loaded to common fluorine-containing contact lenses and the system exhibits a desired release profile.
2. Methods and Materials
2.1 Materials
Ciprofloxacin, trimethylamine, methylene chloride, heptafluorobutyric acid, perfluoropentanoic acid, fluorescein isothiocyanate (FITC) and perfluoroheptanoic acid were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification. Pseudomonas aeruginosa strain 19660 was purchased from ATCC. A telomerase transformed human corneal epithelial cell line (hTCEpi) [66] was used for in vitro studies.
2.2. Synthesis of fluorous-tagged FITC and ciprofloxacin derivatives
2.2.1. Synthesis of fluorous-tagged FITC (FITC-F)
A solution of 1H, 1H-perfluorooctylamine (2 mM, 10 mL) in ethanol was added dropwise to a stirred solution of Fluorescein isothiocyanate (FITC, 15.57 mg) in 10 mL of ethanol at room temperature. The solution was stirred for 24 h, and evaporated under reduced pressure. The residue was purified by silica gel flash chromatography eluted with ethyl acetate/MeOH 9/1 to give FITC-F (22 mg, 93%) as a green powder. 1H NMR (500 MHz, acetone-d6) δ 9.05 (s, 2H), 7.98 – 7.89 (m, 2H), 7.26 (dd, J = 8.1, 7.0 Hz, 1H), 6.75 (d, J = 2.5 Hz, 2H), 6.72 (d, J = 4.0 Hz, 1H), 6.70 (d, J = 3.5 Hz, 1H), 6.65 – 6.64 (m, 1H), 6.63 (dt, J = 4.9, 1.8 Hz, 1H), 4.75 (dtd, J = 48.0, 16.4, 6.2 Hz, 2H), 4.47 (q, J = 7.2 Hz, 1H). 13C NMR (126 MHz, Acetone-d6) δ 184.39, 169.11, 169.07, 160.30, 153.35, 153.32, 153.28, 149.98, 146.63, 141.81, 131.30, 130.17, 130.13, 130.09, 128.40, 125.88, 125.17, 125.12, 124.75, 119.22, 119.07, 117.36, 116.63, 113.30, 111.65, 111.58, 111.55, 103.33, 55.44. 19F NMR (471 MHz, CDCl3) δ -81.44 – -81.64 (m, 3F), -117.09 – -117.92 (m, 2F), -122.23 (s, 2F), -122.46 (s, 2F), -123.18 (s, 2F), -123.94 (s, 2F), -126.63 (td, J = 14.6, 6.9 Hz, 2F). MALDI-TOF-MS m/z: [M]+ calcd for C29H17F15N2O5S: 790.06; found: 790.10.
2.2.2. Synthesis of fluorous-tagged ciprofloxacin derivatives compound 1–4
Ciprofloxacin (250 mg, 0.75 mmol) and triethylamine (139 μL, 1 mmol) were stirred in anhydrous methylene chloride (5 mL) at 0 °C for 15 min. Heptafluorobutyric acyl chloride (259.8 mg, 1.12 mmol) was added dropwise into the mixture under nitrogen atmosphere. The suspension was stirred at room temperature for 12 h, and evaporated under reduced pressure. The residue was purified by silica gel flash chromatography eluted with ethyl acetate/acetone/dichloromethane (v:v:v = 10:1:20) to give 1 (173.9 mg, 33%) as a white powder. 1H NMR (500 MHz, CDCl3) δ 8.73 (s, 1H), 8.01 (d, J = 12.7 Hz, 1H), 7.38 (d, J = 7.0 Hz, 1H), 3.96 (dd, J = 8.9, 4.7 Hz, 4H), 3.56 (s, 1H), 3.45 – 3.36 (m, 4H), 1.42 (q, J = 6.6 Hz, 2H), 1.21 (t, J = 5.0 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 177.15, 166.83, 156.47, 154.70, 152.70, 147.81, 145.03. 139.05, 120.82, 112.95, 112.77, 108.40, 105.50, 50.17, 49.42, 45.95, 43.55, 35.49, 8.43.19F NMR (470 MHz, CDCl3) δ -79.58 (t, J = 9.5 Hz, 3F), -111.46 – -111.53 (m, 2F), -121.14 – -121.21 (m, 2F), -125.51 – -125.57 (m, 2F). MS (ESI) m/z: [M + H]+ calcd for C21H18F8N3O4 =528.12; found 528.09.
Compound 2 was similarly obtained in 178.9 mg, 31% as a yellow powder. 1H NMR (500 MHz, Acetone-d6) δ 8.70 (s, 1H), 7.93 (d, J = 13.1 Hz, 1H), 7.79 (d, J = 7.4 Hz, 1H), 4.06 – 3.94 (m, 4H), 3.88 (dd, J = 7.1, 3.5 Hz, 1H), 3.61 – 3.52 (m, 4H), 1.48 (d, J = 6.0 Hz, 2H), 1.39 – 1.30 (m, 2H). 13C NMR (126 MHz, Acetone-d6) δ 177.89, 166.64, 156.59, 155.42, 153.44, 149.00, 145.87, 145.79, 140.35, 130.20, 129.37, 127.17, 120.96, 112.31, 112.12, 108.59, 107.80, 50.73, 50.05, 46.41, 44.04, 36.61, 8.49. 19F NMR (471 MHz, CDCl3) δ -80.86 (t, J = 9.8 Hz, 3F), -110.94 (t, J = 12.2 Hz, 2F), -121.09 (dd, J = 12.7, 6.9 Hz, 2F), -121.86 (dddd, J = 13.4, 10.0, 6.7,3.4 Hz, 2F), -124.50 – -124.62 (m, 2F). MS (ESI) m/z: [M+H]+ calcd for C22H18F10N3O4 =578.11; found 578.08.
Compound 3 was similarly obtained in 202 mg, 40% yield as a pale yellow powder. 1H NMR (500 MHz, CDCl3) δ 8.63 (s, 1H), 7.86 (d, J = 12.7 Hz, 1H), 7.34 (d, J = 7.0 Hz, 1H), 3.93 – 3.71 (m, 4H), 3.61 – 3.51 (m, 1H), 3.47 – 3.28 (m, 4H), 2.68 (dd, J = 9.6, 6.2 Hz, 2H), 2.49 (ddd, J = 17.6, 14.4, 7.8 Hz, 2H), 1.40 (q, J = 6.7 Hz, 2H), 1.20 (q, J = 6.5 Hz, 2H). 13C NMR (126 MHz,CDCl3) δ 176.91, 168.77, 167.31, 154.64, 152.64, 147.63, 145.46, 145.37, 139.06, 120.04,119.98, 112.50, 112.31, 107.84, 105.24, 49.89, 49.36, 45.23, 41.67, 35.53, 26.57, 26.40, 26.23,24.38, 8.32. 19F NMR (470 MHz, CDCl3) δ -80.62 (t, J = 9.9 Hz, 3F), -110.68 (t, J = 13.4 Hz,2F), -120.60 (s, 2F), -121.00 (dd, J = 15.2, 8.0 Hz, 2F), -121.14 (dd, J = 12.7, 6.9 Hz, 2F), -122.67 (s, 2F), -125.81 – -125.92 (m, 2F). MS (ESI): [M+H] + calcd for C22H22F6N3O4 = 506.1,Found 506.2.
Compound 4 was similarly obtained in 203 mg, 30% yield as a yellow powder. 1H NMR (500 MHz, CDCl3) δ 8.77 (s, 1H), 8.05 (d, J = 12.7 Hz, 1H), 7.38 (d, J = 6.6 Hz, 1H), 3.96 (d, J = 11.8 Hz, 4H), 3.55 (s, 1H), 3.40 (s, 4H), 1.42 (d, J = 5.9 Hz, 2H), 1.22 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 177.23, 166.85, 156.57, 154.73, 152.73, 147.87, 144.95, 139.08, 121.04, 120.98, 113.10, 112.92, 111.37, 110.84, 110.62, 108.56, 105.50, 50.26, 49.45, 46.00, 43.62, 35.48, 8.45. 19F NMR (470 MHz, CDCl3) δ -85.32 (s, 3F), -118.30 (t, J = 18.5 Hz, 2F), -121.05 – -121.10 (m, 2F). MS (ESI): [M+H] + calcd for C24H18F14N3O4 = 678.1, Found 678.1.
2.3. X-ray photoelectron spectroscopy (XPS)
XPS was used to determine the surface atomic concentrations of C1s, N1s, O1s, F1s, Si2p of five types of commercial contact lenses, Comfilcon A, Narafilcon A, Lotrafilcon B, Ocufilcon D, and Delefilcon A. Briefly, lenses were dried in air and cut into pieces of ∼ 5 × 5 mm2. Each piece was glued onto a stainless steel holder and loaded into a PHI 5700 X-ray photoelectron spectrometer, equipped with a monochromatic AlKα X-ray source (hν = 1486.7 eV) at a take-off angle (TOA) of 45° from the film surface.[67]
2.4. Loading of molecules into commercial contact lenses
A list of the commercial contact lenses tested is presented in Table 1. All lenses were air dried and weighed. The weights of Comfilcon A, Narafilcon A, Lotrafilcon B, Ocufilcon D and Delefilcon A lenses are 16.3 ± 0.3, 15.6 ± 0.1, 20.7 ± 0.3, 15.7 ± 0.2, and 21.5 ± 0.4 mg, respectively. Compounds used including FITC-F, FITC, Ciprofloxacin (Cip), fluorous-tagged ciprofloxacin (F-Cip 1–4) are shown in Figure 1. Briefly, commercial lenses were individually immersed in wells of a 24 well plate, each containing 1 mL PBS solution of FITC-F (15.6 μM), FITC (15.6 μM), Cip (100 μM) or F-Cip (100 μM), and incubated for 18 h. Then the lenses were washed with PBS three times for 5 min each. All incubation and wash solutions were collected and the amount of each compound was quantified by spectrophotometry. Standard curves were generated with seven standard samples with known concentrations for each individual compound of interest. Specifically, fluorescent compounds FITC and FITC-F were quantified by fluorescence emission at 520 nm (λex = 485 nm), and Cip and F-Cip were quantified by absorbance at 275 nm. The amount of each compound in the above collected solutions was then measured with the standard curve. The amount of the compound incorporated into lenses was determined by subtracting the amount in solution from the total amount added initially.
Table 1.
Information on the contact lenses tested.
| Trade Name | Biofinity | Air Optix | Biomedics 55 | Acuvue TruEye | Dailies Total 1 |
|---|---|---|---|---|---|
| US Adopted Names | Comfilcon A | Lotrafilcon B | Ocufilcon D | Narafilcon A | Delefilcon A |
| Material | Silicone hydrogel | Silicone hydrogel | Hydrogel | Silicone hydrogel | Silicone hydrogel core, non-silicone hydrogel surface |
| Surface fluorine (%) | 3.7 | 1.05 | 0 | 0 | 0 |
Figure 1.
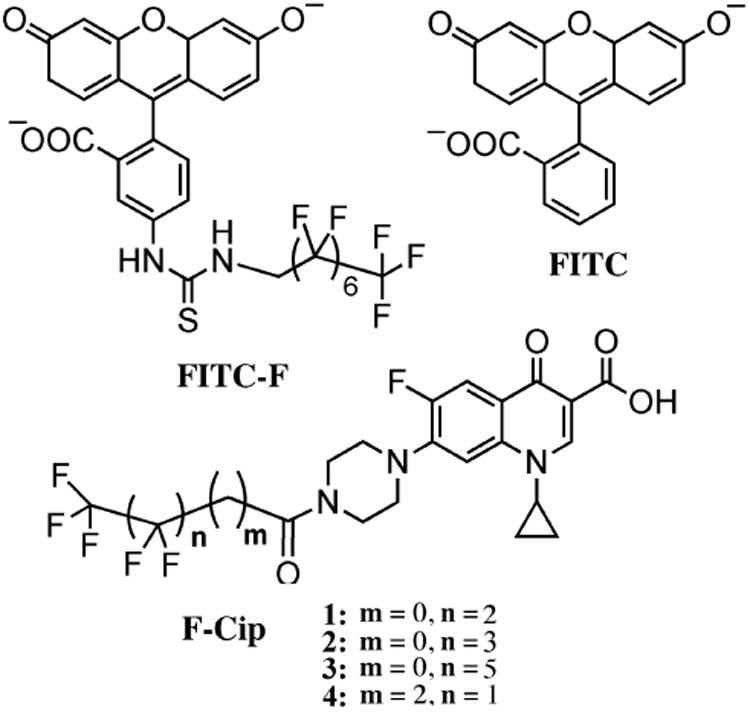
Structures of FITC-F, FITC, and fluorous-tagged ciprofloxacin (F-Cip).
2.5. Lens transparency after modification
To determine the transparency of fresh and modified Comfilcon A contact lenses using light transmission, contact lenses were cut into small disks with a diameter of 6 mm and placed into wells of a UV-transparent 96-well plate, and a wavelength scan from 220 to 800 nm was conducted using a plate reader (BMG LabTech FLUOstar Omega plate reader, Germany).[28]
2.6. Time course release of individual compounds from contact lenses
The modified Comfilcon A lenses were placed into individual wells of a 24 well plate with 1 mL PBS in each well at room temperature. The PBS solution was replaced with fresh PBS every hour for up to 8 h or 15 h, or every ten minutes for 2 h or 6 h. The amount of fluorescent compound released into the collected PBS aliquots was quantified spectrophotometrically based on the above standard curve. The amount of F-Cip released into the collected PBS aliquots was quantified using LC-MS (LCQ Deca XP plus with Surveyor LC, Thermo Fisher, Waltham, MA) with electrospray ionization in positive ion mode. Samples (5 μL each) were loaded onto the analytical column at 200 μL/min (Kinetex XB-C18, 2.1 × 50 mm, 2.6 μm, Phenomenex) with the following gradient: 0-3 min, 10-75% (B); 3-7.5 min, 75-91% (B). The mobile phase consists of (A) water containing 0.1% formic acid and (B) acetonitrile containing 0.1% formic acid. Mass spectrometric parameters were as follows: 250 °C capillary temperature, 45 units sheath gas, 10 units aux gas and 4.5 kV spray voltage. Selective ion monitoring (SIM) was used for detecting F-Cip 1 and 2, and the quantification was based on the respective standard calibration curve. The detection limit for F-Cip 1 and 2 is approximately 1.5 pg based on S/N ratios.
2.7. Antibacterial activity of F-Cip
Laboratory strain Pseudomonas aeruginosa (P. aeruginosa) ATCC 19660 was used to test the antimicrobial activity of modified ciprofloxacin. The strain was inoculated and grown at 37 °C for 18 h. The bacterial suspension (300 μL) was added into 100 mL of nutrient broth and incubated for 2 h to reach a log growth phase. The resulting bacterial suspension was adjusted to a concentration of ∼107 cfu/mL and 100 μl were placed into each well of a 96 well plate. Fifty microliters of F-Cip solution of various concentrations in triplicate were added into the 96 well plate and incubated at 37 °C for 18 h. The inhibition of microbial growth was determined by measuring the absorbance at 620 nm. The concentration that inhibited 50% of bacterial growth (IC50) was calculated based on absorbance readings from three independent experiments.
2.8. In vitro antibacterial efficacy of modified contact lenses
Modified Comfilcon A contact lenses were cut into small disks with a diameter of 6 mm and placed into wells of a 96 well plate, each with 100 μL of bacterial suspension prepared as described above in duplicate. Non-modified lenses taken directly for their packaging were used as control. The plate was incubated at 37 °C for 18 h. The inhibition of microbial growth was determined by measuring the absorbance at 620 nm. The bacterial inhibition was calculated based on absorbance and the mean values from three independent experiments were plotted.
2.9 Cytotoxicity of F-Cip and modified contact lenses
Telomerase modified human corneal epithelial cells were seeded into wells of a 96 well plate with 100 μL KGM-2 medium (Lonza Ltd, Switzerland) and incubated at 37 °C until ∼80% confluence. Ten microliters of ciprofloxacin and fluorous-tagged ciprofloxacin 1 and 2 were added to the wells with final concentrations of 10, 5, 2.5, 1.25, 0.63, 0.31, 0.16, 0.08, 0.04, 0.02 μM. Cells treated with benzalkonium chloride (0.05%) and without any treatment served as controls. The plate was incubated at 37 °C for 18 h, and cell viability was determined using a cell counting kit-8 assay.
To determine the cytotoxicity of contact lenses, control and modified Comfilcon A lenses were cut into small disks with a diameter of 6 mm and placed individually into wells of the 96 well plate in duplicate followed by incubation at 37 °C for 18 h. All lenses were taken out, and cell viability was determined using a cell counting kit-8 assay (Dojindo Molecular Technologies, Inc. Japan).
2.10. Ex vivo antimicrobial efficacy of modified contact lenses using porcine eyes
Fresh porcine eyes were purchased from Sioux-Preme Packing Co. (Chicago, IL). The pigs were slaughtered for commercial use and not specifically for the purpose of this study. The eyes were used because their size is similar to that of human eyes, allowing the use of commercially available contact lenses. Upon arrival, eyelids and connective tissues were removed and the porcine eyes were sanitized using 5% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO) for 1 h then washed with PBS three times. Six scratch wounds with a 3×3 crosshatch pattern and a length of ∼1 cm were created on each cornea using a 27G needle. The eyes were placed into holders and then 100 μL of bacterial solution containing 106 CFU (prepared as in Section 2.7) applied to each cornea. The eyes were left at room temperature in a biosafety cabinet for 5 h. After rinsing with 10 mL of PBS, control and modified Comfilcon A contact lenses were placed individually onto the eyes. Eyes without any lenses and eyes treated with 0.3% ciprofloxacin solution every 15 min for 1 h served as controls. One eye without scratches but incubated with bacteria served as an additional control. A custom-made apparatus was used to flow culture medium over the surface of the porcine eyes. This apparatus consisted of a fluid reservoir containing DMEM (Life Technologies, Inc. Carlsbad, CA) and small diameter tubing, positioned to create a flow rate of four 25 μL drops per min onto individual porcine eyes that were placed directly underneath the tube outlet. The eyes were left for 12 h, and then rinsed with 10 mL PBS. The corneas were dissected and cut into pieces using a sterile scalpel followed by homogenizing using a LabGen 125 homogenizer (Cole-Parmer, Vernon Hills, IL) for 1.5 min in 2 mL PBS. Five hundred microliters of the homogenate solution from each sample was added into individual tubes with 6 mL nutrient broth, and the mixtures were shaken at 250 rpm at 37 °C for 10 h. Then 100 μL of the bacterial suspension from each sample was taken out and added into a 96-well plate in triplicate, and absorbance at 620 nm was measured and plotted as percentage of bacterial growth inhibition compared to the corneas infected with bacteria but without any treatments.
2.11. Statistical analysis
Data are expressed as mean ± SD. Where applicable, statistical analysis was performed using one-way or two-way ANOVA followed by Tukey's test where significance was found with p < 0.05.
3. Results and Discussion
3.1. Loading of FITC-F into contact lenses
Fluorophore FITC and fluorous-tagged FITC (FITC-F, Figure 1) were used to test loading onto various types of contact lenses, taking advantage of their simple detection by fluorescence. Among the five types of contact lenses tested (Table 1), Comfilcon A, Narafilcon A, and Lotrafilcon B are silicone hydrogels, Ocufilcon D is a conventional hydrogel, and Delefilcon A is made of a mixture of both materials. Notably, among these 5 types of lens materials, Comfilcon A and Lotrafilcon B contain fluorocarbons in their formulations with a surface fluorine content of 3.70% and 1.05%, respectively (Table 1).
As shown in Figure 2, Comfilcon A attracted the greatest amount of FITC-F (10.1 ± 0.9 nmol per lens), which was significantly higher than the other four types of lenses. Lotrafilcon B attracted 6.7 ± 1.9 nmol FITC-F per lens, which was more than the other three types, and the difference reached statistical significance compared to Ocufilcon D lenses (1.4 ± 0.8 nmol/lens). Comfilcon A attracted more FITC-F than Lotrafilcon B, which correlates to the surface fluorine concentration (3.7% vs 1.05%, Table 1). Furthermore, for Comfilcon A and Lotrafilcon B lenses, the amount of FITC-F loaded onto lenses was significantly more than the amount of FITC, but this was not the case for the other three types of lenses. The amount of FITC-F loaded was ∼9 times greater than the amount of FITC for Comfilcon A lenses (10.1 ± 0.9 vs. 1.1 ± 1.2 nmol/lens), and ∼5 times for Lotrafilcon B lenses (6.7 ± 1.9 vs. 1.3 ± 1.3 nmol/lens), respectively. The structural difference between FITC-F and FITC is the fluorocarbon chain (Figure 1). This result demonstrates that the fluorescent dye with a fluorous tag can be efficiently loaded to the widely used commercial, fluorine-containing contact lenses.
Figure 2.
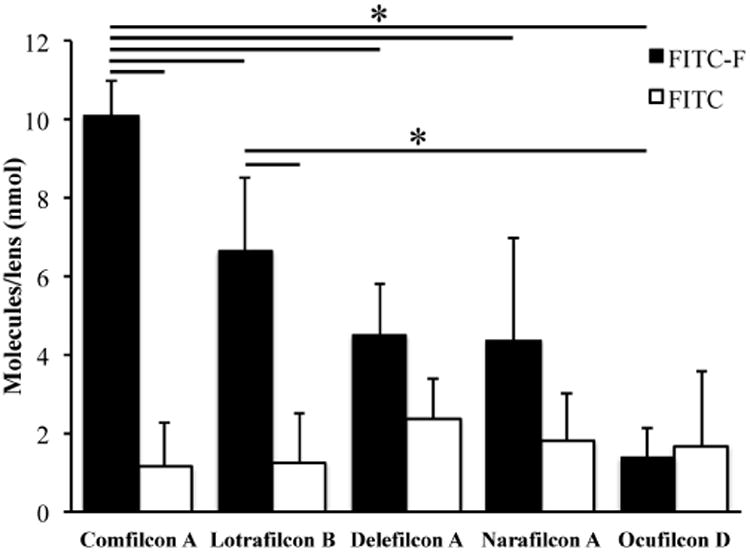
The amount of FITC-F (■) and FITC (□) loaded onto Comfilcon A, Lotrafilcon B, Delefilcon A, Narafilcon A and Ocufilcon D lenses. Data are from at least three independent experiments and are expressed as mean ± SD. Two-way ANOVA was performed followed by Tukey's test where significance was found. * p<0.05.
3.2. Release of FITC-F from Comfilcon A lenses
Comfilcon A lenses were chosen for testing the release profile because they were loaded with the greatest amount of FITC-F. For solution exchange rate of 1 mL/h, the release of unmodified FITC showed a typical burst release profile, and reached plateau with ∼60% release within the first two hours (Figure 3C). The remaining 40% was likely on the lenses. Burst release is the major hurdle for clinical applications of current contact lens based drug delivery systems. Significantly, the release of FITC-F showed a sustained profile with a nearly linear release for the first 6 h with a release rate of 10.5% per hour (Figure 3A), and reached ∼90% at 15 h. Upon increasing the solution exchange rate to 6 mL/h, as expected, the release of unmodified FITC quickly reached plateau within 50 min (Figure 3D). Meanwhile, the release of FITC-F showed a sustained profile with a nearly linear release for the first 120 min with a release rate of 26.5% per hour (Figure 3B), and reached plateau at 270 min. These results showed the release rate increased with faster solution exchange rate, which confirms that the release profiles from contact lens drug delivery model systems are dependent on the experimental setup, and care must be taken to compare results from studies with different setups.[68] The reported tear turnover rate (∼100 μL/h) in human subjects varies significantly,[69-72] and is much lower than the rates used in our experiment. It should be pointed out that the solution in our experiment was changed hourly while tear turnover in the eye is a continuous process, which could result in a different drug release profile. Therefore, it is not feasible to predict the release profile of this system in vivo only based on the solution exchange rates.
Figure 3.
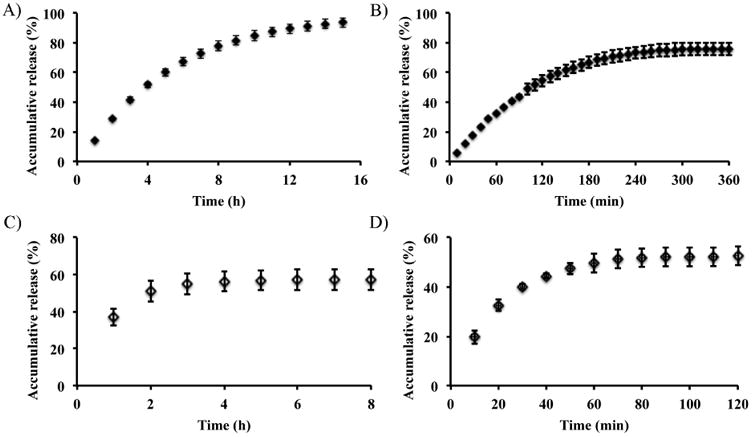
Release of FITC-F (A, B) and FITC (C, D) from Comfilcon A lenses with a solution exchange rate of 1 mL/h (A, C) and 6 mL/h (B, D), respectively. Data are expressed as mean ± SD (n≥3).
The focus of this work is to demonstrate the feasibility of using fluorous chemistry for loading molecules into contact lenses and controlling their release. Overall, our data showed that the release of fluorous-tagged molecules from fluorine-containing lenses exhibited a sustained release profile while non-fluorous-tagged molecules showed a burst release profile. Notably, the structural difference between FITC-F and FITC is the fluorocarbon chain. Therefore, this result demonstrates that fluorous interactions are the driving force in the releasing/retaining of molecules with a fluorous tag from fluorocarbon-containing polymers.
3.3. In vitro antimicrobial activity of fluorous tagged ciprofloxacin
The above results encouraged us to test the feasibility of using this method to load a drug, e.g. antibiotic ciprofloxacin (Cip) in this case, into a contact lens and using it as a drug delivery system. Thus, a series of fluorous-tagged ciprofloxacin (F-Cip, compounds 1–4) molecules were synthesized, differing in the length of the linker (number of CH2 groups) and the number of fluorinated carbons (See Figure 1 for their structures). Table 2 lists the IC50 values of F-Cip 1-4 against P. aeruginosa 19660. The IC50 value of Cip was 0.13 mM, which is similar to the reported values in the literature[73]. Compared to Cip, F-Cip with only 3 and 4 perfluorocarbon atoms (1 and 2) showed a higher IC50 value (0.63–0.69 μM). The IC50 values of F-Cip 3 and 4 were greater than the highest concentration we tested (40 μM), indicating that both lost their antimicrobial activity. The structural difference between F-Cip 1, 2, and 3 is the number of fluorinated carbons, which is 3, 4, and 6 respectively. The result indicated that the longer the fluorinated carbon chain, the less was its antimicrobial efficacy against P. aeruginosa 19660. The attachment of a fluorocarbon chain to Cip may undermine the drug's mechanism of action by decreasing its ability to penetrate bacterial cell membrane and/or binding to its target due to the unique properties of fluorocarbons (i.e. neither hydrophilic nor hydrophobic), therefore, the longer the chain, the larger the effect. Furthermore, although F-Cip 4 has two fluorinated carbons, it also has two extra carbons between the fluorinated carbon chain and its parent compound Cip, which negatively affected its antimicrobial activity. The above brief screening identified compounds 1 and 2 as the lead compounds, which were used in subsequent studies.
Table 2.
IC50 values of fluorous-tagged ciprofloxacin (F-Cip) and Cip against pseudomonas aeruginosa (PA) ATCC 19660. (n≥3)
| No. | No. of CH2 groups | No. of fluorocarbons | IC50 (μM) |
|---|---|---|---|
| 1 | 0 | 3 | 0.63 ± 0.15 |
| 2 | 0 | 4 | 0.69 ± 0.62 |
| 3 | 0 | 6 | > 40 |
| 4 | 2 | 2 | > 40 |
| Cip | 0.13 ± 0.035 |
3.4. Cytotoxicity of F-Cip
The cytotoxicity of 1, 2 and Cip was tested against a telomerase modified human corneal epithelial cell line.[66] Cells exhibited ∼100% viability after incubation with F-Cip 1 and 2, and Cip with concentrations up to 10 μM compared to cells without any treatment. Benzalkonium chloride is known to be cytotoxic [74-76] and killed ∼100% cells and served as control. There was no significant difference among Cip, 1 and 2, indicating that modification of Cip with a fluorous tag did not change its cytotoxicity.
3.5. Loading of F-Cip into Comfilcon A lenses
As Comfilcon A lenses present the greatest amount of fluorine in the formulation, this type of lens was used for the loading experiment of F-Cip. As shown in Figure 5, the amount of 1, 2, 3, and 4 loaded onto contact lenses was 67.96 ± 11.50, 68.72 ± 5.28, 108.36 ± 5.95, and 83.31 ± 8.33 nmol/lens, which was approximately 19, 19, 30, and 23 times more than the amount of Cip loaded on the lenses (3.56 ± 4.51 nmol/lens), respectively. There were no statistically significant differences for the loading of the F-Cip 1, 2, and 4 onto the contact lenses; however, all of them were significantly higher than the amount of Cip. This result demonstrates that the presence of even a short fluorous tag on the molecule greatly enhances its immobilization onto the fluorocarbon-containing contact lenses. The amount of 3 loaded on lenses was significantly more than that of 1, 2, and 4, demonstrating that longer fluorocarbon chain (i.e. stronger interactions between molecules and the lens) facilitated the loading onto lenses.
Figure 5.
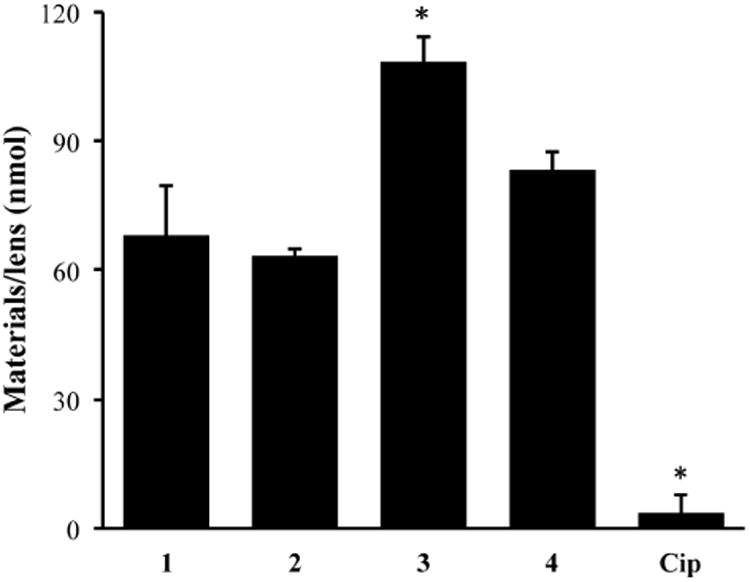
The amount of F-Cip and Cip loaded onto Comfilcon A lenses. Data are from three independent experiments and are expressed as mean ± SD. One-way ANOVA was performed followed by Tukey's test where significance was found. *p<0.05.
It is also remarkable that after modification, the lenses maintained their transparency. As shown in Figure 6, within the visible light range (350 – 750 nm), the light transmission of modified lenses remained ∼ 100% compared to fresh lenses without modification. Therefore, the current simple method has addressed the previously reported issue of loss of transparency of ciprofloxacin-loaded lenses that often exhibited precipitates [14].
Figure 6.
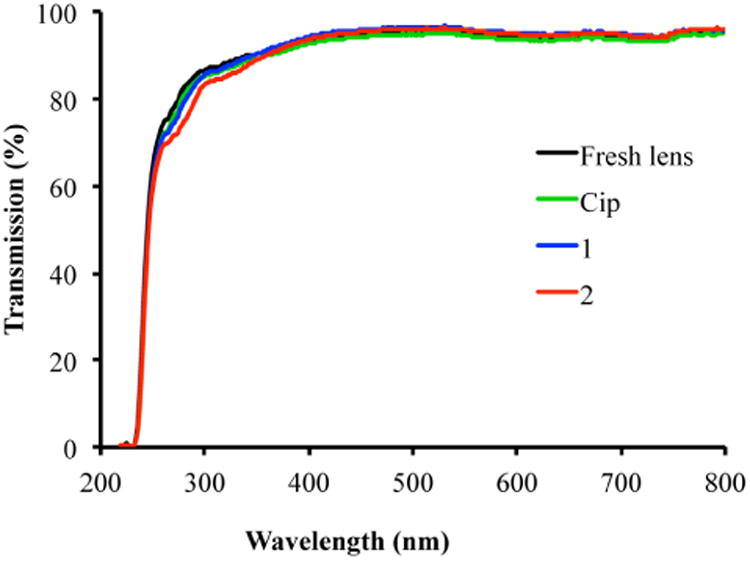
Lens transparency within a wavelength range of 220-800 nm. Fresh lens: black line; Cip: Comfilcon A lenses loaded with ciprofloxacin, green line; 1: Comfilcon A lenses loaded with F-Cip 1, blue line; 2: Comfilcon A lenses loaded with F-Cip 2, red line.
3.6. Release of F-Cip from Comfilcon A lenses
Figure 7 shows the release profile of F-Cip 1, 2, and Cip from Comfilcon A lenses over a period of 8 h with a solution exchange rate of 1 mL/h. Cip exhibited a typical burst release profile, that is, most release occurred within the first hour and no more release after 3 h. The plateau was reached at ∼60% release, and the remaining 40% were likely on the lenses. Significantly, both F-Cip 1 and 2 exhibited a sustained release profile that was very similar to the release profile of FITC-F as shown in Figure 3. Although the percentage release of F-Cip 1 and 2 within the 8 h period was lower than that of Cip, the amount of F-Cip released was 6 times of that of Cip because the amount of F-Cip loaded onto lenses (∼68 nmol/lens) was much more than that of Cip (3.56 ± 4.51 nmol/lens) as shown in Figure 5. The release profiles of 1 and 2 were very similar indicating that only one CF2 group difference in the structures of these two molecules may not affect the overall fluorous interactions between F-Cip and contact lenses, which was also supported by the comparable amounts of 1 and 2 loaded onto Comfilcon A lenses.
Figure 7.
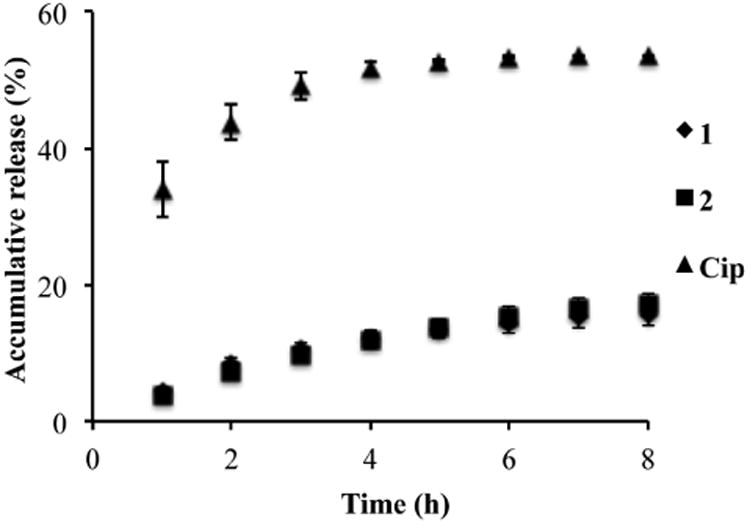
Release of 1 (◆), 2 (■) and Cip (▲) from Comfilcon A lenses over a course of 8 h. Data are expressed as mean ± SD. (n=3)
3.7. In vitro antimicrobial activity of F-Cip-loaded contact lenses
The antimicrobial activity of 1- and 2- as well as Cip-loaded Comfilcon A lenses was tested against P. aeruginosa 19660. As shown in Figure 8, the lenses loaded with 1 and 2 exhibited 99.3% and 93.6% growth inhibition, which was significantly higher than the 35.2% by Cip loaded lenses. Although 1 and 2 exhibited IC50 values that were ∼5 times higher than ciprofloxacin (Table 2), the amount of 1 and 2 loaded onto Comfilcon A lenses was ∼19 times greater than that of Cip (Figure 5), which may account for the significantly higher efficacy of 1-and 2-loaded lenses than Cip-loaded lenses. It is worth noting that in vivo bacteria are also partially cleared from the eye with the continuous tear turnover, which may present a less challenging condition compared to the experimental condition tested here in which bacteria were maintained in nutrient broth without other clearance mechanisms. Therefore, it is significant that this drug-loaded delivery system exhibited nearly complete killing of bacteria under such demanding conditions.
Figure 8.
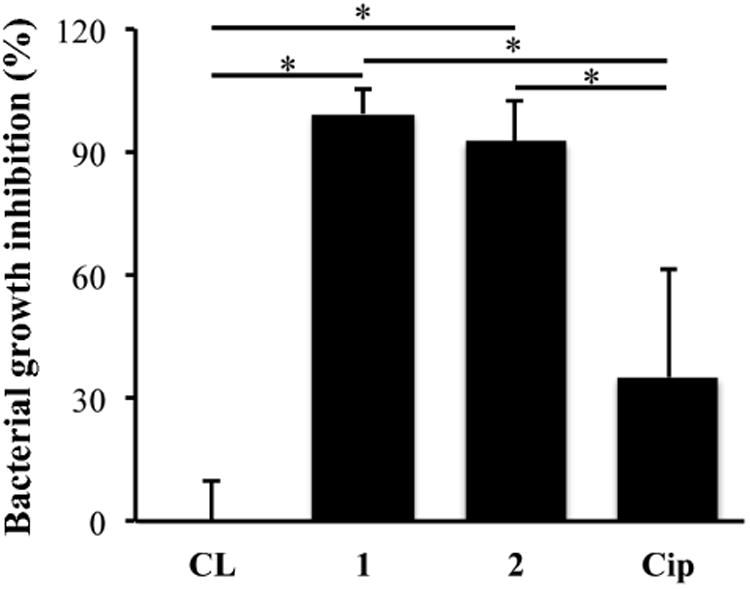
Antimicrobial activity of F-Cip 1 and 2 and Cip loaded Comfilcon A lenses against P. aeruginosa 19660. Data are from three independent experiments expressed as mean ± SD. Oneway ANOVA was performed followed by Tukey's test where significance was found. *p<0.05.
The performance of a drug delivery system is also dependent upon the drug release profile from contact lenses because the released drug has to reach a threshold concentration over a desired period of time. As tear clearance is a continuous process in the eye, the release of drugs from contact lenses needs to be fast enough to overcome the clearance. This requirement is challenging for many drug delivery systems as there would be only a very small portion of drug, if any, to be released after their short burst release period, thus no antimicrobial activity would be observed. To mimic the in vivo conditions, we designed an ex vivo porcine eye infection model to test the antimicrobial efficacy of the modified contact lenses with a flow of culture medium representing tear turnover (See section 3.9).
3.8. Cytotoxicity of F-Cip-loaded contact lenses
The cytotoxicity of 1, 2 and Cip loaded lenses was tested against hTCEpi cells. Cells exhibited ∼100% viability after incubation with lenses loaded with F-Cip 1 and 2, and unmodified Cip compared to cells incubated with untreated lenses, and there was no significant difference among them, indicating that loaded drug did not exhibit a toxic effect on this cell line (Figure 9).
Figure 9.
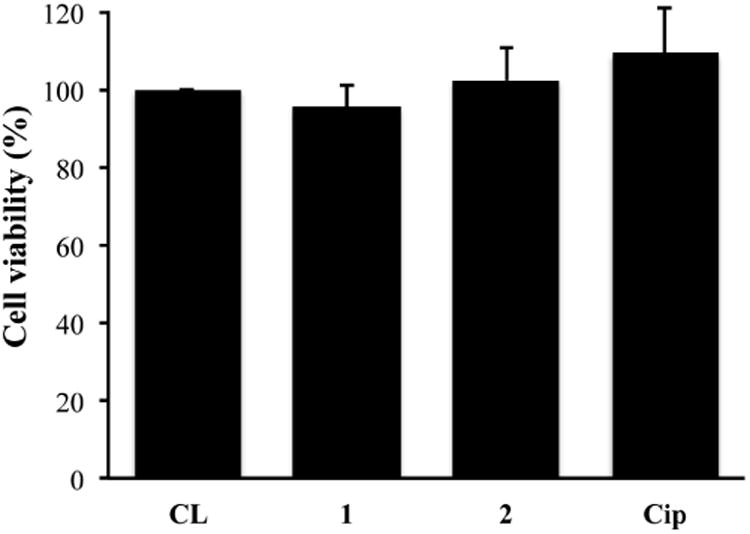
Cytotoxicity of F-Cip and Cip loaded Comfilcon A lenses against hTCEpi cells. Data are from three independent experiments and expressed as mean ± SD. One-way ANOVA was performed and no significance was found.
3.9. Ex vivo infection model and antimicrobial activity of F-Cip-loaded contact lenses
Porcine eyes have been adopted for studies that test commercial contact lenses due to their similar size and anatomy with human eyes.[77-83] Few studies have been carried out on ocular infections using ex vivo porcine eye models that had a mechanism to mimic the tear turnover.[78, 79, 83] Herein we designed an ex vivo porcine eye infection model, in which culture medium was dripped onto each eye at a rate of four 25 μL drops/min to represent the tear turnover in vivo. Using this system, we tested the antimicrobial activity of F-Cip 1 and unmodified Cip loaded Comfilcon A lenses against P. aeruginosa 19660. Eyes that were infected with bacteria but not exposed to contact lenses served as control. As shown in Figure 10, for eyes with contact lenses modified with F-Cip 1, there was significant bacterial growth inhibition (86.8 ± 13.9 %) compared to the eyes with unmodified lenses (labeled as CL) and lenses modified with Cip, which showed 29.7 ± 12.7% and 37.5 ± 8.2% bacterial growth inhibition, respectively. This result supported our finding in the in vitro antimicrobial assay. The eyes infected with bacteria and treated with 0.3% ciprofloxacin solution showed 98.1 ± 4.3% bacterial growth inhibition, which was higher than the ones wearing F-Cip modified lenses although this difference did not reach statistical significance (p=0.70). In clinical practice, a high concentration (0.3%) of ciprofloxacin solution is commonly used to treat eye infections and frequent administration is necessary. For example, treatment of relatively mild bacterial conjunctivitis requires one to two drops four times a day, while treatment of sight threatening bacterial ulcers requires drops every fifteen minutes for the first six hours.[84] It should be noted that the amount of ciprofloxacin in the solution that was used for treatment was more than 100 times of the amount of F-Cip 1 (67.96 ± 11.50 nmol/lens, see Figure 5), yet these two systems showed a comparable efficacy. Therefore, our approach could significantly reduce the amount of drug needed to treat eye infections. For the eyes without scratch wounds, the number of bacteria detected was much less than that for the control eyes indicating that bacteria did not penetrate the intact corneal epithelium and those that grew on top of the ocular surface were most likely washed away during the washing step. Overall, these results showed that contact lenses loaded with F-Cip exhibited antimicrobial activity in an ex vivo model that represents tear clearance in vivo.
Figure 10.
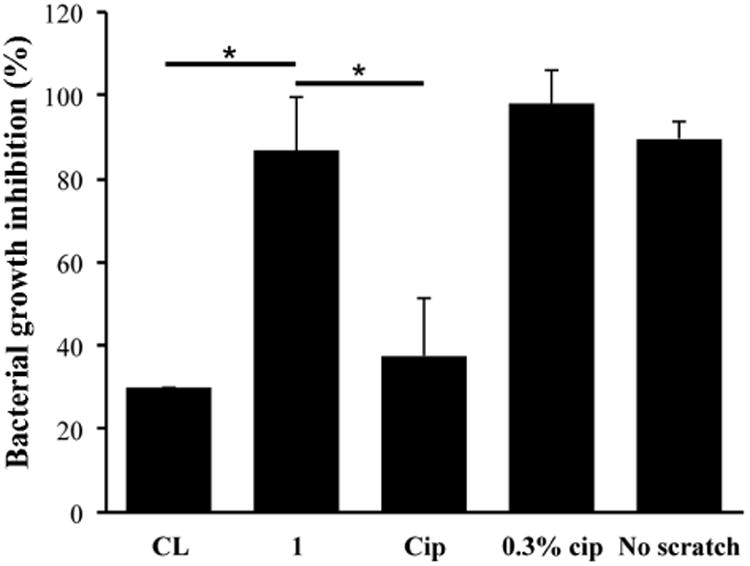
Antimicrobial efficacy of modified Comfilcon A lenses in an ex vivo porcine eye infection model. CL: unmodified lenses. 1: lenses modified with F-Cip 1. Cip: lenses modified with Cip. 0.3% Cip: 0.3% ciprofloxacin solution administered in the form of eye drops. No scratch: intact porcine eyes. Statistical analysis was performed using one-way ANOVA followed by Tukey's test comparing to 1 where significance was found. *p<0.05. (n=3)
In summary, we modified both hydrophilic (FITC) and hydrophobic (ciprofloxacin) molecules with fluorinated carbon chains and tested their loading into contact lenses. We found that more F-FITC was loaded onto fluorine-containing Comfilcon A lenses compared to FITC and compared to other non-fluorine-containing contact lenses. Similarly, there was significantly more F-Cip loaded onto Comfilcon A lenses compared to Cip. F-FITC and F-Cip exhibited sustained release from Comfilcon A lenses while unmodified molecules showed typical burst release profiles. Lenses loaded with F-Cip exhibited antimicrobial efficacy against P. aeruginosa 19660 in vitro and ex vivo, while not exhibiting cytotoxicity against human corneal epithelial cells.
3.10. Proposed role of fluorous chemistry
In our system, the molecular interactions between the molecules and contact lenses consist of hydrophobic, hydrophilic, hydrogen bonding, ionic, and fluorous interactions. As shown in Figure 2 and 5, the loading of fluorous-tagged molecules is much greater than that of their non-fluorous-tagged counterparts, indicating that the fluorous interactions play a significant role. Fluorous interactions are a combination of bonding between fluorinated molecules, which includes several chemical bonding such as F…F, C-F…H, C-F…π, C-F…C=O, etc.,[54] among which the F…F non-covalent interactions that are known to be the strongest attractive force.[85] Extensive theoretical and experimental studies are still ongoing to understand the nature of fluorous interaction force.[85] In general, the fluorous attractive force increases with the number and proximity of the interacting F-atoms in the molecule, and is also affected by the surface dipolar interactions.[86] Attachment of the fluorous tag to the molecules creates the fluorous interactions between the molecules and the fluorous side chains of the contact lens polymers as well as the fluorous interactions among the molecules, thus greatly increases the loading of the fluorous-tagged molecules.
The release data for FITC-F and F-Cip 1 and 2 were fitted to the Peppas equation for the first 60% of the normalized drug release using the following equation [87, 88]:
Where Mt is the drug released at time t, M0 is the total amount of drug released, k is the rate constant, and n is the release exponent. The n values were 0.78-0.92 with R2 = 0.987-0.996. Although in the classical Higuchi diffusion model, the release exponent is 0.5,[88] deviation of the release exponent between 0-1 is often reported, which can be attributed to the polymer material geometry and anomalous transport mechanism.[87, 88] The release rate constants, ranging 0.4 – 1.2 min-1/2 in our system, are strongly affected by the molecular interactions of the system. Specifically, the attachment of the fluorous tag to the molecules creates the fluorous interactions among the molecules as well as between the molecules and the fluorous side chains of the contact lens polymers. The combination of these two effects greatly slows down the release of the molecules from the contact lens matrix.
4. Conclusion
In conclusion, we demonstrated that fluorous interactions could be utilized to greatly enhance loading of fluorous-tagged molecules into fluorine-containing delivery systems. The antibiotic-loaded delivery system exhibited nearly complete growth inhibition of bacterium P. aeruginosa in vitro and ex vivo while having no cytotoxicity towards host cells and no effect on the optical quality of the lenses. Furthermore, our results encourage further investigation of the antimicrobial properties and cytotoxicity of fluorous-tagged ciprofloxacin loaded contact lenses and other fluorous-based biomaterial systems in vivo for better understanding of their actions and their potential applications in the clinic.
Supplementary Material
Figure 4.
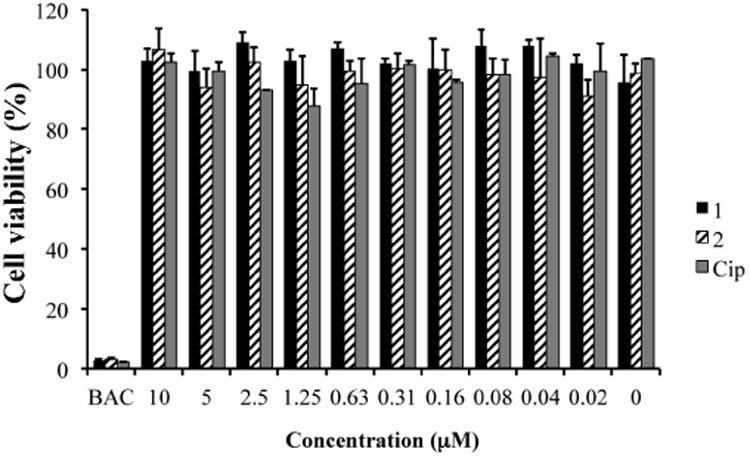
Cytotoxicity of F-Cip 1, 2 and Cip against human telomerase corneal epithelial cells (hTCEpi) with a series of concentrations. BAC: benzalkonium chloride as control. Data are expressed as mean ± SD (n=3).
Acknowledgments
This work was supported by the National Institutes of Health [EY13175], National Science Foundation [DMR-1508722], the University of Houston GEAR grant and the New Faculty Research Program Award.
Footnotes
Supplementary data: 1H, 13C, and 19F NMR spectra of FITC-F, and F-Cip 1-4 can be found at.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3:275–87. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 2.Lang JC. Ocular drug-delivery conventional ocular formulations. Adv Drug Deliv Rev. 1995;16:39–43. [Google Scholar]
- 3.White CJ, Tieppo A, Byrne ME. Controlled drug release from contact lenses: a comprehensive review from 1965-present. J Drug Deliv Sci Technol. 2011;21:369–384. [Google Scholar]
- 4.Dutta D, Willcox MDP. Antimicrobial Contact Lenses and Lens Cases: A Review. Eye Contact Lens-Sci Clin Pra. 2014;40:312–324. doi: 10.1097/ICL.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 5.Hsu KH, Gause S, Chauhan A. Review of ophthalmic drug delivery by contact lenses. J Drug Deliv Sci Technol. 2014;24:123–135. [Google Scholar]
- 6.Phan CM, Subbaraman L, Jones L. Contact lenses for antifungal ocular drug delivery: a review. Expert Opin Drug Deliv. 2014;11:537–546. doi: 10.1517/17425247.2014.882315. [DOI] [PubMed] [Google Scholar]
- 7.Ciolino JB, Dohlman CH, Kohane DS. Contact Lenses for Drug Delivery. Semin Ophthalmol. 2009;24:156–160. doi: 10.1080/08820530902802161. [DOI] [PubMed] [Google Scholar]
- 8.Bengani LC, Hsu KH, Gause S, Chauhan A. Contact lenses as a platform for ocular drug delivery. Expert Opin Drug Deliv. 2013;10:1483–1496. doi: 10.1517/17425247.2013.821462. [DOI] [PubMed] [Google Scholar]
- 9.Ruben M, Watkins R. Pilocarpine dispensation for soft hydrophilic contact-lens. Br J Ophthalmol. 1975;59:455–458. doi: 10.1136/bjo.59.8.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu JK, Li XS, Sun FQ. In vitro and in vivo evaluation of ketotifen fumarate-loaded silicone hydrogel contact lenses for ocular drug delivery. Drug Deliv. 2011;18:150–158. doi: 10.3109/10717544.2010.522612. [DOI] [PubMed] [Google Scholar]
- 11.Winterton LC, Lally JM, Sentell KB, Chapoy LL. The elution of poly (vinyl alcohol) from a contact lens: The realization of a time release moisturizing agent/artificial tear. J Biomed Mater Res Part B. 2007;80B:424–432. doi: 10.1002/jbm.b.30613. [DOI] [PubMed] [Google Scholar]
- 12.Jain MR. Drug delivery through soft contact lenses. Br J Ophthalmol. 1988;72:150–154. doi: 10.1136/bjo.72.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soluri A, Hui A, Jones L. Delivery of Ketotifen Fumarate by Commercial Contact Lens Materials. Optom Vis Sci. 2012;89:1140–1149. doi: 10.1097/OPX.0b013e3182639dc8. [DOI] [PubMed] [Google Scholar]
- 14.Hui A, Boone A, Jones L. Uptake and release of ciprofloxacin-HCl from conventional and silicone hydrogel contact lens materials. Eye Contact Lens-Sci Clin Pra. 2008;34:266–271. doi: 10.1097/ICL.0b013e3181812ba2. [DOI] [PubMed] [Google Scholar]
- 15.Karlgard CCS, Wong NS, Jones LW, Moresoli C. In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. Int J Pharm. 2003;257:141–151. doi: 10.1016/s0378-5173(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Chauhan A. Dexamethasone transport and ocular delivery from poly(hydroxyethyl methacrylate) gels. Int J Pharma. 2008;353:205–222. doi: 10.1016/j.ijpharm.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 17.Li CC, Chauhan A. Ocular transport model for ophthalmic delivery of timolol through p-HEMA contact lenses. J Drug Deliv Sci Technol. 2007;17:69–79. [Google Scholar]
- 18.Alvarez-Lorenzo C, Hiratani H, Gomez-Amoza JL, Martinez-Pacheco R, Souto C, Concheiro A. Soft contact lenses capable of sustained delivery of timolol. J Pharm Sci. 2002;91:2182–2192. doi: 10.1002/jps.10209. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Lorenzo C, Yanez F, Barreiro-Iglesias R, Concheiro A. Imprinted soft contact lenses as norfloxacin delivery systems. J Control Release. 2006;113:236–244. doi: 10.1016/j.jconrel.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Lorenzo C, Yanez F, Concheiro A. Ocular drug delivery from molecularly-imprinted contact lenses. J Drug Deliv Sci Technol. 2010;20:237–248. [Google Scholar]
- 21.White CJ, Byrne ME. Molecularly imprinted therapeutic contact lenses. Expert Opin Drug Deliv. 2010;7:765–780. doi: 10.1517/17425241003770098. [DOI] [PubMed] [Google Scholar]
- 22.White CJ, McBride MK, Pate KM, Tieppo A, Byrne ME. Extended release of high molecular weight hydroxypropyl methylcellulose from molecularly imprinted, extended wear silicone hydrogel contact lenses. Biomaterials. 2011;32:5698–5705. doi: 10.1016/j.biomaterials.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 23.Yanez F, Martikainen L, Braga MEM, Alvarez-Lorenzo C, Concheiro A, Duarte CMM, Gil MH, de Sousa HC. Supercritical fluid-assisted preparation of imprinted contact lenses for drug delivery. Acta Biomater. 2011;7:1019–1030. doi: 10.1016/j.actbio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Hui A, Sheardown H, Jones L. Acetic and Acrylic Acid Molecular Imprinted Model Silicone Hydrogel Materials for Ciprofloxacin-HCl Delivery. Materials. 2012;5:85–107. doi: 10.3390/ma5010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tieppo A, White CJ, Paine AC, Voyles ML, McBride MK, Byrne ME. Sustained in vivo release from imprinted therapeutic contact lenses. J Control Release. 2012;157:391–397. doi: 10.1016/j.jconrel.2011.09.087. [DOI] [PubMed] [Google Scholar]
- 26.Malaekeh-Nikouei B, Vahabzadeh SA, Mohajeri SA. Preparation of a Molecularly Imprinted Soft Contact Lens as a New Ocular Drug Delivery System for Dorzolamide. Curr Drug Deliv. 2013;10:279–285. doi: 10.2174/1567201811310030004. [DOI] [PubMed] [Google Scholar]
- 27.Guidi G, Korogiannaki M, Sheardown H. Modification of Timolol Release From Silicone Hydrogel Model Contact Lens Materials Using Hyaluronic Acid. Eye Contact Lens-Sci Clin Pra. 2014;40:269–276. doi: 10.1097/ICL.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 28.Hui A, Willcox M, Jones L. In Vitro and In Vivo Evaluation of Novel Ciprofloxacin-Releasing Silicone Hydrogel Contact Lenses. Invest Ophthalmol Vis Sci. 2014;55:4896–4904. doi: 10.1167/iovs.14-14855. [DOI] [PubMed] [Google Scholar]
- 29.Ciolino JB, Stefanescu CF, Ross AE, Salvador-Culla B, Cortez P, Ford EM, Wymbs KA, Sprague SL, Mascoop DR, Rudina SS, Trauger SA, Cade F, Kohane DS. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials. 2014;35:432–439. doi: 10.1016/j.biomaterials.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maulvi FA, Lakdawala DH, Shaikh AA, Desai AR, Choksi HH, Vaidya RJ, Ranch KM, Koli AR, Vyas BA, Shah DO. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J Control Release. 2016;226:47–56. doi: 10.1016/j.jconrel.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Paradiso P, Chu V, Santos L, Serro AP, Colaco R, Saramago B. Effect of plasma treatment on the performance of two drug-loaded hydrogel formulations for therapeutic contact lenses. J Biomed Mater Res Part B. 2015;103:1059–1068. doi: 10.1002/jbm.b.33287. [DOI] [PubMed] [Google Scholar]
- 32.Hagiwara K, Hasebe T, Hotta A. Effects of plasma treatments on the controlled drug release from poly(ethylene-co-vinyl acetate) Surf Coat Technol. 2013;216:318–323. [Google Scholar]
- 33.Gulsen D, Li CC, Chauhan A. Dispersion of DMPC liposomes in contact lenses for ophthalmic drug delivery. Curr Eye Res. 2005;30:1071–1080. doi: 10.1080/02713680500346633. [DOI] [PubMed] [Google Scholar]
- 34.dos Santos JFR, Alvarez-Lorenzo C, Silva M, Balsa L, Couceiro J, Torres-Labandeira JJ, Concheiro A. Soft contact lenses functionalized with pendant cyclodextrins for controlled drug delivery. Biomaterials. 2009;30:1348–1355. doi: 10.1016/j.biomaterials.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Garhwal R, Shady SF, Ellis EJ, Ellis JY, Leahy CD, McCarthy SP, Crawford KS, Gaines P. Sustained Ocular Delivery of Ciprofloxacin Using Nanospheres and Conventional Contact Lens Materials. Invest Ophthalmol Vis Sci. 2012;53:1341–1352. doi: 10.1167/iovs.11-8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan XY, Marcano DC, Shin CS, Hua X, Isenhart LC, Pflugfelder SC, Acharya G. Ocular Drug Delivery Nanowafer with Enhanced Therapeutic Efficacy. ACS Nano. 2015;9:1749–1758. doi: 10.1021/nn506599f. [DOI] [PubMed] [Google Scholar]
- 37.Robertson DM. The Effects of Silicone Hydrogel Lens Wear on the Corneal Epithelium and Risk for Microbial Keratitis. Eye Contact Lens-Sci Clin Pra. 2013;39:67–72. doi: 10.1097/ICL.0b013e31827c5b73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saini A, Rapuano CJ, Laibson PR, Cohen EJ, Hammersmith KM. Episodes of Microbial Keratitis With Therapeutic Silicone Hydrogel Bandage Soft Contact Lenses. Eye Contact Lens-Sci Clin Pra. 2013;39:324–328. doi: 10.1097/ICL.0b013e31829fadde. [DOI] [PubMed] [Google Scholar]
- 39.Shovlin JP. Ocular surface health with contact lens wear. Cont Lens Anterior Eye. 2013;36:S14–S21. doi: 10.1016/S1367-0484(13)60005-3. [DOI] [PubMed] [Google Scholar]
- 40.Konda N, Willcox M. Review of Inflammation and Infection in Contact Lens Wears: Risk Factors and Association with Single Nucleotide Polymorphisms. J Ocular Bio. 2013;1:9. [Google Scholar]
- 41.Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low Dk hydrogel extended contact lens wear: A meta-analysis. Optom Vis Sci. 2007;84:247–256. doi: 10.1097/OPX.0b013e3180421c47. [DOI] [PubMed] [Google Scholar]
- 42.Stapleton F, Stretton S, Papas E, Skotnitsky C, Sweeney DF. Silicone hydrogel contact lenses and the ocular surface. Ocul Surf. 2006;4:24–43. doi: 10.1016/s1542-0124(12)70262-8. [DOI] [PubMed] [Google Scholar]
- 43.Farandos NM, Yetisen AK, Monteiro MJ, Lowe CR, Yun SH. Contact Lens Sensors in Ocular Diagnostics. Adv Healthc Mater. 2015;4:792–810. doi: 10.1002/adhm.201400504. [DOI] [PubMed] [Google Scholar]
- 44.Chana KY, Cho P, Boost M. Microbial adherence to cosmetic contact lenses. Cont Lens Anterior Eye. 2014;37:267–272. doi: 10.1016/j.clae.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Ray M, Lim DK. Rare Polymicrobial Keratitis Involving Chryseobacterium meningosepticum and Delftia acidovorans in a Cosmetic Contact Lens Wearer. Eye Contact Lens-Sci Clin Pra. 2013;39:192–193. doi: 10.1097/ICL.0b013e3182448881. [DOI] [PubMed] [Google Scholar]
- 46.Yao H, Liao Y, Lingley AR, Afanasiev A, Lahdesmaki I, Otis BP, Parviz BA. A contact lens with integrated telecommunication circuit and sensors for wireless and continuous tear glucose monitoring. J Micromech Microeng. 2012;22:10. [Google Scholar]
- 47.Singh S, Satani D, Patel A, Vhankade R. Colored Cosmetic Contact Lenses: An Unsafe Trend in the Younger Generation. Cornea. 2012;31:777–779. doi: 10.1097/ICO.0b013e31823cbe9c. [DOI] [PubMed] [Google Scholar]
- 48.Sauer A, Bourcier T. French Study Grp Contact Lenses. Microbial keratitis as a foreseeable complication of cosmetic contact lenses: a prospective study. Acta Ophthalmol. 2011;89:E439–E442. doi: 10.1111/j.1755-3768.2011.02120.x. [DOI] [PubMed] [Google Scholar]
- 49.Chu MX, Shirai T, Takahashi D, Arakawa T, Kudo H, Sano K, Sawada S, Yano K, Iwasaki Y, Akiyoshi K, Mochizuki M, Mitsubayashi K. Biomedical soft contact-lens sensor for in situ ocular biomonitoring of tear contents. Biomed Microdevices. 2011;13:603–611. doi: 10.1007/s10544-011-9530-x. [DOI] [PubMed] [Google Scholar]
- 50.Yao HF, Shum AJ, Cowan M, Lahdesmaki I, Parviz BA. A contact lens with embedded sensor for monitoring tear glucose level. Biosens Bioelectron. 2011;26:3290–3296. doi: 10.1016/j.bios.2010.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malik A, Claoue C. Transport and interaction of cosmetic product material within the ocular surface: Beauty and the beastly symptoms of toxic tears. Cont Lens Anterior Eye. 2012;35:247–259. doi: 10.1016/j.clae.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Dutta D, Ozkan J, Willcox MDP. Biocompatibility of Antimicrobial Melimine Lenses: Rabbit and Human Studies. Optom Vis Sci. 2014;91:570–581. doi: 10.1097/OPX.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 53.Horvath IT, Rabai J. Facile catalyst separation without water - fluorous biphase hydroformylation of olefins. Science. 1994;266:72–75. doi: 10.1126/science.266.5182.72. [DOI] [PubMed] [Google Scholar]
- 54.Berger R, Resnati G, Metrangolo P, Weber E, Hulliger J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem Soc Rev. 2011;40:3496–3508. doi: 10.1039/c0cs00221f. [DOI] [PubMed] [Google Scholar]
- 55.Hayama T, Yoshida H, Yamaguchi M, Nohta H. Fluorous affinity-based separation techniques for the analysis of biogenic and related molecules. J Pharm Biomed Anal. 2014;101:151–160. doi: 10.1016/j.jpba.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 56.Anton D. Surface-fluorinated coatings. Adv Mater. 1998;10:1197–1205. [Google Scholar]
- 57.Hugel HM, Jackson N. Special Feature Organo-Fluorine Chemical Science. Appl Sci-Basel. 2012;2:558–565. [Google Scholar]
- 58.McKeen L. Fluorinated Coatings and Finishes Handbook. William Andrew; 2007. [Google Scholar]
- 59.Ahrens ET, Flores R, Xu HY, Morel PA. In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol. 2005;23:983–987. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- 60.Biro GP, Blais P. Perfluorocarbon blood substitutes. Crit Rev Oncol Hematol. 1987;6:311–374. doi: 10.1016/s1040-8428(87)80018-5. [DOI] [PubMed] [Google Scholar]
- 61.Castro CI, Briceno JC. Perfluorocarbon-Based Oxygen Carriers: Review of Products and Trials. Artif Organs. 2010;34:622–634. doi: 10.1111/j.1525-1594.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 62.Janjic JM, Ahrens ET. Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip Rev-Nanomed Nanobiotechnol. 2009;1:492–501. doi: 10.1002/wnan.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rapoport N. Phase-shift, stimuli-responsive perfluorocarbon nanodroplets for drug delivery to cancer. Wiley Interdiscip Rev-Nanomed Nanobiotechnol. 2012;4:492–510. doi: 10.1002/wnan.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reznik N, Williams R, Burns PN. Investigation of vaporized submicron perfluorocarbon droplets as an ultrasound contrast agent. Ultrasound Med Biol. 2011;37:1271–1279. doi: 10.1016/j.ultrasmedbio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Santos CM, Kumar A, Kolar SS, Contreras-Caceres R, McDermott A, Cai CZ. Immobilization of Antimicrobial Peptide IG-25 onto Fluoropolymers via Fluorous Interactions and Click Chemistry. ACS Appl Mater Interfaces. 2013;5:12789–12793. doi: 10.1021/am404591n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 67.Karlgard CCS, Sarkar DK, Jones LW, Moresoli C, Leung KT. Drying methods for XPS analysis of PureVision (TM), Focus (R) Night & Day &(TM) and conventional hydrogel contact lenses. Appl Surf Sci. 2004;230:106–114. [Google Scholar]
- 68.Tieppo A, Boggs AC, Pourjavad P, Byrne ME. Analysis of release kinetics of ocular therapeutics from drug releasing contact lenses: Best methods and practices to advance the field. Cont Lens Anterior Eye. 2014;37:305–313. doi: 10.1016/j.clae.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Eter N, Gobbels M. A new technique for tear film fluorophotometry. Br J Ophthalmol. 2002;86:616–619. doi: 10.1136/bjo.86.6.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Occhipinti JR, Mosier MA, Lamotte J, Monji GT. Fluorophotometric measurement of human tear turnover rate. Curr Eye Res. 1988;7:995–1000. doi: 10.3109/02713688809015145. [DOI] [PubMed] [Google Scholar]
- 71.Webber WRS, Jones DP. Continuous fluorophotometric method of measuring tear turnover rate in humans and analysis of factors affecting accuracy. Med Biol Eng Comput. 1986;24:386–392. doi: 10.1007/BF02442693. [DOI] [PubMed] [Google Scholar]
- 72.Webber WRS, Jones DP, Wright P. Fluorophotometric measurements of tear turnover rate in normal healthy persons - evidence for a circadian rhythm. Trans Ophthalmol Soc UK. 1987;1:615–620. doi: 10.1038/eye.1987.95. [DOI] [PubMed] [Google Scholar]
- 73.Pendland SL, Neuhauser MM, Garey KW, Prause JL, Jung R. Comparative killing rates of gatifloxacin and ciprofloxacin against 14 clinical isolates: impact of bacterial strain and antibiotic concentration. Diagn Microbiol Infect Dis. 2002;44:59–61. doi: 10.1016/s0732-8893(02)00422-4. [DOI] [PubMed] [Google Scholar]
- 74.Tsai TY, Chen TC, Wang IJ, Yeh CY, Su MJ, Chen RH, Tsai TH, Hu FR. The Effect of Resveratrol on Protecting Corneal Epithelial Cells from Cytotoxicity Caused by Moxifloxacin and Benzalkonium Chloride. Invest Ophthalmol Vis Sci. 2015;56:1575–1584. doi: 10.1167/iovs.14-15708. [DOI] [PubMed] [Google Scholar]
- 75.Onizuka N, Uematsu M, Kusano M, Sasaki H, Suzuma K, Kitaoka T. Influence of Different Additives and Their Concentrations on Corneal Toxicity and Antimicrobial Effect of Benzalkonium Chloride. Cornea. 2014;33:521–526. doi: 10.1097/ICO.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 76.Iwasawa A, Ayaki M, Niwano Y. Cell viability score (CVS) as a good indicator of critical concentration of benzalkonium chloride for toxicity in cultured ocular surface cell lines. Regul Toxicol Pharmacol. 2013;66:177–183. doi: 10.1016/j.yrtph.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 77.Tankam P, Won J, Canavesi C, Cox I, Rolland JP. Optical Assessment of Soft Contact Lens Edge-Thickness. Optom Vis Sci. 2016;93:987–996. doi: 10.1097/OPX.0000000000000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jaishankar D, Buhrman JS, Valyi-Nagy T, Gemeinhart RA, Shukla D. Extended Release of an Anti-Heparan Sulfate Peptide From a Contact Lens Suppresses Corneal Herpes Simplex Virus-1 Infection. Invest Ophthalmol Vis Sci. 2016;57:169–180. doi: 10.1167/iovs.15-18365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vermeltfoort PBJ, van Kooten TG, Bruinsma GM, Hooymans AMM, van der Mei HC, Busscher HJ. Bacterial transmission from contact lenses to porcine corneas: An ex vivo study. Invest Ophthalmol Vis Sci. 2005;46:2042–2046. doi: 10.1167/iovs.04-1401. [DOI] [PubMed] [Google Scholar]
- 80.Pino CJ, Haselton FR, Chang MS. Seeding of corneal wounds by epithelial cell transfer from micropatterned PDMS contact lenses. Cell Transplant. 2005;14:565–571. doi: 10.3727/000000005783982783. [DOI] [PubMed] [Google Scholar]
- 81.Leonardi M, Leuenberger P, Bertrand D, Bertsch A, Renaud P. First steps toward noninvasive intraocular pressure monitoring with a sensing contact lens. Invest Ophthalmol Vis Sci. 2004;45:3113–3117. doi: 10.1167/iovs.04-0015. [DOI] [PubMed] [Google Scholar]
- 82.Iguchi I, Kamiyama K, Imamichi M, Ohashi T, He JC, Wang XG, Imanishi J. Influence of dynamic contact of hard contact lens materials on corneal epithelial cells examined by rose bengal staining. Curr Eye Res. 1996;15:647–652. doi: 10.3109/02713689609008905. [DOI] [PubMed] [Google Scholar]
- 83.He YG, McCulley JP, Alizadeh H, Pidherney M, Mellon J, Ubelaker JE, Stewart GL, Silvany RE, Niederkorn JY. A pig model of acanthamoeba keratitis - transmission via contaminated contact lenses. Invest Ophthalmol Vis Sci. 1992;33:126–133. [PubMed] [Google Scholar]
- 84.Bartlett JD, Jaanus SD. Clinical Ocular Pharmacology. 5th. Butterworth-Heinemann; St. Louis, MO, USA: 2008. [Google Scholar]
- 85.Omorodion H, Twamley B, Platts JA, Baker RJ. Further Evidence on the Importance of Fluorous-Fluorous Interactions in Supramolecular Chemistry: A Combined Structural and Computational Study. Cryst Growth Des. 2015;15:2835–2841. [Google Scholar]
- 86.Lee HJ, Jamison AC, Lee TR. Surface Dipoles: A Growing Body of Evidence Supports Their Impact and Importance. Accounts Chem Res. 2015;48:3007–3015. doi: 10.1021/acs.accounts.5b00307. [DOI] [PubMed] [Google Scholar]
- 87.Peppas NA. Analysis of fickian and non-fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–111. [PubMed] [Google Scholar]
- 88.Siepmann J, Peppas NA. Higuchi equation: Derivation, applications, use and misuse. Int J Pharm. 2011;418:6–12. doi: 10.1016/j.ijpharm.2011.03.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


