Abstract
Background
Despite increasing use of infliximab (IFX) in children with Crohn’s disease (CD) and ulcerative colitis (UC), long-term durability and safety of IFX beyond 1 year is limited in pediatric inflammatory bowel disease.
Methods
We performed a 10-year single-center retrospective cohort study of 188 patients initiating IFX at <21 years of age with 1-year minimum follow-up. Data were retrieved from medical records. IFX outcomes were defined as sustained remission in the absence of dose modification (sustained durable remission), recaptured response, and treatment failure. Adverse events, anti-infliximab antibodies (ATI), and role of concomitant low-dose oral methotrexate (<10 mg/wk) on IFX durability were analyzed. Univariate associations and survival analysis were performed.
Results
As of the last follow-up, 39% of patients with CD and 29% of patients with UC achieved sustained durable remission and another 60% recaptured and maintained response. For CD, 88% remained on IFX at 1 year, 80% at 2 years, and 82% at 5 years. In UC, 70% avoided colectomy at 1 year. Of IFX failures, 25% with CD and 11% with UC developed ATI. The most common adverse event causing cessation of therapy was infusion reactions. Treatment limiting recurrent infections occurred in <1%, and 1 patient developed lymphoproliferative disease. Low-dose methotrexate did not influence any IFX outcomes.
Conclusions
IFX is safe and effective for long-term maintenance therapy in pediatric patients with inflammatory bowel disease. IFX dose intensification can optimize durability and overcome loss of response. Loss of response is likely affected by development of ATI. Higher doses of oral methotrexate may be needed to optimize IFX.
Keywords: inflammatory bowel disease, infliximab, pediatric, Crohn’s disease, ulcerative colitis, anti-infliximab antibody
Inflammatory bowel disease (IBD) is a chronic immune-mediated inflammatory disorder of the gastrointestinal tract. Approximately, 20% to 30% of patients present with IBD during childhood or adolescence, and the prevalence of pediatric IBD has increased over the past few decades.1–3 Medical therapies available for pediatric IBD are limited. However, with the introduction of biologics and approved use of infliximab (IFX) for pediatric patients with IBD, more opportunities to induce and maintain remission have become available.
IFX is a chimeric IgG-1 monoclonal antibody with specificity and affinity for tumor necrosis factor α. It was the first biologic to be approved for the use in pediatric patients with moderate to severely active Crohn’s disease (CD) or ulcerative colitis (UC) refractory to conventional therapies. However, data on the long-term efficacy and safety of IFX in the pediatric IBD population beyond 1 year are limited.4–10
It is estimated that 20% to 50% of patients with IBD who initially respond to IFX induction lose response by approximately 1 year.4–7,11 The underlying mechanism for secondary loss of response to IFX is multifactorial. Immunogenicity, meaning development of anti-drug antibodies resulting in increased drug clearance, is an important mechanism that is receiving a lot of attention as of late. Scheduled and not episodic IFX therapy is emphasized to prevent the development of antibodies to IFX (ATI), infusion reactions, and most importantly loss of efficacy. The addition of concomitant immunomodulators, specifically thiopurines, reduces ATI and decreases clearance of IFX, which is reflected in higher trough levels and sustains steroid-free clinical remission in adult patients with CD.12,13 Similar results are seen with use of combination therapy of IFX and methotrexate (MTX) in adult patients with rheumatoid arthritis.14 A complete understanding of the role of concomitant MTX in pediatric IBD is still unclear. An increasing proportion of gastroenterologists treating younger patients with IBD, males in particular, have transitioned to this regimen after reports of hepatosplenic T-cell lymphoma in young males treated with combination thiopurines and IFX.15
The ability to recapture response in secondary nonresponders to standard IFX dosing by dose intensification is well known in adult patients with IBD. More data are needed in the pediatric age group to determine the long-term outcomes of IFX dosing modifications.9,10 In this study, we evaluated the long-term durability and safety of IFX maintenance therapy in a cohort of pediatric patients with CD and UC followed at a single tertiary care pediatric IBD center. We additionally examined the predictors of IFX outcomes and the role of concomitant low-dose MTX on IFX durability.
MATERIALS AND METHODS
Study Population
We performed a retrospective chart review of patients with IBD who received at least 1 dose of IFX at the Cedars Sinai Pediatric IBD Center from January 2001 to December 2012. Patients who initiated IFX therapy at ≤21 years of age, with at least 1 year of follow-up, were included in this study. Data retrieved included demographic data, immunomodulator history, indication for IFX treatment, baseline disease location (small bowel, large bowel, or both), disease behavior (nonpenetrating nonstricturing, penetrating, or stricturing), disease duration, duration of IFX therapy, use of concomitant immunomodulators, response to IFX, and IFX dose intensification outcomes. Information regarding clinical disease activity, such as Harvey Bradshaw Index for CD and Pediatric Ulcerative Colitis Activity Index for UC, and biomarkers of inflammation, such as C-reactive protein and erythrocyte sedimentation rate, in addition to albumin were obtained for patients at both baseline and time of dose intensification. The development of immunogenicity, reason for treatment failures, and adverse events were recorded. Adverse events for the purpose of this study were grouped into immunogenicity related, infectious, neurologic, and neoplastic. This study was approved by the CSMC Ethics Review Board.
Definition of Study Groups
Our study cohort was initially divided into patients with CD and UC who achieved sustained durable remission (SDR) and those who did not. SDR was defined as remission with standard maintenance IFX dosing of 5 mg/kg administered at a frequency of every 7 to 8 weeks. Patients who were primary nonresponders (PNR), defined as failure to respond to IFX during the induction period (by week 14), were excluded from this comparison.
Subsequently, patients who lost response to IFX maintenance and underwent IFX dose intensification, defined as IFX dose increase to 10 mg/kg and/or frequency change to every 6 weeks or less, were divided into recaptured responders (RRs) and nonrecaptured responders (NRRs). RRs were defined as patients who recaptured remission after IFX dose intensification. The decision of IFX dose intensification was determined by the treating clinician based on physician’s global assessment, which was standardly noted in the clinic note. Evidence of immunogenicity was also recorded at time of loss of response and compared in each of the groups. Immunogenicity was categorized as infusion reaction, presence of ATI, delayed hypersensitivity reaction, lupus-like reaction, and/or psoriaform reactions.
IFX failures defined those patients who discontinued therapy. Reason for IFX discontinuation was categorized into loss of efficacy in the absence of or after dose intensification (NRRs), immunogenicity related, noncompliance, or other treatment-limiting adverse events.
Statistical Analysis
Continuous variables were reported as medians with interquartile range. T test and Wilcoxon rank sum test were used to compare differences in continuous variables between groups, and the chi-square test was used to compare categorical variables. Kaplan–Meier analysis was used to evaluate long-term durability of IFX by representing response to IFX over time. Differences between survival curves were compared using log-rank test. P-values <0.05 were considered statistically significant.
RESULTS
Patient Demographics
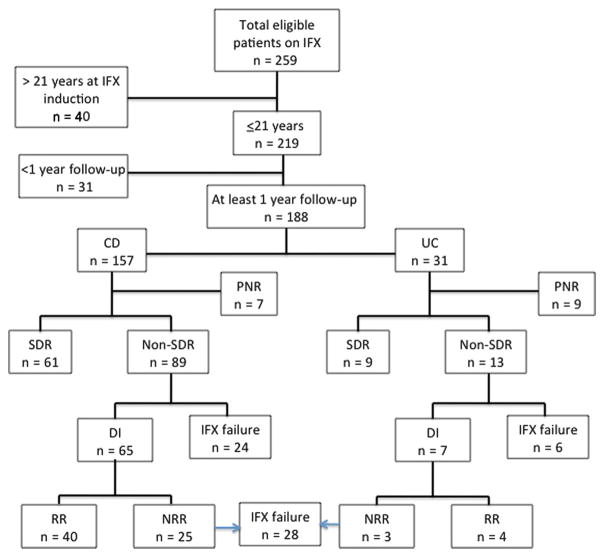
A total of 259 patients with IBD who received at least 1 dose of IFX were identified at the Cedars Sinai Pediatric IBD Center (Fig. 1). Forty patients (15%) started IFX at greater than 21 years of age, whereas 31 patients (12%) had not reached their 1-year follow-up. Therefore, 188 patients (73%) were ≤21 years of age at initiation of IFX and were followed up for at least 1 year and included in this analysis. Of these 188 patients, 157 (84%) were diagnosed with CD and 31 (16%) with UC (Table 1). The majority of patients with CD had disease localized to both the small and large bowel (70%) and 3% had isolated perianal disease at baseline. A total of 16% had penetrating perianal disease, 4% had internal penetrating disease, while 18% of patients had stricturing disease. Most patients with CD (71%) initiated IFX secondary to intolerance or failure of immunomodulator therapy. A significantly greater proportion of patients with UC were steroid refractory as primary indication for IFX therapy as compared with patients with CD (P = 0.0007; 42% versus 14%, respectively). Steroid refractory was defined as patients who failed to respond or had inadequate response to corticosteroid therapy. Forty-four percent of patients with CD were transitioned from thiopurines to MTX at or shortly after IFX initiation. Additionally, 65% of patients with UC versus only 35% of patients with CD (P = 0.007) were induced with IFX monotherapy. As detailed in Table 1, the median duration of disease (P = 0.04) and median duration of IFX therapy (P = 0.05) as of last follow-up in patients with CD was greater than that in patients with UC.
FIGURE 1.
Flow diagram of total number of eligible patients on IFX. Patients who were ≤21 years of age with at least 1-year follow-up were included in this study. Patients with CD and UC were divided into those who SDR, defined as remission on standard IFX maintenance therapy of 5 mg/kg administered at a frequency of 7 to 8 weeks. Patients who did not respond to standard maintenance dosing (non-SDR) were divided into those who required IFX dose intensification (DI), defined as IFX dose increase to 10 mg/kg and/or frequency change to every 6 weeks or less, and IFX failures who stopped IFX. Patients who discontinued IFX within the time of induction (by week 14) were defined as PNR. Patients who had DI were divided into RRs and NRRs. RRs were defined as patients who recaptured remission after IFX dose intensification.
TABLE 1.
Clinical Characteristics of Study Cohort
| CD (n = 157) | UC (n = 31) | P | |
|---|---|---|---|
| Male gender, n (%) | 81 (52) | 12 (39) | 0.20 |
| Median (IQR) age at diagnosis (yr) | 11 (8–13) | 12 (9–14) | 0.80 |
| Median (IQR) duration of disease at the time of IFX induction (mo) | 11 (4–30) | 17 (6–27) | 0.45 |
| Median (IQR) age at IFX induction (yr) | 13 (10–15) | 14 (10–15) | 0.85 |
| Primary indication for IFX, n (%) | |||
| First line therapy | 24 (15) | 2 (6) | 0.15 |
| Intolerant or failed immunomodulator | 111 (71) | 16 (52) | 0.08 |
| Steroid refractory | 22 (14) | 13 (42) | 0.0007 |
| Immunomodulator use before IFX induction, n (%) | |||
| Thiopurines | 104 (66) | 17 (55) | 0.37 |
| MTX | 3 (2) | 0 | 0.42 |
| Median (IQR) duration of immunomodulator before IFX induction (mo) | 10.8 (6–24) | 12.8 (7–19) | 0.67 |
| Immunomodulator at the time of IFX induction, n (%) | |||
| None | 55 (35) | 20 (65) | 0.007 |
| Thiopurines | 33 (21) | 9 (29) | 0.22 |
| MTX | 69 (44) | 2 (6) | 0.08 |
| Steroid use during IFX induction, n (%) | 57 (36) | 20 (65) | 0.004 |
| Median (IQR) duration of disease at the last follow-up (mo) | 60 (35–90) | 47 (32–57) | 0.04 |
| Median (IQR) duration of IFX at the last follow-up (mo) | 30 (17–51) | 25 (15–34) | 0.05 |
IFX Efficacy Outcomes
Crohn’s Disease
Of the 150 patients with CD who responded to IFX induction, 61 (41%) achieved SDR at the time of last follow-up (29 [18–48] months), with standard IFX dosing of 5 mg/kg every 7 to 8 weeks. Median age at diagnosis and IFX initiation were similar in both SDR and non-SDR groups (11 years). Although 70% of patients in both groups had disease in both small and large bowel, twice as many patients in the non-SDR group had perianal disease (SDR 8 versus non-SDR 17, P = 0.09) and 15% had stricturing phenotype at baseline as compared with only 3% in the SDR group (P = 0.006). The primary indication for IFX induction was intolerance or failure of previous immunomodulator therapy in both groups (SDR 62% versus non-SDR 79%, P = 0.03). A smaller percentage of patients initiated IFX as first-line therapy (SDR 16% versus non-SDR 13%, P = 0.68) or were steroid refractory (SDR 22% versus non-SDR 8%, P = 0.02). A greater proportion of patients in SDR group were on corticosteroids at the time of IFX induction (46% versus 26%, P = 0.02). Approximately 40% of patients in both SDR and non-SDR groups were transitioned to concomitant MTX therapy during IFX induction. At the time of last follow-up, the median duration of IFX therapy was similar in both SDR and non-SDR groups (29 [18–48] months versus 30 [13–55] months, respectively, P = 0.89).
Ulcerative Colitis
Of the 22 patients with UC who responded to IFX induction, 9 (41%) remained in SDR at the time of last follow-up (27 [18–34] months). Primary indication for IFX in 67% of SDR patients was intolerance or failure of thiopurines, whereas 38% of patients in non-SDR group were intolerant or failed previous immunomodulators (P = 0.19); 62% versus 22% were steroid refractory (P = 0.07), respectively. The majority of patients were not on concomitant immunomodulator therapy at IFX initiation in both groups, and at the time of last follow-up, median duration of IFX was similar (27 [18–34] months versus 22 [12–25] months, P = 0.26).
Dose Intensification Outcomes
Crohn’s Disease
Sixty-five of the 89 patients with CD (73%) not achieving SDR underwent dose intensification. Of these 65 patients, 37 (57%) had IFX dose escalation to 10 mg/kg while 28 (43%) had both IFX dose and frequency escalation to 10 mg/kg every 6 weeks or less. Forty (62%) of these 65 patients RR after dose intensification (Fig. 1). Indications for IFX therapy in both RR and NRR groups were similar, with more than 70% of patients failing previous immunomodulator therapy. Disease was localized to both small bowel and large bowel in 70% of patients in each group, with no significant difference in disease behavior at baseline between the RR and NRR groups. There was also no significant difference in proportion of patients receiving concomitant MTX therapy in each group (45% versus 36%, P = 0.47). Median time to dose intensification was longer in RR (18 [10–37] months) as compared with the NRR group (11 [7–28] months) (P = 0.047). At time of dose intensification, median C-reactive protein, erythrocyte sedimentation rate, albumin, and Harvey Bradshaw Index were compared in each group and no difference was observed (Table 2). ATI levels were drawn at the time of dose intensification in 38 of 65 (58%) patients, and significantly more patients in the NRR had detectable ATI versus the RR group (73% versus 22%, P = 0.002).
TABLE 2.
Outcomes of Dose Intensification in Patients With CD
| At the Time of DI | RRs (n = 40) | NRRs (n = 25) | P |
|---|---|---|---|
| Median (IQR) time to DI after IFX initiation (mo) | 18 (9–37) | 11 (7–28) | 0.047 |
| Median (IQR) CRP (mg/mL) | 1.3 (0.4–3) | 0.8 (0.5–3.7) | 0.46 |
| Median (IQR) ESR (mm/h) | 26 (4–21) | 31 (16–54) | 0.26 |
| Median (IQR) albumin (g/dL) | 4.2 (3.8–4.3) | 4.0 (3.6–4.2) | 0.26 |
| Median (IQR) BSA (m2) | 1.5 (1.3–1.6) | 1.4 (1.2–1.6) | 0.14 |
| Median (IQR) HBI | 5 (3–6) | 5.5 (3–8) | 0.28 |
| ATI detected (%) | 5/23 (22) | 11/15 (73) | 0.002 |
| Median (IQR) duration of IFX (mo) | 48 (28–87) | 24 (10–41) | 0.001 |
BSA, body surface area; CRP, C-reactive protein; DI, dose intensification; ESR, erythrocyte sedimentation rate; HBI, Harvey Bradshaw Index.
Ulcerative Colitis
Seven (54%) of the 13 patients meeting non-SDR definition underwent IFX intensification, with 4 patients (57%) undergoing only dose escalation to 10 mg/kg and 3 patients (43%) had both dose and frequency escalation. Fifty-seven percent of patients (4 of 7) met criteria for RR (Fig. 1). None of these patients with UC were started on concomitant MTX therapy at the time of IFX induction. No statistical difference was found between the RR versus NRR groups with regards to median C-reactive protein, erythrocyte sedimentation rate, albumin, and Pediatric Ulcerative Colitis Activity Index at the time of dose intensification (Table 3). As compared with the CD groups, only 1 patient in RR group and 2 patients in NRR group had ATI levels drawn at the time of dose intensification, and only 1 patient in NRR group had the presence of ATI.
TABLE 3.
Outcomes of Dose Intensification in Patients With UC
| At the Time of DI | RRs (n = 4) | NRRs (n = 3) | P |
|---|---|---|---|
| Median (IQR) time to DI after IFX initiation (mo) | 11 (9–15) | 7 (6–14) | 0.42 |
| Median (IQR) CRP (mg/mL) | 0.6 (0.4–0.7) | 0.5 (0.5–0.6) | 0.49 |
| Median (IQR) ESR (mm/h) | 21 (16–23) | 10 (6–36) | 0.38 |
| Median (IQR) Albumin (g/dL) | 3.7 (3.7–4.0) | 2.6 (2.6–3.5) | 0.15 |
| Median (IQR) BSA (m2) | 1.4 (1.4–1.5) | 1.3 (1.0–1.4) | 0.16 |
| Median (IQR) PUCAI | 55 (30–55) | 35 (25–45) | 0.44 |
| ATI detected (%) | 0/1 (0) | 1/2 (50) | 0.39 |
| Median (IQR) duration of IFX (mo) | 51 (34–75) | 15 (12–18) | 0.05 |
BSA, body surface area; CRP, C-reactive protein; DI, dose intensification; ESR, erythrocyte sedimentation rate; PUCAI, Pediatric Ulcerative Colitis Activity Index.
IFX Durability
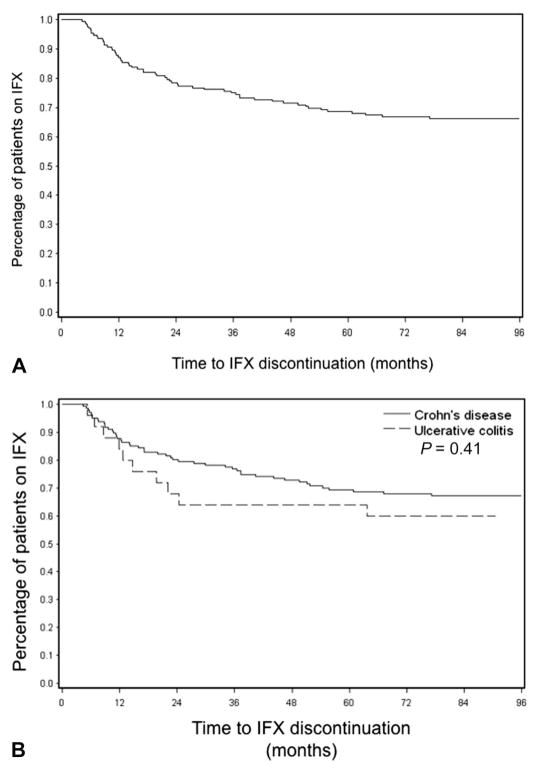
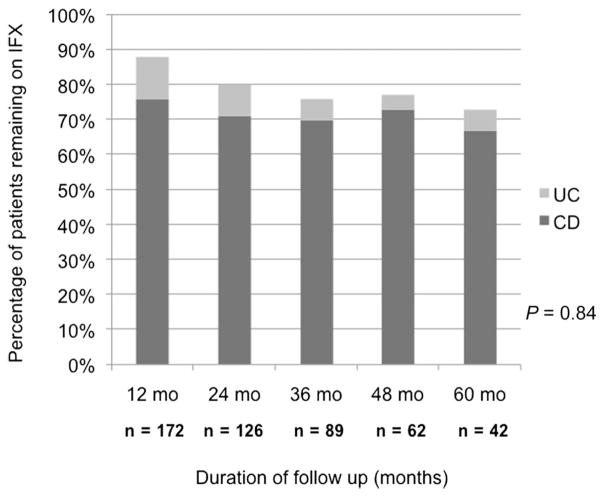
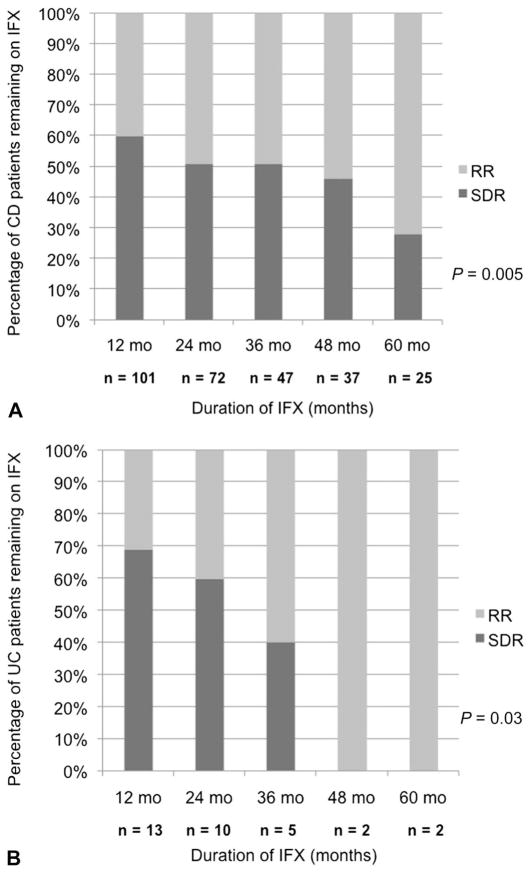
As shown in Figure 1, only 16 (8%) of patients with IBD discontinued IFX within the induction period (PNR) and never received maintenance IFX. Figure 2 represents the Kaplan–Meier survival curve for time to IFX discontinuation in all patients with IBD (Fig. 2A) and CD versus UC (Fig. 2B) excluding PNR. Only 13% of patients discontinued IFX by 12 months, and 22% discontinued therapy by 24 months. Figure 3 illustrates that the proportion of patients with CD and UC remaining on IFX is similar at each annual follow-up (P = 0.84). Of the 42 patients with IBD who were followed up for at least 60 months from the time of induction, 31 (74%) remained on IFX (83.5 [67–94] months). Figure 4 demonstrates what proportion of patients with CD and UC remaining on IFX were SDR versus RR at each year of available follow-up. Over a 5-year period, there seems to be a significant difference in proportion of patients in SDR versus RR for both CD and UC (P = 0.005 and P = 0.03, respectively). Over time, the proportion of SDR decreases and the rate of RR increases.
FIGURE 2.
Kaplan–Meier curve representing time to discontinuation of IFX in all patients with IBD (A) and in patients with CD versus UC (B), excluding PNR. There is no significant difference in time to IFX discontinuation between CD and UC (P = 0.41).
FIGURE 3.
IFX durability demonstrated by proportion of patients remaining on IFX at each annual follow-up.
FIGURE 4.
IFX durability demonstrated by proportion of patients with CD (A) and UC (B) remaining on IFX who were SDR versus RR at each year of available follow-up.
IFX Failures
Crohn’s Disease
Seven (4%) patients with CD met criteria for PNR. One patient had worsening disease activity during IFX induction and proceeded to surgery, then started adalimumab. Six patients had infusion reactions within the induction period and were transitioned to adalimumab. Four of these patients had ATI levels assessed and 3 were detectable.
For the 49 patients with CD who discontinued therapy as of the last follow-up, median duration of IFX was 17 months (10–37 months) (Table 4). Twenty-four patients (40%) stopped IFX befor dose intensification for the following reasons: delayed hypersensitivity reaction (n = 1), psoriaform rash (n = 1), lymphoproliferative disease (n = 1), paresthesias (n = 1), infusion reaction (n = 5), detectable ATI (n = 5), worsening of disease (n = 6), and patient-directed discontinuation of IFX (n = 4). The other 25 patients stopped IFX after dose intensification for the following reasons: persistent or worsening disease activity (n = 11), lupus-like reaction (n = 1), recurrent perianal abscess (n = 1), infusion reactions (n = 3), and ATI development postintensification (n = 9).
TABLE 4.
Reasons for IFX Discontinuation in Patients With CD
| Reason for IFX Discontinuation | N (%) |
|---|---|
| Lack of efficacy | |
| Primary nonresponse | 7 (12) |
| Secondary loss of response | |
| No dose intensification | 6 (11) |
| Despite dose intensification | 11 (19) |
| Immunogenicity | |
| Infusion reactions | 8 (14) |
| Detectable ATI | 14 (25) |
| Delayed hypersensitivity reaction | 1 (2) |
| Lupus-like reaction | 1 (2) |
| Psoriaform rash | 1 (2) |
| Recurrent infections | 1 (2) |
| Paresthesias | 1 (2) |
| Neoplasia | |
| Lymphoproliferative disease | 1 (2) |
| Noncompliance | 4 (7) |
Of the 56 total patients with CD who discontinued IFX therapy at any point after first induction dose (PNR and IFX failures), 40 (71%) transitioned to adalimumab. Eight of these 40 patients failed adalimumab therapy. Of these, 3 patients are now on certolizumab, 3 patients are on natalizumab, 1 patient is on MTX monotherapy, and 1 underwent surgery.
Of the 16 patients not transitioned to adalimumab, 6 (11%) were treated with thiopurine monotherapy, 3 (5%) with MTX monotherapy, 1 (2%) developed lymphoproliferative disease and so was continued on mesalamine only, 5 (9%) had surgical resection, and 1 (2%) discontinued therapy by choice.
Ulcerative Colitis
Nine of 31 (29%) patients with UC were PNR and all underwent colectomy. Of the remaining 22 patients, 9 (29%) discontinued IFX as of the last follow-up, with median duration of therapy of 12.7 months (9–20 months) (Table 5). Of these 9 patients, 6 stopped drug without undergoing dose intensification. Reasons for discontinuation included: presence of ATI (n = 1), infusion reaction (n = 1), difficulty with intravenous access (n = 1), development of seizures then transitioned to mesalamine (n = 1), discontinued IFX by choice of patient then started thiopurine (n = 1), and persistent disease activity, eventually required colectomy after trial of adalimumab (n = 1). Three of these 6 patients transitioned to adalimumab and continued to respond. The other 3 patients stopped IFX after dose intensification. One of these patients developed an infusion reaction and was transitioned to adalimumab. Another patient had positive ATI and was then maintained on a thiopurine only, whereas 1 patient had evidence of persistent disease activity and went on to colectomy.
TABLE 5.
Reasons for IFX Discontinuation in Patients With UC
| Reason for IFX Discontinuation | N (%) |
|---|---|
| Lack of efficacy | |
| Primary nonresponse | 9 (49) |
| Secondary loss of response | 1 (6) |
| No dose intensification | 1 (6) |
| Despite dose intensification | |
| Immunogenicity | 2 (11) |
| Infusion reactions | 2 (11) |
| Detectable ATI | |
| Seizures | 1 (6) |
| Noncompliance | 2 (11) |
Concomitant Low-dose MTX
Of the 157 patients with CD, 69 (44%) were started on MTX at the time of IFX induction and 5 of these patients were PNR. Fifty-six (81%) of the 69 patients remained on low-dose oral MTX, defined as less than 10 mg every week, throughout the duration of IFX therapy. MTX was initiated after IFX induction in 38 (24%) patients with CD and median time to starting MTX was 14 months (6–24 months) from time of IFX induction. Only thirty-six patients (23%) remained on IFX monotherapy throughout the duration of therapy. The remaining 14 of 157 (9%) patients did not receive concomitant MTX therapy but were on a thiopurine when IFX was initiated, 3 of whom remained on this combination at the time of last follow-up. There was no statistical difference between patients in SDR (P = 0.41) or RR (P = 0.10) when comparing patients on IFX monotherapy with patients on combination therapy with low-dose oral MTX from time of IFX induction. Median time to loss of response, defined as the time to dose intensification or IFX discontinuation, and median duration of IFX therapy were also similar between the 2 groups (P = 0.16).
Only 2 of 31 (6%) patients with UC were started on low-dose oral MTX at the time of induction, limiting the analysis of this group. Four patients (13%) were started on MTX therapy after IFX induction (median time to initiation of MTX was 11.4 [8–13] months), whereas 7 patients (23%) remained on IFX monotherapy throughout the duration of treatment. Nine patients (29%) received thiopurines at the time of IFX initiation, and 2 of these patients remained on this combination at the time of last follow-up.
DISCUSSION
IFX is effective in the induction and maintenance of remission in pediatric and adult-onset IBD. There are limited data, however, on durability of IFX in pediatric patients with IBD beyond 1 year. We presented a 10-year single-center experience with IFX in a cohort of pediatric patients with IBD evaluating long-term efficacy and safety of IFX. In our cohort, 87% of patients remained on IFX at 1 year postinitiation and 75% at the 2 year follow-up. In those patients followed up beyond 2 years, there was a stabilization of proportion of patients still on IFX even out to 5 years.
When specifically evaluating IFX durability in patients with CD, results for patients remaining on IFX therapy at 1 and 2 years (88% and 80%, respectively) are comparable with previous studies.4,6–8 However, in our cohort of patients with CD, a larger percentage of patients (72%) remained on IFX therapy at 5 years postinitiation, where an estimated 50% of patients were likely to discontinue IFX in previous studies.4,6–8 Within the UC group, 71% (22 of 31) of patients avoided colectomy at 1 year after treatment with IFX. This is similar to what has been previously reported in a larger multicenter cohort study by Hyams et al.5
As would be expected, more severe disease at baseline, such as stricturing disease and perianal involvement, was associated with loss of response to IFX. It was also notable that more patients with UC in the non-SDR group were steroid refractory. This may suggest these patients had more refractory disease, were nonresponsive to steroids, and may have benefited from earlier initiation of a second-line agent, such as IFX.
Primary reasons for IFX discontinuation in our cohort were loss of IFX efficacy despite dose intensification and development of immunogenicity with presence of ATI and infusion reactions. Low IFX trough levels and development of ATI is associated with a reduced duration of response to IFX.16,17 This is seen in our study where a higher rate of ATI was present in patients who were unable to recapture response with IFX dose intensification. However, only a subset of patients who were classified as secondary nonresponders had ATI and IFX trough levels assessed, and there was no clear association between trough levels predose intensification in the RR and the NRR groups.
IFX dose intensification, increasing IFX dosing from 5 to 10 mg/kg, and/or increasing the frequency of infusions to every 6 weeks or less has been shown to overcome loss of response to maintenance therapy.9,10 In our study, close to 60% of patients were able to recapture and maintain response after dose intensification. In patients with CD who eventually lost response after dose intensification, 40% of patients gained an additional 12 months of remission before discontinuing IFX. Additionally, over a 5-year period, we demonstrated a rise in the proportion of RR versus patients in SDR in both CD and UC groups, suggesting an attenuation in response to standard IFX dosing over time and ability to recapture response after dose intensification. There is clear benefit in IFX dose intensification in the face of loss of response.
Adverse events were limited in our patient population; however, one patient with CD and Wilson’s disease, treated concomitantly with thiopurines and infliximab, developed Epstein-Barr virus-associated lymphoproliferative disease.18 As previously reported, the patient is now off immunosuppressive therapies and her IBD is managed with mesalamine alone. The majority of our patients with CD who lost response to IFX were transitioned to an alternate anti–tumor necrosis factor α agent, specifically adalimumab. Of the patients who were transitioned to adalimumab, 82% remained on this therapy as of the last follow-up. This was similar in patients with UC, where 60% of patients who discontinued IFX therapy were transitioned to adalimumab and 83% of these patients remained on adalimumab at the time of last follow-up. With the retrospective design of this study, the duration of follow-up varied between patients, but median duration on adalimumab was more than 1 year. Support for the strategy of switching to adalimumab after intolerance to IFX has been reported,19–21 but long-term data in pediatrics are limited. Further studies evaluating durability and efficacy of adalimumab in this cohort of patients is warranted.
Concomitant use of immunomodulators, specifically thiopurines, decreases immunogenicity to IFX, increases trough levels, and is associated with greater therapeutic efficacy.12,13 However, with reports of hepatosplenic T-cell lymphoma described predominantly in young males with CD treated with thiopurines and IFX,15,22,23 clinical practice has transitioned for some clinicians to using concomitant low-dose oral MTX in patients with IBD on IFX based on a demonstrated pharmacokinetic advantage in the rheumatoid arthritis literature.24 In our cohort of patients with CD, many were transitioned off of thiopurines and onto concomitant MTX at the time of IFX induction. An additional 24% of patients were transitioned to concomitant MTX after IFX induction, with a limited number of patients remaining on thiopurines and IFX (<10%). In the subset of patients in our cohort treated with oral MTX at a dose of <10 mg/wk, there was not a significant difference in IFX durability or efficacy between this group and patients with CD on IFX monotherapy. Additionally, ATIs were only assessed in a small number of patients and did not seem to be significantly different between the 2 groups. It is likely that interindividual variation in MTX metabolism may contribute to the lack of efficacy and higher doses may be needed to achieve a reduction in immunogenicity to IFX. Further studies evaluating adequate dosing of concomitant MTX and the possible role of MTX therapeutic drug monitoring are warranted.
There are several limitations to our study. We recognize that these data are reflective of only a single Pediatric IBD Center, which may contribute to potential practice and referral bias. However, the data are similar to what is reported by Hyams et al4,5,7 and a single provider may result in less variation in care. Additionally, the smaller sample size in the UC group and the subset of patients on concomitant MTX therapy versus IFX monotherapy may have limited our power to detect meaningful differences for those outcomes. Larger studies are currently underway to examine this further. We also recognize that ATI and IFX trough levels were only assessed in a subset of patients when there was suspicion for loss of response; therefore, these available data may be biased. However, we did see a significant difference in those that responded to dose intensification when ATIs were undetectable. Availability of ATI and IFX trough levels for all patients in our prospective study will better characterize loss of response and efficacy of IFX dose intensification.
Our study demonstrates that IFX is safe and effective for long-term maintenance therapy in pediatric patients with IBD. IFX dose intensification can optimize durability and overcome loss of response. Loss of response is likely affected by underlying disease severity and development of immunogenicity. Low-dose concomitant MTX may not be effective in reducing immunogenicity to IFX, and higher doses or dosing based on therapeutic MTX levels will need to be evaluated further.
Acknowledgments
Supported by the National Institutes of Health/National Center for Advancing Translational Science UCLA CTSI Grant Number UL1TR000124.
Footnotes
M.C. Dubinsky received an independent investigator scientific grant from Janssen for this study and is a consultant for Janssen. Other authors have no conflicts of interest to disclose.
References
- 1.Benchimol EI, Guttmann A, Griffiths AM, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58:1490–1497. doi: 10.1136/gut.2009.188383. [DOI] [PubMed] [Google Scholar]
- 2.Kugathasan S, Judd RH, Hoffmann RG, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr. 2003;143:525–531. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 3.Baldassano RN, Piccoli DA. Inflammatory bowel disease in pediatric and adolescent patients. PCNA, Gastroenterol Clin North Am. 1999;28:445–458. doi: 10.1016/s0889-8553(05)70064-9. Review. [DOI] [PubMed] [Google Scholar]
- 4.Hyams JS, Lerer T, Griffiths A, et al. Long-term outcome of maintenance infliximab therapy in children with Crohn’s disease. Inflamm Bowel Dis. 2009;15:816–822. doi: 10.1002/ibd.20845. [DOI] [PubMed] [Google Scholar]
- 5.Hyams JS, Lerer T, Griffiths A, et al. Outcome following infliximab therapy in children with ulcerative colitis. Am J Gastroenterol. 2010;105:1430–1436. doi: 10.1038/ajg.2009.759. [DOI] [PubMed] [Google Scholar]
- 6.Assa A, Hartman C, Weiss B, et al. Long-term outcome of tumor necrosis factor alpha antagonist’s treatment in pediatric Crohn’s disease. J Crohns Colitis. 2013;7:369–376. doi: 10.1016/j.crohns.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Hyams J, Walters TD, Crandall W, et al. Safety and efficacy of maintenance infliximab therapy for moderate-to-severe Crohn’s disease in children: REACH open-label extension. Curr Med Res Opin. 2011;27:651–662. doi: 10.1185/03007995.2010.547575. [DOI] [PubMed] [Google Scholar]
- 8.Gonzaga JE, Ananthakrishnan AN, Issa M, et al. Durability of infliximab in Crohn’s disease: a single-center experience. Inflamm Bowel Dis. 2009;15:1837–1843. doi: 10.1002/ibd.20974. [DOI] [PubMed] [Google Scholar]
- 9.Lin KK, Velayos F, Fisher E, et al. Durability of infliximab dose intensification in Crohn’s disease. Dig Dis Sci. 2012;57:1013–1019. doi: 10.1007/s10620-011-1969-3. [DOI] [PubMed] [Google Scholar]
- 10.Chaparro M, Panes J, Garcia V, et al. Long-term durability of infliximab treatment in Crohn’s disease and efficacy of dose “escalation” in patients losing response. J Clin Gastroenterol. 2011;45:113–118. doi: 10.1097/MCG.0b013e3181ebaef9. [DOI] [PubMed] [Google Scholar]
- 11.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 12.Vermeire S, Noman M, Van Assche G, et al. The effectiveness of concomitant immunosuppressive therapy to suppress formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226–1231. doi: 10.1136/gut.2006.099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombel JF, Sanborn WJ, Reinisch W, et al. Infliximab, azathioprine or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 14.Breedveld F, Weisman M, Kavanaugh A, et al. A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 15.Mackey AC, Green L, Liang LC, et al. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265–267. doi: 10.1097/MPG.0b013e31802f6424. [DOI] [PubMed] [Google Scholar]
- 16.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 18.Gidrewicz D, Lehman D, Rabizadeh S, et al. Primary EBV infection resulting in lymphoproliferative disease in a teenager with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2011;52:103–105. doi: 10.1097/MPG.0b013e3181e80410. [DOI] [PubMed] [Google Scholar]
- 19.Mozziconacci N, Vermeire S, Laharie D, et al. Efficacy of a third anti-TNF monoclonal antibody in Crohn’s disease after a failure of two other anti-TNFs. Gastroenterology. 2008;134:A663. doi: 10.1111/j.1365-2036.2009.04130.x. [DOI] [PubMed] [Google Scholar]
- 20.Sandborn WJ, Hanauer S, Loftus EV, Jr, et al. An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn’s disease. Am J Gastroenterol. 2004;99:1984–1989. doi: 10.1111/j.1572-0241.2004.40462.x. [DOI] [PubMed] [Google Scholar]
- 21.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 22.Kotlyar DS, Osterman MT, Diamond RH, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:36–41. doi: 10.1016/j.cgh.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Rosh J, Gross T, Mamula P, et al. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn’s disease. A cautionary tale? Inflamm Bowel Dis. 2007;13:1024–1030. doi: 10.1002/ibd.20169. [DOI] [PubMed] [Google Scholar]
- 24.Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]