Abstract
Dopamine (DA) and orexin neurons play important roles in reward and food intake. There are anatomical and functional connections between these two cell groups, where orexin peptides stimulate DA neurons in the ventral tegmental area and DA inhibits orexin neurons in the hypothalamus. However, the cellular mechanisms underlying DA action on orexin neurons remain incompletely understood. Therefore, the effect of DA on inhibitory transmission to orexin neurons was investigated in rat brain slices using whole cell patch clamp technique. We found that DA modulated the frequency of spontaneous and miniature IPSCs (mIPSCs) in a concentration dependent, bidirectional manner. Low (1 μM) and high concentrations (100 μM) of DA decreased and increased IPSC frequency, respectively. These effects did not accompany a change in mIPSC amplitude and persisted in the presence of G protein signaling inhibitor GDPβS in the pipette, suggesting that DA acts presynaptically. The decrease in mIPSC frequency was mediated by D2 receptors, whereas the increase required co-activation of D1 and D2 receptors and subsequent activation of phospholipase C. In summary, our results suggest that DA has complex effects on GABAergic transmission to orexin neurons, involving cooperation of multiple receptor subtypes. The direction of dopaminergic influence on orexin neurons is dependent on the level of DA in the hypothalamus. At low levels DA disinhibits orexin neurons whereas at high levels it facilitates GABA release, which may act as negative feedback to curb the excitatory orexinergic output to DA neurons. These mechanisms may have implications for consummatory and motivated behaviours.
Keywords: patch clamp, lateral hypothalamus, IPSC, phospholipase C, rat
Introduction
The lateral hypothalamus (LH), including the perifornical area, plays important roles in reward and feeding through direct connections with the reward circuitry (Kenny 2011). The LH is known to be a site of action for dopamine (DA) to modulate consummatory and motivated behaviours. DA is released in the LH during meals (Meguid et al 1995) and inhibits food intake (Leibowitz et al 1986;Yang et al 1997), likely acting as a satiety signal. Pharmacological studies suggest that both D1-like and D2-like receptors (D1Rs and D2Rs) are functionally expressed in the LH and differentially modulate food and ethanol consumption. D1R activation promotes, while D2R activation inhibits food and ethanol intake (Chen et al 2013). Additionally, blockade of D2Rs in the LH induces DA release in the nucleus accumbens, resulting in robust conditioned place preference and locomotor activity (Morutto and Phillips 1998;Parada et al 1995). Therefore, DA receptors in the LH play a significant role in controlling reward-related behaviours.
One of the key mediators of reward and feeding function in the LH, and a candidate target for DA action, are orexin neurons. Orexin neuropeptides are primarily expressed in the LH and stimulate food intake (Sakurai et al 1998), in particular palatable food (Clegg et al 2002;Thorpe et al 2005). Orexins are also involved in neuronal and behavioural responses to drugs of abuse (Borgland et al 2006;Boutrel et al 2005;Georgescu et al 2003;Harris et al 2005;Narita et al 2006), which may be mediated by DA neurons in the ventral tegmental area (VTA) (Kenny 2011). On one hand, orexins have been shown to activate VTA DA neurons (Borgland et al 2006;Korotkova et al 2003). On the other hand, high concentrations of DA inhibit orexin neurons via D2Rs (Li and van Den Pol 2005;Yamanaka et al 2006), while D1R activation modulates excitatory transmission to orexin neurons (Rao et al 2008). There is also evidence that orexin mRNA expression is increased by D1R activation and decreased by D2R activation (Chen et al 2013). These studies suggest that DA actions on orexin neurons have important implications for the LH function in reward.
It is generally accepted that D1Rs and D2Rs are coupled to G proteins with αs and αi/o subunits, respectively, and consequently have opposite effects on adenylyl cyclase activity (Beaulieu and Gainetdinov 2011). However, in addition to these canonical signaling cascades, DA receptors can activate distinct cellular signaling pathways by interacting with each other or with other receptors (Perreault et al 2014). The physiological significance of these receptor interactions in reward and addiction has been suggested (Perreault et al 2014), while it remained unknown whether orexin neurons are modulated by DA in this manner. Here, we investigated the effect of DA on fast inhibitory transmission to orexin neurons and found that DA has complex dose-dependent effects mediated by the interaction of multiple subtypes of DA receptors.
Materials and Methods
Ethics standards
All experiments were conducted following the Canadian Council on Animal Care guidelines and approved by the Memorial University Institutional Animal Care Committee. Male Sprague Dawley rats (3–4 week old) were obtained from the breeding colony at Memorial University and housed in a light and temperature controlled room (L:D=12:12 h, lights on at 7:00 am, room temperature 22 ± 1°C). A total of 114 cells from 89 rats were used in these experiments.
In vitro electrophysiology
Rats were deeply anesthetized with halothane or isoflurane inhalation and decapitated. 250μm coronal brain slices were generated in ice-cold buffer solution containing (in mM): 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 0.5 CaCl2, 25 NaHCO3, 25 glucose, 30 sucrose, 3 pyruvic acid, 1 ascorbic acid. Slices were incubated at 33–34°C for 30–45 min and then at room temperature until recording in artificial cerebral spinal fluid (ACSF) (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2 CaCl2, 25 NaHCO3, 10 glucose, 1 ascorbic acid. Both solutions were continuously bubbled with O2 (95%) and CO2 (5%).
A hemisected brain slice (levels corresponding to −2.64 to −3.24 mm from bregma in the Rat Stereotaxic Coordinates (Paxinos and Watson 2005)) was placed in the recording chamber and continuously perfused with 33–34°C ACSF at 1.5–2.0 mL/min. A differential interference contrast microscope (DM LFSA; Leica Microsystems) was used to select neurons in the LH dorsal to the fornix (including the perifornical area) and with a soma diameter of 10–20 μm. Whole-cell patch-clamp recording was performed using a Multiclamp 700B amplifier and pClamp 9.2 or 10.3 software (Molecular Devices, Sunnyvale, CA).
Once whole cell access was obtained, a series of hyperpolarizing and depolarizing current steps (300 or 600 ms each) was applied to characterize the electrophysiological properties of the cell. To aid in the detection of GABAergic inhibitory postsynaptic current (IPSC), the internal solution was composed of (in mM): 132 KCl, 2 MgCl2, 0.2 EGTA, 10 HEPES, 4 Na2-ATP, 0.3 Na2-GTP, pH 7.3, which reversed the polarity of picrotoxin-sensitive GABAergic currents (referred to as IPSCs) recorded at −70 mV. Filled recording electrodes had a tip resistance of 3–7 MΩ. In a subset of recordings, biocytin (1–2 mg/ml) was added to the internal solution to label cells. DNQX (10 μM) and D-AP5 (50 μM) were present in the bath to block glutamatergic transmission and isolate spontaneous IPSCs. To record miniature IPSCs (mIPSCs), tetrodotoxin (1 μM) was further added to block action potential-driven synaptic transmission. For the GDPβS experiment, GDPβS (2 mM) replaced Na2-GTP in the internal solution and was allowed to diffuse into the cell following break-in for at least 10 minutes before recording the baseline.
Membrane currents were filtered at 1 kHz, digitized at 5 kHz and stored for offline analysis. A 20 mV, 100 ms hyperpolarizing pulse was applied every 20–60 s, and the steady state and capacitive currents were monitored as measures of input resistance and series/access resistance, respectively. Cells that showed significant change (>20%) in these parameters were excluded from analysis. Occasionally, clusters of high frequency mIPSCs (typically 5–50 Hz) appeared, lasting up to several seconds and clearly standing out from the background. Cells displaying these clusters interfered with the experiments and were therefore not analyzed.
Post-hoc immunohistochemistry
After recordings, brain slices with biocytin-filled cells were immediately placed in 4% paraformaldehyde or 10% formalin and fixed for >16h at 4°C. To distinguish orexin neurons from other LH neurons that are melanin concentrating hormone (MCH)-expressing or non-orexin/non-MCH, fixed slices were individually incubated with a cocktail of goat anti-orexin A (1:1000–3000; sc-8070, Santa Cruz Biotechnology) and rabbit anti-MCH (1:1000–2000; G-070–47, Phoenix Pharmaceuticals) for 3 days at 4°C. This was followed by secondary antibodies (1:500; Cy3 or Alexa594-conjugated donkey anti-goat, Cy2 or Alexa488-conjugated donkey anti-rabbit and AMCA-conjugated streptavidin). Stained slices were examined under epifluorescence microscope to identify co-localization of orexin A or MCH with biocytin.
Data Analysis
mIPSCs and spontaneous IPSCs were detected using Minianalysis 6.0 (Synaptosoft, Decatur, GA). The data are expressed as mean ± S.E.M. The frequency or amplitude of mIPSCs during the baseline and peak drug effect was compared by paired t-test. A change in these parameters by greater than 15% was considered significant. For group comparisons, the drug effect was normalized to its respective baseline for each cell and then compared using unpaired t-test or one-way ANOVA with Dunnett’s post-hoc test. P < 0.05 was considered significant.
Chemical Compounds
All drugs were bath perfused at final concentrations by diluting 1000× stock in the ACSF immediately before use. DA solutions included 1 mM ascorbic acid and were light protected to minimize oxidation. The maximum final concentration of DMSO (vehicle) was 0.2%. SKF81297, quinpirole, SCH23390, and sulpiride were purchased from Tocris Bioscience (Minneapolis, MN); DA, U73122, H89, biocytin, DNQX, D-AP5, and GDPβS from Sigma Aldrich (St. Louis, MO); edelfosine from Santa Cruz Biotechnology, Inc. (Dallas, TX); Rp-cAMPs from Enzo Life Sciences (Farmingdale, NY); and tetrodotoxin from Alomone Labs (Jerusalem, Israel).
Results
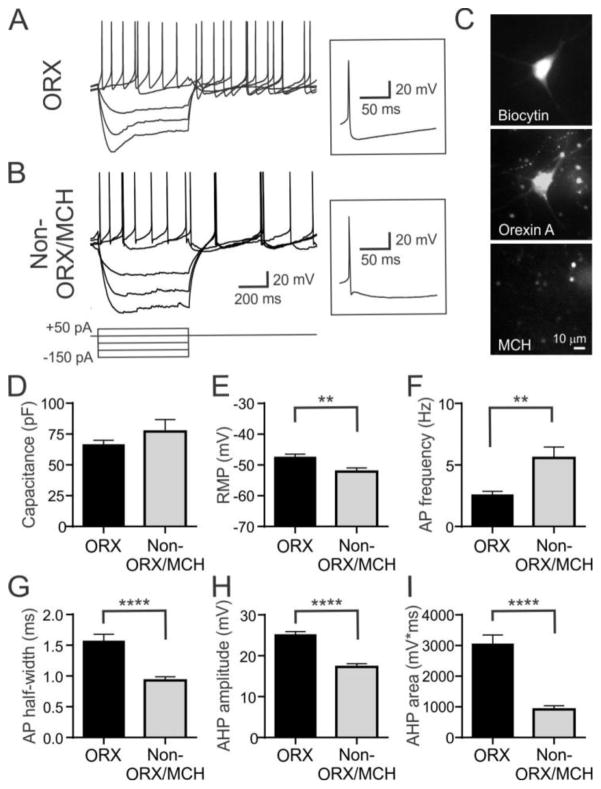
The LH contains several types of neurons in addition to orexin neurons. Therefore, electrophysiological properties unique to orexin neurons were characterized. Typically, orexin-immunopositive neurons are spontaneously active, display H-current upon hyperpolarization and rebound depolarization following relief from hyperpolarization that is often capped with an action potential(Eggermann et al 2003;Parsons et al 2012) (Fig. 1A,C). MCH neurons have a similar soma size and anatomical distribution, and also play a role in food intake regulation (Kawano et al 2002). However, these cells are electrophysiologically distinct from orexin neurons, as characterized by hyperpolarized resting membrane potential and lack of H-current or rebound depolarization (Alberto et al 2011). In addition, we found neurons that are immunonegative for orexin and MCH (non orexin/non-MCH, n=15) but are similar to orexin-immunopositive neurons (n=18) in terms of spontaneous firing, H-current, and membrane capacitance (Fig. 1B,D). Nonetheless, these non-orexin/non-MCH neurons were easily distinguishable from orexin neurons by the presence of fast and medium afterhyperpolarizing potentials (AHP) (Fig. 1B, inset), since orexin neurons display a single pronounced AHP (Fig. 1A, inset). Furthermore, non-orexin/non-MCH neurons had a significantly more negative resting membrane potential (Fig. 1E). Despite this, the firing frequency was higher in non-orexin/non-MCH neurons (Fig. 1F), which may be due to shorter action potential half-width and smaller AHP amplitude and area (Fig. 1G–I). Therefore, H-current, spontaneous firing, and uniphasic AHP can be used to reliably predict orexin phenotype (100% success rate, n=47). Based on these results, only cells with characteristics typical of orexin neurons were included in the present study.
Figure 1. Identification of orexin neurons.
A and B) Typical electrophysiological characteristics of orexin neurons (A, ORX) and non-orexin/non-MCH LH neurons (B, Non-ORX/MCH) in response to current injections at a similar resting membrane potential (−50mV). Both cell types display Ih and spontaneous firing. Inset: orexin neurons show uniphasic afterhyperpolarizing potential (AHP) while non-orexin/non-MCH neurons display fast and medium AHPs distinguishable by a notch following action potential repolarization.
C) Posthoc immunohistochemistry demonstrating a biocytin-filled cell co-labelled with orexin A but not MCH. Note that incubation of brain slices in vitro before fixation results in blobbing of dendrites at the severed end, which appear as small dots and are readily distinguishable from the soma in orexin and MCH staining.
D–I) Comparison of electrophysiological properties of orexin and non-orexin/non-MCH neurons in the LH. RMP, resting membrane potential; AP, action potential; AHP, afterhyperpolarizing potential.
**:p<0.01, ****:p<0.0001
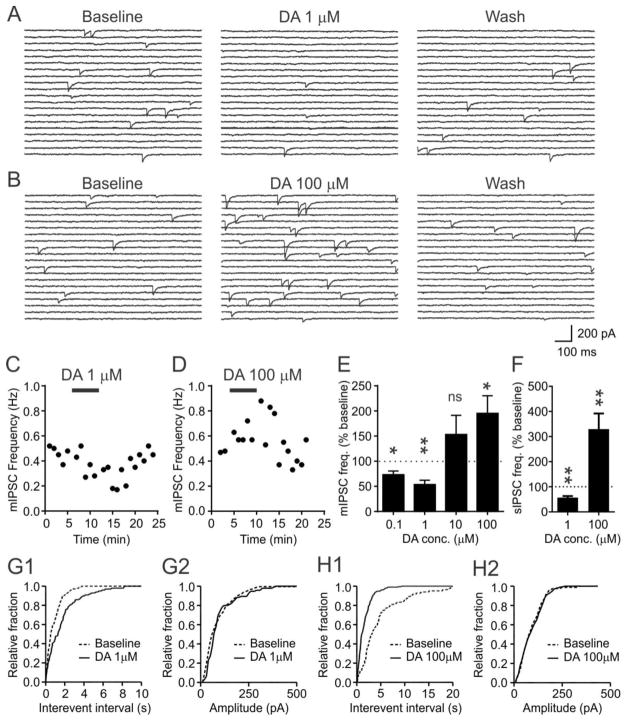
To determine the effects of DA on inhibitory transmission, mIPSCs were investigated allowing us to distinguish between pre- and postsynaptic sites of DA action. In orexin neurons, the baseline frequency of mIPSCs was 0.62 ± 0.08 Hz (n=47). At low concentrations, DA decreased mIPSC frequency (Fig. 2A,C,E,G1): 0.1 μM had a significant inhibitory effect (n=6, p=0.0147, paired t-test), as did 1 μM (n=7, p=0.0031, paired t-test; one outlier excluded due to high baseline frequency, defined as > mean + 2 S.D.). In contrast, a higher concentration of DA (100 μM) significantly increased mIPSC frequency (n=6, p=0.0124, paired t-test; Fig. 2B,D,E, H1). 10 μM DA also increased mIPSC frequency (defined as >15% increase) in the majority of cells tested (6 of 9), but the remaining 3 cells showed a decrease. When grouped, no significant change was detected (n=9, p=0.189, paired t-test; Fig. 2E). DA had similar effects on spontaneous IPSC frequency (Fig. 2F), inducing a decrease at 1μM (n=6, p=0.0041, paired t-test, one cell was excluded as an outlier (> mean + 2 S.D.)) and an increase at 100 μM (n=5, p=0.0052, paired t-test). mIPSC amplitude was insensitive to DA regardless of the concentration tested (Fig. 2G2, H2). These findings suggest that DA has concentration-dependent bidirectional effects on the frequency of IPSCs.
Figure 2. Dopamine modulates GABAergic transmission to orexin neurons.
A and B) Representative voltage clamp traces showing the effect of dopamine (DA) on mIPSC at different concentrations as indicated.
C) Representative time-effect plot showing that a low concentration of DA reversibly inhibits mIPSC frequency.
D) A high concentration of DA increases mIPSC frequency.
E) The direction of DA effect is concentration-dependent. Dotted line denotes the baseline level (100%).
F) Effect of DA on the frequency of spontaneous IPSCs (sIPSC).
G–H) Cumulative plot of the inter-event interval (G1, H1) and amplitude (G2, H2) of mIPSCs during baseline and DA application. Inter-event interval, but not amplitude, is modulated by DA.
*:p<0.05, **:p<0.01, ns: not significant vs. baseline.
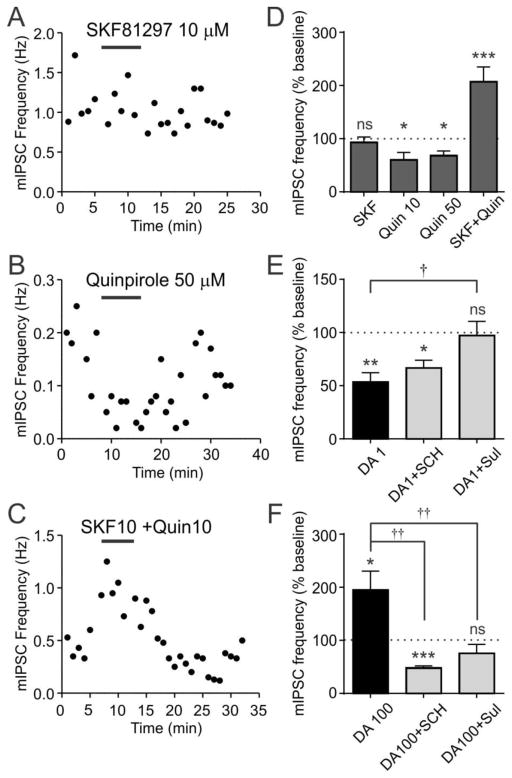
To understand the cellular mechanism underlying these DA effects, we investigated the DA receptor subtypes involved. We found that the D1R agonist SKF81297 (SKF, 10 μM) had no effect on mIPSCs (n=8, p=0.4004, paired t-test; Fig. 3A,D), while the D2R agonist quinpirole (Quin) decreased mIPSC frequency (50 μM: n=5, p=0.0344, paired t-test; 10 μM: n=5, p=0.0455, paired t-test; 10 μM vs. 50 μM: p=0.7148, unpaired t-test; Fig. 3B,D). Interestingly, when the two agonists were applied together (SKF+Quin, 10 μM each), there was an significant increase in mIPSC frequency (n=12, p=0.0001, paired t-test; Fig. 3C,D).
Figure 3. Different DA receptor subtypes mediate presynaptic mIPSC modulation.
A–C) Time-effect plots showing the effect of SKF81297 (SKF, 10 μM), Quinpirole (Quin, 50 μM) and co-application of SKF and Quin (10 μM each) on mIPSC frequency, as indicated by horizontal bars.
D) Summary graph showing the effects of DA receptor agonists.
E) Effect of 1 μM DA is blocked by sulpiride (DA1+Sul) but not SCH23390 (DA1+SCH). 1 μM and 100 μM DA data in E and F are also shown in Fig. 2E.
F) Effect of 100 μM DA is reversed by SCH23390 (DA100+SCH) and blocked by sulpiride (DA100+Sul).
*:p<0.05, **:p<0.005, ***:p<0.0005, ns: not significant vs. baseline.
†:p<0.05, ††:p<0.005 between groups.
Because Quin mimicked the decrease in mIPSC frequency induced by low concentrations of DA, D2Rs may be responsible for the inhibitory effect. Thus, 1 μM DA was tested in the presence of the D2R antagonist sulpiride. As shown in Fig. 3E, sulpiride (10 μM) abolished the effect of DA (n=4, p=0.0403 vs. 1 μM DA, one-way ANOVA). In contrast, the D1R antagonist SCH23390 (SCH, 10 μM) did not block the DA effect (n=5, p=0.4375 vs. 1 μM DA, one-way ANOVA), suggesting that the inhibition is mediated by D2Rs.
A high concentration of DA (100 μM) was also tested in the presence of DA receptor antagonists. We found that the facilitating effect of 100 μM DA was abolished by sulpiride (10 μM) (n=5, p=0.0052 vs. 100 μM DA, one-way ANOVA; Fig. 3F), while reversed to a reduction in mIPSCs by 10 μM SCH (n=5, p=0.0013 vs. 100 μM DA, one-way ANOVA; Fig. 3F). Taken together with the co-application of DA receptor agonists (SKF+Quin), which has an effect similar to that of 100 μM DA, it appears that the facilitation of mIPSC frequency surprisingly requires simultaneous activation of D1Rs and D2Rs.
Although a change in mIPSC frequency is indicative of presynaptic modulation, there may also be postsynaptic effects. To investigate this, GDPβS (2 mM) was included in the recording pipette to block G-protein signaling in the postsynaptic cell under investigation. We have shown previously that this concentration of GDPβS completely blocks G-protein coupled nociceptin receptor signaling in orexin neurons (Parsons et al 2012). Even in the presence of GDPβS, Quin (50 μM) decreased mIPSC frequency as it did in the absence of GDPβS (n=5, p=0.9489 vs. Quin; Fig. 4A). Likewise, SKF+Quin (10 μM each) induced an increase in mIPSC frequency in the presence of GDPβS (n=5, p=0.3283 vs. SKF+Quin; Fig. 4A). This agrees with a lack of DA effect on mIPSC amplitude (Fig. 2F2,G2 and Fig. 4B; 1 μM DA, n=7, p=0.4524; 100 μM DA, n=5, p=0.4252; all comparisons vs. baseline, paired t-test). Furthermore, DA receptor agonists failed to alter mIPSC amplitude ((Quin 10–50 μM, n=7, p=0.4376; SKF+Quin 10 μM each, n=7, p=0.4881; all comparisons vs. baseline, paired t-test; Fig. 4B). Therefore, GABAergic transmission to orexin neuron is modulated by presynaptic DA receptors.
Figure 4. Effect of DA is presynaptic.
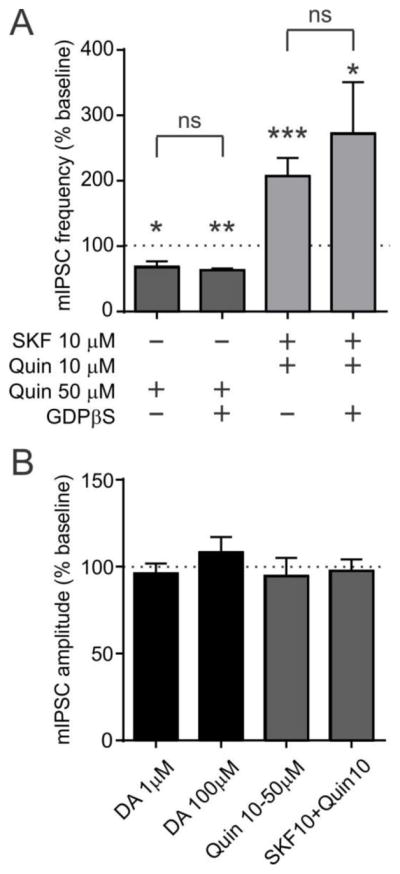
A) Effect of SKF and Quin in the presence or absence of GDPβS in the recording pipette. Agonist data in Fig. 3D is shown as a comparison.
*:p<0.05, **:p<0.005, ***:p<0.0005 vs. baseline frequency.
ns: not significant between groups.
B) DA or DA agonists have no effect on mIPSC amplitude.
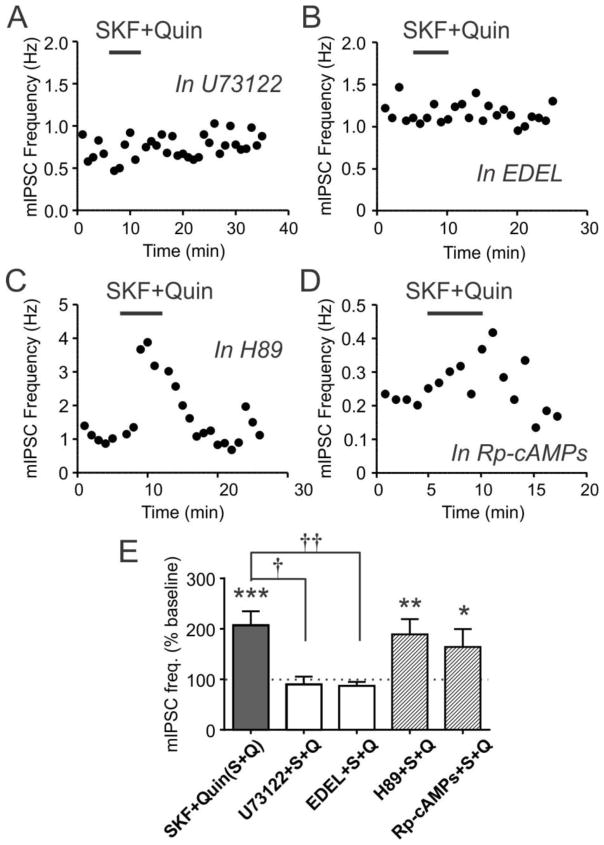
Since the facilitation of mIPSCs with high DA concentrations requires co-activation of D1Rs and D2Rs, we determined whether this involved unconventional signaling. Typically, D1Rs and D2Rs regulate adenylyl cyclase activity via Gs and Gi/o, respectively (Beaulieu and Gainetdinov 2011). However, these receptors can together activate the Gq-phospholipase C (PLC) pathway (Beaulieu and Gainetdinov 2011;Hasbi et al 2010). To test this, we used the PLC inhibitors U73122 and edelfosine. In brain slices incubated with U73122 (10 μM), the effect of SKF+Quin (10 μM each) was abolished (n=6; p=0.0104 vs. SKF+Quin without inhibitor; Fig. 5A,E). Similarly, edelfosine (10 μM) blocked the SKF+Quin effect (n=6; p=0.0084 vs. SKF+Quin without inhibitor; Fig. 5B,E). The fact that the two structurally-unrelated inhibitors have the same effect strongly suggests that PLC is implicated. In contrast, treating slices with the protein kinase A (PKA) inhibitor H89 (10 μM) did not prevent SKF+Quin from inducing a significant increase in mIPSC frequency (n=5; p=0.9684 vs. SKF+Quin without inhibitor; Fig. 5C,E). Similarly, Rp-cAMPs (100 μM), which inhibits cAMP-dependent activation of PKA, also failed to prevent SKF+Quin from increasing mIPSC frequency (n=5; p=0.6236 vs. SKF+Quin without inhibitor; Fig. 5D,E). These results suggest that co-activation of D1Rs and D2Rs activates the PLC pathway, but not the conventional PKA pathway, to facilitate GABAergic transmission to orexin neurons.
Figure 5. D1–D2 receptor co-signaling is mediated by phospholipase C.
A and B) Time-effect plot of SKF+Quin co-application in the presence of PLC inhibitors U73122 and edelfosine (EDEL).
C and D) Effect of SKF+Quin co-application in the presence of PKA inhibitors H89 and Rp-cAMPs.
E) Summary graph showing the effect of SKF+Quin in PLC and PKA inhibitors. SKF+Quin data in Fig. 3D is shown as a comparison.
*:p<0.05, **:p<0.01, ***:p<0.0005 vs. baseline frequency.
†:p<0.05, ††:p<0.01 between groups.
Discussion
The present study demonstrates that DA induces bidirectional effects on GABA release from inhibitory terminals synapsing onto orexin neurons. The effects are reversible and concentration-dependent. Low concentrations of DA decrease mIPSC frequency, which is blocked or mimicked by D2 receptor antagonist and agonist respectively, suggesting that DA acts via D2Rs. In contrast, high concentrations of DA increase mIPSC frequency. This may partially account for the inhibition of orexin neurons by high DA concentrations (20 – 300 μM) as shown previously (Li and van Den Pol 2005;Yamanaka et al 2006). In all, the majority of orexin neurons tested responded to low concentrations of DA (0.1 or 1 μM) with reduced mIPSC or spontaneous IPSC frequency (18 of 20 cells, 90%), whereas 100 % of cells tested (11 of 11 cells) responded to a high concentration of DA (100 μM) with a consistent increase in these parameters. Therefore, these bidirectional synaptic responses to DA appear to be a common property of orexin neurons,
The D2R antagonist sulpiride blocks the increase in mIPSCs induced by a high concentration of DA whereas the D1R antagonist SCH reverses the increase to a reduction. Therefore, it is likely that the increase is mediated by both D1Rs and D2Rs. In the presence of SCH, DA activates only D2Rs, which decrease mIPSC frequency. In contrast, in the presence of sulpiride, DA only activates D1Rs, inducing no effect. This lack of D1R effect on IPSCs in orexin neurons is in contrast with EPSCs where D1Rs facilitate transmission (Rao et al 2008), suggesting that DA regulates inhibitory and excitatory inputs to orexin neurons by different mechanisms.
We found no evidence for a postsynaptic locus of DA action that modulates mIPSCs in orexin neurons. Blocking G-protein signaling in the postsynaptic neuron with GDPβS did not block the effects of DA on mIPSCs. Thus, both D1Rs and D2Rs appear to be functionally expressed on presynaptic inhibitory terminals. Some GABA terminals may only express D2Rs and are thus suppressed by DA; however, at least some terminals should co-express D1Rs and D2Rs for co-signaling to occur. At these synapses, bidirectional control of GABA release by DA may be possible. The dose-dependency of bidirectional DA effects could be due to the proportion of D1Rs and D2Rs in states of high or low affinity for DA (Dunnett et al 2005). Specifically, at GABAergic synapses to orexin neurons, D2Rs appear to have higher affinity for DA than D1Rs, so that co-signaling is only observed at high DA concentrations when both receptors are activated.
Because the effects of DA were observed when action potentials were blocked by tetrodotoxin, it can be concluded that the effects are on the release process downstream or independent of action potential-driven Ca2+ entry. D2Rs are generally linked to the Gi/o protein that suppresses the adenylyl cyclase-cAMP-PKA pathway (Beaulieu and Gainetdinov 2011). Since PKA can facilitate transmitter release (Capogna et al 1995;Hirasawa and Pittman 2003), suppression of this pathway is a plausible mechanism by which D2Rs inhibit GABA release. On the other hand, our results suggest that PLC mediates D1R/D2R co-signaling to increase mIPSCs frequency. PLC could activate PKC and intracellular Ca2+ release (Beaulieu and Gainetdinov 2011) to increase GABA release onto orexin neurons. This unconventional PLC-mediated DA signaling has been described in other brain regions where DA receptor heteromers consisting of D1–D2 or D2–D5 subtypes signal through PLC and intracellular Ca2+ release (Hasbi et al 2010).
The origins of these DA-sensitive GABAergic inputs to orexin neurons are unknown; however, possible candidates exist both within the hypothalamus and elsewhere. Within the LH, MCH neurons and leptin receptor-expressing neurons co-express GABA and make direct contact with orexin neurons (Elias et al 2008;Guan et al 2002;Louis et al 2010). A subpopulation of neuropeptide Y and proopiomelanocortin neurons in the arcuate nucleus also express GABA (Hentges et al 2009;Horvath et al 1997), some of which may express leptin receptors and innervate the LH (Elias et al 1998). GABA neurons in the preoptic area also innervate orexin neurons (Sakurai et al 2005). Extrahypothalamic origins of GABAergic inputs to orexin neurons include the central amygdaloid nucleus (Nakamura et al 2009), reticular thalamus (Barone et al 1994) and basal forebrain (Henny and Jones 2006). Any of these GABAergic inputs to orexin neurons are potentially modulated by DA and could serve as mediators for the interaction between energy homeostasis, arousal and reward systems.
GABA and DA can signal satiety in the LH, as GABAA and DA agonists attenuate feeding when injected into this area (Stanley et al 2011;Yang et al 1997). It is possible that DA promotes satiety as its concentration rises in the LH, partially by facilitating GABAergic transmission to orexin neurons through the mechanism described in this study. Our results suggest that the direction of dopaminergic influence on orexin neurons is dependent on the level of DA release in the hypothalamus. At low levels, DA disinhibits orexin neurons from GABA, which in turn may further stimulate VTA DA neurons. In contrast, facilitation of GABA release by high levels of DA in the LH may act as negative feedback to prevent overexcitation of orexin and DA neurons. Such bidirectional control of orexin neurons, achieved by DA receptor interactions and unconventional signaling, may be physiologically significant for the control of feeding and reward.
Acknowledgments
This study was funded by the Canadian Institutes of Health Research (CIHR), Research and Development Corporation and Natural Science and Engineering Research Council (NSERC). VL is a recipient of CIHR/RDC Scholarship. We thank Mr. Christian Alberto for technical assistance. Authors have no competing interests to declare.
Abbreviations
- AHP
afterhyperpolarizing potential
- DA
dopamine
- D1R
D1-like receptor
- D2R
D2-like receptor
- GABA
gamma-aminobutyric acid
- GDPβS
guanosine 5′-[β-thio]diphosphate
- MCH
melanin concentrating hormone
- mIPSC
miniature inhibitory postsynaptic current
- PKA
protein kinase A
- PLC
phospholipase C
- Quin
quinpirole
- SCH
SCH23390
- SKF
Reference List
- 1.Alberto CO, Trask RB, Hirasawa M. Dopamine acts as a partial agonist for alpha2A adrenoceptor in melanin-concentrating hormone neurons. J Neurosci. 2011;31:10671–10676. doi: 10.1523/JNEUROSCI.6245-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barone FC, Cheng JT, Wayner MJ. GABA inhibition of lateral hypothalamic neurons: role of reticular thalamic afferents. Brain Res Bull. 1994;33:699–708. doi: 10.1016/0361-9230(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu JM, Gainetdinov RR. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacological Reviews. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 4.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capogna M, Gahwiler BH, Thompson SM. Presynaptic enhancement of inhibitory synaptic transmission by protein kinases A and C in the rat hippocampus in vitro. J Neurosci. 1995;15:1249–1260. doi: 10.1523/JNEUROSCI.15-02-01249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YW, Morganstern I, Barson JR, Hoebel BG, Leibowitz SF. Differential Role of D1 and D2 Receptors in the Perifornical Lateral Hypothalamus in Controlling Ethanol Drinking and Food Intake: Possible Interaction with Local Orexin Neurons. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- 9.Dunnett SB, Bentivoglio M, Bjorklundm A, Hokfelt T. Handbook of Chemical Neuroanatomy, Dopamine 2005 [Google Scholar]
- 10.Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, Jones BE, Muhlethaler M. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23:1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 12.Elias CF, Sita LV, Zambon BK, Oliveira ER, Vasconcelos LA, Bittencourt JC. Melanin-concentrating hormone projections to areas involved in somatomotor responses. J Chem Neuroanat. 2008;35:188–201. doi: 10.1016/j.jchemneu.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan JL, Uehara K, Lu S, Wang QP, Funahashi H, Sakurai T, Yanagizawa M, Shioda S. Reciprocal synaptic relationships between orexin- and melanin-concentrating hormone-containing neurons in the rat lateral hypothalamus: a novel circuit implicated in feeding regulation. Int J Obes Relat Metab Disord. 2002;26:1523–1532. doi: 10.1038/sj.ijo.0802155. [DOI] [PubMed] [Google Scholar]
- 15.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 16.Hasbi A, O’Dowd BF, George SR. Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Current Opinion in Pharmacology. 2010;10:93–99. doi: 10.1016/j.coph.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henny P, Jones BE. Innervation of orexin/hypocretin neurons by GABAergic, glutamatergic or cholinergic basal forebrain terminals evidenced by immunostaining for presynaptic vesicular transporter and postsynaptic scaffolding proteins. J Comp Neurol. 2006;499:645–661. doi: 10.1002/cne.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29:13684–13690. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirasawa M, Pittman QJ. Nifedipine facilitates neurotransmitter release independently of calcium channels. Proc Natl Acad Sci USA. 2003;100:6139–6144. doi: 10.1073/pnas.0936131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- 21.Kawano H, Honma S, Honma A, Horie M, Kawano Y, Hayashi S. Melanin-concentrating hormone neuron system: the wide web that controls the feeding. Anato Sci Int. 2002;77:149–160. doi: 10.1046/j.0022-7722.2002.00027.x. [DOI] [PubMed] [Google Scholar]
- 22.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12:638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 23.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leibowitz SF, Shor-Posner G, Maclow C, Grinker JA. Amphetamine: effects on meal patterns and macronutrient selection. Brain Res Bull. 1986;17:681–689. doi: 10.1016/0361-9230(86)90200-5. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, van Den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons. J Neurosci. 2005;25:173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis GW, Leinninger GM, Rhodes CJ, Myers MG., Jr Direct Innervation and Modulation of Orexin Neurons by Lateral Hypothalamic LepRb Neurons. J Neurosci. 2010;30:11278–11287. doi: 10.1523/JNEUROSCI.1340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meguid MM, Yang ZJ, Koseki M. Eating induced rise in LHA-dopamine correlates with meal size in normal and bulbectomized rats. Brain Res Bull. 1995;36:487–490. doi: 10.1016/0361-9230(95)92128-3. [DOI] [PubMed] [Google Scholar]
- 28.Morutto SL, Phillips GD. Interactions between sulpiride infusions within the perifornical region of the lateral hypothalamus and the nucleus accumbens on measures of locomotor activity and conditioned place preference. Behav Pharmacol. 1998;9:345–355. [PubMed] [Google Scholar]
- 29.Nakamura S, Tsumori T, Yokota S, Oka T, Yasui Y. Amygdaloid axons innervate melanin-concentrating hormone- and orexin-containing neurons in the mouse lateral hypothalamus. Brain Res. 2009;1278:66–74. doi: 10.1016/j.brainres.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 30.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parada MA, Puig dP, Hoebel BG. Rats self-inject a dopamine antagonist in the lateral hypothalamus where it acts to increase extracellular dopamine in the nucleus accumbens. Pharmacol Biochem Behav. 1995;52:179–187. doi: 10.1016/0091-3057(95)00086-c. [DOI] [PubMed] [Google Scholar]
- 32.Parsons MP, Burt J, Cranford A, Alberto C, Zipperlen K, Hirasawa M. Nociceptin induces hypophagia in the perifornical and lateral hypothalamic area. PLoS One. 2012;7:e45350. doi: 10.1371/journal.pone.0045350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; 2005. [Google Scholar]
- 34.Perreault ML, Hasbi A, O’Dowd BF, George SR. Heteromeric Dopamine Receptor Signaling Complexes: Emerging Neurobiology and Disease Relevance. Neuropsychopharmacology. 2014;39:156–168. doi: 10.1038/npp.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao Y, Lu M, Ge F, Marsh DJ, Qian S, Wang AH, Picciotto MR, Gao XB. Regulation of Synaptic Efficacy in Hypocretin/Orexin-Containing Neurons by Melanin Concentrating Hormone in the Lateral Hypothalamus. J Neurosci. 2008;28:9101–9110. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 37.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Stanley BG, Urstadt KR, Charles JR, Kee T. Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake. Physiology & Behavior. 2011;104:40–46. doi: 10.1016/j.physbeh.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 39.Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl) 2005;182:75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- 40.Yamanaka A, Muraki Y, Ichiki K, Tsujino N, Kilduff TS, Goto K, Sakurai T. Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J Neurophysiol. 2006;96:284–298. doi: 10.1152/jn.01361.2005. [DOI] [PubMed] [Google Scholar]
- 41.Yang ZJ, Meguid MM, Chai JK, Chen C, Oler A. Bilateral hypothalamic dopamine infusion in male Zucker rat suppresses feeding due to reduced meal size. Pharmacol Biochem Behav. 1997;58:631–635. doi: 10.1016/s0091-3057(97)00022-1. [DOI] [PubMed] [Google Scholar]